Abstract
Purpose
An interlaboratory comparison of radiation dosimetry was conducted to determine the accuracy of doses being used experimentally for animal exposures within a large multi-institutional research project. The background and approach to this effort are described and discussed in terms of basic findings, problems and solutions.
Methods
Dosimetry tests were carried out utilizing optically stimulated luminescence (OSL) dosimeters embedded midline into mouse carcasses and thermal luminescence dosimeters (TLD) embedded midline into acrylic phantoms.
Results
The effort demonstrated that the majority (4/7) of the laboratories was able to deliver sufficiently accurate exposures having maximum dosing errors of ≤ 5%. Comparable rates of ‘dosimetric compliance’ were noted between OSL- and TLD-based tests. Data analysis showed a highly linear relationship between ‘measured’ and ‘target’ doses, with errors falling largely between 0–20%. Outliers were most notable for OSL-based tests, while multiple tests by ‘non-compliant’ laboratories using orthovoltage x-rays contributed heavily to the wide variation in dosing errors.
Conclusions
For the dosimetrically non-compliant laboratories, the relatively high rates of dosing errors were problematic, potentially compromising the quality of ongoing radiobiological research. This dosimetry effort proved to be instructive in establishing rigorous reviews of basic dosimetry protocols ensuring that dosing errors were minimized.
Keywords: dosimetry, OSL/TLD dosimeters, animal models
Introduction
Over the past several decades the federal government has been supporting, via contracts and grants, large multi-institutional, multi-investigator-related radiobiological research programs (NIAID 2005, NIAID 2009, NIAID 2012, NIST 2011, BER 2015, Maidment 2011). The vast majority of these research programs involve active laboratory work requiring the use of ionizing radiation (IR) and associated IR-emitting devices to irradiate specific test subjects, and the subsequent monitoring and assessment of IR-induced responses within those ‘targets’. These biological targets can, and often do, span the entire spectrum of biological organization, going from elemental molecules of the cell, to vital tissues of the body, to whole animals. The IR-induced responses are equally diverse, but dependent on defined, highly variable IR exposure conditions, predetermined by investigative objectives and strategies. Some of the key exposure variables include, but are not limited to: total dose and rate of exposure of IR, the quality of IR, and a host of biologic variables. Certainly the latter includes both the basic nature of the biologic entity and its’ targeted volume (Zoetelief et al. 1984). Measurements of the IR-induced responses of those biologic targets can vary as well, sometimes extensively, but generally in proportion to the total magnitude, the intensity, and the duration of the exposing IR. Accordingly, the accuracy and precision (reproducibility) of the bioresponse-initiating IR exposures are, in general, critical to the successful outcomes of any/all radiobiological investigations, especially in terms of achieving reliable, reproducible results that are useful to the scientific community (Desrosiers et al. 2013). The latter sentiment has been embraced by investigators, program managers, and the radiobiological community at large. This is evident by the numerous conferences and workshops that have been devoted to this subject (Desrosiers et al. 2013, Murphy 2011, NIST 2011, RadCore 2010, Yoshizumi et al. 2011).
A multi-institutional NIAID-sponsored research project entitled “Studies of immunosenescence and other late effects of acute ionizing radiation exposures in atomic bomb survivors” was initiated September 30 2009 and continues to date (NIAID 2009). The research project contractually links the Radiation Effects Research Foundation (RERF) in Hiroshima Japan and the National Institute of Allergy and Infectious Diseases (NIAID), along with nine collaborating institutions (five US institutions and four Japanese institutions), and has as its primary objective the study of the long-term health effects of atomic radiation exposures on a defined Japanese population and the use of RERF’s unique repositories of longitudinal clinical and biologic data, radiation exposure estimates, and biospecimens to define more precisely the changing nature of immune capacity and function with age and with (or without) prior acute radiation exposure and associated injury.
Although the major scientific thrust of this NIAID-funded program is ‘epidemiological’ in nature (i.e., retrospective, clinical, sample-based analyses) and involves the analyses of stored biosamples from atomic bomb survivors, supplemental, supporting experimental radiation studies using small animal (mice) models are being conducted by a number of the collaborating researchers. It was recognized by the program managers early on of the need to confirm and verify the accuracy of the IR doses being proposed for use in animal experiments at the various participating institutions. As such, dosimetric confirmations of the experimental radiation exposures were considered to be fundamental to the proper performance of any/all radiobiologic investigations. Accordingly, a radiation dosimetry exercise was initiated early in 2010 for these IR dose confirmations (Seed 2011). The goals of this exercise were two-fold, namely to: 1) standardize the basic dosimetric approach among the laboratories (performing animal-based experiments); 2) assure that the radiation doses being delivered to experimental animals were consistent, with a minimal accuracy of 95% or better (or conversely, a maximum error of 5% or less). In this regard, the proper use of IR generating systems (IR-irradiators) required full periodic dosimetric assessments in order to assure consistent, accurate and precise IR exposures. But beyond the later requirements, was the need to ensure consistency between laboratories so that the data coming from individual institutions could be compared in a meaningful manner. This report attempts to summarize the primary findings of this ‘dosimetry exercise’, while highlighting strengths and weaknesses of the work effort, and suggested areas of improvement for comparable, future efforts by others.
Materials and Methods
General
The ‘dosimetry exercise’ developed as a result of the early recognition that in order to effectively execute the planned animal-based radiobiological studies across the various participating institutions, oversight and guidance on IR dosimetry related issues were crucial to programmatic success. The sheer complexity of the project itself and the involvement of numerous investigators working at different institutions under different conditions were the principal drivers of these initial concerns. Accordingly, plans for a coordinated dosimetry testing and support effort (i.e., the ‘dosimetry exercise’) were laid during the initial ‘kick-off’ meeting at NIAID/NIH in Bethesda, MD, December 9 2009 (RERF-NIAID 2009).
Survey of available resources
A major ‘action item’ resulting from discussions of ‘experimental radiation exposures of mice’ during the initial contractor’s meeting related to the need to try to ‘harmonize’ as much as possible, IR exposure protocols within the various laboratories. This would however require surveying the various participating laboratories and gathering details on IR irradiators and IR exposure protocols. A survey was subsequently initiated to collect information on the following four areas: 1) type, model, and operating conditions the IR irradiator being used and/or proposed for use (i.e., IR type, manufacturer, model, basic operating conditions and other comments); 2) basic parameters of the planned exposures (range of doses; exposure rate, exposure array, stationary/rotating, unilateral/bilateral/multilateral); 3) dosimetry performed to date for the impending animal exposures (dosimetry performed, reliance on manufacturer’s specifications, dosimeters employed, doses measures, phantoms employed); and 4) availability and access to a qualified staff radiation dosimetrist at the facility.
The survey was forwarded by email to each of the co-investigators at the seven participating institutions (four Japanese laboratories, plus three US laboratories) who were planning animal-based experiments. The information collected in this initial survey, and in subsequent follow-up queries to the participating laboratories, is listed in Table I.
Table I.
Participating laboratories, irradiators and operating/exposure conditions.
| No. Lab |
Lab/ Location |
Irradiator type |
Operating conditions |
Dose (Gy) |
Dose- rate (Gy/ Min) |
Exposure array |
Staff Rad physicist available |
Prior dosimetry performed |
|---|---|---|---|---|---|---|---|---|
| 1 | Keio U /Tokyo, JP | X-ray- orthovoltage
|
Energy- 150 kVp Current- 20mA HLV- 9mm Filters- Al(0.5) + Cu (0.2mm) | 0.5,1,2, 4 | 1 | Round acrylic/ 12 cubicles/ rotating/IR source fixed relative to target | No | Yes, ionization chamber-based dosimetry. Yes, reliant on manufacturer’s specs |
| 2 | Tokushim a U /Tokushi ma, JP | X-ray- orthovoltage
|
Energy- 150 kVp Current- 20mA HLV- not given Filters- Al( 0.5)+ Cu(0.1) | 1,4,10 | 1 | Round/acryl- ic/12 cubicle/ rotating w. IR/IR source fixed rel. to target | No | Yes, TLD-based Dosimetry. Not reliant on manufacturer’s specs |
| 3 | Chiba U /Chiba, JP | X-rays/ orthovoltage
|
Energy- 150 kVp Current- 20mA HLV- 7mm Filters- Al( 0.5)+ Cu(0.1) | 1,2, 4 | 1 | Round/ acrylic/ 12 cubicles/ rotating w. IR/IR source fixed rel.to rotating target | No | No prior dosimetry. Reliant on older dosimetry & manufacturer’s specs |
| 4 | U Arizona /Tucson, AZ, USA | Gamma rays/
|
|
1,4 |
|
Raddisk circular/ xx cubicles/ rotating w. IR/IR source fixed rel. to rotating target | Yes | No prior dosimetry. No, not reliant on manufacturer’s specs |
| 5 | MSKCC /New York, NY, USA | Gamma rays/ 1. 137Cs MDS/Nordion / (Best Theratronics). /Gamma cell- 40 | Energy- 662 KeV | 1,4,7 | 1 | Round, acrylic/ 12 cubicles/ Stationary, fixed bilateral IR source | Yes | Yes, TLD-based Dosimetry. No, not reliant on manufacturer’s specs |
| 6 | NIHS /Tokyo, JP | Gamma rays/ 137Cs Nordion/CRS/ Gamma cell- 40 | Energy-662 KeV | 1,4,8 | 0.88 | Flat, round acrylic/12 cubicles/stati onary w. IR/bilateral IR exposures | No | Yes, TLD-based dosimetry. No, not reliant on manufacturer’s specs |
| 7 | U Georgia / Athens, GA, USA | Gamma rays/ 60Co Nordion/Gam ma cell-220 137Cs JL Shepard/Mark 1–68A |
|
|
|
|
Yes | No prior dosimetry Yes, initially reliant on manufacturer’s specs |
Outsourcing of dosimetry assessments
While the ‘survey of resources’ was being collected, it was clear from the initial discussions that among the participating laboratories there was an extraordinarily wide range of available resources, experience, and capabilities relative to radiation dosimetry specifically and to radiation biology in
Therefore, in addition to the survey, we proceeded to find a suitable ‘out-source’ for the project’s dosimetric needs and requirements. Following consultations with the Dosimetry Group (Dr Vitaly Nagy) at the US Armed Forces Radiobiology Institute (Bethesda, MD) and with the Radiation Dosimetry Laboratory (Dr Terry Yoshizumi) at Duke University (Durham, NC), along with the principal investigators and program managers at RERF, an additional commercial dosimetry laboratory, Landauer Inc. (Glenwood, IL) was contacted (and latter contracted) for specific dosimetric services. Midway through the contract, another major dosimetry facility, namely the Medical Radiation Research Center (MRRC) at the University of Wisconsin (Madison, WI), under the direction of Dr Larry DeWerd, was contacted, and again, services contracted.
Approaches to the ‘dosimetry exercise’
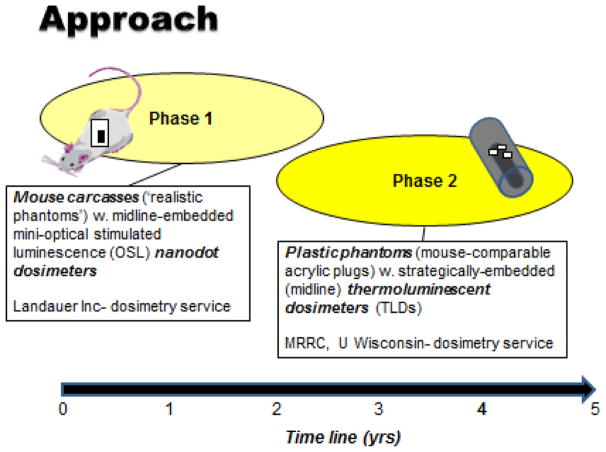
Initially the plan was to perform dosimetry testing using optically stimulated luminescence (OSL) dosimeters embedded midline into mouse carcasses (Figure 1, Phase 1) (Note- later in the project, Phase 2, TLD dosimeters embedded midline into plastic phantoms). All participating laboratories were asked to perform a preset range of test IR exposures with their irradiators using OSL dosimeters (InLight NanoDottm ; Al2O3) that were supplied and later analyzed by Landauer Inc. (Landauer Inc, 2 Science Road, Glenwood, Illinois 60425 USA). Landauer Inc. is fully accredited and licensed ionizing radiation calibration facility for OSL-based, radiation monitoring services and technologies. Briefly, the OSL dosimeters supplied by Landauer Inc. represent a new generation of highly sensitive and reliable radiation dosimeters are designed for use in single point radiation assessments over a wide range of potential energies and engineered to be conveniently read out by small, table-top optical luminescence detectors- the microStar®. These OSL dosimeters offer the advantages of a complete reanalysis if required, requires no preparation prior to use and has a labeled sensitivity that is built into the dosimeter 2D bar code for rapid, accurate reading. In addition, prior to shipping (to the participating laboratories) all dosimeters were prescreened for radiation sensitivity and for reproducibility of measurement accuracy (+/− 3%). ( http://www.landauer.com/uploadedFiles/InLight_nanoDot_FN.pdf). Further details concerning the nature, analytics and calibrations of these OSL dosimeters can be obtained by the reference articles by Akselrod (Akselrod 2011) and Yukihara and McKeever (Yukihara and McKeever 2008, 2011).
Figure 1.
Two basic dosimetry testing approaches, in turn two phases, have been employed for the RERF/NIAID-funded project: Phase 1, OSL dosimeters embedded surgically into mouse carcasses; Phase 2, TLD dosimeters embedded into plastic mouse phantoms.
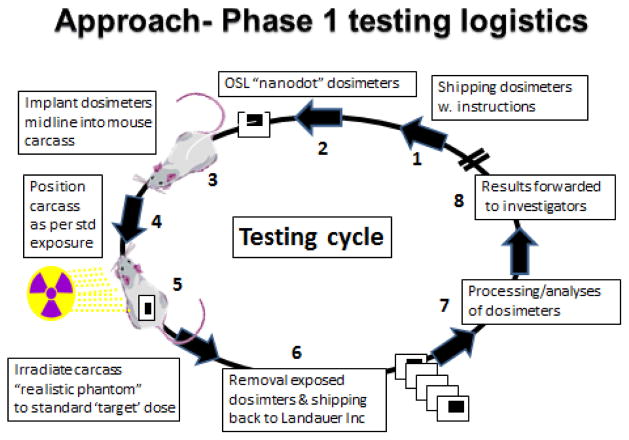
Guidance was provided to each of the laboratories as to how the OSL dosimeters were to be handled. Briefly, prescreened InLight NanoDottm dosimeters were encased within mini watertight plastic ‘zip-lock’ pouches and surgically embedded into the abdominal cavities of freshly sacrificed mice of standard size and weight (e.g., ~25 g weight, ~6.5cm in length and ~2.5cm wide). The mouse carcasses with embedded OSL dosimeters were then positioned within the IR exposure chamber as would be the experimental IR-exposed test animals. For these exposures, it was recommended that at least two (of the three) test doses be between 1–4 Gy, while the third test dose could be either higher or lower depending on the individual laboratory’s needs (the IR doses evaluated by the various laboratories ranged from 0.5 to 10 Gy). Duplicate tests were to be performed for each of the radiation doses tested. Following test IR exposures, the mouse carcass-embedded dosimeters were retrieved, repackaged and forwarded, via a courier delivery service, to the Landauer dosimetry laboratory for analyses. Dosimeters were analyzed individually using a Landauer® InLight® microStar reader and standard OSL calibration curves based on: a) 0.662 MeV 137Cs gamma rays (for either 137Cs- or 60Co irradiators); or b) 140 kVp x-ray beam (with 8.3 mm Al filter at the ‘half value layer’ or HVL) in the case of orthovoltage x-ray irradiators. The ‘read-out’ process by the microStar reader is based on a light emitting diode array that stimulates light emission from the OSL detector material that in turn is detected and measured by a photomultiplier tube using a ultrasensitive photon counting system. The light released during optical stimulation is directly proportional to the radiation dose and the intensity of the stimulation light. The calibration factor formula and dose calculations used for the microStar reader are as previously published (Yukihara and McKeever 2011) and based, in part, on the early work by Moscovitch (Moscovitch 1993, 1999) that described dose algorithms for personal luminescence-based dosimetry. Full dosimetric reports were generated and forwarded to the participating investigators. Figure 2 illustrates basic logistics of the dosimetry test cycle using Phase 1 elements.
Figure 2.
Key elements and logistics of the dosimetry testing for Phase 1
As a result of anomalous results coming from a select number of laboratories using orthovoltage x-ray units and based on solicited recommendations of several radiation dosimetrists, the decision was made to switch over to more traditionally used thermoluminescent dosimeters (TLD) with lower photon energy-dependence in combination with plastic (acrylic), mouse-like phantoms (Figure 1, Phase 2). As indicated above, the MRRC at the University of Wisconsin (MRRC, University of Wisconsin, 1111 Highland Avenue, Madison, WI 53705) was contracted for this phase of the dosimetry work. Briefly, the TLD used were LiF:MgTi 1mm3 microcubes (TLD-100™, Thermo Scientific RMP, Franklin MA), processed using the Cameron method (Cameron et al. 1964), and read out using a Harshaw 5500 TLD reader. Prior to shipping, all of the TLD were fully characterized and sorted for their precision reliability. The plastic mouse phantoms with embedded TLD were calibrated by MRRC using three distinct irradiator types, namely: a) a gamma ray emitting 137Cs irradiator (Hopewell G10 137Cs, Hopewell Designs, Inc., Alpharetta, GA); b) a gamma ray emitting 60Co irradiator (Theratronics T-1000 60Co, Best Theratronics, Ottawa, Ontario, Canada); and c) orthovoltage photon X-ray emitting unit (Advanced X-ray CP320 orthovoltage unit). With these irradiators, the MRRC facility delivered National Institute of Standards and Technology (NIST)-traceable air kerma irradiations to the plastic phantoms using NIST-calibrated ion chambers and with calculations of known absorbed doses using Monte Carlo simulations. Air kerma-to-absorbed dose conversion factors were calculated for each beam energy used.
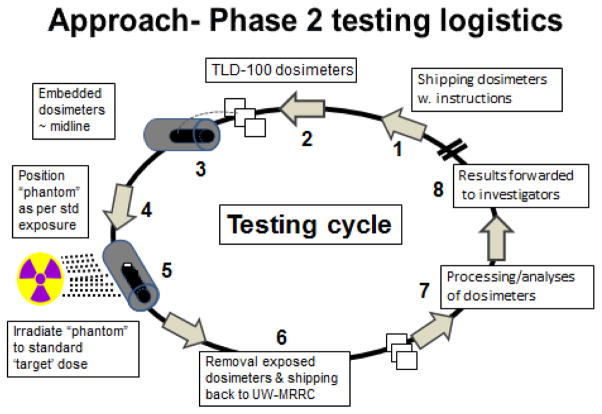
The dosimetry test package shipped to individual labs included six plastic phantoms, each with three pre-tested TLD-100tm microtubes (LiF:Mg Ti - 1×1×1 mm cubes; Thermo Scientific RMP, Franklin, MA) that were inserted midline (geometric center) into the body of the phantoms. Additional TLD (3) were included in the test package and used as ‘unirradiated controls’. The participating laboratories were asked to irradiate three phantoms at each of the two ‘target’ doses, namely 1 and 4 Gy using their standard exposure protocol employed for their experimental animals. Following these exposures, the irradiated phantoms were collected (along with the ‘unirradiated control TLD) and sent back to the MRRC for subsequent analyses. Once the dosimeters were received by the MRRC, they were processed using read-out methods described above. Results were reported to the investigators in terms of the measured midline doses (absorbed dose in Gy in water) and the standard deviations of those measurements. Average doses, along with the percent variances from target doses were reported as well. Figure 3 illustrates basic logistics of the dosimetry test cycle using Phase 2 elements.
Figure 3.
Key elements and logistics of the dosimetry testing for Phase 2
Data management
All dosimetry data coming from specific laboratories were incorporated into a centralized database, the ‘REDCap’ database system, and is currently available for further analyses by the project’s investigators and program managers alike.
Results
Surveys of irradiators and operating conditions
The project’s participating laboratories and associated investigators who performed animal-based experiments employed a full range of irradiators. These irradiators included six gamma ray units (4 137Cs units and 2 60Co units, with one of the latter 60Co units decommissioned midstream during the project). In addition, there were four orthovoltage X-ray machines (2 of X-ray units remained in active service for the duration of the project, while one was decommissioned and another one became inactive relative to the project’s work) (Table I).
Periodic surveys of the irradiators used by the project’s investigators in their research facilities, along with basic operating parameters, were conducted and results compiled (Table 1). A range of both estimated radiation doses and dose-rates, namely 0.5–10 Gy and 0.9–1.3 Gy per minute of exposure respectively, were utilized experimentally by the different laboratories for exposures carried out alternatively either uni-directionally, bi-directionally or rotationally. Similarly, different types of exposure arrays were employed allowing for the constraint and irradiation of either single- or multiple animals. Despite the periodic surveys, verification of specific operating conditions of these irradiators was occasionally problematic, due to the failure by certain laboratories to provide specific and detailed answers to a number of survey-related questions (e.g., lack of specific details not only concerning basic irradiator operation [HVL values, exposure rates, etc.] but also how the animals were handled during given exposures [nature of the constraining devices, numbers of animals irradiated per exposure run, etc.]).
Dosimetry and dosimetrists
Survey of the participating laboratories revealed that less than half (3/7 or ~43%) of the participating laboratories had a qualified dosimetrist on staff to assist in dosimetry. Nevertheless, the survey suggested that the majority of laboratories (5/7 or ~71%) had performed preliminary, experiment-specific radiation dosimetry on the irradiators. A sizable fraction (3/7 or ~43%) of the laboratories did however, acknowledge that historical data on their irradiators were used to setup initial exposure conditions (Table I).
OSL-based dosimetry tests
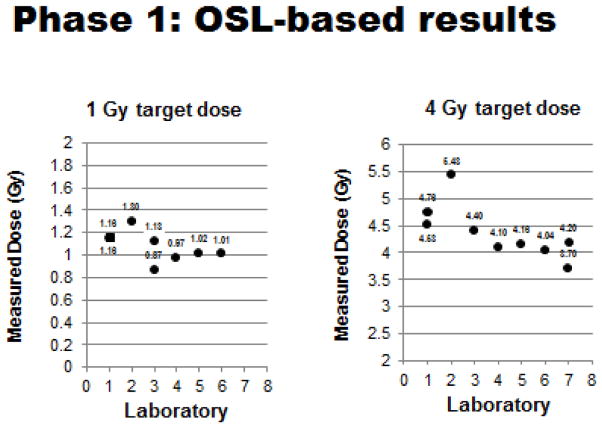
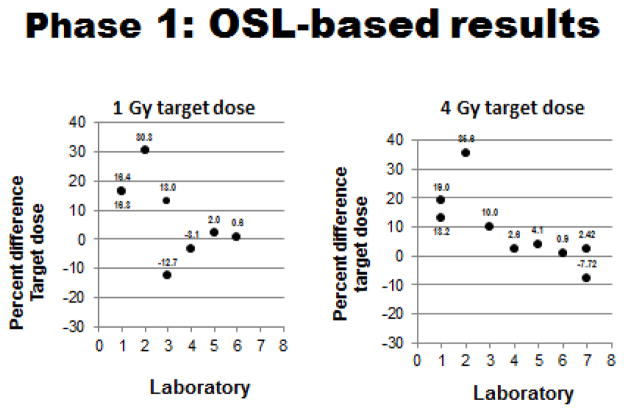
The initial phase (Phase 1/OSL-based) of the ‘dosimetry exercise’ was completed during the first ~2.5 years of the program’s contract. Results of Phase 1 can be summarized as follows: 1) 100% all of participating laboratories (7/7) performed the requisite OSL-based tests using cadaver mice that had been implanted (midline) with OSL dosimeters prior to exposure to predetermined radiation doses (generally 1–4 Gy, but some laboratories chose to test both higher and lower radiation doses as well). Results of these OSL-based tests are shown in Figures 4, 5 and 6 and summarized in Table II; 2) ~57% (4/7) of the laboratories ‘passed’ this initial test and demonstrated a capacity to deliver sufficiently accurate doses to specific biologic targets (mouse midlines) that were within the desired 95–105% range of the estimated doses.
Figure 4.
Phase 1 OSL-based testing: Measured doses relative to 1 & 4 Gy target doses for each participating laboratory. (Plotted doses are averages of measured doses with standard deviations generally ≤ 0.05, except for SDs of 1 Gy Lab #3 value of 0.20 and 4 Gy Lab #7 value of 0.54).
Figure 5.
Phase 1 OSL-based testing: Dose errors (% errors) relative to 1 & 4 Gy target doses for each participating laboratory. (Plotted doses are averages of measured % errors with standard deviations generally ≤ 5.0, except for SDs of 1 Gy values for Lab #1 of 5.8, Lab #3 of 20.4 & 10.8).
Figure 6.
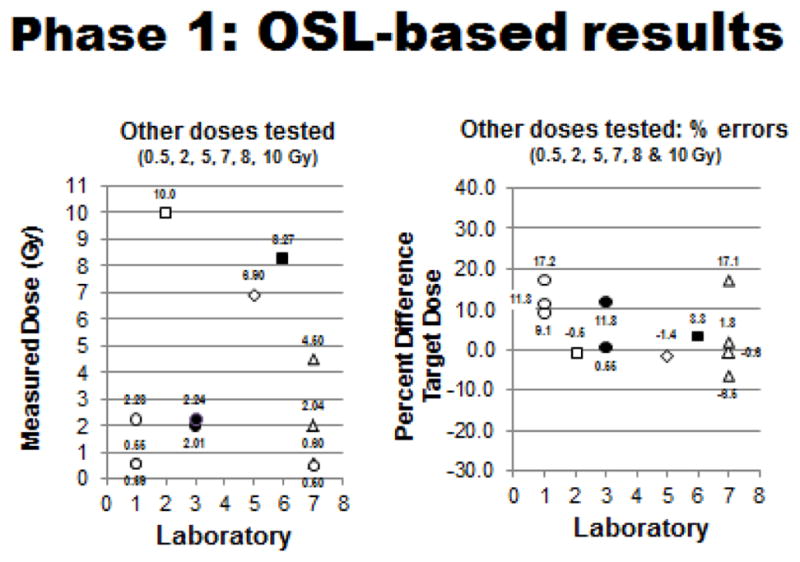
Phase 1 OSL-based testing: Measured doses (left panel) and dosing errors (right panel) relative to all other target doses tested (0.5, 2, 5, 7, 8, & 10 Gy) for each participating laboratory. (Plotted doses are averages of measured doses with standard deviations generally ≤ 0.05, except for SDs of Lab #2 at 10 Gy of 0.20, Lab #5 at 7 Gy of 0.15, and Lab #6 at 8 Gy of 0.09: plotted % errors are average values with standard deviations generally ≤ 5.0, except for SDs of Lab #1 at 0.5 Gy of 9.1/17.2 and at 2.0 Gy of 11.3).
Table II.
Summary of dosing errors of Phases 1 & 2 testing
| Lab No. | Rad Type | OSL Dosimetry | TLD Dosimetry | Comment(s) | Impact(s) |
|---|---|---|---|---|---|
| 1 | X-ray | >5% errors | >5% errors | Reanalysis recommended | Accuracy / reproducibility of IR dose-dependent bioresponses moderately compromised |
| 2 | X-ray | >5% errors | >5% errors | Dosimetric goal(s) achieved | Accuracy / reproducibility of IR dose-dependent bioresponses significantly compromised |
| 3 | X-ray | >5% errors | ≤5% errors | Dosimetric goal(s) achieved | Accuracy / reproducibility of IR dose-dependent bioresponses improved / appropriate |
| 4 | 137Cs/60Co | ≤5% errors | ≤5% errors | Dosimetric goal(s) achieved | Accuracy / reproducibility of IR dose-dependent bioresponses appropriate / enhanced |
| 5 | 137Cs | ≤5% errors | >5% errors | Reanalysis recommended | Accuracy / reproducibility of IR dose-dependent bioresponses minimally compromised |
| 6 | 137Cs | ≤5% errors | ≤5% errors | Dosimetric goal(s) achieved | Accuracy / reproducibility of IR dose-dependent bioresponses appropriate / enhanced |
| 7 | 60Co/137Cs | ≤5% errors | ≤5% errors | Dosimetric goal(s) achieved | Accuracy / reproducibility of IR dose-dependent bioresponses appropriate / enhanced. |
For the four ‘passing’ laboratories equipped with either 137Cs or 60Co gamma irradiators, the average dosing errors across all target doses tested were: −0.26%, 1.55%, 1.58% and 4.16%, respectively. By contrast, the three ‘non-passing’ laboratories (3/7 or ~43%), all using orthovoltage X-rays, were unable to demonstrate consistently this exposure capability, despite multiple testing cycles. The average estimated errors for these laboratories across all radiation doses tested were significantly higher, namely 14.3%, 21.8% and 6.7%. The reason for the latter inability (i.e., to achieve dosing errors of 5% or less) remains uncertain.
TLD-based dosimetry tests
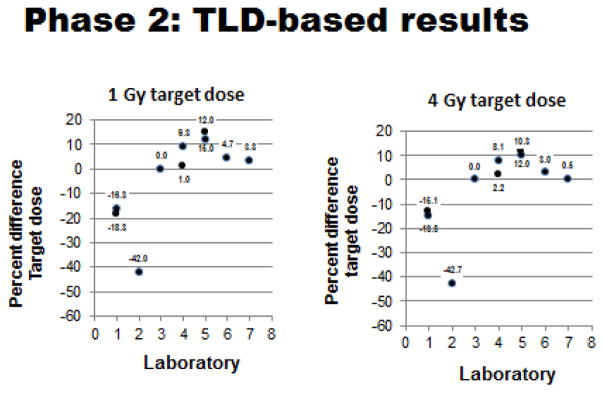
In light of the OSL-dosimetry-based results, a second, slightly different dosimetric strategy was developed and implemented. In this ‘secondary phase’ of dosimetric testing, older, more conventional ‘thermoluminescent dosimeters’ (TLD) and associated technologies were used (Figure 1). Results of the TLD-based dosimetry tests can be summarized as follows: 1) all seven participating laboratories (100%) performed the test exposures as requested, with several laboratories performing additional follow-up analyses; 2) four of the 7 laboratories (~57%) achieved the original goal of having maximum radiation dosing errors of 5% or less. The results of the TLD-based tests are shown in Figures 7 & 8 and summarized in Table II. In contrast to the OSL-based tests of Phase 1, these positive results (i.e., estimated dosing errors of ≤ 5%) included laboratories equipped not only with gamma ray units but also an X-ray unit as well. Based on these TLD-results, a single laboratory successfully reduced exposure errors from above 5% to 5% or less, while another laboratory (with a prior record of successful tests using OSL dosimeters) had slightly elevated exposure errors (i.e., exposure errors >5%).
Figure 7.
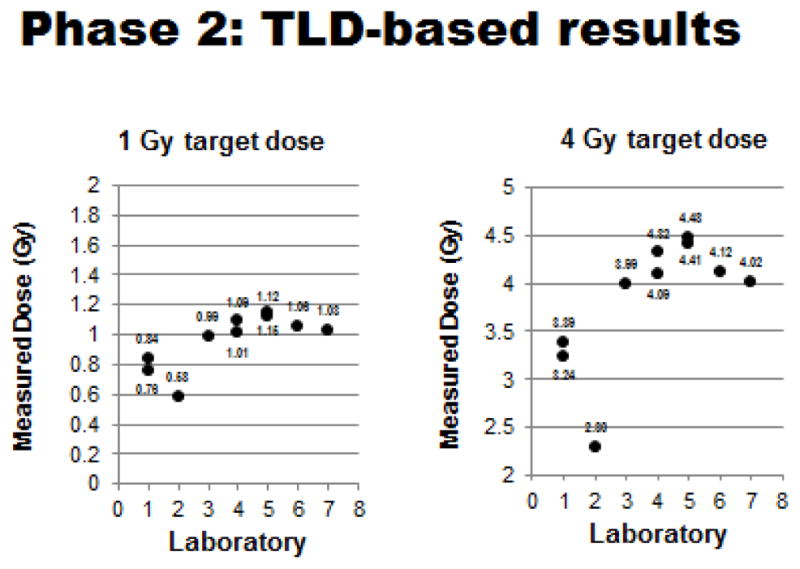
Phase 2 TLD-based testing: Measured doses relative to 1 & 4 Gy target doses for each participating laboratory. (Plotted doses are averages of measured doses with standard deviations generally ≤ 0.05, except for SDs of 1 Gy Lab #3 value of 1.99 and 4 Gy Lab #1 value of 0.55, Lab #3 of 1.05 and Lab #7 of 0.06).
Figure 8.
Phase 2 TLD-based testing: Dose errors relative to 1 & 4 Gy target doses for each participating laboratory. (Plotted doses are averages of measured % errors with standard deviations generally ≤ 5.0, except for SDs of 4 Gy value for Lab #1 of 13.9).
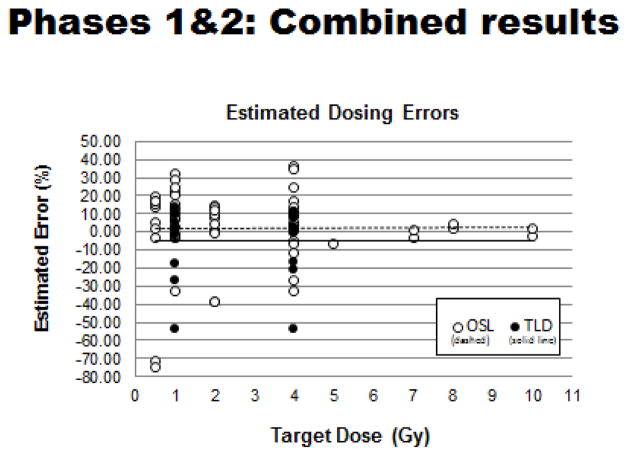
Combined results of OSL- and TLD-based dosimetry tests
Analysis of the data has shown an expected, highly linear relationship between the ‘measured dose’ versus the ‘target dose’ for all tests done to date. The predominance of dosing errors fell between 0–20% across the entire range of radiation doses tested (Figures 9 & 10). Outliers were most notable for the Phase 1 tests that employed OSL dosimeters and that multiple tests by ‘non-compliant’ laboratories using largely orthovoltage x-ray units contributed heavily to the noted wide variation in dosing errors. In sum, the ‘dosimetry exercise’ has indicated that four of the seven laboratories (~57%) were able to reach the original dosimetric goal of having maximum dosing errors of 5% or less (Table II).
Figure 9.
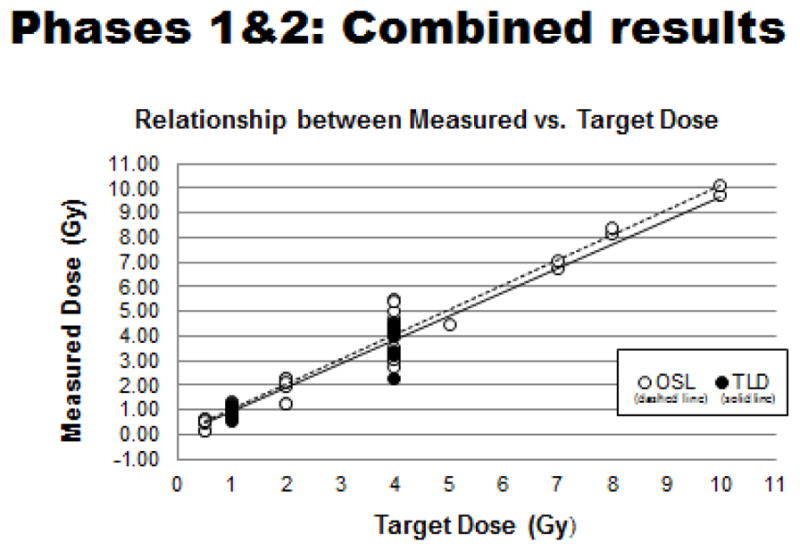
Combined OSL- and TLD-based tests: Relationship between measured radiation doses and related target doses.
Figure 10.
Combined OSL- and TLD-based tests: Extent of dosing errors (% errors) over full range of testing measurements.
Discussion
Observations
A dosimetric exercise for a large, multi-institutional radiobiological research project was initiated by early in 2010 to certify the accuracy of ionizing radiation doses used for animal-based experiments by various investigators working on the same scientific project at several different research institutions located in both the United States and in Japan. While driven by rather simple and straight forward objectives (e.g., to test and to document the reliability and accuracy of radiation exposures delivered to the Project’s experimental animals) this task proved to be much more difficult than originally anticipated. The original goal was to have all of the participating laboratories (7 laboratories in total; 3 US labs, plus 4 Japanese labs) ‘compliant’ in terms of demonstrating minimum exposure accuracies of 95% or greater (or conversely, maximum dosing errors of 5% or less). However, in the final analysis, only 4 of laboratories (4/7 or ~57%) had achieved this goal. Average dosing errors across the specific radiation doses tested recorded by the three non-compliant laboratories using TLD-based dosimetry were 11.5%, −20.6% and −53.6% respectively: this markedly contrasted with the minimal dosing errors of −0.9%, 1.8%, 3.8% and 5.0% for the laboratories deemed ‘compliant’, using again TLD-based dosimetry. In general, the aggregate data (of the average error values) from all laboratories showed, as expected, a highly linear relationship over the full range of radiation doses (target doses ranging from 0.5 to 10 Gy). Such linearity of both the aggregate error data, as well as data from individual laboratories, suggests a systematic source rather than a random score of errors.
It is of interest to note the somewhat comparable outcomes of previously reported studies concerning ‘inter-laboratory dosimetry testing’ of radiation sources used both clinically and experimentally (Zoetelief et al 1985; Yegingil et al 2012; Roue et al 2004). The relatively high rates of radiation dosing errors that we report here for experimental irradiators within select laboratories appear not to be an uncommon finding (Zoetelief et al. 1985).
Problems and solutions
Dosing errors associated with the use of historical dosimetry data and the lack of consultative assistance by qualified ‘in-house’ radiation physicists contributed significantly to the large errors reported during the initial testing phase that employed OSL dosimeters implanted into midsections of mouse cadavers. One laboratory, for example, had planned on using an older 60Co gamma irradiator available to them as a departmental resource and although operational protocols of the irradiator were well-documented, the source strength and associated dosimetry had not been updated. The solution to the latter was simply a matter of garnering the assistance of their local radiation/health physicist in order to recalibrate the source strength and updating general operational protocols for the irradiator and subsequently retesting the instrument. Once these corrective actions were taken, the dosimetry ‘problem’ was rectified; e.g., the dosing errors declined appreciably from ~47% to ~6%, over the course of two dosimetry testing cycles.
In the Commission of European Communities (EC)/European Union Late Effects Project (EULEP) report by Zoetelief et al in 1985, potential, underlying source of these IR dosing errors (specifically for experimental X-ray sources) were addressed and detailed, with four major causation groups identified, namely 1) x-ray output (e.g, beam filtration, voltage waveform), ii) scatter (e.g., size/shape of beam, proximity of phantom to other scattering materials), iii) depth dose (e.g., focus-to-surface distance, position in beam relative to central axis) and iv) miscellaneous (e.g., positioning phantom/animal in beam). However, the report concluded that the basis of IR dosing errors resides mainly at the level of basic IR irradiator setup and associated dosimetry testing, beginning with the use of properly calibrated and standardized ionizing chambers. Further, above cited EC/EULEP report (as well a follow up report by Zoetelief et al 2001) provides procedural guidelines for such preliminary dosimetry testing and basic requirements of experimental animal irradiations with x-rays.
Another major problem that presented early in the testing phase (Phase 1) using OSL-based dosimetry involved laboratories using orthovoltage x-ray units and claiming (via the initial surveys) photon beam energies to be in the range of 150 kVp. Exposure errors recorded with OSL dosimeters implanted into mouse carcasses were uniformly on the high side, suggesting perhaps that they were over responding to relatively low energy x-rays, a phenomenon that has been well-documented with maximum photon energies falling below ~125 KeV (MS Akselrod, personal communication): e.g., at ~100 KeV of photon energy OSL dosimeter signals can be ~1.5 times higher than those OSL signals recorded at substantially higher photon energies, i.e., 150 KeV or greater (Akselrod 2011). Although the dosimetry service provider, Landauer Inc., had attempted to correct for the use of relatively low energy X-rays (OSL signal correction was for 125 KeV) based on the information (or lack thereof) they received back from participating laboratories, these corrections appeared to be insufficient. Rather than requiring the laboratories with orthovoltage x-ray units to establish substantially higher, more uniform beam energies amenable for the OSL-based dosimetry, an entirely new dosimetry strategy was adopted. This strategy utilized older, more conventional, and slightly less energy-dependent, thermoluminescence dosimeters embedded into midlines of mouse-sized, acrylic phantoms. This switch in dosimeter type required that a new service provider (the Medical Radiation Research Center at the University of Wisconsin) be contracted. The results of these TLD-based tests were revealing, although not entirely unexpected, in that the suspected OSL-based dosimetry problem was not entirely corrected with the switch to the TLD dosimeters: two of the three laboratories with orthovoltage x-ray units still reported unacceptably large dosing errors, while the remaining laboratory using orthovoltage x-rays did show significant improvement and managed to reach the original dosimetry goal of having dosing errors of 5% or less. Further, an additional surprise came from a laboratory using a 137Cs irradiator that after registering acceptable dosing errors of ≤5% using OSL dosimeters, reported significantly higher and clearly now non-compliant (in terms of original dosimetric goals) dosing errors in the range of ~10–14% during the later tests with TLD dosimeters. This laboratory acknowledged the dosimetry problem, initiated a full internal dosimetric review and subsequent corrective changes. Unfortunately, this laboratory declined the offer of having their irradiator retested by the MRRC/UW group.
The ‘bottom-line’ here is that when the final results of Phase 1 (OSL-based dosimetry) and Phase 2 (TLD-based dosimetry) tests were compared, similar fractions of ‘compliant’ and ‘non-compliant’ laboratories (~57% vs ~43% respectively) were noted, suggesting a commonality of test results, independent of the type of dosimeter applied.
Lessons learned and recommendations
Lessons were indeed both taught and learned as a consequence of this ‘dosimetry exercise’. First, a full range of proficiencies were noted among the participating laboratories: several were able to achieve reasonably precise exposures in a consistent manner from the very start of project, while others were able to achieve this dosimetry proficiency only after significant reassessments and readjustments of their dosimetry. Unfortunately, a few laboratories were simply unable to obtain the initially established goals, due possibly to the lack of dosimetry resources available to them, especially in terms of having a ‘in-house’ radiation dosimetrists on staff, or of having the essential funds to bring in, on a temporary basis, skilled staff or contractors in order to perform the necessary corrective dosimetric assessments and adjustments. One laboratory that initially failed the initial OLS-based dosimetry tests and lacked ‘in-house’ dosimetry support, found it necessary to bring in external service experts from the irradiator’s manufacturer: as a result, later dosimetry tests proved successful, with dosing errors substantially reduced to a bare minimum. The ‘take-home’ lesson here is that all prospective investigators proposing irradiated animal-based experiments need to carefully assess their own institutional resources in order to be able to perform full and proper dosimetry and to provide written assurances of these capabilities and resources within in their proposals prior to funding.
Further because of the noted dosimetric difficulties experienced by laboratories equipped with orthovoltage x-ray units, it is suggested, although quite hesitantly, that these IR-generating devices are perhaps not well suited and place investigators at a disadvantage when conducting animal-based, radiobiological studies. Gamma ray emitting 137Cs and 60Co sources, by contrast, have more predictable and uniform beam properties. As pointed out by one of RERF’s internal reviewers (in a pre-journal submission review), x-ray units produce a “….wide spectrum of photon energies that depend not only ….on the nominal peak kilovoltage of the electrical waveform….”, but also on a number of other critical factors, including “….the actual applied peak kilovoltage, the rectification of the waveform, the nature of the target inside the X-ray tube and the angle between the electron beam and target surface, and the inherent and added beam filtration.” Bearing in mind these factors, it is quite understandable why the orthovoltage x-ray units might be less predictable than the gamma ray generating IR sources when used in combination with energy-sensitive responses of OSL dosimeters.
Still another lesson is that all investigators need to recognize the fundamental importance of having a well-developed and properly executed dosimetry program for radiobiological studies. Relative to this project, the later ‘lesson’ specifically entailed not only a periodic developing, reviewing and updating IR exposure SOPs, but also a continued effort to perform ‘in house’ dosimetry and the certification of the accuracy and reliability of radiation dose estimates. The final lesson for individual investigators and program managers alike is that all essential details of experimental irradiation and associated dosimetry need to be incorporated into any/all progress reports and journal publications.
Acknowledgments
Funding
This work was supported by a National Institute of Allergy and Infectious Disease/National Institutes of Health contract (No. HHSN272200900059C) to the Radiation Effects Research Foundation, Hiroshima, Japan.
The authors would like to thank the following individuals for their assistance, guidance and discussions in various phases of this work: Dr Vitaly Nagy, Dosimetry Group, US Armed Forces Radiobiology Research Institute of the Uniform Services University, Bethesda, MD; Dr Terry Yoshizumi, Radiation Dosimetry Laboratory, Medical Center, Duke University, Durham, NC; Dr Wendell Lutz, University of Arizona, Tucson, AZ; Ms Lauren Young, Memorial Sloan Kettering Cancer Center, New York, NY; Drs. Mirela Kir, Mark Akselrod, and Mr Luke Carr, Dosimetry Laboratory, Landauer Inc., Glenwood, IL.
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- 1.Akselrod MS. Fundamentals of materials, techniques and instrumentation for OSL and FNTD dosimetry. In: Rosenfeld AB, Kron T, d’Errico F, Moscovitch M, editors. Concepts and Trends in Medical Radiation Dosimetry; AIP Conference Proceedings-1345; 2010 September 15–18; Wollongong, Australia. New York: AIP Publishing; 2011. pp. 274–302. [Google Scholar]
- 2.BER. Research: Biological Systems Science Division, US Department of Energy, Office of Science, Biological and Environmental Research. Radiobiology: Low Dose Radiation Research Program. 2015 [Cited/web posted 2015 March 31]. Available from: http://science.energy.gov/ber/research/bssd/
- 3.Cameron JR, Zimmerman D, Kenney G, Buch R, Bland R, Grant R. Thermoluminescent Radiation Dosimetry utilizing LiF. Health Phys. 1964;10:25–29. doi: 10.1097/00004032-196401000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Desrosiers M, DeWerd L, Deye J, Lindsay P, Murphy MK, Mitch M, Macchiarini F, Stojadinovic S, Stone H. The Importance of Dosimetry Standardization in Radiobiology. J Res Nat Inst Stand Tech. 2013;118:403–418. doi: 10.6028/jres.118.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kazi AM, MacVittie TJ, Lasio G, Lu W, Prado KL. The MCART Radiation Physics Core: The Quest for Radiation Dosimetry Standardization. Health Phys. 2014;106(1):97–105. doi: 10.1097/HP.0b013e3182a2a987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maidment BW. NIAID Rad/Nuc Research Program- Radiation dose is more than a number workshop; 15–15 September 2011; 2011. [Cited/web posted 2012 August 21]. Available from: http://www.nist.gov/pml/div682/grp02/upload/FT4MaidmentR1.pdf. [Google Scholar]
- 7.Moscovitch M. Dose Algorithms for Personal thermoluminescence dosimetry. Rad Prot Dosimetry. 1993;47:373–380. [Google Scholar]
- 8.Moscovitch M. Personal Dosimetry using Lif:Mg,Cu,P. Rad Prot Dosimetry. 1999;85:49–56. doi: 10.1093/oxfordjournals.rpd.a005983. [DOI] [PubMed] [Google Scholar]
- 9.Murphy MK. Physics framework- Why is dosimetry and standardization important in radiobiology? – The Physics framework. NIAID/NCI/NIST Workshop on Radiation Dosimetry Standardization for Radiobiology; September 15 – 16, 2011; NIST; 2011. [Cited/web posted 2012 August 21]. Available from: http://www.nist.gov/pml/div682/grp02/upload/FT2Murphy.pdf. [Google Scholar]
- 10.NIAID. National Institute of Allergy and Infectious Diseases (NIAID): Radiation Nuclear Countermeasures: Strategic Planning. 2005 [Cited/web posted 2005 September 14]. Available from: http://www.niaid.nih.gov/topics/radnuc/program/pages/strategicplanning.aspx.
- 11.NIAID. NIAID Announces New Award to Study the Effects of Radiation and Aging on the Human Immune System, NIH News. 2009 [Cited/web posted 2009 November 12]. Available from: http://www.niaid.nih.gov/news/newsreleases/archive/2009/Pages/RERF.aspx.
- 12.NIAID. NIH/NIAID Radiation and Nuclear Medical Countermeasures Development Program. National Institutes of Health, National Institute of Allergy and Infectious Diseases; 2012. [Cited/web posted 2012 September]. Available from: http://www.niaid.nih.gov/topics/BiodefenseRelated/RadiationCountermeasures/Documents/Rad_Nuc_Counter_Tagged.pdf. [Google Scholar]
- 13.NIST. National Institute of Standards and Technology (NIST). Dosimetry Standardization for Radiobiology, NIST workshop; September 15–16, 2011; 2011. [Cited/web posted 2011 June 10]. Available from: http://www.nist.gov/pml/div682/grp02/dosimetry-standardization-for-radiobiology.cfm. [Google Scholar]
- 14.RADCore 2010. RADCore small animal dosimetry workshop. Duke University; Durham, NC: 2010. Organized/chaired: M Robbins, D Bourland, and T Yoshizumi, May 20, 2010. [Google Scholar]
- 15.RERF-NIAID. Kickoff Meeting for research contract entitled ‘Studies Immunosenescence and Other Late Effects of Acute Ionizing Radiation Exposure in A-bomb Survivors’; December 9 2009; Bethesda, MD 20892: Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases, National Institutes of Health; 2009. [Google Scholar]
- 16.Roué A1, Van Dam J, Dutreix A, Svensson H. The EQUAL-ESTRO external quality control laboratory in France. Cancer Radiother. 2004;8(Spl 1):S44–49. [PubMed] [Google Scholar]
- 17.Seed TM. Standardization methodologies: a dosimetry exercise for the NIAID-RERF project. Dosimetry Standardization for Radiobiology, NIST workshop; September 15–16, 2011; 2011. [Cited/web posted 2012 August 21]. Available from: http://www.nist.gov/pml/div682/grp02/upload/FT14Seed.pdf. [Google Scholar]
- 18.Yegingil Z, DeWerd LA, Davis SD, Hammer C, Kunugi K. Photon beam audits for radiation therapy clinics: a pilot mailed dosimeter study in Turkey. Radiat Prot Dosimetry. 2012;148(2):249–257. doi: 10.1093/rpd/ncr017. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizumi T, Brady SL, Robbins ME, Bourland JD. Specific issues in small animal dosimetry and irradiator calibration. Int J Radiat Biol. 2011;87:1001–1010. doi: 10.3109/09553002.2011.556178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yukihara EG, McKeever SW. Optically stimulated luminescence (OSL) dosimetry in medicine. Phys Med Biol. 2008;53(20):351–379. doi: 10.1088/0031-9155/53/20/R01. [DOI] [PubMed] [Google Scholar]
- 21.Yukihara EG, McKeever SWS. Optically Stimulated Luminescence: Fundamentals and Applications, Chapter 3 Personal dosimetry, Sections 3.3.2, 3.3.3 and 3.5.2.3. John Wiley and Sons Inc; Hoboken, NJ: 2011. [Google Scholar]
- 22.Zoetelief J, Hennen LA, Broerse JJ. Some practical aspects of dosimetry and dose specification for whole body irradiation. In: Broerse JJ, Macvittie TJ, editors. Response of different species to total body irradiation. Dordrecht, NL: Martinus Nijhoff Publishers; 1984. pp. 3–28. [Google Scholar]
- 23.Zoetelief Broerse JJ, Davies RW. Protocol for X-ray dosimetry, EULEP Report EUR 9507 EN, Supplements VII & VIII. Commission of the European Communities, Office for Official Publications of the European Communities; Luxembourg: 1985. pp. 71–76. [Google Scholar]
- 24.Zoetelief J, Broerse JJ, Davies RW, Octave-Prignot M, Rezvani M, Vergara JC, Toni MP. Protocol for X-ray dosimetry in radiobiology. Int J Radiat Biol. 2001;77(7):817–835. doi: 10.1080/09553000110050605. [DOI] [PubMed] [Google Scholar]