Abstract
Interleukin-1 receptor antagonist (IL1ra) has demonstrated efficacy in a wide range of animal models of neuronal injury. We have previously published a randomised controlled study of IL1ra in human severe TBI, with concomitant microdialysis and plasma sampling of 42 cytokines and chemokines. In this study, we have used partial least squares discriminant analysis to model the effects of drug administration and time following injury on the cytokine milieu within the injured brain. We demonstrate that treatment with rhIL1ra causes a brain-specific modification of the cytokine and chemokine response to injury, particularly in samples from the first 48 h following injury. The magnitude of this response is dependent on the concentration of IL1ra achieved in the brain extracellular space. Chemokines related to recruitment of macrophages from the plasma compartment (MCP-1) and biasing towards a M1 microglial phenotype (GM-CSF, IL1) are increased in patient samples in the rhIL1ra-treated patients. In control patients, cytokines and chemokines biased to a M2 microglia phenotype (IL4, IL10, MDC) are relatively increased. This pattern of response suggests that a simple classification of IL1ra as an ‘anti-inflammatory’ cytokine may not be appropriate and highlights the importance of the microglial response to injury.
Keywords: Chemokines, brain trauma, microglia, inflammation, neurochemistry
Introduction
Traumatic brain injury (TBI) is a complex disease with several related pathological mechanisms. The role of both acute and chronic inflammation as potential drivers of tissue damage and subsequent repair is increasingly recognised.1 The role of microglia in this process, both as a source of inflammatory cytokines and chemokines, as well as targets and effectors of inflammation is an important area of active research in the pre-clinical setting.2 Several methods are available for exploring the role of microglia in human disease in post-mortem tissue including mRNA expression, microglial morphology and immunohistochemistry.3 This has led to the concept of a spectrum of microglial activity such that the same cell can take on a range of phenotypes with distinct functions based on the inflammatory milieu in which it resides.4 M1, or ‘classically activated’ macrophages, are stimulated by IFNg and TNF and produce, TNF, IL6 and IL23. They are thought to potentiate inflammation and are traditionally thought of as damaging to tissue. M2, or alternatively acting macrophages, are further subdivided into M2a, ‘tissue repair’, and M2b, ‘regulatory’, subtypes. M2a macrophages are stimulated by IL4 and IL13. M2b macrophages are stimulated by IL10 and produce both IL10 and TGF. Furthermore, this biasing of microglial activation may be a dynamic process and the balance between microglial subtypes may be a determinant of tissue outcome.5 In vivo, in human studies, methods for determining the underlying phenotype of microglia are limited. Positron Emission Tomography (PET) with the radioligand11C-(R)-PK11195, which binds the peripheral benzodiazepine receptor (also known as the mitochondrial 18 kDa translocator protein or TSPO), has been used as a marker of microglial activation within the brain, but this does not distinguish between phenotypic subtypes.6
One approach to uncovering the relative bias between macrophage subtypes is to measure the neurochemical milieu within the brain and attempt to characterise the cytokines and chemokines that influence microglial phenotype or are produced by specific subtypes. We have previously described a cerebral microdialysis method for measuring a wide range of cytokines and chemokines within the human brain following trauma, which demonstrated a stereotyped production of mediators following injury.7 In observational studies, it is not possible to attribute causation to the production of any given mediator. To this end, we have previously described a randomised phase II study of recombinant human interleukin-1 receptor antagonist (rhIL1ra), combined with cerebral microdialysis and plasma sampling to measure 42 cytokines and chemokines in both brain and blood.8 rhIL1ra is a putative neuroprotective agent that has been demonstrated to attenuate neuronal injury a wide variety of animal models including trauma.9–12 Our previous study demonstrated safety, plasma and brain penetration of rhIL1ra following subcutaneous administration. Principal Component Analysis of the original dataset demonstrated a different pattern of cytokines and chemokines between the control and intervention groups.8
In the present study, we have used more advanced multivariate projection techniques to specifically address the question as to the downstream effects of inhibition of the interleukin-1 receptor following TBI in humans. Partial Least Squares Discriminant Analysis (PLS-DA) has been used to determine the cytokines and chemokines that differ between the control and rhIL1ra administered patient groups. This method specifically identifies the cytokines and chemokines that differ between the control and rhIL1ra groups to gain further insights into the effects of rhIL1ra on microglial recruitment and phenotypes.13
Patients and methods
Patients and sampling
Our previous publication provides a detailed description of the clinical aspects of this dataset.8 Twenty patients with severe diffuse brain injury were recruited (10 males, 10 females) with an age range of 18–61 years. The protocol was approved by the ‘Cambridgeshire (2) Local Research Ethics Committee’ (06/Q0108/64) and by the appropriate regulatory authorities. Assent was taken from patient’s next of kin in accordance with ethical approvals. In brief, the study recruited patients with severe diffuse traumatic brain injury within the first 24 h of injury. Patients were randomly allocated to control or intervention groups. In the intervention arm patients were given 100 mg subcutaneous rhIL1ra once daily for five days. In the control arm, no drug was given. Patients were monitored using cerebral microdialysis using a CMA71, 100 kDa molecular weight cut-off catheter (M Dialysis AB, Stockholm, Sweden), via a cranial access device (Technicam, Newton Abbot, UK), perfused at 0.3 µl/min using a CMA106 pump (M Dialysis AB). The perfusate consisted of 3.5% Human Albumin Solution made up in CNS perfusion fluid (M Dialysis AB), formulated by Ipswich Hospital NHS Trust Pharmacy Manufacturing Unit (Ipswich, UK). Plasma samples were taken concurrently at 1 h before and after the drug administration. Plasma samples were also taken from patients in the control arm at the equivalent time following injury.
Cytokine analysis
All samples were analysed using the Milliplex Multi-Analyte Profiling Human Cytokine/Chemokine 42 analyte premixed kit (Millipore, St Charles, MI, USA) using the manufacturer’s instructions as described previously.7 The 42 cytokines and chemokines assayed are detailed in Table 1. Owing to the ultra-low flow rates inherent in microdialysis, to allow sufficient volume for the assay, microdialysates from a 6-h time period were pooled immediately before analysis. Plasma samples had sufficient volume for analysis without dilution or pooling. All samples were assayed in duplicate wells (25 µL per well) and the mean of the ensuing results was used. The plates were read using a Luminex 200 analyser (Luminex Corporation, Austin, TX, USA) running STarStation software (Applied Cytometry Systems, Sheffield, UK). Cytokine concentrations were calculated by reference to an eight-point five-parameter logistic standard curve for each cytokine. Microdialysate and plasma samples were run on separate plates as the buffers and background (control) wells for these samples differ within the assay protocol.
Table 1.
Cytokines and chemokines employed in PLS-DA model.
| Cytokines and chemokines | Abbreviation | Microdialysate model | Plasma model |
|---|---|---|---|
| Epidermal growth factor | EGF | ✓ | |
| Eotaxin | Eotaxin | ✓ | ✓ |
| Basic fibroblast growth factor | FGF-2 | ✓ | ✓ |
| Fms-related tyrosine kinase 3 ligand | FLT-3 ligand | ✓ | |
| Fractalkine/ CX3CL | Fractalkine | ✓ | ✓ |
| Granulocyte colony stimulating factor | G-CSF | ✓ | ✓ |
| Granulocyte-monocyte colony stimulating factor | GM-CSF | ✓ | ✓ |
| GRO/CXCL3 | GRO | ✓ | ✓ |
| Interferon alpha-2 | IFNa2 | ✓ | ✓ |
| Interferon gamma | IFNg | ✓ | ✓ |
| Interleukin-1 alpha | IL-1a | ✓ | ✓ |
| Interleukin-1 beta | IL-1b | ✓ | ✓ |
| Interleukin-2 | IL-2 | ✓ | |
| Interleukin-3 | IL-3 | ||
| Interleukin-4 | IL-4 | ✓ | |
| Interleukin-5 | IL-5 | ✓ | |
| Interleukin-6 | IL-6 | ✓ | ✓ |
| Interleukin-7 | IL-7 | ✓ | ✓ |
| Interleukin-8 | IL-8 | ✓ | ✓ |
| Interleukin-9 | IL-9 | ✓ | |
| Interleukin-10 | IL-10 | ✓ | ✓ |
| Interleukin 12 subunit beta | IL-12 p40 | ✓ | ✓ |
| Interleukin-12 | IL-12 p70 | ✓ | ✓ |
| Interleukin-13 | IL-13 | ✓ | |
| Interleukin-15 | IL-15 | ✓ | ✓ |
| Interleukin-17 | IL-17 | ✓ | |
| Chemokine (C-X-C motif) ligand 10 | IP-10 | ✓ | ✓ |
| Monocyte Chemotactic Protein 1 | MCP-1 | ✓ | ✓ |
| Monocyte Chemotactic Protein3 | MCP-3 | ✓ | ✓ |
| Macrophage Derived Chemoattractant | MDC | ✓ | ✓ |
| Macrophage Inflammatory Protein-1alpha | MIP-1a | ✓ | ✓ |
| Macrophage Inflammatory Protein-1beta | MIP-1b | ✓ | ✓ |
| Platelet Derived Growth Factor AA | PDGF AA | ✓ | ✓ |
| Platelet Derived Growth Factor AB/BB | PDGF ABBB | ✓ | ✓ |
| RANTES | RANTES | ✓ | ✓ |
| Soluble CD40 Ligand | sCD40L | ✓ | ✓ |
| Soluble Interleuking-2 Receptor | sIL-2Ra | ✓ | ✓ |
| Transforming Growth Factor alpha | TGFa | ✓ | ✓ |
| Tumour Necrosis Factor alpha | TNFa | ✓ | ✓ |
| Tumour Necrosis Factor beta | TNFb | ||
| Vascular Endothelial Growth Factor | VEGF | ✓ | ✓ |
Partial least squares discriminant analysis of cerebral and plasma cytokines
Statistical analyses were carried out using the SPSS20 (SPSS, Chicago, IL, USA) for Windows and SIMCA 13.0 (Umetrics AB, Sweden) for Windows. We have published our detailed method for the analysis of high dimensional microdialysis and plasma datasets using multivariate projection methods.13 We have previously presented an unsupervised model of these data, using Principal Component Analysis (PCA), to demonstrate that there is a separation between control and intervention groups without any modification of the dataset.8 All variables were mean centred and scaled to unit variance before calculation of the model. This ensures that a given variable does not contribute excessively to the model simply because of a high absolute measured concentration.
In order to specifically provide an assessment of the downstream effects of rhIL1ra on other cytokines and chemokines, we refined our model in several ways in order to extract the most useful biological information from the dataset. First, we employed two supervising variables. PLS-DA is a regression extension of PCA in which additional supervising (Y) variables are used to identify sources of variation in a pre-specified domain.14 We have previously demonstrated that there is a stereotyped temporal sequence of cytokine and chemokine expression following TBI,7 and the downstream consequences of rhIL1ra are necessarily superimposed on this background response to trauma, and therefore we incorporated Time as a supervising variable. The Randomisation (Control = 0, Intervention = 1) group was used as a second supervising variable to specifically explore the effects of rhIL1ra.
Second, a small proportion of cytokines and chemokines appear at a concentration close to the limit of sensitivity of the assay used. In this case, several missing values appear across the range of samples and lead to spurious variation within the dataset. To avoid skewing the model as a result of these values, any cytokine or chemokine that had more than 50% missing values across all observations was excluded. The excluded cytokines and chemokines differed between the cerebral and plasma datasets (Table 1).
Third, as microdialysate- and plasma-derived IL1ra concentration differs between the two groups, we excluded IL1ra data from this analysis to avoid biasing our model. Thus, the principal components derived are not affected by rhIL1ra and reflect the downstream consequences of its administration.
Separate PLS-DA models were derived based on these parameters for the plasma and cerebral cytokine and chemokine datasets using the same method.
Concentration changes in individual cytokines and chemokines
The key advantage of utilising the PLS-DA technique is that it incorporates modest changes in cytokine and chemokine levels across the range of measured mediators and provides a composite measure of the response to IL1ra administration. We have also provided z-scores for the change in mean cytokine and chemokine concentrations across the two patient groups in Table 2. These are calculated as

Z-scores are provided for each time window analysed in the PLS-DA model.
Table 2.
Z-scores for change in mean microdialysis cytokine concentration between control and intervention groups.
| Cytokines and chemokines | Time following injury (hours) |
|||
|---|---|---|---|---|
| 0–48 | 48–96 | 96–144 | 144–192 | |
| Eotaxin | 0.81 | 0.11 | −0.33 | −0.50 |
| FGF-2 | 1.21a | −0.76 | −0.35 | −0.86 |
| FLT-3 ligand | −0.15 | −0.31 | −0.46 | −0.57 |
| Fractalkine | 1.65a | 0.43 | −0.38 | −0.35 |
| G-CSF | −0.10 | −0.38 | −0.68 | −0.73 |
| GM-CSF | 1.26a | 0.26 | −0.18 | −0.10 |
| GRO | −0.06 | −0.35 | −0.32 | −0.58 |
| IFNa2 | 0.98 | 0.10 | −0.37 | −0.36 |
| IFNg | −0.08 | −0.68 | −1.04c | −1.65! |
| IL-1a | 0.24 | 0.28 | −0.49 | −0.43 |
| IL-1b | 0.13 | −0.20 | −0.42 | −7.24 |
| IL-1ra | 2.37b | 1.47a | 2.93b | 7.43b |
| IL-4 | −0.74 | −0.79 | −0.72 | −0.61 |
| IL-6 | 0.07 | −0.27 | −0.33 | −0.38 |
| IL-7 | 2.94b | 2.25b | 0.40 | 1.35a |
| IL-8 | −0.28 | −0.31 | −0.43 | −0.49 |
| IL-9 | 1.54a | −1.78c | −1.70c | −2.68d |
| IL-10 | −0.21 | −0.43 | −0.43 | −0.36 |
| IL-12 p40 | −0.73 | −0.86 | −1.22c | −0.81 |
| IL-12 p70 | −0.35 | −0.71 | −0.57 | −0.46 |
| IL-13 | 0.75 | −0.06 | −0.23 | −0.52 |
| IL-15 | −0.39 | −0.36 | −0.61 | −0.64 |
| IP-10 | −0.19 | −0.15 | −0.47 | −0.33 |
| MCP-1 | 0.99 | 0.44 | −0.17 | 0.74 |
| MCP-3 | −0.16 | −0.19 | −0.64 | −0.56 |
| MDC | −0.89 | −0.71 | −0.78 | −0.77 |
| MIP-1a | 0.93 | −0.20 | −0.41 | −0.59 |
| MIP-1b | 1.49a | −0.16 | −0.54 | −0.50 |
| PDGF AA | 0.77 | 0.88 | −0.08 | 0.11 |
| PDGF ABBB | 0.38 | 0.61 | −0.20 | 0.41 |
| RANTES | 0.52 | 0.15 | −0.35 | −0.47 |
| sCD40L | −0.84 | −0.76 | −1.05c | −0.74 |
| sIL-2Ra | −0.90 | −1.18c | −1.37c | −1.23c |
| TGFa | 0.55 | −0.06 | −0.25 | 0.21 |
| TNFa | 1.05a | −0.21 | −0.18 | −0.33 |
| VEGF | 0.52 | −0.22 | −0.33 | −0.44 |
Note: Z-scores were calculated (as described in Patients and methods section) for the 35 cytokines and chemokines that were incorporated into the PLS-DA model (Table 3) as well as for IL1ra.
z-scores between 1 and 2.
z-scores greater than 2.
z-scores between −1 and −2.
z-scores lower than −2.
Area under receiver operating characteristic curve for predictive power of PLS-DA model
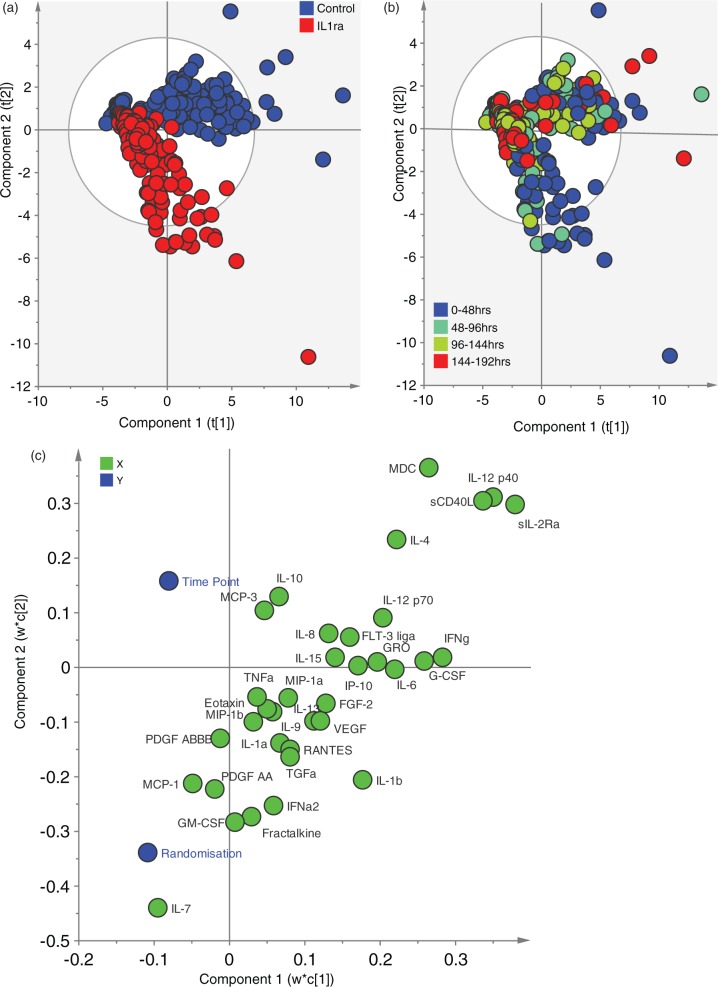
In order to provide an objective measure of the accuracy of the PLS-DA model in predicting the randomisation group, the first 2 PCs illustrated in Figure 1(a) were used to calculate a receiver operating characteristic (ROC) curve (1-specificity vs sensitivity) (Figure 4).
Figure 1.
Partial least squares discriminant analysis of microdialysis data. The model is supervised by two variables: time and randomisation. IL1ra data were excluded to avoid skewing the model and any cytokine/chemokine with more than 50% missing values was also excluded, leaving 35 variables in total. Table 1 lists the cytokines and chemokines in full. (a) Scores plot coloured by randomisation group. The scores plot demonstrates a separation between the two groups of data points. It is apparent that principal component 2 is responsible for the separation between control and IL1ra treated patients with a greater negative score on this axis responsible for a greater ‘IL1ra treated’ phenotype. (b) Scores plot coloured by time of observation. The same scores plot as in (a) has been re-coloured by time point from which the observation arose. This demonstrates that those observations with the greatest negative loading on PC2 are most likely to come from the earliest time points following injury (0–48 h). (c) Loading Bi-plot. This plot shows both the supervising (Y) and observed (X) variables on the same plot. Each cytokine and chemokine loading provided a coefficient by which the scaled variable is multiplied, and the sum of these scores provides the value plotted in the scores plot. In this way, it can be inferred that cytokines/chemokines with a positive loading on PC2 are responsible for the ‘control’ phenotype apparent in (a), while cytokines/chemokines with a negative loading on PC2 are responsible for a ‘IL1ra treated’ phenotype in Figure 1(a).
Figure 4.

Receive operating characteristic curve derived from first two principal components of microdialysate PLS-DA model. The ROC curve plots 1-specificity vs sensitivity, utilising the first two PCs illustrated in Figure 1 in order to predict the randomisation group from which the observation is derived. The AUC = 95%, i.e. excellent accuracy.
Comparison of patient cerebral IL1ra concentration based on median split of principal component scores
In order to investigate whether the concentration of IL1ra in the brain microdialysates could be responsible for the separation between observations seen in PC2 in the PLS-DA model, we identified the time point with the greatest separation between control and intervention patients, namely the first 48-h pool, consistent with the early IL1 beta response to TBI.7 We therefore carried out a median split of the patients in the intervention group based on their PC2 score. We then compared the concentration of IL1ra achieved within the brain between the two groups of patients that showed the least and greatest response on PC2. The two groups are compared using the Mann–Whitney U-test.
Results
The clinical aspects of this study, including full details of age, demographics and plasma and microdialysate concentrations of all cytokines and chemokines assayed are as published.8 In brief, the mean ages of patients in each group were 41.8 years (control) and 36.0 years (intervention group). The concentrations of IL1ra achieved (control vs intervention) were 67 pg/ml vs 243 ng/ml in plasma, and 27.6 pg/ml vs 123.6 pg/ml in microdialysate 6 h following dosage of rhIL1ra.
rhIL1ra promotes cytokines and chemokines related to macrophage recruitment and M1 activation
The PLS-DA derived model identified eight principal components (Table 3). The first two components explained 44.2% of the variation in the measured cytokines and chemokines (X-block). Colouring the observations by the patient’s randomisation group clearly demonstrates a subset of observations that discriminate between the two groups (Figure 1(a)). The latent variable, PC2, is responsible for this separation between control and intervention patients’ observations. Inspection of the loading plot (Figure 1(c)) for this principal component reveals the cytokines and chemokines that make up this latent variable. The loading is the coefficient by which the scaled variable is multiplied by to calculate the PC2 score. Thus, cytokines and chemokines with a positive coefficient on PC2 push observations into a ‘control’ phenotype while those with a negative coefficient push observations into a ‘rhIL1ra treated’ phenotype.
Table 3.
Principal components generated within microdialysate PLS-DA model.
| Principal component | R2X | R2X Cumulative | R2Y | R2Y Cumulative | Q2 Cumulative |
|---|---|---|---|---|---|
| 1 | 34% | 34% | 7.9% | 8.9% | 7.3% |
| 2 | 10.1% | 44.2% | 22.5% | 30.4% | 30.1% |
| 3 | 5.5% | 49.7% | 12.5% | 42.9% | 41.5% |
| 4 | 4.7% | 54.3% | 3.9% | 46.8% | 43.2% |
| 5 | 5.9% | 60.3% | 2.5% | 49.3% | 44.1% |
| 6 | 5.2% | 65.5% | 1.8% | 51% | 43.8% |
| 7 | 3.1% | 68.6% | 2.1% | 53.1% | 45% |
| 8 | 2.2% | 70.8% | 1.2% | 54.3% | 43.5% |
Note: The table lists the outputs from the PLS-DA model for each component generated. R2X is the amount of variation explained in the X-block, i.e. the amount of variation in cytokine and chemokine data that is explained by the individual principal component. R2X cumulative is the cumulative amount of variation explained in the cytokine and chemokine data as additional principal components are added in. R2Y is the amount of variation explained in the Y-block, i.e. the amount of variation due to time and randomisation category. R2Y cumulative is the cumulative amount of variation due to time and randomisation as each principal component is added in. Q2 cumulative is a measure of the calibration of the model.
rhIL1ra modulates the cytokine and chemokine milieu within the first 48 h following injury
The observations can also be coloured by the time following injury (Figure 1(b)). This demonstrates that those observations that discriminate between the two groups to the greatest degree are those from the first 48 h following injury. Furthermore, there is an interaction between time and randomisation such that the rhIL1ra-treated group has observations that have a greater ‘early’ time point phenotype. The bi-plot (Figure 1(c)) demonstrates that time point and randomisation status appear at opposite ends of PC2, suggesting that increasing time point following injury is related to control patients.
rhIL1ra treatment has a distinct effect on the plasma cytokine and chemokine system
The PLS-DA model derived from the concordant plasma cytokine and chemokine data is shown in Figure 2. The first two components explain 16.4% of the variation in the X-block (Table 4), less than half as much of the variation explained in the microdialysate-derived model. Nevertheless, PC1 discriminates between control and intervention observations, as illustrated in the scores plot (Figure 2(a)). Figure 4 illustrates the ROC curve for the predictive power of the first 2PCs in predicting the group from which each observation came. The AUC = 95% confirms the excellent accuracy of this model. A qualitatively different set of cytokines and chemokines discriminate between control- and rhIL1ra-treated patient observations. Notably, many chemokines that are associated with macrophage recruitment such as MIP1a, MCP3, Fractalkine and GM-CSF are not found at higher plasma concentration in the rhIL1ra treated group, unlike in the microdialysis data PLS-DA model. However, MCP-1 and IL1β are found at higher concentration in plasma from patients treated with rhIL1ra, as in microdialysate.
Figure 2.

Partial least squares discriminant analysis of plasma data. The plasma data was treated in the same way as the microdialysis data, in that the model is supervised by two variables: time and randomisation. IL1ra data were excluded to avoid skewing the model and any cytokine/chemokine with more than 50% missing values was also excluded, leaving 35 variables in total. These differed slightly from those in the microdialysis derived data model. (a) Scores plot coloured by randomisation group. The scores plot demonstrates a separation between the control- and IL1ra-treated observations. In this model, PC1 is responsible for the separation between groups. (b) Loading Bi-plot. This plot shows both the supervising (Y) and observed (X) variables on the same plot. The loading plot demonstrates which cytokines and chemokines are responsible for the separation seen in Figure 2(a). Variables that load negatively on PC1 are responsible for pushing observations towards an IL1ra-treated phenotype while those that load positively push observations towards a control phenotype. There are qualitative differences between the loading plots in microdialysate and plasma data implying that there are cerebral-specific effects of IL1ra treatment.
Table 4.
Principal components generated within plasma PLS-DA Model.
| Principal component | R2X | R2X Cumulative | R2Y | R2Y Cumulative | Q2 Cumulative |
|---|---|---|---|---|---|
| 1 | 9.4% | 9.4% | 24.2% | 24.2% | 24% |
| 2 | 7.0 % | 16.4% | 7.4% | 31.6% | 25.8% |
| 3 | 19.1% | 35.5% | 2.5% | 34.1% | 28% |
| 4 | 6.4% | 41.9% | 3.3% | 37.4% | 29.4% |
| 5 | 3.4% | 45.3% | 3.0% | 40.4% | 29.4% |
| 6 | 6.4% | 51.6% | 1.4% | 41.8% | 29.4% |
Note: The table lists the outputs from the PLS-DA model for each component generated in the plasma. The notation is identical to that in Table 3.
The concentration of IL1ra achieved within the brain extracellular space relates to the degree of downstream effect
A median split between PC2 scores for these observations from the first 48 h splits the rhIL1ra-treated group of patients into two subsets, which have been termed ‘least responders’ for those with small negative PC2 scores and ‘greatest responders’ for those with higher negative PC2 scores. Figure 3 shows a box and whisker plot of the IL1ra concentrations measured within the brain in the two subsets of patients with least and greatest response, based on PC2. A Mann–Whitney U-test demonstrates a significant difference in the concentration of IL1ra in the brain, between observations with a greater or lesser PC2 score.
Figure 3.
Comparison of microdialysate IL1ra in IL1ra-treated patients’ observations from first 48 h: Median split by magnitude of PC2 score. The 10 patients in the IL1ra-treated group are divided into two groups (greatest responders and least responders) based on a median split based on PC2 score in the observations from the first 48 h following injury. The microdialysate IL1ra values in these two groups are plotted in the boxplot above and demonstrate a statistically significant difference in concentrations (Mann–Whitney U-test, p = 0.008).
Discussion
The data presented demonstrate the downstream effects of rhIL1ra administration on the cytokine and chemokine response to TBI, namely a dose-dependent biasing towards mediators related to the M1 microglial phenotype. Cytokines and chemokines act in complex cascades in pathologies such as TBI, such that univariate correlations between any given mediator and a clinical parameter can be misleading.13 In this study, we have adopted an approach that explores the patterns of response to TBI using multivariate projection methods and how this can be modified by a specific intervention. In this way, we have sought to identify the mediators that are responsible for the differences in patterns between the control and rhIL1ra treated patients, and put this into an appropriate context relating to the cellular response to injury.
Microdialysis data from TBI patients is intrinsically ‘noisy’ as a result of the inherent variability due to individual patient specific factors and catheter location. This makes it difficult to extract patterns of response from a complex dataset which incorporates several mediators of interest. Traditional statistical methods that rely on calculating a mean and standard deviation across a patient group (e.g. Student’s t-test, z-scores) are limited by a large variance in each group and it is therefore difficult to draw meaningful comparisons between treatment groups. Similarly, methods, such as pathway analysis, have not proven fruitful in the current dataset, although future studies that collect a larger dataset with more patients may allow analyses of this sort. We have previously discussed in detail the benefits of multivariate projection methods for data of this type.13 First, in a complex inter-related biological cascade, univariate correlations do not imply that the biomarker with greatest correlation with a given outcome is responsible for this outcome.15 For this reason, in our present small study of 20 patients, it would be premature to correlate patient related factors (such as Glasgow Outcome Score, which rates a patient’s functional capabilities) to individual cytokine or chemokine concentrations. Second, by exploring the sources of variation within the dataset, in each individual observation (single patient, single time point), the relative levels of all the cytokines and chemokines are related. For this reason, although the z-scores (whereby the mean for the rhIL1ra-treated group is standardised against the control group) in Table 2 are modest for each mediator, the latent variable identified in the PLS-DA model, which incorporates contributions from all the cytokines and chemokines in the dataset relates clearly to rhIL1ra administration (Figures 1(a), 3 and 4).
In order to focus on the specific role of antagonism at the IL1 receptor (IL1R) by rhIL1ra, we have sought to refine the data on which we base our PLS-DA model. We have pre-specified the randomisation category and time as supervising variables to focus on the sources of cytokine and chemokine variation that differ between observations in these supervising variables. As we have previously demonstrated that rhIL1ra passes into the blood and brain extracellular fluid (ECF),8 we sought to exclude IL1ra from the model, as this can potentially skew the model as it generates variation between the control and intervention observations that is due to drug administration rather than its downstream effects.
As the PLS-DA model specifically explores sources of variation within the multivariate dataset, we sought to exclude potential sources of spurious variation. One limitation of utilising antibody-based quantitative analyses such as that utilised in the Luminex™ assay, is defining a true ‘zero’ value. If an analysed species is close the lower limit of sensitivity of the assay, there is a tendency to ascribe a set ‘lowest’ value derived from the lowest value on the standard curve or designate the value as missing. Mathematically, this appears as a large source of variation within the dataset, when it is an idiosyncrasy of the analytical method. To avoid this effect, we have excluded any species with >50% of missing values from the model (Table 1). We accept that the 50% cut-off is an arbitrary one and may potentially exclude biologically significant mediators that happen to be close to the lower limit of quantitation; however, we feel this increases the robustness of the model and simplifies its interpretation.
The resulting PLS-DA models therefore encapsulate the sources of variation within the dataset that are attributable to the downstream effects of rhIL1ra treatment and time. Interpretation of the model relies on identifying the PC that discriminates between the relevant observations and inspecting the cytokines and chemokines that contribute to that PC. The polarity (positive or negative) of loadings on the bi-plot are interchangeable and are a consequence of the coding of the supervising variables and therefore interchangeable. Cytokines and chemokines that load at opposite ends (positive versus negative) of an axis are contributing to an observation’s score in opposite directions and the absolute magnitude on the loading plot reflects the strength of this contribution. In this way, an assessment of the contribution of a given cytokine and chemokine to a ‘control’ or ‘rhIL1ra’ treated pattern of response can be inferred.
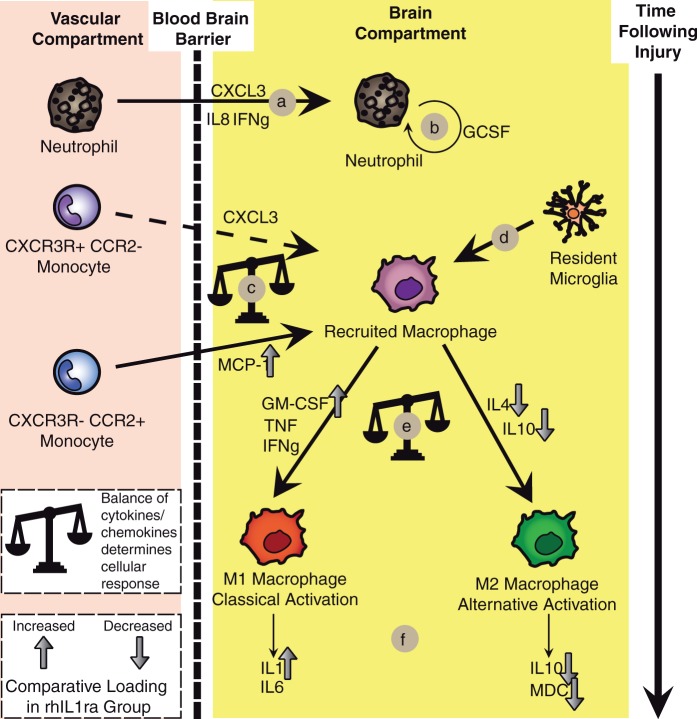
Several cells within the central nervous system can both produce and respond to cytokines and chemokines. In Figure 5, we have sought to summarise the key cellular events following TBI, based on the existing literature. This allows us to superimpose the effects of rhIL1ra treatment on several cytokines and chemokines to infer the potential consequences of IL1ra treatment on the cellular response to injury. Although IL1ra is conventionally regarded as an ‘anti-inflammatory’ cytokine,16 our data demonstrate that chemokines related to macrophage recruitment from the vascular compartment (e.g. MCP-1) are increased in brain ECF following rhIL1ra treatment. Furthermore, cytokines and chemokines related to ‘Classical’ activation (M1) of macrophages (GM-CSF, IL1β) are increased in the rhIL1ra treated group while those related to ‘Alternative’ activation (M2) of macrophages (IL4, IL10, MDC) are reduced in the rhIL1ra-treated group. Superficially, this appears counterintuitive; however, it illustrates that the simplistic division of the inflammatory response into ‘pro-’ and ‘anti-’ inflammatory is not valid. In a complex disease such as TBI, inflammation should be thought of as the endogenous response to injury and as such recruitment of inflammatory cells that are able to take on a range of functional phenotypes4 can play a wide range of roles following injury, both protective and damaging. Furthermore, as in this study (Figure 1(b)) and in previous observational studies,7,13 there is a modification of the cytokine and chemokine milieu over time such that the same cellular components may take on differing functions over time.
Figure 5.
Summary of cellular responses following traumatic brain injury. A model of the key cellular events following TBI has been built up from the literature including the role of several cytokines and chemokines. The effects of rhIL1ra treatment are superimposed, as implied by the loading values from Figure 1(c). Mediators with loading values close to zero (±0.1) are not plotted. (a) Neutrophil recruitment cytokines and chemokines are apparent early following TBI (load negatively on PC1 in Figure 2(e))5,41,42. (b) G-CSF is responsible for neutrophil expansion and is also apparent in the early phase following TBI43. (c) Distinct monocyte subsets which are capable of differentiating into M1 (CCR2+, CX3CR−) and M2 (CCR2−, CX3CR+) macrophages are present within the blood and can be differentiated by a variety of cell surface markers.19 CCR2− and CX3CR+ monocytes are thought to be less responsive to recruitment and this line is therefore dashed, compatible with GRO (CXCL3) loading close to 0 in Figure 2(c). Following rhIL1ra administration, MCP-1 is increased to a greater degree suggesting recruitment of monocytes with a propensity to M1 phenotypic differentiation.19,21 (d) Resident microglia are also recruited following injury although it is not known to what degree recruited macrophages are from resident or blood-borne precursors.25 (e) Recruited macrophages can subsequently differentiate into a wide spectrum of phenotypically distinct macrophages between the extremes of M1 (classical activation) and M2 (alternative activation) macrophages.4,25 IL4 and IL10 load negatively on PC2 suggesting that the M2 pattern of expression is present to a lesser degree in the rhIL1ra group of patients.4,5,25 M1 macrophage cytokines, such as GM-CSF and IL1β, load to a greater degree within the rhIL1ra treatment group.4,5,25
Inspection of the bi-plot (Figure 1(c)) in the microdialysis-data derived model suggests that there is an interaction between randomisation group and time point. Time and randomisation appear at opposite ends of the loading bi-plot suggesting that increasing time is associated with the pattern seen in control patients. This is confirmed on the scores plots (Figure 1(a) and (b)) which demonstrate that the observations in the IL1ra-treated group that have the greatest separation from control observations are from the first 48 h following injury. Later observations from the IL1ra-treated group, e.g. beyond 96 h, are more similar to those in the control group. Several cytokines have already peaked and may be on a downward trajectory by 48 h following injury7; however, this is the first time point at which we can practicably group patients across the whole study. Patient recruitment is necessarily opportunistic and there is an inevitable delay between the time of injury and the time at which invasive neuromonitoring can be instituted: this is an inescapable limitation in human studies of this sort. All time points are corrected to the time of injury as cytokine and chemokine variations are known to occur stereotypically over time.
A key question in planning future studies of rhIL1ra in TBI is whether a dose–response relationship exists between the concentration of IL1ra achieved in the brain ECF and the response seen in PC2 on observations in the rhIL1ra-treated patients. As observations from the first 48 h following injury were responsible for the greatest separation between control and rhIL1ra treated patterns, these were selected for comparison. Using the median PC2 scores (in the first 48 h following injury), we dichotomised the rhIL-1ra-treated patients into ‘Greatest responders’ and ‘Least responders’, i.e. five patients with the greatest magnitude of PC2 response and five patients with the least magnitude of PC2 response. We then compared IL1ra microdialysate concentration between these two groups in Figure 3 to explore whether higher IL1ra concentrations elicit a greater response on PC2. There is a clear and statistically significant difference between the two groups (Mann–Whitney U, p = 0.008) suggesting that it is not just the class attribution (control vs IL1ra) that is responsible for the separation in observations in the PLS-DA model, but there is also a dose effect with greater IL1ra concentrations associated with a greater magnitude of PC2 score. This dose–response relationship corroborates the conclusion that PC2 encapsulates the downstream effects of IL1ra and also suggests that in future studies of rhIL1ra treatment, a larger dose of administered rhIL1ra can increase the downstream effects of IL1R antagonism, in the subset of patients who achieved a lower concentration of IL1ra in microdialysate. In this way, PC2 can be thought of as a biomarker of IL1ra action in human severe TBI. Although the comparison in Figure 3 is in observations from the first 48 h time epoch, the PLS-DA model that generated PC2 is derived from data across all time points and therefore incorporates the effects of IL1ra throughout the monitoring period.
There is a wide variation in IL1ra concentrations achieved in microdialysate following administration (Figure 3). A range of factors such as local blood flow or blood brain barrier permeability can affect the concentration of IL1ra achieved in the brain. This has implications for extrapolating the findings from the brain in the immediate region of the microdialysis catheter to the brain as a whole, and a potential therapeutic effect. However, conclusions that are specifically related to the downstream effects of IL1ra in the PLS-DA model are still valid. Even if the concentrations measured in the vicinity of catheter tip are not representative of the whole brain, the effects of IL1ra are measured in the same locality. As such, the brain around the catheter acts as a microcosm of the injured brain as a whole, because the concentration of IL1ra in that specific volume of brain has been related to measured variables in PC2 in the same volume of brain.
In addition to exploring the effects of IL1ra in the brain ECF, we also sought to explore the effects in the plasma compartment. As many patients with severe TBI have additional multi-system injuries and complications, one would expect a greater degree of variation in the plasma compartment than in brain. In the plasma PLS-DA model, the first two principal components explain 16.4% of the variation in the measured cytokines and chemokines (Table 4), as compared to 44.2% of the variation in the microdialysate PLS-DA model (Table 3). Although there is no published benchmark for the amount of variation that a given PLS-DA model should explain in order to be valid, we conclude that in the brain ECF, time and randomisation explain more of the variation in the measured cytokines and chemokines than in plasma. Furthermore, TBI is widely regarded as a heterogeneous disease,17 and the finding that just two principal components explain more than 44% of the variation in a model based on a large number (35) of variables suggests that time and randomisation are dominant features in the resulting cytokine and chemokine response to TBI.
The plasma PLS-DA model is illustrated in Figure 2 and PC1 demonstrates an effective separation between control and IL1ra patients (Figure 2(a)). Several cytokines and chemokines are increased in the rhIL1ra-treated group in both plasma and microdialysate (e.g. MCP-1, IL1β, IL1α, and RANTES) while others are raised in microdialysate, but not plasma (e.g. Fractalkine, GM-CSF). This qualitative difference between the plasma and brain ECF compartments suggests distinct responses to rhIL1ra treatment that highlight the importance of interrogating the correct biological compartment. It also suggests that the microdialysate response is brain specific and is not a passive leak of mediators across the blood brain barrier.
A key question is the biological consequences of the differences in cytokine and chemokine patterns that are revealed in the PLS-DA model. Various cell types are capable of producing and responding to these mediators. Figure 5 provides a summary of the cellular responses to TBI18 based on the current TBI literature in humans and animal models. The consequences of rhIL1ra treatment, as evidenced by differences in loading on PC2 in the PLS-DA model (Figure 1(c)), are superimposed on this model. Taken together, this study’s evidence suggests that rhIL-1ra does not behave as a stereotypic “anti-inflammatory” cytokine. Instead, following rhIL1ra treatment, the chemokines increased are those conventionally associated with recruitment of macrophages from the periphery and their biasing into an M1 phenotype. The current methodology, although suggestive of a bias in microglial response, cannot definitively prove that microglia are the source or target of the microdialysis-derived cytokines and chemokines, and this conclusion is an extrapolation from our understanding of the role of these mediators in both in vitro and pre-clinical studies. This fundamental limitation may always be present in human studies in that current research techniques do not allow a functional distinction between microglial subtypes to be made in vivo, in human patients. Nevertheless, we have tried to provide a framework for understanding the effects of rhIL1ra action, notwithstanding this notable caveat. There is functional heterogeneity in the circulating monocyte pool, and the balance between MCP-1 and GRO (CXCL3) may dictate recruitment of distinct subsets of monocytes into injured tissues (Figure 4(c)) and impact on the macrophage subtype that emerges.19 MCP-1, the human homologue of murine CCL2, has been extensively studied and is thought to be a requisite chemokine in monocyte recruitment to the brain following a diverse range of neural insults.20,21 CCR2- monocytes are thought be less responsive to inflammatory signals (dashed line in Figure 5) and this is consistent with the low levels of GRO (CXCL3) loading seen in Figure 1(c). Monocytes differentiate into functionally heterogeneous macrophages within tissues showing a spectrum of activity that can be induced by particular combinations of cytokines.5 Interestingly, CCR2+ monocytes are not necessarily pre-destined to take on a damaging role following recruitment and may have a protective role on the brain vasculature22 and may even lead to M2 polarisation in some contexts.23,24 This complexity of response illustrates the difficulty in understanding the role of such a wide range of mediators and cells that can have a plethora of actions. Of note, as well as increases in mediators associated with the M1 macrophage subtype (IL1, GM-CSF), there is a concordant reduction in mediators associated with an M2 macrophage subtype (IL4, IL10, MDC). This pattern of response is much more informative than isolated changes in a given mediator. We would suggest that exploration of the patterns of cytokine and chemokine response provide a much fuller picture of the neuro-inflammatory response to injury. Also, the increase in monocyte recruitment and differentiation into classically activated macrophages is usually regarded as pro-inflammatory and damaging5,25 suggesting that either rhIL1ra has a deleterious effect in human TBI in contradistinction to rodent models, or that these rhIL1ra-induced ‘pro-inflammatory’ responses can lead to a reparative neuronal environment. In an appropriate context, even cytokines considered damaging, such as MCP-1,26 can mediate a neuroprotective effect.27,28 The present study’s findings thus argue against a simplistic dichotomous categorisation of the immune response as pro- or anti-inflammatory. Moreover, in the literature, the M1/M2 paradigm of macrophage activation is undergoing reappraisal. While this paradigm has historically provided a useful framework for selected immune responses, a less straightforward picture is now emerging that challenges the traditional M1/M2 grouping, with complex, mixed and dynamic macrophage phenotypes becoming evident as a result of recent research.29 This is further complicated by the finding that non-humoral factors, such as phagocytosis of apoptotic cells, can also induce an M2 phenotype.30 In this way, as well as arguing against a dichotomy of inflammatory response into ‘pro-’ and ‘anti-’ inflammatory, care must also be taken in making a simple, and erroneous, dichotomy between M1 and M2 microglial responses. Although terms such as M1 or M2 microglial activation provide useful intellectual shorthand for a blueprint of response, in the same way that we must consider patterns of cytokine and chemokine response, there are likely to be patterns of microglial response that have the same diversity and complexity. Second, there is in vitro evidence that fractalkine, a chemokine loads to a large degree on PC2, in addition to its well defined role as a monocyte chemoattractant, may also act directly on neurones to reduce apoptosis and cell loss.31–34 Lastly, and perhaps most interestingly, is the interaction between the innate and adaptive immune systems and the possibility for classically activated macrophages to promote beneficial auto-reactivity to neuronal antigens.35,36 This is a feature of the neuro-inflammatory response to TBI that is, as yet, poorly explored. rhIL1ra has been trialled in other forms of neuronal injury in humans and the exact mechanism by which rhIL1ra modifies neuronal injury in TBI may implications for clinical trials of rhIL1ra in ischaemic stroke and subarachnoid haemorrhage.37–39
In order to corroborate and validate these findings, there is a need to utilise an alternative methodology for exploring microglial responses to TBI. The PET ligand [11C]-(R)-PK11195 provides a method for quantifying microglial activation6,40 following neurological insults. Combining both microdialysis and PET methodology provides a mechanism for linking the cellular and humoral responses to injury.
While there are advantages in using multivariate projection methods to infer patterns of cytokines and chemokines in a given circumstance, it is not possible using this method to ascribe a ‘statistical significance’. It is only by placing the data in an appropriate biological model based on the existing literature that one can infer the consequences of the loadings on a given PC.
Conclusions
The use of PLS-DA has revealed the downstream consequences of rhIL1ra treatment following severe TBI in humans. There is a dose-dependent modification of the pattern of neuro-inflammatory response that is consistent with an increase in monocyte recruitment and a biasing of microglial responses to a M1 (classically activated) phenotype. This response is brain specific and is not the result of passive leak of mediators from the plasma compartment.
The data presented provide a testable hypothesis, namely, rhIL1ra treatment increases microglial activation following severe TBI. Our data also suggest that a greater biological effect can be achieved by increasing the concentration of IL1ra in the brain ECF, and delivering the drug as early as possible following injury to encompass the first 48 hours following injury.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AH is supported by a joint Medical Research Council/ Royal College of Surgeons of England Clinical Research Training Fellowship (G0802251). AH and MRG are supported by Raymond and Beverly Sackler Fellowships. MRG is supported by the Royal College of Surgeons of England Research Fellowship. KLHC is supported by the National Institute for Health Research Biomedical Research Centre, Cambridge (Neuroscience Theme; Brain Injury and Repair Theme). DKM and JDP are supported by National Institute for Health Research Senior Investigator Awards. PJH is supported by the Academy of Medical Sciences/Health Foundation Senior Surgical Scientist Fellowship, the National Institute for Health Research Brain Repair Centre Collaborative and the National Institute for Health Research Professorship. Study Support was provided by the Medical Research Council (grant number G0600986 ID 79068) and the National Institute for Health Research Brain Repair Centre Collaborative.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PJH and JDP are directors of Technicam. AH and MRG have received specialist training in multivariate projection methods from CAMO Software AS (Oslo, Norway).
Authors’ contributions
AH devised and conducted the study, and is corresponding author. MRG was involved with the statistical methodology and manuscript review. KLHC was involved with laboratory aspects of the study and manuscript review. JDP and DKM were involved with designing the study and manuscript review. PJH devised the study and is principal investigator for the clinical aspects of the study.
References
- 1.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Frontier Neurol 2013; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das M, Mohapatra S, Mohapatra SS. New perspectives on central and peripheral immune responses to acute traumatic brain injury. J Neuroinflammat 2012; 9: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 2013; 39: 3–18. [DOI] [PubMed] [Google Scholar]
- 4.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–686. [DOI] [PubMed] [Google Scholar]
- 6.Cagnin A, Kassiou M, Meikle SR, et al. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics 2007; 4: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmy A, Carpenter KL, Menon DK, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab 2011; 31: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmy A, Guilfoyle MR, Carpenter KL, et al. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab 2014; 34: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clausen F, Hanell A, Israelsson C, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 2011; 34: 110–123. [DOI] [PubMed] [Google Scholar]
- 10.Clausen F, Hanell A, Bjork M, et al. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 2009; 30: 385–396. [DOI] [PubMed] [Google Scholar]
- 11.Tehranian R, Andell-Jonsson S, Beni SM, et al. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma 2002; 19: 939–951. [DOI] [PubMed] [Google Scholar]
- 12.Lazovic J, Basu A, Lin HW, et al. Neuroinflammation and both cytotoxic and vasogenic edema are reduced in interleukin-1 type 1 receptor-deficient mice conferring neuroprotection. Stroke; a journal of cerebral circulation 2005; 36: 2226–2231. [DOI] [PubMed] [Google Scholar]
- 13.Helmy A, Antoniades CA, Guilfoyle MR, et al. Principal component analysis of the cytokine and chemokine response to human traumatic brain injury. PLoS One 2012; 7: e39677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulesteix AL, Strimmer K. Partial least squares: a versatile tool for the analysis of high-dimensional genomic data. Brief Bioinform 2007; 8: 32–44. [DOI] [PubMed] [Google Scholar]
- 15.Helmy A, De Simoni MG, Guilfoyle MR, et al. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol 2011; 95: 352–372. [DOI] [PubMed] [Google Scholar]
- 16.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol 2005; 5: 629–640. [DOI] [PubMed] [Google Scholar]
- 17.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma 2008; 25: 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyoneva S, Ransohoff RM. Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol Sci 2015. 36(7): 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19: 71–82. [DOI] [PubMed] [Google Scholar]
- 20.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babcock AA, Kuziel WA, Rivest S, et al. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci: The official journal of the Society for Neuroscience 2003; 23: 7922–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gliem M, Mausberg AK, Lee JI, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol 2012; 71: 743–752. [DOI] [PubMed] [Google Scholar]
- 23.Miro-Mur F, Perez-de-Puig I, Ferrer-Ferrer M, et al. Immature monocytes recruited to the ischemic mouse brain differentiate into macrophages with features of alternative activation. Brain Behav Immun 2015. (in press). [DOI] [PubMed] [Google Scholar]
- 24.Chu HX, Broughton BR, Kim HA, et al. Evidence That Ly6C(hi) monocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. Stroke 2015; 46: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 25.Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J Innate Immun 2012; 4: 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semple BD, Bye N, Rancan M, et al. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J Cereb Blood Flow Metab 2010; 30: 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stowe AM, Wacker BK, Cravens PD, et al. CCL2 upregulation triggers hypoxic preconditioning-induced protection from stroke. J Neuroinflam 2012; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eugenin EA, D'Aversa TG, Lopez L, et al. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem 2003; 85: 1299–1311. [DOI] [PubMed] [Google Scholar]
- 29.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep 2014; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soki FN, Koh AJ, Jones JD, et al. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J Biol Chem 2014; 289: 24560–24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desforges NM, Hebron ML, Algarzae NK, et al. Fractalkine mediates communication between pathogenic proteins and microglia: implications of anti-inflammatory treatments in different stages of neurodegenerative diseases. Int J Alzheimer's Dis 2012; 2012: 345472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizuno T, Kawanokuchi J, Numata K, et al. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res 2003; 979: 65–70. [DOI] [PubMed] [Google Scholar]
- 33.Denes A, Thornton P, Rothwell NJ, et al. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun 2010; 24: 708–723. [DOI] [PubMed] [Google Scholar]
- 34.Noda M, Doi Y, Liang J, et al. Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. J Biol Chem 2011; 286: 2308–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox AL, Coles AJ, Nortje J, et al. An investigation of auto-reactivity after head injury. J Neuroimmunol 2006; 174: 180–186. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz M, London A, Shechter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience 2009; 158: 1133–1142. [DOI] [PubMed] [Google Scholar]
- 37.Smith CJ, Denes A, Tyrrell PJ, et al. Phase II anti-inflammatory and immune-modulating drugs for acute ischaemic stroke. Expert Opin Investigat Drugs 2015; 24: 623–643. [DOI] [PubMed] [Google Scholar]
- 38.Emsley HC, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatr 2005; 76: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh N, Hopkins SJ, Hulme S, et al. The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J Neuroinflam 2014; 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price CJ, Wang D, Menon DK, et al. Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke 2006; 37: 1749–1753. [DOI] [PubMed] [Google Scholar]
- 41.Eyles JL, Roberts AW, Metcalf D, et al. Granulocyte colony-stimulating factor and neutrophils – forgotten mediators of inflammatory disease. Nat Clin Practice Rheumatol 2006; 2: 500–510. [DOI] [PubMed] [Google Scholar]
- 42.Clark RS, Schiding JK, Kaczorowski SL, et al. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma 1994; 11: 499–506. [DOI] [PubMed] [Google Scholar]
- 43.McLoughlin RM, Witowski J, Robson RL, et al. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest 2003; 112: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]