Abstract
Objective
Because models of attention-deficit/hyperactivity disorder (ADHD) therapeutics emphasize benefits of both enhanced dopaminergic and noradrenergic signaling, strategies to enhance D1 and alpha2A agonism may yield enhanced clinical and cognitive responses. The study tested the hypothesis that combined effects of a dopamine and noradrenergic agonist, d-methylphenidate extended-release (DMPH), with guanfacine (GUAN), an alpha2A receptor agonist, would be clinically superior to either monotherapy, and have equal tolerability.
Method
An 8-week, double-blind, three-arm comparative trial randomized 7- to 14-year-olds with DSM-IV ADHD to GUAN (1-3 mg/day), DMPH (5-20 mg/day), or the combination (COMB) with fixed-flexible dosing. Outcome measures were the ADHD Rating Scale IV (ADHD-RS-IV) and the Clinical Global Impression-Improvement (CGI-I) Scale. Adverse events and safety measures were obtained.
Results
207 participants were randomized and received drug. Analyses showed significant treatment group main effects for ADHD-RS-IV ADHD total (p = .0001) and inattentive symptoms (p = .0001). COMB demonstrated small but consistently greater reductions in ADHD-RS-IV Inattentive subscale scores versus monotherapies (DMPH: p = .05; f2 = .02; and GUAN: p = .02; f2 = .02), and was associated with a greater positive response rate by CGI-I (p = .01). No serious cardiovascular events occurred. Sedation, somnolence, lethargy, and fatigue were greater in both guanfacine groups. All treatments were well tolerated.
Conclusion
COMB showed consistent evidence of clinical benefits over monotherapies, possibly reflecting advantages of greater combined dopaminergic and alpha2A agonism. Adverse events were generally mild to moderate, and COMB treatment showed no differences in safety or tolerability.
Keywords: ADHD, children, guanfacine, methylphenidate, αalpha2A
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is the most commonly diagnosed mental disorder in children, with a prevalence of 9%;1 some 2.8 million US children receive psychostimulant medication. ADHD shows diagnostic persistence (30-70%) and morbidity into adulthood.2-4 ADHD outcomes vary regardless of treatment: when persistent, ADHD impacts nearly all domains of functioning.3-6
Current ADHD medications, including psychostimulants and guanfacine, robustly ameliorate ADHD symptoms in the short term, with “responder” rates of 65 - 75% 7,8 and 50-67.5% 9,10,11 for stimulants and guanfacine, respectively. 12,13 Despite symptom reduction with monotherapies, there is little evidence to show that medications change long-term trajectories of either symptoms or academic, psychiatric, and social outcomes, 14-19 although some acute 17,20 and follow-up studies find modest academic gains. 21,22 The discordance between symptom reduction from standard treatments and continued impaired functioning long-term highlights the importance of identifying treatments that better remediate proximal causes of negative outcomes.
Closer examination of ADHD monotherapy outcomes reveals likely explanations for lack of disorder-modifying effects. Besides substantial number of non-responders and those with intolerable side effects, too few patients experience “normalization” of ADHD with monotherapy. 7,24 Normalization rates vary by domain: symptomatic normalization with methylphenidate was observed in 58% of children with ADHD in one report, 50% of participants showed normalization of inattention, yet only 30% demonstrated normalized academic efficiency.20 In the Multimodal Treatment of ADHD study (MTA), even with rigorous dose optimization, only 56% achieved symptomatic remission. 23 Such findings contrast with recommendations that remission of ADHD should be a goal. 7
Recommendations for greater symptom reduction spring from observations that residual symptoms are associated with negative outcomes in school, social, and emotional domains. 2,24 A variety of clinical and contextual factors influence outcomes, but relevant to medication treatment, cognitive functioning has also emerged as important. 25-28
Medication effects on cognitive control, encompassing working memory (WM) and inhibitory control, are crucial to consider, given the robust association of their deficits with ADHD, 29,30 and influence on outcomes.25-28 Effects of stimulants on cognition in ADHD are more variable than symptom reduction. 31-33 Data on cognitive effects of guanfacine monotherapy in pediatric ADHD34are sparse: no data are available on cognitive effects of combined stimulant plus alpha agonists.
Neurochemical models of ADHD and its treatment emphasize catecholaminergic influences on prefrontal cortical functioning (PFC). 35,36 Stimulants are thought to improve PFC function via increasing endogenous dopamine (DA) stimulation, especially at D1 receptors, 37 and increasing norepinephrine (NE) at α2-adrenergic receptors. 37 Guanfacine appears to exert beneficial PFC effects through selective post-synaptic α2A–agonism. 38 Agonists at both D1 and α2A receptors produce inverted U-shaped WM functions. 39,40 In some models, optimal DA stimulation of cortical D1 receptors paired with D2 inhibition of nigral-striatal activity are theorized to underlie stimulant benefits, 41 augmented by noradrenergic effects on reaction time and switching deficits. 42 Taken together, these models suggest that treatments with optimized D1 and α2A agonism may yield superior effects on clinical and cognitive targets. 36
The purpose of this study was to test the hypothesis that clinical and cognitive responses in ADHD to combined treatment robustly enhancing both DA and NE would be superior to monotherapies.35,40 Therefore, we assessed the relative efficacy of a psychostimulant (d-methylphenidate extended-release [DMPH]), versus an α2A agonist guanfacine (GUAN), versus their combination (COMB) on ADHD symptoms, clinical response rates, and cognitive outcomes in an 8-week randomized, blinded, comparative trial, using a hybrid within-between subjects design with sequential addition of medications to maximize power. Effects on cognition and electroencephalography (EEG) correlates are presented in separate reports (Bilder et al, under review; Loo et al, under review).
METHOD
Participants
The sample consisted of children and adolescents aged 7 to 14 years old who were diagnosed with ADHD. All participants were enrolled in the Translational Research to Enhance Cognitive Control (TRECC) ADHD study (ClinicalTrials.gov Identifier: NCT00429273). Participants were recruited from clinic referrals, radio and newspaper advertisements, community organizations (CHADD; www.chadd.org), local schools, and primary care physicians. Parents and participants provided written informed permission and assent. All study procedures were approved by the University of California, Los Angeles (UCLA) institutional review board and overseen by a data safety and monitoring board.
Inclusion criteria were 1) male or female aged 7-14 years; 2) DSM-IV ADHD (any subtype) diagnosed by semi-structured diagnostic interview (Kiddie-Schedule for Affective Disorders and Schizophrenia-PL [K-SADS-PL]) 43 and clinical interview; and 3) Clinical Global Impression—Severity (CGI-S) score ≥ 4 for ADHD.
Exclusion criteria were 1) autistic disorder, chronic tic disorder, psychosis, bipolar disorder, or structural heart defects; 2) current major depression or panic disorder; 3) systolic or diastolic blood pressure > 95th or <5th percentile for age and body mass index (BMI); 4) medical condition contraindicating stimulants or alpha agonists; 5) need for chronic use of other central nervous system (CNS) medications.
Study Design
The study was an 8-week randomized, eight-week double-blind randomized controlled trial with four treatment conditions: Placebo only, DMPH: d-methylphenidate extended-release (5-20 mg/day) + placebo; GUAN: guanfacine (1-3 mg/day) + placebo; COMB: treated combination of guanfacine and DMPH. We employed a hybrid within-between subject experimental design, in which each participant was assessed sequentially in at least two different treatment conditions, and maximally to three of the four treatment conditions. Due to ethical and practical considerations, the order of the treatment conditions was not counterbalanced; instead, participants were randomized in a 1:1:1 ratio to three different treatment sequences covering the periods baseline, weeks 1-4 and 5-8:
Sequence 1: Placebo ---- Placebo + DMPH.
Sequence 2: Guan ---- Guan + Placebo.
Sequence 3: Guan ---- Guan + DMPH.
Thus, during the first four weeks, two thirds of the participants received guanfacine (sequences 2 and 3) while one third of the participants were given a placebo (sequence 1); beginning in week 5, participants initially given guanfacine added either DMPH (sequence 3) or a placebo (sequence 2) to their regimen, while those who started on placebo added DMPH (sequence 1).
Guanfacine (immediate release form, as guanfacine extended release was not available at beginning of study) and placebo dosing mirrored titration schedules in prior trials. 9,10 Dosing clinicians were blind to group assignment. Initial dosing was one capsule (0.5 mg or placebo) twice daily for week 1; in week 2 dose increased to 1 mg twice daily as tolerated; week 3 doses advanced to 1.5 mg twice daily as tolerated. If Clinical Global Impression-Improvement (CGI-I) ratings were either “1” or “2” (much or very much improved), no dose increase was made; no dose increases were allowed after week 3. Optimal guanfacine dose was determined by CGI-I ratings, ADHD Rating Scale-Version IV (ADHD-RS-IV) 45, and side effects at end of week 3, by consensus agreement of two independent study clinicians, and was defined as lowest dose providing maximal improvement while minimizing adverse events. A minimum total daily dose of 1.0 mg was required for continuation.
DMPH (dual-beaded, extended-release formulation) dosing followed published experience. 44 Two dose schedules were based upon baseline weight. Participants <25 kg received one capsule (5 mg or placebo) of DMPH once daily for week 5; week 6 was advanced to 10 mg DMPH daily; and week 7 moved to 15 mg daily. For participants ≥25 kg, DMPH began with 10 mg DMPH once daily; week 6 advanced to 15 mg daily; week 7 moved to 20 mg daily. No dose increases were allowed after week 7. Low, medium, and high stimulant doses were assessed weekly for behavioral response and tolerability to determine the “optimal” DMPH according to the identical process described above
We note that this differs by degree from a traditional 3-arm longitudinal design with each arm receiving a specific treatment over the entire study period, but this is a hybrid sequential within-between subjects design. This design arose from the clinical and ethical need to keep trial length and placebo-only exposure to a minimum. However, the within-subject component also increases statistical power. Moreover, because prior reports show maximal benefit from both treatments within 3-4 weeks, this design allows us to compare the medication conditions (placebo, GUAN, DMPH, COMB) by combining all participants and time-points for which that condition occurred, after adjusting for overall drift.
Outcome Measures
We utilized ADHD behavioral ratings from independent evaluators and parents, vital signs, electrocardiograms (EKG), adverse event recordings, a cognitive battery (Bilder et al, under review), and other secondary measures, including repeated EEGs (Loo et al, under review).
Symptom Assessments
A blinded clinician without knowledge of adverse events completed the CGI-S and ADHD-RS-IV at baseline and at the end of each within-subject condition or last visit based on parent, participant, and other available data. A treating clinician completed the CGI-I at baseline and each week.
Side Effect Assessments
Side effects were measured via a structured instrument, using a modification of the Physical Symptoms Checklist,46 and open-ended clinician inquiry.
Clinical Endpoints
The primary clinical efficacy variables for treatment were the three scores from the ADHD-RS-IV (Inattentive and Hyperactive-Impulsive subscales, and total score) and the CGI-I. Our primary definition of treatment response was CGI-I 1 or 2 (“very much improved” or “much improved”) versus non-responder. We also examined a second response criterion, adding a reduction in ADHD-RS-IV total scores of ≥30% from baseline to Week 8 to the initial CGI-I criterion. 14
Statistical Analyses
All analyses were conducted in SAS 9.2. For the longitudinal models, we used Proc Mixed, with a compound symmetric covariance structure to account for repeated measurements within participants. The data were modeled with treatment condition (DMPH, GUAN, COMB, or untreated/placebo [UN/PBO]) and time as within-subject effects, and age as a participant-level covariate. Note that in this model, the placebo effect is confounded with the time effect and cannot be estimated separately, as the change from baseline to week 4 for participants in Sequence 1 (the group with multiple measurements in the untreated/placebo condition) could be equally explained by effects of re-testing or placebo effects. The pairwise comparisons between the different treatment conditions are 1 degree of freedom contrasts of the condition effect in this model. All tests used a two-sided significance level of α=.05. For the pairwise comparisons of the treatment conditions, we report Cohen's f2 effect size difference, which is recommended for analyses involving F tests or analysis of variance (ANOVA) models.47 Small, medium, and large f2 values are traditionally defined as .02, .15, and .35, respectively.
For the analyses of treatment response, we used Pearson chi-squared tests, comparing the rates in the three treatment sequences (ending with GUAN alone, DMPH alone, or COMB), along with number needed to treat (NNT)48 as the effect size. For NNT, small, medium, and large effects are defined as 9, 4, and 2, respectively. 48
RESULTS
Demographics and Disposition
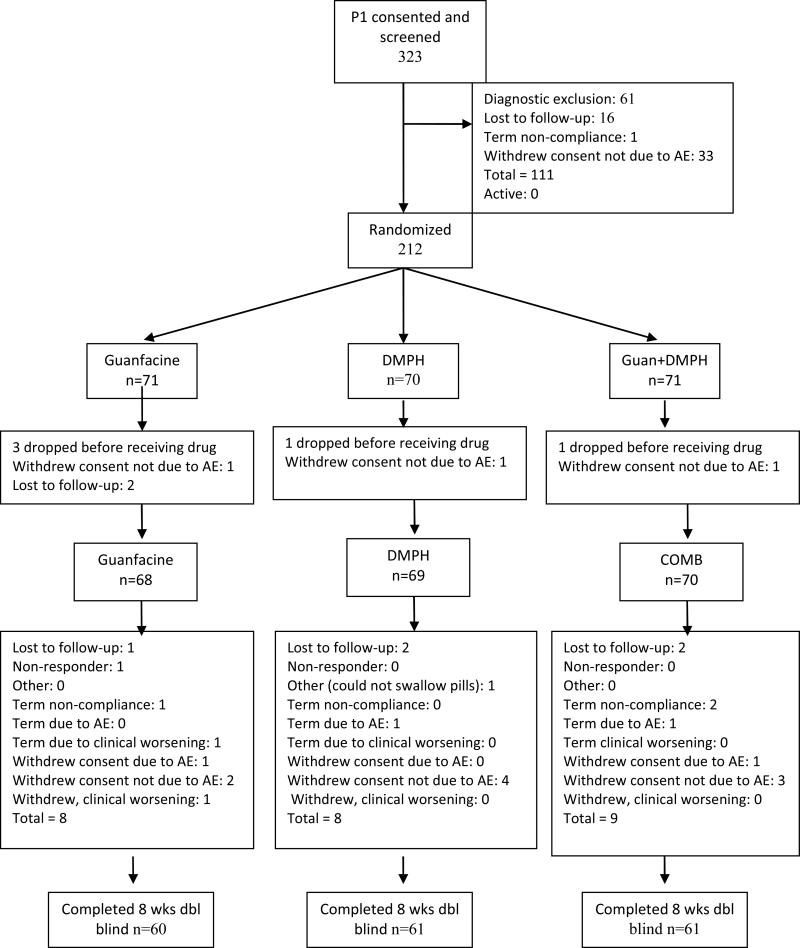
A total of 212 participants were deemed eligible and randomized (Figure 1). Of those, 207 received at least one dose of study drug and form the safety and intent-to-treat samples (Table 1). Mean age was 10.0 (SD ± 2.1 years) and 142 (68%) were male. No significant group differences were found for age, IQ, sex, ADHD subtype, height, weight, % oppositional defiant disorder (ODD) comorbidity, or baseline ADHD-RS-IV scores. Mean final (week 8) daily doses of DMPH were 16.0 (± 3.9) mg for DMPH-only and 15.1 (± 4.8) mg for COMB. Mean final daily doses of guanfacine were 2.2 (± 0.7) mg for GUAN-only and 2.4 (± 0.6) mg for COMB. Mean mg/kg daily doses of guanfacine were 0.06 (± 0.03) mg/kg/d for both guanfacine groups. Exposures to the two treatments were comparable to prior studies.9-11,20,32,49,50 A total of 87.8% of the randomized sample completed the 8-week trial.
Figure 1.
Disposition (Consolidated Standards of Reporting Trials [CONSORT]) for all enrolled study participants. Note: AE = adverse event; COMB = combination; DMPH = d-methylphenidate extended-release; GUAN = guanfacine.
Table 1.
Participant Demographics by Assigned Treatment Groupa
| GUAN | DMPH | COMB | Total | ||
|---|---|---|---|---|---|
| (n=68) | (n=69) | (n=70) | (n=207) | ||
| Age, mean (SD), yrs | 10.1±2.1 | 10.1±2.0 | 9.9±2.2 | 10.0 (2.1) | |
| Sex, n (%) | Male | 45(66.2) | 46(66.7) | 51(72.9) | 142(68) |
| Race/Ethnicity, n (%) | |||||
| White | 51(75.0) | 51(73.9) | 41(58.6) | 143 (69) | |
| Black | 7(10.3) | 10(14.5) | 19(27.1) | 36(17) | |
| Asian, Pacific Islander | 7(10.3) | 4(5.8) | 5(7.1) | 16 (8) | |
| Other | 3(4.4) | 4(5.8) | 5(7.1) | 12 (6) | |
| Hispanic | 16(23.5) | 10(14.5) | 18(25.6) | 44 (21.3) | |
| Weight, mean (SD) kg | 39.8(17.5) | 41.6(16.2) | 40.0(16.7) | 40.5 (16.7) | |
| Height, mean (SD), cm | 140.6(14.4) | 142.9(13.6) | 141.2(14.4) | 141.6 (14.1) | |
| Full Scale IQ | 102.6(14.2) | 101.5(13.3) | 102.9(13.0) | 102.4 (13.5) | |
| ADHD Subtype, n (%) | |||||
| Inattentive | 28 (41) | 33 (48) | 31 (44) | 92 (44) | |
| Hyperactive/Impulsive | 1 (2) | 2 (3) | 2 (3) | 5 (2) | |
| Combined | 38 (56) | 32 (46) | 35 (50) | 105 (51) | |
| ADHD-RS-IV Baseline, mean (SD) | |||||
| Inattentive | 21.3 (.7) | 21.2 (.7) | 20.3 (.7) | ||
| Hyperactive/Impulsive | 15.9 (.8) | 17.2 (.8) | 14.7 (.8) | ||
| Combined | 37.1 (1.2) | 35.3 (1.2) | 35.1 (1.2) | ||
| Comorbidity, n (%) | |||||
| ODD | 27 (40) | 24 (35) | 17 (24) | 68 (33) | |
Note: ADHD = attention-deficit/hyperactivity disorder; ADHD-RS-IV = ADHD Rating Scale IV; COMB = combination; DMPH = d-methylphenidate extended-release; GUAN = guanfacine; ODD = oppositional defiant disorder.
Forms efficacy and safety samples. No significant differences between groups, all p >.05
Efficacy Measures
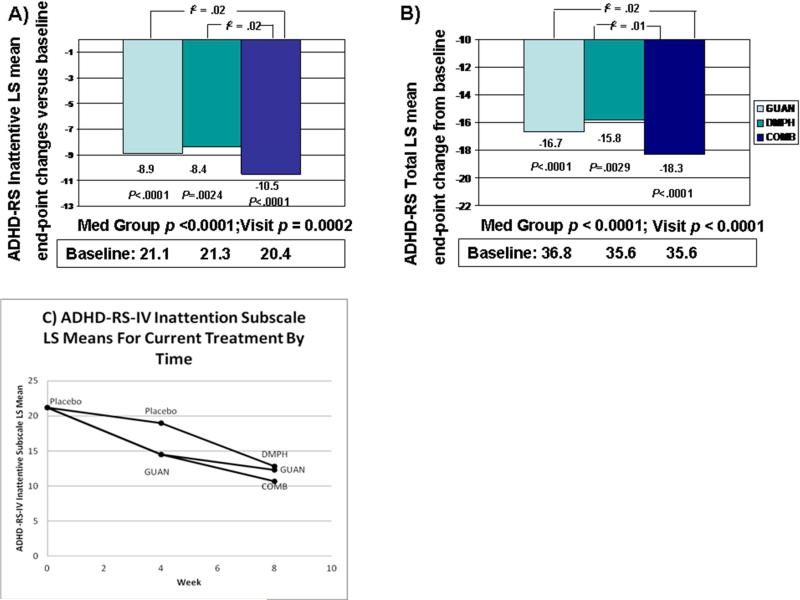
Results of the mixed model analyses are presented in Figure 2 (A, B, C), and Table 2 (additional symptom data in Table S1, available online). There was strong agreement between the raw means and model estimates, suggesting excellent model fit. The corresponding figures show the changes in estimated means by treatment condition from baseline and over time. All participants showed improvement in their ADHD-RS-IV total score over time, independent of treatment condition as demonstrated by the significant effect for visit (F=18.90, df=1, p<.0001), and significant effects of treatment condition (F=10.14, df=3, p<.0001). Examination of the ADHD-RS-IV Inattentive subscale scores also showed improvements with significant effects for visit (F=14.20, df=1, p=.0002) and differences by treatment condition (F=11.28, df=3, p<.0001). Similar effects were seen in ADHD-RS-IV Hyperactive-Impulsive subscale scores for visit (F=12.42, df=1, p = .0005) and for treatment condition (F=4.09, df=3, p=.0069).
Figure 2.
A) Changes in least squares means for Attention-Deficit/Hyperactivity Disorder (ADHD) Rating Scale IV (ADHD-RS-IV) Inattentive subscale scores from baseline to week 8 for the three randomized treatment groups; B) Changes in least squares means for ADHD-RS-IV Total scores from baseline to week 8 for the three randomized treatment groups; C) Least square mean ADHD-RS-IV Inattention subscale scores by current treatment condition from baseline to week 8. Note: COMB = combination; DMPH = d-methylphenidate extended-release; GUAN = guanfacine.
Table 2.
Pairwise Comparisons of Least Squares Mean Attention-Deficit/Hyperactivity Disorder Rating Scale IV (ADHD-RS-IV) Scores by Assigned Treatment Conditions
| ADHD-RS Total Score | Estimated Difference | F | P value | Effect size f2 |
|---|---|---|---|---|
| COMB vs Placebo | −10.66±1.99 | 28.68 | <.0001 | 0.12 |
| COMB vs GUAN | −2.67±1.35 | 3.93 | .049 | 0.02 |
| COMB vs DMPH | −2.89±1.56 | 3.44 | .065 | 0.01 |
| GUAN vs DMPH | −0.21±1.31 | 0.03 | .87 | 0.0001 |
| GUAN vs Placebo | −7.99±1.22 | 42.43 | <.0001 | 0.18 |
| DMPH vs Placebo | −7.77±1.70 | 20.98 | <.0001 | 0.01 |
| Inattention Subscale | Estimated Difference | F | P value | Effect size f2 |
|---|---|---|---|---|
| COMB vs Placebo | −5.89±1.15 | 26.08 | <.0001 | 0.11 |
| COMB vs GUAN | −1.79±0.79 | 5.21 | .023 | 0.02 |
| COMB vs DMPH | −1.74±0.90 | 3.75 | .054 | 0.02 |
| GUAN vs DMPH | 0.049±0.76 | 0 | .95 | 0 |
| GUAN vs Placebo | −4.10±0.71 | 33.26 | <.0001 | 0.14 |
| DMPH vs Placebo | −4.14±0.99 | 17.41 | <.0001 | 0.01 |
| Hyperactive-Impulsive Subscale | Estimated Difference | F | P value | Effect size f2 |
|---|---|---|---|---|
| COMB vs Placebo | −5.10±1.12 | 20.75 | <.0001 | 0.09 |
| COMB vs GUAN | −1.10±0.75 | 2.15 | .14 | 0.01 |
| COMB vs DMPH | −1.37±0.87 | 2.45 | .12 | 0.01 |
| GUAN vs DMPH | −0.27±0.72 | 0.14 | .71 | 0.01 |
| GUAN vs Placebo | −4.0±0.69 | 33.59 | <.0001 | 0.14 |
| DMPH vs Placebo | −3.73±0.92 | 16.32 | <.0001 | 0.01 |
Note: COMB = combination; DMPH = d-methylphenidate extended-release; GUAN = guanfacine.
To examine the specific nature of the treatment effects, one degree of freedom contrasts were used to compare each pair of medication conditions (Table 2). COMB showed superiority for total ADHD-RS-IV total score versus GUAN (t = −1.99, p = .049, two-tailed; f2 = .02), but did not differ statistically from DMPH (t = −1.84, p=.066; f2 = .01). For ADHD-RS-IV Inattentive subscale scores, COMB again showed superiority over GUAN (t = −2.28, p = .02, two-tailed; f2 = .02) as well as a trend of greater improvement than DMPH (t = −1.94, p = .05; f2 = .02); both differences yielded small but consistent effect size estimates. Examination of estimated marginal means shows that compared to the initial placebo-only condition and controlling for time, ADHD-RS-IV Total scores are substantially lower in the GUAN (− 46%), DMPH (− 44%), and COMB (− 51%) conditions.
There were significant differences in treatment response (CGI-I “very much improved” or “much improved”) for the three treatment sequences, with rates of 81% for Sequence 1 (ending with DMPH alone), 69% for Sequence 2 (ending with GUAN alone), and 91% for Sequence 3 (COMB) (x2 = 8.55, df = 2, p = .01) (NNT 4.6: COMB versus GUAN; NNT 10: COMB versus DMPH). Using the combined definition of treatment response based on CGI-I and end-of-trial ADHD-RS-IV total scores gave rates of 62%, 63%, and 75%, respectively (NNT 8.5: COMB versus GUAN; NNT 7.5: COMB versus DMPH).
Safety Assessments
Treatment-emergent adverse events (TEAEs) with a frequency of ≥ 10% occurring at any time are shown in Table 3. Overall rates for any TEAE (mild, moderate, and severe) were high, but did not differ between groups. No serious AEs occurred during the trial. Most TEAEs were mild to moderate in severity. Discontinuation at any time due to TEAEs was low and equivalent across groups: 1.5% (1/68) in GUAN, 1.5% (1/69) in DMPH, and 2.9% (2/70) in COMB. Discontinuation at any time due to lack of efficacy or clinical worsening was uncommon, with only 2.9% (2/68) in GUAN, none in DMPH, and 1.4% (1/70) in COMB discontinuing due to these outcomes.
Table 3.
All Treatment-Emergent Adverse Events Occurring During the Double-Blind Phase by Randomized Treatment Groups
| Adverse Event | COMB (N=70) | DMPH (N=69) | GUAN (N=68) | P Value |
|---|---|---|---|---|
| Any adverse event | 69 (98.6) | 66 (95.7) | 66 (97.1) | .93 |
| Neuropsychiatric Disorders | ||||
| Headache | 23(32.9) | 23(33.3) | 34(50.0) | .10 |
| Irritability | 18(25.7) | 12(17.4) | 15(22.1) | .49 |
| Sedation | 16(22.9) | 4(5.8) | 12(17.6) | .02 |
| Somnolence | 15(21.4) | 3(4.3) | 16(23.5) | .01 |
| Affect lability | 8(11.4) | 14(20.3) | 7(10.3) | .16 |
| Gastrointestinal Disorders | ||||
| Abdominal pain | 16(22.9) | 18(26.1) | 19(27.9) | .83 |
| Abdominal pain upper | 10(14.3) | 10(14.5) | 11(16.2) | .97 |
| Sleep Disturbance | ||||
| Insomnia 24(34.3) 20(29.0) 18(26.5) .53 | ||||
| Metabolism and Nutrition Disorders | ||||
| Decreased appetite 25(35.7) 31(44.9) 15(22.1) .01 | ||||
| General Disorders | ||||
| Lethargy | 16(22.9) | 9(13.0) | 23(33.8) | .02 |
| Fatigue | 12(17.1) | 5(7.2) | 22(32.4) | .001 |
| Vascular Disorders | ||||
| Dizziness | 7(10.0) | 6(8.7) | 8(11.8) | .87 |
Note: COMB = combination; DMPH = d-methylphenidate extended-release; GUAN = guanfacine.
Cardiovascular indices at week 8 showed small changes from baseline. Mean sitting pulse rate declined by −4.1 (12.6) bpm for GUAN, increased 3.6 (15.7) bpm for DMPH, and 4.6 (16.7) bpm for COMB. Mean systolic blood pressure declined by −2.2 (13.8) mm Hg for GUAN, increased by 7.3 (11.5) mm Hg for DMPH, and 3.5 (14.1) mm Hg for COMB. Mean diastolic blood pressure declined by −3.8 (11.4) mm Hg for GUAN, increased by 5.8 (10.4) mm Hg for DMPH, and 3.7 (9.9) mm Hg for COMB. Changes in the QTcB were minor, with a decrease of −2.7 (16.4) msec for GUAN, an increase of 9.9 (22.5) msec in the DMPH group, and a decrease of −4.4 (20.8) msec for COMB. No participant demonstrated a QTcB interval of ≥500 msec or an increase of >60 msec during the trial. No clinically significant laboratory findings were observed.
DISCUSSION
This study represents a novel test of a theory-driven combination of two drugs with distinct mechanisms of action for ADHD versus standard monotherapies. It is the first report to our knowledge to directly test the benefits of the combination to monotherapies in a sample not ascertained to be partially stimulant responsive.49,50 Results from this comparative trial provide modest but cogent evidence for the clinical superiority of the combination treatment of a stimulant and an α2A agonist for the treatment of ADHD. On clinical global measures of response and ADHD symptoms (especially inattentive symptoms), combination treatment in most comparisons showed additional benefits without any evidence of diminished tolerability, safety concerns, or increased side effect burden. The symptomatic benefits of combination treatment could yield improvements in longer-term outcomes, given the role of residual ADHD symptoms on functioning.2,24 Results with this combination, based on neurochemical models of ADHD, add support for the “dual” model of the importance of optimized dopaminergic and noradrenergic effects in ADHD therapy.35,62
However, a deeper discussion of the effects of combination treatment is needed to support a determination of “superiority” over monotherapies. These comparisons focus solely on acute clinical effects; our initial interest in combination was its hypothesized superiority for both clinical and cognitive outcomes. A more comprehensive comparison admittedly requires joint consideration of broader effects, and at an individual level. EEG effects suggest COMB exhibited greater normalizing effects than the monotherapies, by decreasing theta and increasing beta frequency band power (Loo et al, under review). That said, on clinical outcomes alone, in keeping with our hypotheses, COMB showed small but consistently greater reductions in ADHD-RS-IV Inattentive subscale scores to both monotherapies, and surpassed GUAN on ADHD-RS-IV total score comparison, again showing consistent, albeit small, effect size differences on these dimensional endpoints. Despite the small separation on symptom scores versus GUAN, COMB emerged robustly superior by a 22% difference to GUAN on responder comparisons (NNT = 4.6), a moderate effect, with DMPH intermediate (NNT = 10). Importantly, tolerability did not differ by condition.
Can these small effect size differences be clinically significant? As pointed out by Kraemer and Kupfer48, treatments with equivalent risk and burden may be deemed clinically superior even with effect size differences far less than the small to medium effects we have documented for COMB versus both monotherapies. For comparison, our approximate 3-point least squares mean difference between COMB and the monotherapies is equivalent to the 3-point difference reported in the MTA between medication management versus behavior treatment groups on teacher Swanson, Nolan, and Pelham (SNAP) total scores.
Supporting a role for α2A modulation in ADHD treatment, our findings add to extant data on efficacy of an α2A agonist, guanfacine. Guanfacine has emerged as approved ADHD monotherapy for children and adolescents with ADHD with moderate efficacy. 9,10,11 Another less selective α2 agonist, clonidine, has benefits on ADHD symptoms from controlled trials in children with ADHD with 51 and without tic disorders52 and as an adjunctive treatment in children with ADHD partially responsive to stimulants.54,55 Not surprisingly, guanfacine alone showed efficacy in reducing total ADHD symptoms (ADHD-RS total − 46% from baseline) in our trial, with a very good clinical response rate (69% improved by CGI-I), nearly identical to a flexibly-dosed monotherapy trial in adolescents (68%).11
The two pivotal trials of guanfacine extended release for ADHD using a randomized, fixed-dose design also showed remarkably comparable reductions in total ADHD symptom reduction by ADHD-RS (45% and 49%), with global responder rates of 54 - 56% and 50 – 56%.9,10 Overall, our efficacy data with guanfacine appears on par with the monotherapy guanfacine extended release trials, with our greater global response rates likely reflecting our younger sample and our use of a fixed-flexible titration scheme, optimizing on clinical response. Notably, the one pivotal trial of guanfacine extended release that reported analyses by ADHD subtype found that those participants with ADHD, Inattentive subtype (26% of all participants) in their sample showed no statistically significant benefit of guanfacine.10 Such a finding suggests that guanfacine addition in our sample, with its higher proportion (44%) of participants with inattentive ADHD, would be predicted to yield less additive benefit. In that light, our observed COMB effects could represent an underestimate of the beneficial effects of COMB in individuals with combined ADHD, and also support our focus on change in ADHD-RS-IV Inattentive symptoms where COMB showed greater separation from both monotherapies.
DMPH monotherapy showed expected clinical benefits with moderate reductions in ADHD symptoms and an intermediate clinical global response rate of 81% between the two comparison arms. Our observed responder rate is comparable to similar trials12 including the 79% responder rate in a comparative stimulant trial. 53 Yet the addition of a selective α2A to DMPH boosted comparative efficacy versus DMPH on most measures. Pairwise comparisons of DMPH to the COMB condition found COMB to be statistically superior to DMPH on ADHD-RS-IV inattentive symptoms and global response rates with small to moderate effect size differences.
While overall response rates by CGI-I in our trial were high, examination of the other commonly applied combined categorical + dimensional response rate (CGI-I 1 or 2, “very much” or “much” improved, and >30% reduction in ADHD-RS-IV from baseline) is informative. Both monotherapies yielded expected improvement rates of 62% and 63%, with COMB boosting response to 75% (NNT 7.5 and 8.5, versus DMPH and GUAN, respectively). The impact of these differences in “excellent” responses, though admittedly small to moderate effects, need to be examined over time to discern their clinical significance. However, at the very least, they underscore the continued challenge in ADHD therapeutics to achieve more robust, trajectory-changing outcomes.
The clinical implications of our results suggest that combination of DMPH and GUAN over 8 weeks is associated with substantial clinical benefits on ADHD symptoms in the short term, and is more successful at approaching contemporary goals for ADHD treatment. Our data, taken together with data from positive combined trials showing beneficial effects of guanfacine and clonidine addition to stimulants in ADHD partial responders,49,50 and combined treatment in children with ADHD and chronic tic disorders,51 provide solid evidence for the clinical benefits of combined stimulant and α–adrenergic agonist treatment.
Our results also support the acceptable safety profile of COMB treatment. Adverse events observed were consistent with the literature for both monotherapies.44,56,57 Guanfacine, as a monotherapy and as COMB, was associated with a greater frequency of sedation, fatigue, and somnolence. Most such adverse events were mild or moderate in severity, and very few participants discontinued due to any adverse events. There was no signal that COMB was associated with a greater adverse event burden, nor with any unique, serious adverse events.
The cardiovascular effects of the treatments were also consistent with the literature. Taken together with the largest extant studies of stimulants plus an α2A agonist, available data does not find evidence for added risk of this combination in the treatment of healthy children and adolescents, although expected effects of bradycardia and hypotension have been observed 49-51,56-57, and clinical recommendations should include mention of risk of syncope with α2A agonists. Therefore, the acute safety database for combination stimulant plus an α2 agonist and initial longer-term data56,57 argues for acceptable safety and very good tolerability.
Implications for ADHD Biology
The primary hypothesis behind the COMB strategy rested on the consistent observations that cognitive control functions, especially WM and inhibitory control, are modulated by dopamine and noradrenaline. 35-37 Theories of ADHD continue to emphasize deficient or “imbalanced” dopamine systems, 41,59 and possibly noradrenergic dysregulation. 35 While the core pathophysiology of ADHD is unknown, such catecholamine abnormalities are thought to underlie ADHD differences in PFC function, reward processing, and motivation. Arsten has advanced the notion that DA and NE post-synaptic effects, at optimal levels, exert differing and complementary positive influences on information processing in the PFC.35 Our data on the effects of COMB treatment on clinical outcomes, especially on inattentive ADHD symptoms, appear to support the above models of ADHD and WM biology, and effects of catecholamine agonists. However, these data do not confirm a dopamine or noradrenergic ADHD “deficit,” and we acknowledge that stimulants also have noradrenergic effects, complicating interpretation. Furthermore, the absence of pro-cognitive effects of guanfacine (Bilder et al, under review) raise the possibility that it may also be acting presynaptically, or insufficiently engaging the target of prefrontal α2A receptors.
Although there are many strengths of the study, including blinded comparison of three treatments, an ADHD sample not selected for stimulant refractoriness, and flexible dosing to optimize benefit, our findings have several limitations. Larger group sizes would have enabled more conclusive tests of treatment differences. Our study design began guanfacine first, with the addition of a stimulant second, which may yield differences versus those study designs adding guanfacine to ongoing stimulants, and like any sequential design, may blur timing of individual treatment effects when combined. Furthermore, drug dosage was optimized by clinical response rather than cognitive response or biomarker, which might have better separated the treatments. Lastly, we note that CGI-I ratings were made by treating clinicians, who were privy to side effect information.
In summary, results from our study suggest modest but consistent additional benefit from a carefully applied combination of a psychostimulant with a selective α2A agonist, guanfacine, on ADHD symptoms and global clinical responses, but should also serve to encourage further research to identify a range of treatment strategies using other possible approaches to successfully improve the long-term trajectory of ADHD.
Supplementary Material
Clinical Guidance.
A comparison of combination treatment with guanfacine and dexmethylphenidate versus monotherapy with guanfacine or dexmethylphenidate in children and adolescents with ADHD showed that combination treatment was associated with greater improvement, particularly by categorical ratings, during the 8-week trial.
Benefits of combined treatment may extend beyond children with ADHD who are partially responsive to stimulant monotherapy, based on this study.
Combined treatment with an alpha agonist + stimulant is one approach which may be more likely to achieve the goal of “remission” in ADHD therapy.
Regular monitoring of cardiovascular parameters and warning parents about risks of syncope and dehydration is recommended when treating with both a stimulant and alpha agonist, but available data suggests no significant changes in overall safety.
Combination treatment is generally well tolerated by most children and adolescents.
Acknowledgments
This work is supported by the National Institute of Mental Health (NIMH) Research Center grant P50MH077248, “Translational Research to Enhance Cognitive Control” (J.T.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Hellemann and Sugar served as the statistical experts for this research. The authors would like to thank the children and parents for their participation.
Supplemental material cited in this article is available online.
Disclosure: Dr. McCracken has received consultant honoraria from Dart Neuroscience and Think Now, Inc. Dr. McGough has received consultant honoraria from Neurovance; research support from Purdue, material research support for investigator initiated studies from NeuroSigma and Shire; book royalties from Oxford University Press; and data and safety monitoring board honoraria from Sunovion. He has provided expert testimony for Shire. Dr. Bilder has received consulting income or honoraria from EnVivo Pharmaceuticals, Forum Pharmaceuticals, Lumos Labs, Maven Research, Neurocog Trials Inc., OMDUSA, LLC, Snapchat, Takeda-Lundbeck, and ThinkNow Inc. He has received research support from the National Institute of Mental Health, the John Templeton Foundation, and Johnson and Johnson. Drs. Loo, Levitt, Del'Homme, Cowen, Hellemann, Sugar, and Mss. Sturm and Whelan report no biomedical financial interests or potential conflicts of interest.
Clinical trial registration information: Single Versus Combination Medication Treatment for Children With Attention Deficit Hyperactivity Disorder (Project1); http://clinicaltrials.gov/; NCT00429273.
Contributor Information
Dr. James T. McCracken, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA..
Dr. James J. McGough, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA..
Dr. Sandra K. Loo, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA..
Dr. Jennifer Levitt, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA..
Dr. Melissa Del'Homme, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA..
Dr. Jennifer Cowen, Semel Institute for Neuroscience and Human Behavior at UCLA..
Ms. Fiona Whelan, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA..
Dr. Gerhard Hellemann, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA..
Dr. Catherine Sugar, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA.; UCLA School of Public Health.
Dr. Robert M. Bilder, David Geffen School of Medicine at University of California, Los Angeles (UCLA), and the Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA.; UCLA College of Letters and Science.
REFERENCES
- 1.Wolraich ML, McKeown RE, Visser SN, et al. The prevalence of ADHD: Its diagnosis and treatment in four school districts across two states. J Atten Disord. 2014;18:563–75. doi: 10.1177/1087054712453169. [DOI] [PubMed] [Google Scholar]
- 2.Biederman J, Petty CR, Lomedico A, Faraone SV. Predictors of persistent ADHD: an 11-year follow-up study. J Psychiatric Res. 2011;45:150–155. doi: 10.1016/j.jpsychires.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012;73:941–950. doi: 10.4088/JCP.11m07529. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Adler LA, Barkley R, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood. Results from the National Comorbidity Survey Replication. Biol Psychiatry. 2005;57:1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45:192–202. doi: 10.1097/01.chi.0000189134.97436.e2. [DOI] [PubMed] [Google Scholar]
- 6.Klein RG, Mannuzza S, Loazagasti MA, et al. Clinical and functional outcomes of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2013;69:1295–303. doi: 10.1001/archgenpsychiatry.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steele M, Pensen PS, Quinn DM. Remission versus response as the goal of therapy in ADHD: a new standard for the field? Clinical Therapeutics. 2006;28:1892–1902. doi: 10.1016/j.clinthera.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Heal DJ, Smith SL, Findling RL. Stanford C and Tannock R, editor. ADHD: current and future therapeutics. Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment. Current Topics in Behavioral Neurosciences. 2012;9:361–390. doi: 10.1007/7854_2011_125. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 10.Salle FR, McGough J, Wigal T, et al. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:155–65. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 11.Wilens TE, Robertson B, Sikirica V, et al. A randomized, placebo-controlled trial of guanfacine extended release in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:916–925. doi: 10.1016/j.jaac.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 12.MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–86. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 13.Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43:802–11. doi: 10.1097/01.chi.0000128791.10014.ac. [DOI] [PubMed] [Google Scholar]
- 14.Gadow KD. Effects of stimulant drugs on academic performance in hyperactive and learning disabled children. J Learn Disabilities. 1983;16:291–299. doi: 10.1177/002221948301600509. [DOI] [PubMed] [Google Scholar]
- 15.Jacobvitz DJ, Sroufe A, Stewart M, Leffert N. Treatment of attentional and hyperactivity problems in children with sympathomimetic drugs: a comprehensive review. J Am Acad Child Adolesc Psychiatry. 1990;29:677–688. doi: 10.1097/00004583-199009000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Hechtman L, Abikoff H, Klein RC, et al. Academic achievement and emotional status of children with ADHD treated with long-term methylphenidate and mutimodel psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43:812–819. doi: 10.1097/01.chi.0000128796.84202.eb. [DOI] [PubMed] [Google Scholar]
- 17.Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46:989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- 18.Abikoff H, Hechtman L, Klein RG, et al. Social functioning in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43:820–9. doi: 10.1097/01.chi.0000128797.91601.1a. [DOI] [PubMed] [Google Scholar]
- 19.Hoza B, Gerdes AC, Mrug S, et al. Peer-assessed outcomes in the multimodal treatment study of children with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol. 2005;34:74–86. doi: 10.1207/s15374424jccp3401_7. [DOI] [PubMed] [Google Scholar]
- 20.Rapport MD, Denney C, DuPaul GJ, Gardner MJ. Attention deficit disorder and methylphenidate: Normalization rates, clinical effectiveness, and response prediction in 76 children. J Am Acad Child Adolesc Psychiatry. 1994;33:882–893. doi: 10.1097/00004583-199407000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Prasad V, Brogan E, Mulvaney C, Grainge M, Stanton W, Sayal K. How effective are drug treatments for children with ADHD at improving on-task behavior and academic achievement in the classroom? A systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2013;22:203–216. doi: 10.1007/s00787-012-0346-x. [DOI] [PubMed] [Google Scholar]
- 22.Schleffer RM, Brown TT, Fulton BD, Hinshaw SP, Levine P, Stone S. Positive association between attention-deficit/hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123:1273–9. doi: 10.1542/peds.2008-1597. [DOI] [PubMed] [Google Scholar]
- 23.Swanson JM, Kraemer HC, Hinshaw SP, et al. Clinical relevance of the primary findings of the MTA: success rates based on the severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Biederman J, Mick E, Faraone SV. Normalized functioning in youths with persistent attention-deficit/hyperactivity disorder. J Pediatrics. 1998;133:544–551. doi: 10.1016/s0022-3476(98)70065-4. [DOI] [PubMed] [Google Scholar]
- 25.Biederman J, Monuteaux MC, Doyle AE, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychology. 2004;72:757–766. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- 26.Miller M, Ho J, Hinshaw SP. Executive functions in girls with ADHD followed prospectively into young adulthood. Neuropsychology. 2012;26:278–87. doi: 10.1037/a0027792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: evidence for selective effects on ADHD symptom domains. J Abnorm Psychol. 2005;114:706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- 28.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 30.Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J Child Psychol Psychiatry. 2008;49:958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2006;30:1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Konrad K, Günther T, Hanisch C, Herpertz-Dahlmann B. Differential effects of methylphenidate on attentional functions in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:191–8. doi: 10.1097/00004583-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Kollins SH, Lopez FA, Vince BD, et al. Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21:111–120. doi: 10.1089/cap.2010.0064. [DOI] [PubMed] [Google Scholar]
- 34.Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacology, Biochemistry and Behavior. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solanto M. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Research. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 37.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten A. The Alpha-2AAdrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–251. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 39.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 40.Levy F. Dopamine versus noradrenaline: inverted-U effects and ADHD theories. Aust NZ J Psychiatry. 2009;43:101–108. doi: 10.1080/00048670802607238. [DOI] [PubMed] [Google Scholar]
- 41.Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatr. 1997;36:381–393. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- 42.Frank MJ, Santamaria A, O'Reilly RC, Willcutt E. Testing computational models of dopamine and noradrenaline dysfunction in attention/deficit hyperactivity disorder. Neuropsychopharmacology. 2007;32:1538–1599. doi: 10.1038/sj.npp.1301278. [DOI] [PubMed] [Google Scholar]
- 43.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 44.McGough JJ, Pataki CS, Suddath R. Dexmethylphenidate extended-release capsules for attention deficit hyperactivity disorder. Expert Rev Neurotherapeutics. 2005;5:437–441. doi: 10.1586/14737175.5.4.437. [DOI] [PubMed] [Google Scholar]
- 45.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. Guilford Press; New York: 1998. [Google Scholar]
- 46.Abikoff H, McGough J, Vittiello B, et al. Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity disorder and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2005;44:418–427. doi: 10.1097/01.chi.0000155320.52322.37. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J. Statistical Power Analysis for the Behavioral Sciences, Second Edition. Lawrence Erlbaum Associates; Mahwah, NJ: 1988. [Google Scholar]
- 48.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Wilens T, Bukstein O, Brams M, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:74–85. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Kollins SH, Jain R, Brams M, et al. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2011;127:e1406–1413. doi: 10.1542/peds.2010-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tourette's Syndrome Study Group Treatment of ADHD in children with tics. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 52.Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder: report of a double-blind placebo-controlled crossover therapeutic trial. J Am Acad Child Psychiatry. 1985;24:617–629. doi: 10.1016/s0002-7138(09)60065-0. [DOI] [PubMed] [Google Scholar]
- 53.Elia J, Borcherding BG, Rapoport JL, Keysor CS. Methylphenidate and dextroamphetamine treatments of hyperactivity: Are there true non-responders? Psychiatry Res. 1991;36:141–155. doi: 10.1016/0165-1781(91)90126-a. [DOI] [PubMed] [Google Scholar]
- 54.Hazell PL, Stewart JE. A randomized controlled trial of clonidine added to psychostimulants for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;42:886–94. doi: 10.1097/01.CHI.0000046908.27264.00. [DOI] [PubMed] [Google Scholar]
- 55.Palumbo DR, Sallee FR, Pelham WE, Bukstein OG, Daviss WB, McDermott MP. Clonidine for attention-deficit/hyperactivity disorder: I. efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47:180–186. doi: 10.1097/chi.0b013e31815d9af7. [DOI] [PubMed] [Google Scholar]
- 56.Spencer TJ, Greenbaum M, Ginsburg LD, Murphy WR. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:501–510. doi: 10.1089/cap.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sallee FR, Lyne A, Wigal T, McGough JJ. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity. J Child Adolesc Psychopharmacol. 2009;19:215–26. doi: 10.1089/cap.2008.0080. [DOI] [PubMed] [Google Scholar]
- 58.Daviss WB, Patel NC, Robb AS, et al. Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry. 2008;47:189–98. doi: 10.1097/chi.0b013e31815d9ae4. [DOI] [PubMed] [Google Scholar]
- 59.Volkow ND, Wang GJ, Newcorn J, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.