Abstract
Background and Purpose
White matter (WM) injury during stroke increases the risk of disability and gloomy prognosis of post-stroke rehabilitation. However, modeling of WM loss in rodents has proven to be challenging.
Methods
We report improved WM injury models in male C57BL/6 mice. Mice were given either endothelin-1 (ET-1) or L-N5-(1-iminoethyl)ornitine (L-NIO) into the periventricular white matter (PVWM), in the corpus callosum (CC), or in the posterior limb of internal capsule (PLIC). Anatomical and functional outcomes were quantified on day 7 post injection.
Results
Injection of ET-1 or L-NIO caused a small focal lesion in the injection site in the PVWM. No significant motor function deficits were observed in the PVWM lesion model. We next targeted the PLIC by using single or double injections of L-NIO and found that this strategy induced small focal infarction. Interestingly, injection of L-NIO in the PLIC also resulted in gliosis, and significant motor function deficits.
Conclusions
By employing different agents, doses, and locations, this study shows the feasibility of inducing brain WM injury accompanied with functional deficits in mice. Selective targeting of the injury location, behavioral testing, and the agents chosen to induce WM injury are all keys to successfully develop a mouse model and subsequent testing of therapeutic interventions against WM injury.
Keywords: Corpus callosum, Demyelination, Ischemia, Lysophosphatidylcholine, Motor functions, NOS inhibitor, Posterior limb internal capsule, Vasoconstriction
3. Introduction
White matter (WM) injury is common in stroke patients and is correlated with prognosis and functional recovery post-rehabilitation. In patients with minor stroke, WM lesions increased the risk of a recurrent stroke [1–3]. Although the exact mechanism by which WM lesions occur is not fully understood, they may be triggered by cerebral ischemia or hypoperfusion in penetrating arteries deep in the WM [4–6]. Corpus callosum (CC), periventricular WM (PVWM), and the posterior limb of internal capsule (PLIC) are the most affected area under acute neurologic conditions [7–9]. For more information on topics such as pathophysiology of WM injury, WM hypersensitivity, and genetics variants leading to stroke and WM injury, refer to some of the reviews [10–15] and original research articles published recently [16,17].
Given the advances in the imaging techniques, the role of WM injuries in the pathophysiology of various neurologic disorders, especially stroke, is being recognized. The necessity of a better understanding of the mechanisms of WM injuries is now essential to further provide better options on disease management. Animals, such as rodents, have been important in the development of pre-clinical translational models and the potential discovery of novel therapeutics. In the rodent model of WM injury, lesion size and functional deficits are dependent upon the drug that has been used, the injection site, and the strain of animal [4,18–21]. Therefore, the understanding of the drugs and the location of the lesion in animal models permit us to closely imitate the effects of behavioral and neurological deficits that occur in patients with WM injury.
Lesions of the WM can be induced focally in rodents by the injection of any of the several potential vasoconstricting or demyelinating agents reported or globally by ligating the bilateral common carotid arteries [22–26]. The rational for using one or the other agents/methods would be based on the questions being asked. For example, if one is more interested in ischemic component then vasoconstricting agents or use of bilateral carotid artery ligation models would be better suited. On the other hand, use of demyelinating agents could be used to test therapies that can potentially be used to prevent loss of WM and stimulate regeneration. Based upon the involvement of CC, PVWM, and PLIC in acute neurologic conditions, these are the most common parts of the brain targeted to induce WM injury [18,20,21,24,25,27]. In this study, we selected different strategies to induce ischemic WM injury in PVWM and PLIC by using the vasoconstrictors endothelin-1 (ET-1) or L-N5-(1-iminoethyl)ornitine (L-NIO). These updated protocols improved the existing models and resulted in reproducible WM injuries along with appreciable functional deficits. Use of these models could allow testing of various interventions leading to better outcomes during rehabilitation.
4. Materials And Methods
4.1 Mice and Reagents
All experiments were performed in accordance with the NIH and the University of Florida guidelines for the care and use of animals for experimental procedures, and the protocols were approved by University of Florida’s Institutional Animal Care and Use Committee. Adult male C57BL/6J (3–3.5 months old; 25.0 ± 2.0g) mice from our breeding facility were used in this study. ET-1 or L-NIO (Enzo Life Science Inc., Farmingdale, NY) were freshly prepared in saline (vehicle).
4.2 Mortality and Exclusion Criteria
All mice were randomized and personnel were blinded with experimental groups. Mice exhibiting convulsion (functional) or showing intracranial bleeding (histological) were excluded from the study following our pre-set exclusion criteria. One mouse from saline and L-NIO groups each were excluded. A total of 2 mice (L-NIO single and double = 1 each) died before the 7 day end-point.
4.3 Injection of the Drugs
Mice were randomized and anesthesia was induced by using 3% isoflurane (Vedco Inc., Saint Joseph, MO) that was then maintained at 1.0% to 1.5% for the rest of the procedure. Mice were then positioned in digital stereotaxic instruments (Stoelting Co., Wood Dale, IL) and either saline (control), ET-1 or L-NIO was injected intracranially in the PVWM or PLIC using Hamilton micro-syringes as described earlier with some modifications [21,28]. After the injections, mice were allowed to survive for 7 days and were then evaluated for the functional deficits before being sacrificed.
4.3.1 Single Injection of ET-1 or L-NIO in the PVWM
In the first set of experiments in this study, mice were given a stereotaxic injection of saline (N = 8; 0.5 µL) or ET-1 (10 µg/0.5 µL; N = 11) in the PVWM using the Hamilton micro-syringe at the coordinates of AP 0.5 mm, ML 1.0 mm, and DV 2.0 mm [29]. The injections were made using an auto-injector with a delivery rate of 0.1 µL/min and the needle was then left in place for another 5 min before being gradually retracted. In the second set of experiments, mice received three microinjection of L-NIO (6.75 µg/0.25 µL; N = 6) in subcortical WM as described earlier [21,28], with some modifications, at the following coordinates: the first injection at AP 0.21 mm, ML 0.22 mm, and DV −2.20 mm; the second injection at AP 0.71 mm, ML 0.15 mm, and DV −2.25 mm; and the third injection at AP 1.21 mm, ML 0.22 mm, and DV −2.20 mm.
4.3.2 Single or Double Injection of L-NIO in the PLIC
Since we did not observe any functional deficits following ET-1 or L-NIO injection in PVWM, we performed single or double injection of L-NIO in internal capsule which is a densely packed WM area involved with locomotion and motor functions. In the first set of experiments in this study, the animals were administered saline (N = 5) or L-NIO (6.75 µg/0.25 µL; N = 7) in the PLIC. Coordinates of the injection site were: AP −0.7 mm, ML 2.5 mm, and DV −3.2 mm with inclination at 10°. In the second set of experiments, mice were given double injections of saline (N = 5) or L-NIO (N = 6) in the PLIC using the same dose described above for single injection experiment. The second coordinates used in this experiment were AP −1.1 mm, ML 2.8 mm, and DV −3.5 mm with inclination at 10°.
4.4 Behavioral Tests
Mice were tested for baseline performance prior to surgery and the post-surgery testing was performed on day 7. All animals received at least 30 min of recovery time before being tested for another behavioral assessment. Each animal underwent three trials for each assessment.
4.4.1 Neurologic deficit scores (NDS)
The neurologic deficits induced by these injections were evaluated by a slightly modified 28-point scoring system [30,31]. This system includes body symmetry, gait, climbing, circling behavior, front limb symmetry, compulsory circling, and whisker response tests (not tested in this study). Each test is graded from 0 to 4 thus establishing a maximum deficit of 24 points.
Rotarod
This task was used to evaluate motor coordination and sensorimotor deficits. Prior to the test, mice were trained for three consecutive days. Pre- and post-injury testing was performed with accelerating speed ranging from 5 to 30 rpm as described earlier [31,32]. Each test session was composed of three trials, each with maximum duration of 5 min and an interval of 30 min. Total retention time on the rod for each mouse was automatically collected by the Rotamex computer software.
Grip strength
This task was designed to assess neuromuscular function and muscular strength by sensing the maximal peak force of the mouse forelimb [32–34]. Muscle strength was measured using a Grip Strength Meter (San Diego instruments Inc., San Diego, CA). The maximal force achieved by the animal was recorded and each animal underwent three trials with a 5-min break in between each trial.
Wire-hanging test
This procedure was performed to measure muscular strength as described earlier [32,35]. Mice were suspended with their forelimbs from a wire that was stretched 100 cm apart between two poles at a height of 60 cm. The time taken by the mice to reach either side of the pole or fall down was recorded with a cut-off time of 120 sec. Soft bedding under the wire was used to prevent any suffering to the mice from the fall.
4.5 Histology and Lesion Volume Quantification
After the functional outcomes assessment, mice were perfused transcardially with 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde in PBS. The harvested brains were post-fixed in 4% paraformaldehyde overnight followed by cryoprotection in 30% sucrose. The serial coronal sections at 30 µm obtained on Cryostat (Leica CM 1850; Leica Biosystem, Buffalo Grove, IL) were processed for histological and immunohistochemical analysis.
To determine the lesion volume in WM, sections were stained with luxol fast blue (LFB) and counterstained with Neutral red. The mounted sections were hydrated in distilled water then defatted overnight in 70% ethanol. The next day, sections were placed in 95% ethanol for 5 min followed by staining with 0.1% solvent blue 38 (Luxol fast blue stain; Sigma) in 95% ethanol for 4 to 6 h at 60°C. After rinsing with ethanol and distilled water, sections were differentiated with 0.05% Li2CO3 and 70% ethanol several times until the contrast between the gray matter and WM was clearly detected [36]. Sections were then finally counterstained in 0.1% acidified neutral red solution. Images were acquired using an Aperio ScanScope XT slide scanner (Aperio, Vista, CA). The lesion areas were defined as areas with reduced myelin staining and neutral red-positive cell staining in the injury site. The lesion volume was quantified using Aperio ImageScope software (Aperio). The data in all the graphs are means ± SEM and the difference with P < 0.05 was considered significant between different groups.
4.6 Immunohistochemistry
To further determine the WM loss in the injury site, we utilized silver staining and myelin basic protein (MBP) immunostaining which detects the myelin and axonal fibers. The silver staining was performed by immersing the mounted tissue sections in fresh ammoniacal silver nitrate solution for 45 min as described earlier [37]. Excess (unbound) silver was removed by washing sections in three changes of 0.5% acetic acid. Because gliosis is also concomitant with the demyelination process, CD11b was used as a marker for microglial/macrophages and glial fibrillary acidic protein (GFAP) was used as a marker for astrocytes. To stain MBP, CD11b, or GFAP, mounted sections were washed in 0.01M PBS with 0.1% Triton-X100 (PBS-Tx; 3 × 55 min) and quenched in methanol containing 1% H2O2 for 30 min. After another series of washing (3 × 5 min) in PBS-Tx, sections were blocked with 5% normal horse serum in PBS-Tx at room temperature for 60 min. One of the following primary antibodies was applied overnight at 4°C: 1:500 anti-MBP (Abcam, Cambridge, MA), 1:2000 anti-GFAP (Dako, Glostrup, Denmark), or 1:500 anti-CD11b (AbD Serotec, Raleigh, NC). Sections were washed and incubated in biotinylated secondary horse anti-rabbit for MBP and GFAP, or horse anti-rat for CD11b (1:1000, Vector Laboratories, Burlingame, CA). Conjugation with avidin-biotin complex (Vecstatin Elite ABC kit; Vector Laboratories) was followed by visualization with 3,3-diaminonbenzidine-hydogen peroxidase (Vector Laboratories) according to the manufacturer’s instructions. Sections were then washed in PBS, and dried at room temperature, dehydrated in series of graded alcohol followed by HistoClear.
4.7 Statistical Analysis
The brain section images were acquired by Aperio Scan scope and quantified by Spectrum software (Aperio, Vista, CA). The statistical analyses were performed by Prism software (GraphPad, La Jolla, CA). Neurologic deficit scores were analyzed by the non-parametric Kruskal–Wallis analysis of ranks and are presented as medians with interquartile ranges (25th and 75th percentiles). Other functional outcomes and the lesion volume data were analyzed by one-way ANOVA followed by Newman–Keuls multiple range tests and presented as mean ± SEM. Significant levels were achieved when P < 0.05.
5. Results
5.1 Unilateral Injection of L-NIO in the Subcortical WM Causes Focal Infarction
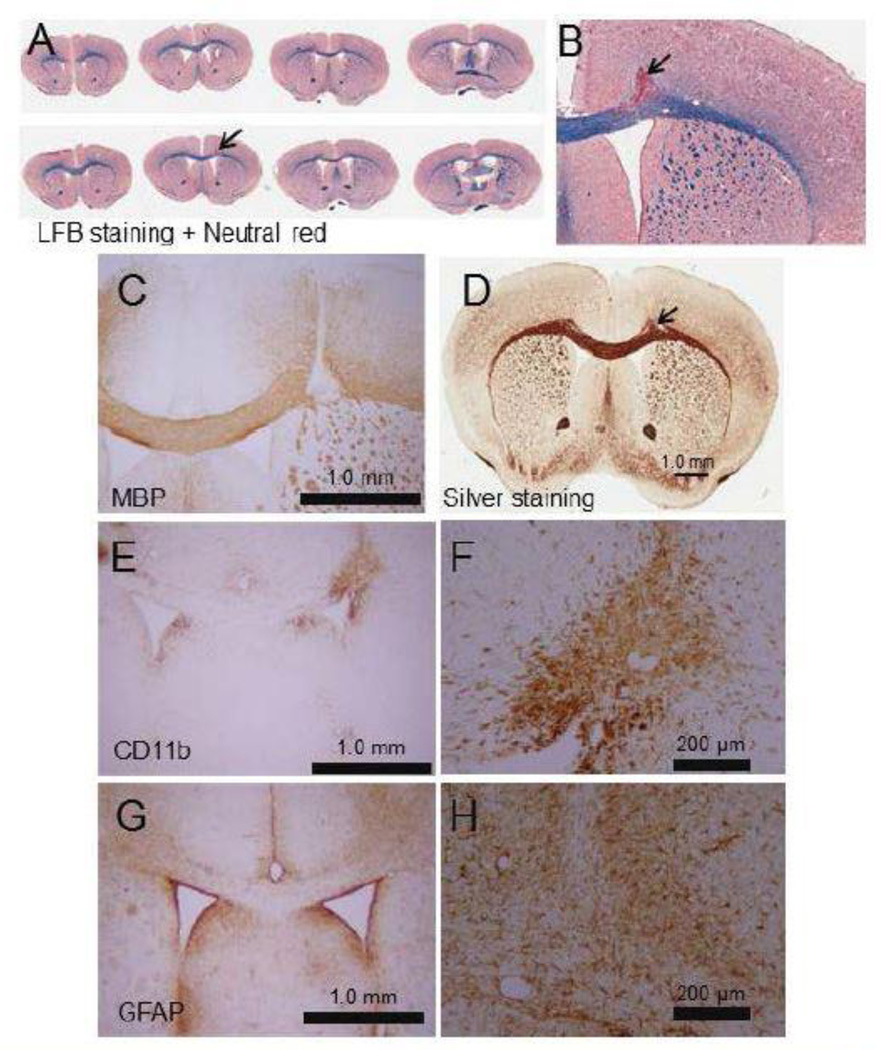
A potent selective endothelial nitric oxide synthase (eNOS) inhibitor, L-NIO, was injected into the mouse subcortical WM to induce an ischemic infarct. The administration of L-NIO induced small focal ischemic damage in the injected sites as shown by LFB (Figures 1A, 1B).
Figure 1.
Representative images showing loss of WM in the PVWM post L-NIO injection. Injection of L-NIO induced focal degeneration of the PVWM as shown by LFB (A and B), MBP (C), and sliver (D) staining Microinjection of L-NIO in the PVWM also induced microglial activation (CD 11b; E and F) in the injured site and astrogliosis (FGAP; G and H) around the injured site. Sclae bars are a 1 mm for lower magnification and 200 µm for higher magnification images.
Mice injected with the vasoconstricting agents revealed extensive tissue damage at the injection site and it is markedly packed with picknotic cell bodies which are surrounded by sparingly condensed cell bodies. Interestingly, mice injected with saline also showed localized picknotic cell bodies along the needle track. Similar mechanical damages induced by the needle and the saline mass have been reported by others (38,39).
The WM injury was further confirmed by performing MBP (Figure 1C) and silver (Figure 1C) staining. Interestingly, microglial activation as monitored by CD11b staining (Figure 1E–F) was increased inside the injury area; whereas, astrogliosis as monitored by GFAP staining (Figure 1G–H) was increased around the injury. This indicates that the injury process induced strong gliosis.
5.2 Vasoconstricting Agents Induced Moderate Damage in the PVWM
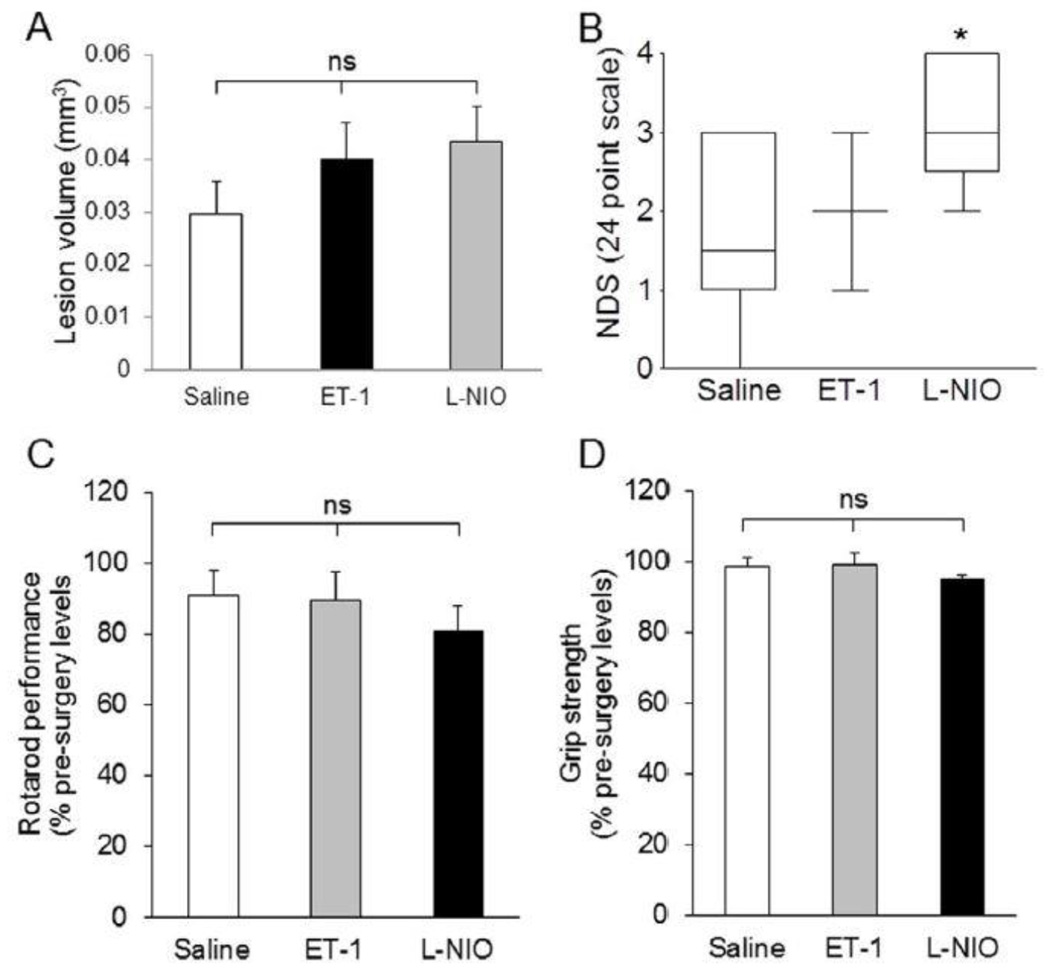
The vasoconstricting agents (ET-1 and L-NIO)-injected groups exhibit no apparent difference in lesion volume (0.040 ± 0.024 mm3 and 0.043 ± 0.017 mm3, respectively) compared with the saline-injected group (0.030 ± 0.020 mm3; Figure 2A). Interestingly, the only significant change in functional outcomes was observed in the NDS of L-NIO-injected group. The NDS observed in different groups was: saline, 1.70 ± 0.33; ET-1, 2.20 ± 0.37; and L-NIO, 3.00 ± 0.31 (Figure 2B). The rotarod test showed that all mice ran for approximately the same compared with their respective baseline before falling on the underlining cushion (Figure 2C). Similarly, we did not observe any significant difference in grip strength of both forelimbs among the groups. The percentage strength from baseline (pre-surgery) of each group was as follows: saline, 98.39% ± 2.73%; ET-1, 99.09% ± 3.34%; and L-NIO, 94.80% ± 1.38% (Figure 2D).
Figure 2.
Anatomical and functional outcomes after LPC, endothelin-1, or L-NIO injections in the PVWM. Although the lesion volume (A) in LPC-injected animals is higher compared with the saline-, ET-1, and L-NIO-treated mice, neurological deficit score was higher in L-NIO-injected groups (NDS; B). Other functional outcomes remained similar among the groups. Data presented as mean ± SEM. * P < 0.05, ** P < 0.01, when compared with the saline group; ns = non-significant.
Thus, it seems that low focal WM loss in the PVWM and subcortical WM did not induce neurological and behavioral deficits, suggesting that other axonal fibers in the CC may alter the local cortical circuitry to compensate for the loss of the WM in those areas. Therefore, in the subsequent experiments we targeted the internal capsule, which contains the corticospinal tracts responsible for locomotor function.
5.3 Unilateral Microinjection of LNIO Causes Discrete Focal WM Loss in the PLIC and Induces Neurological and Behavioral Deficits
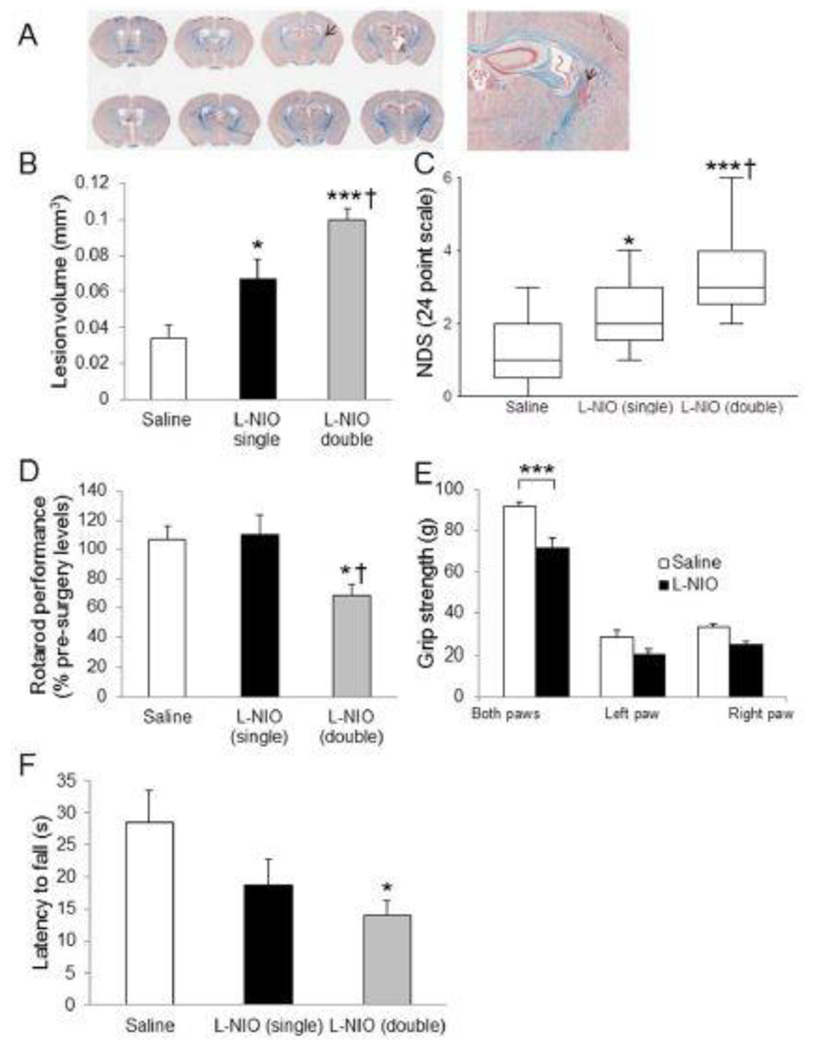
The unilateral microinjection of L-NIO only induced focal WM loss at the site of injection (Figure 3A–F). LFB staining showed infarction and infiltration of cells stained with neutral red at the site of injection (Figure 3A, arrow). Since there was no substantial difference between the lesion volumes following single or double injection of saline, the data from these groups were merged together. Lesion volume analysis demonstrated that a double injection of L-NIO produces bigger lesion volumes compared to saline or a single injection. There was a significant difference between saline and a single injection of L-NIO (0.034 ± 0.007 mm3 and 0.067 ± 0.011 mm3, respectively; P < 0.05; Figure 3B), saline and a double injection of L-NIO (0.034 ± 0.007 mm3 and 0.100 ± 0.006 mm3, respectively; P < 0.001; Figure 3B), and between a single and double injection of L-NIO (0.067 ± 0.011 mm3 and 0.100 ± 0.006 mm3, respectively; P < 0.05). NDS in single- and double-injected L-NIO mice resulted in higher neurological deficit scores compared to saline-injected group (P < 0.05, and 0.001 respectively; Figure 3C). Rotarod performance demonstrated significant differences between saline and double injection (106.7% ± 12.4% vs 68.8% ± 6.9%, P < 0.05; Figure 3D), but not for a single L-NIO injection. Grip strength analysis showed significant differences between L-NIO and saline groups in both paws (71.5 ± 5.1 vs 91.6 ± 2.6, P < 0.001; Figure 3E); however, there was no difference seen in either affected (20.8 ± 1.4 vs 28.2 ± 3.4, P = 0.09) and unaffected arms (24.3 ± 2.1 vs 33.0 ± 1.6, P = 0.09). The wire hanging testing also showed that double L-NIO-injection group had greater latency to fall (12.9 ± 2.5 sec, P < 0.05; Figure 3F) as compared with saline-injected group (28.6 ± 4.9 sec). There was no significant difference between saline and single L-NIO-injection groups.
Figure 3.
A very focal but effective WM loss was induced by L-NIO in the internal capsule. Densely packed cells stained with neutral red display the injured area (A). Lesion volume and NDS (B) show that single and double injections of L-NIO produced significant damage. Other functional outcomes show significant changes associated only with the double injection of L-NIO. Data presented as mean ± SEM. * P < 0.05, *** P < 0.001, when compared with the saline group; † P < 0.05 when compared with the L-NIO single group.
6. Discussion
Our results demonstrate the utility of vasoconstricting agents in mediating WM ischemic stroke and inducing glial responses in mice. Interestingly, both vasoconstricting agents (ET-1 and L-NIO) resulted in similar lesion volume. However, it is surprising that no neurological and locomotor deficits in these mouse models of WM injury were observed. This could be an indication that nearby motor neurons in the cortex and/or other axonal fibers may alter neuronal circuitry to overcome functional deficits as a result of WM loss. Therefore, in another set of experiments we targeted internal capsule and performed single of double injections of L-NIO. This change in dose and target area produced substantial functional and histologic changes.
L-NIO, a potent eNOS inhibitor, is approximately five times more potent as an inhibitor of eNOS compared to any other arginine analogs such as L-NAME [40,41]. The inhibitory effects of L-NIO are rapid in onset and irreversible compared to other arginine analogs [42]. A relatively recent report from Dr. Carmichael’s group demonstrated that microinjection of L-NIO in the cortex and CC induced focal cortical and WM stroke, resulting in axonal fiber loss (axon initial segment) and myelin damage within the CC [28]. Similarly, in our present study also, we showed that both ET-1 and L-NIO injection in the PVWM or subcortical WM in C57BL/6 mice caused a small infarction but did not induce a behavioral deficit. Nevertheless, a double, injection of L-NIO in the PLIC induced neurological and motor deficits in this mouse model of WM injury. Therefore, it is likely that larger lesions in the PLIC are necessary to induce motor deficits.
Some studies, such as Sozmen and colleagues [21] and Horrie co-workers [19], demonstrated that injection of ET-1 in the PVWM and subcortical WM induced axonal degeneration and myelin loss accompanied by gliosis in the injected site. Similarly microinjection of L-NIO in subcortical WM, also resulted in lesion development at the site of injection [28]. Use of demyelinating agents such as LPC has also been reported to induce WM injury. The injection of LPC into the PVWM resulted in WM loss in the site of injection and eventually the injury spread out into the CC [43,24,25]. Similarly, we also observed that LPC injection in PLIC resulted in significant WM injury and functional deficit (unpublished data). However, a significant limitation of LPC-induced injury, at least from stroke and rehabilitation point of view, is the potential for reversibility of the injury [44]. Therefore, LPC injection in WM is not recognized as a preferred ischemic WM stroke model.
To model a subcortical WM stroke, focal microinjection of a vasoconstricting agent into the PVWM or below the frontal cortex is reported. Some studies have shown the effectiveness of discrete injections of ET-1 [19,21] or L-NIO [28] to induce infarction and/or WM loss in the subcortical WM or PVWM. However, information concerning lesions in the PVWM or subcortical WM with behavior analysis was not provided. PVWM lesions are well known to be associated with vascular cognitive impairment and are commonly found in aging and ischemic stroke patients [45]. Reports regarding the relationship between focal PVWM lesions with behavioral deficits in human studies are few and far between. One single report showed that a PVWM lesion may be associated with dysphagia in subcortical stroke patients [46]. However, there is no report on translational studies using animals associated with motor function in the PVWM lesion model. To our knowledge, no previous studies have specifically focused on the effect of vasoconstrictor-induced focal WM loss in the PVWM or CC in rodents in association with their behavioral and or neurological outcomes. Our present data suggests that selective targeting of PVWM might not be sufficient to induce WM injury along with locomotor and/or neurological deficits, although previous reports suggest the involvement of PVWM injury in regulating cognition and plasticity [47,48].
In the present study, we demonstrated that lesions in the PLIC not only induce WM loss but also induce neurological and functional deficits. In a clinical study, Puig et al. [7] have shown that damage to corticospinal motor fibers in the internal capsule resulted in sensorimotor deficit in patients. Our data confirms findings from previous animal studies that have shown that the vasoconstriction in the PLIC caused infarction in the rat internal capsule and induced sensorimotor deficits [18,20]. While considering the use of L-NIO to induce WM stroke, it should be acknowledged that at higher dose L-NIO may exerts toxic effects apart from its inhibitory effect on eNOS.
Both tested agents ET-1 and L-NIO caused focal WM degeneration in PVWM and subcortical WM but did not produce substantial functional deficits. One possible explanation for the lack of functional deficits in such a PVWM lesion may be due to the fact that other intact axonal fibers in the CC are able to compensate for axonal loss and remodel the neuronal circuitry to counterbalance the functional deficit. Nevertheless, we showed that a double injection of L-NIO (a vasoconstricting agent having more relevance for an ischemic WM stroke) in the PLIC induced larger lesion volumes and induced neurological and functional deficits. Thus we conclude that injuring the PLIC by L-NIO may be considered as a viable model for WM ischemic stroke, particularly when post-stroke rehabilitation is of interest.
Acknowledgements
The authors would like to thank all colleagues from the Doré lab and the BRRC members.
Sources of Funding
This work was supported by the Malcom Randall Veterans Affairs Medical Center-Brain Rehabilitation Research Center (BBRC) and by the National Institute of Heath (NS046400 and AT007429).
References
- 1.van Swieten JC, Kappelle LJ, Algra A, van Latum JC, Koudstaal PJ, van Gijn J. Hypodensity of the cerebral white matter in patients with transient ischemic attack or minor stroke: Influence on the rate of subsequent stroke. Ann Neurol. 1992;32(2):177–183. doi: 10.1002/ana.410320209. [DOI] [PubMed] [Google Scholar]
- 2.Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population based study: The Rotterdam Study. Neurology. 1994;44(7):1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 3.Imaizumi T, Inamura S, Nomura T. The severities of white matter lesions possibly influence the recurrences of several stroke types. J Stroke Cerebrovasc Dis. 2014;23(7):1897–1902. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Lin JX, Tomimoto H, Akiguchi I, Wakita H, Shibasaki H, Horie R. White matter lesions and alteration of vascular cell composition in the brain of spontaneously hypertensive rats. Neuroreport. 2001;12(9):1835–1839. doi: 10.1097/00001756-200107030-00015. [DOI] [PubMed] [Google Scholar]
- 5.Sierra C, de la Sierra A, Chamorro A, Larrousse M, Domenech M, Coca A. Cerebral hemodynamics and silent cerebral white matter lesions in middle-aged essential hypertensive patients. Blood Press. 2004;13(5):304–309. doi: 10.1080/08037050410024448. [DOI] [PubMed] [Google Scholar]
- 6.Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120(2):433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puig J, Pedraza S, Blasco G, Daunis IEJ, Prados F, Remollo S, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011;32(5):857–863. doi: 10.3174/ajnr.A2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi GS, Kim OL, Kim SH, Ahn SH, Cho YW, Son SM, et al. Classification of cause of motor weakness in traumatic brain injury using diffusion tensor imaging. Arch Neurol. 2012;69(3):363–367. doi: 10.1001/archneurol.2011.1930. [DOI] [PubMed] [Google Scholar]
- 9.Yeo SS, Choi BY, Chang CH, Kim SH, Jung YJ, Jang SH. Evidence of corticospinal tract injury at midbrain in patients with subarachnoid hemorrhage. Stroke. 2012;43(8):2239–2241. doi: 10.1161/STROKEAHA.112.661116. [DOI] [PubMed] [Google Scholar]
- 10.Assareh A, Mather KA, Schofield PR, Kwok JB, Sachdev PS. The genetics of white matter lesions. CNS Neurosci Ther. 2011;17(5):525–540. doi: 10.1111/j.1755-5949.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matute C, Ransom BR. Roles of white matter in central nervous system pathophysiologies. ASN Neuro. 2012;4(2):e00079. doi: 10.1042/AN20110060. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sozmen EG, Hinman JD, Carmichael ST. Models that matter: white matter stroke models. Neurotherapeutics. 2012;9(2):349–358. doi: 10.1007/s13311-012-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matute C, Domercq M, Pérez-Samartín A, Ransom BR. Protecting White Matter From Stroke Injury. Stroke. 2013;44(4):1204–1211. doi: 10.1161/STROKEAHA.112.658328. [DOI] [PubMed] [Google Scholar]
- 14.Falcone GJ, Malik R, Dichgans M, Rosand J. Current concepts and clinical applications of stroke genetics. Lancet Neurol. 2014;13(4):405–418. doi: 10.1016/S1474-4422(14)70029-8. [DOI] [PubMed] [Google Scholar]
- 15.Fern RF, Matute C, Stys PK. White matter injury: Ischemic and nonischemic. Glia. 2014;62(11):1780–1789. doi: 10.1002/glia.22722. [DOI] [PubMed] [Google Scholar]
- 16.Weaver J, Jalal FY, Yang Y, Thompson J, Rosenberg GA, Liu KJ. Tissue oxygen is reduced in white matter of spontaneously hypertensive-stroke prone rats: a longitudinal study with electron paramagnetic resonance. J Cereb Blood Flow Metab. 2014;34(5):890–896. doi: 10.1038/jcbfm.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J Cereb Blood Flow Metab. 2015;35(7):1145–1153. doi: 10.1038/jcbfm.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frost SB, Barbay S, Mumert ML, Stowe AM, Nudo RJ. An animal model of capsular infarct: endothelin-1 injections in the rat. Behav Brain Res. 2006;169(2):206–211. doi: 10.1016/j.bbr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Horie N, Maag AL, Hamilton SA, Shichinohe H, Bliss TM, Steinberg GK. Mouse model of focal cerebral ischemia using endothelin-1. J Neurosci Methods. 2008;173(2):286–290. doi: 10.1016/j.jneumeth.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecrux C, McCabe C, Weir CJ, Gallagher L, Mullin J, Touzani O, et al. Effects of magnesium treatment in a model of internal capsule lesion in spontaneously hypertensive rats. Stroke. 2008;39(2):448–454. doi: 10.1161/STROKEAHA.107.492934. [DOI] [PubMed] [Google Scholar]
- 21.Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. J Neurosci Methods. 2009;180(2):261–272. doi: 10.1016/j.jneumeth.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakita H, Tomimoto H, Akiguchi I, Lin JX, Miyamoto K, Oka N. A cyclooxygenase-2 inhibitor attenuates white matter damage in chronic cerebral ischemia. Neuroreport. 1999;10(7):1461–1465. doi: 10.1097/00001756-199905140-00013. [DOI] [PubMed] [Google Scholar]
- 23.Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M, et al. Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res. 2002;924(1):63–70. doi: 10.1016/s0006-8993(01)03223-1. [DOI] [PubMed] [Google Scholar]
- 24.Jean I, Lavialle C, Barthelaix-Pouplard A, Fressinaud C. Neurotrophin-3 specifically increases mature oligodendrocyte population and enhances remyelination after chemical demyelination of adult rat CNS. Brain Res. 2003;972(1–2):110–118. doi: 10.1016/s0006-8993(03)02510-1. [DOI] [PubMed] [Google Scholar]
- 25.Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60(6):875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattori Y, Enmi J, Kitamura A, Yamamoto Y, Saito S, Takahashi Y, et al. A novel mouse model of subcortical infarcts with dementia. J Neurosci. 2015;35(9):3915–3928. doi: 10.1523/JNEUROSCI.3970-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka Y, Imai H, Konno K, Miyagishima T, Kubota C, Puentes S, et al. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig: neurological assessment and histological, immunohistochemical, and physiological evaluation of dynamic corticospinal tract deformation. Stroke. 2008;39(1):205–212. doi: 10.1161/STROKEAHA.107.489906. [DOI] [PubMed] [Google Scholar]
- 28.Hinman JD, Rasband MN, Carmichael ST. Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke. 2013;44(1):182–189. doi: 10.1161/STROKEAHA.112.668749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin K. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. Elsevier Academic Press; 2013. [Google Scholar]
- 30.Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998;29(10):2136–2140. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- 31.Singh N, Ma B, Leonardo C, Ahmad A, Narumiya S, Doré S. Role of PGE2 EP1 Receptor in Intracerebral Hemorrhage-Induced Brain Injury. Neurotox Res. 2013;24(4):549–559. doi: 10.1007/s12640-013-9410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57(6):809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Reitmeir R, Kilic E, Kilic U, Bacigaluppi M, ElAli A, Salani G, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134(Pt 1):84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- 34.Glushakov AV, Robbins SW, Bracy CL, Narumiya S, Doré S. Prostaglandin F2alpha FP receptor antagonist improves outcomes after experimental traumatic brain injury. J Neuroinflammation. 2013;10:132. doi: 10.1186/1742-2094-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31(8):1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- 36.Xing B, Li H, Wang H, Mukhopadhyay D, Fisher D, Gilpin CJ, et al. RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp Neurol. 2011;231(2):247–260. doi: 10.1016/j.expneurol.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pistorio AL, Hendry SH, Wang X. A modified technique for high-resolution staining of myelin. J Neurosci Methods. 2006;153(1):135–146. doi: 10.1016/j.jneumeth.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Xue M, Del Bigio MR. Acute Tissue Damage After Injections of Thrombin and Plasmin into Rat Striatum. Stroke. 2001;32(9):2164–2169. doi: 10.1161/hs0901.095408. [DOI] [PubMed] [Google Scholar]
- 39.Roome RB, Bartlett RF, Jeffers M, Xiong J, Corbett D, Vanderluit JL. A reproducible Endothelin-1 model of forelimb motor cortex stroke in the mouse. J Neurosci Methods. 2014;233:34–44. doi: 10.1016/j.jneumeth.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Mulligan MS, Moncada S, Ward PA. Protective effects of inhibitors of nitric oxide synthase in immune complex-induced vasculitis. Br J Pharmacol. 1992;107(4):1159–1162. doi: 10.1111/j.1476-5381.1992.tb13423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rus A, Peinado MA, Blanco S, Del Moral ML. Is endothelial-nitric-oxide-synthase-derived nitric oxide involved in cardiac hypoxia/reoxygenation-related damage? J Biosci. 2011;36(1):69–78. doi: 10.1007/s12038-011-9006-4. [DOI] [PubMed] [Google Scholar]
- 42.McCall TB, Feelisch M, Palmer RM, Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jean I, Allamargot C, Barthelaix-Pouplard A, Fressinaud C. Axonal lesions and PDGF-enhanced remyelination in the rat corpus callosum after lysolecithin demyelination. Neuroreport. 2002;13(5):627–631. doi: 10.1097/00001756-200204160-00018. [DOI] [PubMed] [Google Scholar]
- 44.Jeffery ND, Blakemore WF. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J Neurocytol. 1995;24(10):775–781. doi: 10.1007/BF01191213. [DOI] [PubMed] [Google Scholar]
- 45.Tu Q, Ding B, Yang X, Bai S, Tu J, Liu X, et al. The current situation on vascular cognitive impairment after ischemic stroke in Changsha. Arch Gerontol Geriatr. 2014;58(2):236–247. doi: 10.1016/j.archger.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41(3):482–486. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- 47.Croall ID, Cowie CJ, He J, Peel A, Wood J, Aribisala BS, et al. White matter correlates of cognitive dysfunction after mild traumatic brain injury. Neurology. 2014;83(6):494–501. doi: 10.1212/WNL.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X, Liang Y, Wang J, Chen K, Chen Y, Zhou X, et al. Early frontal structural and functional changes in mild white matter lesions relevant to cognitive decline. J Alzheimers Dis. 2014;40(1):123–134. doi: 10.3233/JAD-131709. [DOI] [PubMed] [Google Scholar]