Abstract
Ultradian release of glucocorticoids is thought to be essential for homeostasis and health. Furthermore, deviation from this pulsatile release pattern is considered to compromise resilience to stress-related disease, even after hormone levels have normalised. In the present study, we investigate how constant exposure to different concentrations of corticosterone affects diurnal and ultradian pulsatility. The rate of recovery in pulsatile hypothalamic-pituitary-adrenal (HPA) activity after withdrawal of exogenous corticosterone is also examined. Finally, the behavioural and neuroendocrine responsiveness to an audiogenic stressor is studied. Adrenally intact male rats were subcutaneously implanted with vehicle, 40% or 100% corticosterone pellets for 7 days. The continuous release of corticosterone from these implants abolished diurnal and ultradian corticosterone variation, as measured with high-frequency automated blood sampling. Pellet removal on post-surgery day 8 allowed rapid recovery of endogenous rhythms in animals previously exposed to daily average concentrations (40%) but not after exposure to high concentrations (100%) of corticosterone. Behavioural and neuroendocrine responsiveness to stress was distinctly different between the treatment groups. Audiogenic stimulation 1 day after pellet removal resulted in a similar corticosterone response in animals previously exposed to 40% corticosterone or vehicle. The 40% pellet group, however, showed less and shorter behavioural activity (i.e. locomotion, risk assessment) to noise stress compared to 100% corticosterone and vehicle-treated animals. In conclusion, unlike the animals impanted with 100% corticosterone, we find that basal HPA axis activity in the 40% group, which had mean daily levels of circulating corticosterone in the physiological range, rapidly reverts to the characteristic pulsatile pattern of corticosterone secretion. Upon reinstatement of the ultradian rhythm, and despite the fact that these animals did not differ from controls in their response to noise stress, they did show substantial changes in their behavioural response to stress.
Keywords: HPA axis, glucocorticoids, ultradian, circadian, stress, behaviour, brain
The diurnal variation in the secretion of glucocorticoids is principally controlled by the hypothalamic suprachiasmatic nucleus and is fine-tuned by autonomic nervous system activity and afferents of the central hypothalamic-pituitary-adrenal (HPA) axis (1, 2). Daily variations in glucocorticoid hormone concentrations are thought to be fundamental for the maintenance of physiology and well being, as deviations from the normal release pattern are considered to enhance vulnerability to stress-related disease (3, 4). These diurnal fluctuations consist of approximately hourly, ultradian glucocorticoid hormone bursts that change in amplitude according the time of day (5–7). Superimposed on these rhythms is the HPA-driven glucocorticoid response to a stressor.
Alterations in glucocorticoid pulsatile patterns can occur and are associated not only with normal transitions in physiological states, but also stress-related disorders. For example, in rodents, lactation suppresses pulsatility, whereas, during ageing, the hourly pattern becomes disorganised (8). In humans, depression is associated with enhanced pulse magnitude thereby abolishing circadian variation in pulse amplitude, resulting in dramatically changed patterns of tissue exposure to hormone (3, 9–11). However, the functional contribution of ultradian glucocorticoid pulses to HPA axis reactivity and stress responsiveness, and the consequences of changes in pulse characteristics for physiology, are largely unknown.
Diurnal rhythmicity can experimentally be abolished by subcutaneous implantation of corticosterone releasing pellets that elevate circadian trough concentrations. Consequently, via negative-feedback mechanisms, this leads to a compensatory lower output of corticosterone from the adrenal cortex at the time of the circadian peak (12–14). Previously, we demonstrated that such flattening of the diurnal corticosterone patterns has substantial effects on glucocorticoid receptor (GR) signalling in the rat hippocampus; although tonic actions of glucocorticoids are apparent (13, 14), we found that the response in expression of glucocorticoid marker genes to acute elevations superimposed on the constant hormone signal is attenuated. Surprisingly, constant exposure to moderate concentrations of corticosterone in some aspects had greater consequences for GR responsiveness than overt hypercorticism (15). These data indicate that the tissue responsiveness to glucocorticoids depends on the pattern of ligand exposure and that a pulsatile pattern is necessary to prevent receptor desensitisation.
However, a number of issues are still unresolved. It remains to be demonstrated that ultradian variations are abolished in the pellet model. Also, it is unknown how fast the negative-feedback action abates after cessation of constant exogenous corticosterone and when the endogenous pulses re-emerge. Observations in rodents and humans with a history of changes in corticosteroid exposure suggest that, even after normalisation of hormone levels, there are residual disturbances in the brain (16, 17). In that respect, although neuroendocrine stress responsiveness is clearly affected under conditions of flattened corticosterone (12, 13, 18, 19), very little data are available on the behavioural response to stress after changes in pulse characteristics.
To this end, adrenally intact male rats were subcutaneously implanted with vehicle, 40% or 100% corticosterone pellets, respectively. As such, animals were exposed to either high, pathological concentrations of corticosterone (100% pellet) or, on average, normal daily concentrations, but in a continuous (40% pellet) rather than pulsatile manner (vehicle pellet). After 7 days of pellet implantation, corticosterone pulsatile profiles were measured in individual animals using high-frequency automated blood sampling (6, 20). Pellets were rapidly removed on day 8 to assess how fast ultradian rhythms recover after cessation of constant corticosterone exposure (washout). As a functional readout, we measured hormonal and behavioural responsiveness to white noise stress.
We demonstrate that different concentrations of constant corticosterone exposure in adrenally intact rats indeed abolish both diurnal and ultradian rhythmicity. We also find that HPA axis activity flexibly recovers from long-term exposure to 40%, but not 100% corticosterone, although, in contrast, the behavioural stress responsiveness is affected after washout in the 40% corticosterone group compared to vehicle.
Materials and methods
Subjects
Experiments were conducted on male Sprague–Dawley rats (Harlan, Bicester, UK) weighing approximately 250 g at the time of surgery. After arrival, animals were group housed (four animals per cage) under standard environmental conditions and a 14 : 10 h light / dark cycle (lights on 05.15 h) and were allowed to acclimatise for a week. Food and water were provided ad lib throughout the experiment. Animal procedures were approved by the University of Bristol Ethical Review Group. Animal care was conducted in accordance with Home Office guidelines, the UK Animals (Scientific Procedures) Act 1986 and the EC Council Directive of November 1986 (86/609/EEC).
Surgery
Surgery was performed essentially as described previously (21). Briefly, animals were anaesthetised with a combination of Hypnorm (0.32 mg/kg fentanyl citrate and 10 mg/kg fluanisone, i.m.; Janssen Pharmaceuticals, Oxford, UK) and diazepam (2.6 mg/kg i.p.; Phoenix Pharmaceuticals, Gloucester, UK). The right jugular vein was cannulated by inserting a polythene cannula (Portex, Hythe, UK) into the vessel. The free end of the cannula was exteriorised through a scalp incision and tunnelled through a protective spring. During the same surgery, 100 mg pellets containing either 40% or 100% corticosterone (40% and 100% corticosterone, respectively; ICN Biomedicals, Irvine, CA, USA) or 100% cholesterol (vehicle; Sigma-Aldrich, St Louis, MO, USA) were subcutaneously implanted between the shoulder blades to obtain constant concentrations of corticosterone in blood (12–14). After surgery, animals were individually housed and kept in the automated blood sampling room. The cannula was attached to a mechanical swivel that rotated through 360° in a horizontal plane and 180° through a vertical plane, allowing the rats to maximise freedom of movement. The cannulae were flushed daily with heparinised saline to maintain patency.
Experimental design
One cohort of animals was used which were subjected to different phases of the experimental design.
Phase 1: basal diurnal and ultradian corticosterone rhythms
The effect of different concentrations of exogenous corticosterone on basal diurnal and ultradian corticosterone rhythms was studied. Six days after pellet implantation, the cannula of each animal was connected to an automated blood sampling (ABS) system, as previously described in detail (6, 20). On post-surgery day 7, blood samples (37 μl aliquots) were automatically collected at 10-min intervals at a dilution of 1 : 5 in heparinised saline. As a result of practical limitations in the number of samples that can be collected with the ABS in the same rat, it was not possible to obtain a full corticosterone profile on this experimental day. Therefore, samples were collected during the trough (07.00 and 10.00 h) and peak (18.00 and 21.00 h) of hormonal release, the times during which strong differences in diurnal and ultradian concentrations have been described extensively (6, 20, 21). Plasma was separated by centrifugation and then stored at −80 °C until processed for corticosterone measurements as described below. Because of practical reasons related to blood sampling, we had dropouts in the vehicle group for the endocrine studies (phase 1 and 2). The obtained corticosterone values in this group, however, are consistent between the experiments and with previously published data (20, 21). Fortunately, these animals were still available for the behavioural studies (phase 3).
Phase 2: Effect of pellet removal (washout) on basal diurnal and ultradian corticosterone rhythms
To investigate whether corticosterone rhythmicity would recover within 1 day of termination of constant corticosterone administration, on day 8 post-surgery, the implanted pellets were removed (washout). Between 08.00–09.00 h, the pellets were removed under brief isoflurane anaesthesia (2–3 min) and animals were reconnected to the ABS at 09.30 h. ABS started at 10.00 and samples were collected every 20 min (10.00–13.00 h) or 10 min (13.00–05.00 h). The impact of exogenous corticosterone replacement on body weight, thymus weight and adrenal gland weight has been reported previously (15).
Phase 3: Stress-induced behavioural and corticosterone responses
Stress-induced corticosterone release and behavioural responsiveness were studied after cessation of constant corticosterone concentrations. Animals were then exposed to white noise stress (99 dB for 10 min) in the morning of day 9 post-surgery (06.00 h). Blood samples were collected every 20 min using the ABS between 05.00 h and 07.20 h. Concurrently, animal home cage behaviour was recorded using cameras mounted above the cage of each animal. Behavioural analysis was performed 10 min before, during and after relative to the onset of the stressor.
Corticosterone measurements
Blood samples were centrifuged for 15 min at 4000 r.p.m. at 4 °C. Plasma was stored at −80 °C until processed using a commercially available radio immuno assay (RIA; MP Biomedicals Inc., Santa Ana, CA, USA). Inter-assay and intra-assay variance was 6.9% and 7.3%, respectively.
Behavioural analysis
The behaviours analysed with a semiautomatic scoring system (The Observer Mobile 4.1; Noldus Information Technology, Wageningen, The Netherlands) were: (i) locomotion (walking, front and back paws change position); (ii) risk assessment (‘seeking’ behaviour while the body is still or stretched); (iii) face washing (grooming of face using front paws); (iv) body grooming (grooming using front and back paws, i.e. scratching); and (v) sitting (e.g. sitting or sleeping). Total activity was calculated by adding the scores of behaviours (i–iv). In addition, the number of (vi) rearing (raising of the rat and then lowering both front paws) and (vii) digging events (moving around of cage bedding with front paws) was counted. The data is presented in either 10-min bins (Table 3) or plotted per minute to study changes in behavioural patterns over time (Fig. 4).
Table 3.
Behavioural Analysis Before, During and After Noise Stress.
| Before stress (10 min) |
During stress (10 min) |
After stress (10 min) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | 40% corticosterone | 100% corticosterone | Vehicle | 40% corticosterone | 100% corticosterone | Vehicle | 40% corticosterone | 100% corticosterone | |
| Sitting (% time) | 90.6 ± 4.1 | 91.6 ± 2.2 | 97.7 ± 1.8 | 41.9 ± 8.3 | 71.4 ± 8* | 43.3 ± 6.3 | 58.3 ± 7.0 | 77.7 ± 9.7 | 83.8 ± 5.6 |
| Total activity (% time) | 9.4 ± 4.1 | 8.4 ± 2.2 | 2.3 ± 1.8 | 58.1 ± 8.3 | 28.6 ± 9* | 56.7 ± 6.3 | 41.7 ± 7.0 | 22.2 ± 9.7 | 16.2 ± 5.6 |
| Locomotion (% time) | 1.0 ± 0.5 | 1.1 ± 0.5 | 0.4 ± 0.3 | 10.9 ± 2.4 | 3.8 ± 1.2* | 7.1 ± 1.5 | 2.3 ± 0.7 | 0.7 ± 0.5 | 0.9 ± 0.4 |
| Risk assessment (% time) | 0.5 ± 0.3 | 0.4 ± 0.3 | 0.4 ± 0.4 | 27.9 ± 7.4 | 12 ± 3.1* | 42 ± 6.0* | 7.3 ± 3.4 | 10.0 ± 5.7 | 6.3 ± 2.9 |
| Face washing (% time) | 1.7 ± 1.1 | 1.9 ± 0.7 | 0.2 ± 0.2 | 17 ± 2.6 | 3.2 ± 1.7** | 6.6 ± 1.6* | 17.6 ± 4.1 | 4.8 ± 1.8** | 4.0 ± 1.9** |
| Body grooming (% time) | 6.2 ± 3.0 | 3.8 ± 1.1 | 1.3 ± 1.0 | 6.0 ± 2.2 | 0.7 ± 0.7* | 1.0 ± 0.4* | 8.2 ± 2.0 | 5.2 ± 3.0 | 2.1 ± 1.3 |
| Rearing (events) | 0 ± 0 | 0 ± 0 | 0.6 ± 0.6 | 7.5 ± 2.1 | 3.7 ± 1.8 | 10.3 ± 2.4 | 0.5 ± 0.3 | 0.4 ± 0.3 | 0.3 ± 0.3 |
| Digging (events) | 0.3 ± 0.3 | 1.3 ± 1.0 | 0 ± 0 | 0.2 ± 0.2 | 2.0 ± 1.0 | 7.5 ± 2.1 | 4.5 ± 2.5 | 1.6 ± 1.4 | 1.1 ± 0.9 |
One-way anova and Tukey’s post-hoc test (*P < 0.05; **P < 0.01 compared to vehicle) indicate differences in stress responsiveness in both the 40% and 100% corticosterone animals compared to vehicle animals. Data represent the mean ± SEM of behavioural parameters scored per 10 min bins before during and after the stressor of vehicle (n = 4), 40% (n = 6) or 100% corticosterone pellets (n = 6).
Fig. 4.
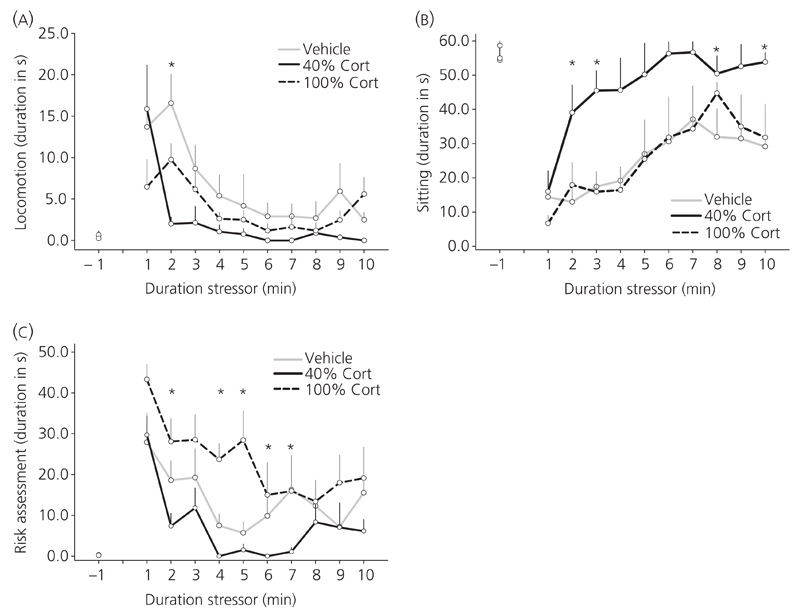
Behavioural responses to 10 min of noise stress (99 dB). Behavioural changes to noise stress are presented in 1-min bins over time in (a) locomotion (F10,60 = 13.4; P < 0.001) (b) sitting (F10,60 = 12.3; P < 0.001) and (c) risk assessment (F3.7,22.0 = 17.9; P < 0.001). Analysis of interaction effect showed that in the 40% animals, the stressor induced significantly less locomotion (F20,120 = 2.8; P < 0.001) and risk assessment (F20,120 = 1.7; P = 0.04) compared to vehicle, indicating less total activity. Data are presented as the mean ± SEM duration in seconds per 1-min bin for vehicle (n = 6), 40% (n = 7) and 100% corticosterone (Cort) pellet animals (n = 7). *P < 0.05 compared to vehicle as tested by two-way repeated measures anova and Bonferroni’s post-hoc test.
Statistical analysis
Statistical analyses were performed using spss, version 16.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± SEM, except for individual corticosterone profiles. For phase 1, two-way anova was used to study the diurnal differences in mean corticosterone and area under the curve (AUC) concentrations between treatment groups. Because of the limited number of samples collected for analysis, pulsar analysis may not be reliable on short time intervals. Therefore, as an indication of ultradian pulsatility, repeated measures anova was used to analyse the effect of time on corticosterone concentrations in the 3-h time frames in morning and evening. For phase 2, the hormone profile for each individual animal was analysed using the pulsar algorithm (22) with previously published G-values (21). The pulse characteristics analysed were: AUC, mean daily corticosterone concentration, number, height (absolute increase from zero baseline), amplitude (absolute increase from respective baseline) and frequency of corticosterone pulses. One-way anova and independent Student’s t-tests were used to test statistical differences in pulse characteristics between corticosterone- and vehicle-treated rats. For phase 3, repeated measures anova was used to indicate time and treatment effects in stress-induced corticosterone profiles. Behavioural data were analysed using one-way anova or two-way repeated measures anova with time and treatment as factors. Where applicable, post-hoc tests were used as indicated. With respect to repeated measures anova, sphericity (e.g. comparable to homogeinity of variance) was tested with Mauchly’s test. In case the conditions were not met, the rather conservative Greenhouse–Geisser correction was used to enable a valid F-ratio. P < 0.05 was considered statistical significant.
Results
Effect of corticosterone pellet implantation on basal diurnal and ultradian corticosterone rhythms
Figure 1 depicts mean (Fig. 1a) and individual morning and evening plasma corticosterone profiles (Fig. 1b–d) of animals from each treatment group measured on day 7 of pellet implantation. Mean corticosterone concentrations and AUC are shown in Table 1. In agreement with previous studies (20, 21), in vehicle-treated animals, we observed very low to nondetectable concentrations in the morning and distinctively higher fluctuating hormone concentrations in the evening (Fig. 1a, b), indicating significant diurnal differences in mean corticosterone concentrations (Table 1; P = 0.03) and AUC (P = 0.04). By contrast, exogenous corticosterone exposure induced clear effects on corticosterone rhythms: both 40% and 100% corticosterone pellet implantation effectively flattened diurnal corticosterone rhythmicity at the same times as elevating basal concentrations in afternoon and/or in the morning (Fig. 1a, c–d). No statistical differences between morning and evening mean corticosterone concentrations or AUC were found within these groups, whereas mean corticosterone concentrations in the 100% group were significantly higher compared to vehicle-treated animals (Table 1; P < 0.05).
Fig. 1.
Effect of subcutaneous corticosterone (Cort) pellet implantation on diurnal and ultradian plasma corticosterone rhythms. Data represent (a) group averages (mean ± SEM) and representative individual plasma corticosterone profiles of rats implanted with (b) vehicle (n = 4), (c) 40% (n = 7) and (d) 100% corticosterone pellets (n = 7). Blood samples were collected at 10-min intervals from 07.00–10.00 h and 18.00–21.00 h on day 7 post-surgery. Significant ultradian fluctuations in corticosterone concentrations were only detected in the evening in vehicle-treated animals (repeated measures anova), indicating successful flattening of ultradian pulses in 40% and 100% corticosterone pellet animals. ***Effect of time with Bonferroni’s post-hoc test: F1.9,5.8 = 9.1; P = 0.02. Grey bar indicates the dark phase.
Table 1.
Morning and Evening Area Under the Curve (AUC) and Mean Corticosterone Concentrations in Rats Implanted with Vehicle, 40% or 100% Corticosterone Pellets.
| Time of day (h) | AUC | Mean corticosterone (ng/ml) | |
|---|---|---|---|
| Vehicle | 07.00–10.00 | 164 ± 44 | 9.2 ± 3.4 |
| 18.00–21.00 | 621 ± 102* | 36.5 ± 13.7* | |
| 40% | 07.00–10.00 | 342 ± 77 | 21.6 ± 5.0 |
| corticosterone | 18.00–21.00 | 323 ± 49 | 19.1 ± 3.8 |
| 100% | 07.00–10.00 | 3269 ± 570** | 180.2 ± 41.0** |
| corticosterone | 18.00–21.00 | 3182 ± 501** | 170.3 ± 37.6** |
Values represent mean ± SEM of AUC and mean corticosterone concentrations in rats exposed to vehicle (n = 4), 40% (n = 7) and 100% corticosterone pellet (n = 7) for 7 days measured in the morning (07.00–10.00) and in the evening (18.00–21.00). Two-way anova (Games–Howell post-hoc test) was used to analyse diurnal differences in AUC (F5,12 = 13.1; P < 0.001) and mean corticosterone concentrations (F5,13 = 11.9; P < 0.001). Vehicle-treated animals showed normal diurnal variation in mean corticosterone concentrations and AUC (*P < 0.05). No diurnal differences were detected in the 40% and 100% corticosterone pellet groups. Corticosterone concentrations and AUC were significantly higher in the 100% corticosterone-treated animals compared to morning and evening concentrations of vehicle animals (**P < 0.05).
As expected, ultradian pulses were only detected in the evening in vehicle-treated animals (effect of time: P = 0.02), whereas the other treatment groups did not show statistically significant differences in the morning or evening (Fig. 1a, b). These results show that both 40% and 100% corticosterone pellet implantation successfully abolished diurnal and ultradian corticosterone rhythms.
Effect of pellet removal (washout) on basal diurnal and ultradian corticosterone rhythms
To determine whether endogenous corticosterone pulsatility recovers within 1 day from constant exogenous corticosterone administration, on day 8 post-surgery, pellets were removed early in the morning. Figure 2 shows group averaged and individual plasma corticosterone profiles over 19 h after removal of pellets and reconnection to the ABS system. In vehicle-treated animals, typical diurnal and ultradian variation was observed with increasing mean corticosterone concentrations and ultradian pulses towards the dark phase (Fig. 2a, b). In 40% corticosterone-treated animals, pellet removal resulted in a rapid fall of corticosterone to very low concentrations and a recovery of ultradian and diurnal rhythms (see below). The onset of detectable ultradian pulses was observed at approximately 16.00 h, suggesting a delay in reoccurance of pulsatility compared to vehicle animals (Fig. 2a–c). Although, in general, a tendency towards decreased corticosterone concentrations was observed, in the 100% corticosterone group, no clear effect of rapid reinstatement of ultradian corticosterone pulsatility was observed (Fig. 2a–d).
Fig. 2.
Effect of corticosterone pellet removal (washout) on diurnal and ultradian plasma corticosterone (Cort) rhythms. Data represent (a) group averages (mean ± SEM) and individual plasma corticosterone profiles measured after pellet removal in rats previously implanted with (b) vehicle (n = 4), (c) 40% (n = 6) or (d) 100% corticosterone pellets (n = 6). Pellets were removed at 08.00 of day 8 post-surgery; rats were reconnected to the ABS at 09.30 h and samples were collected every 20 min (10.00–12.40 h) or 10 min (13.00–05.00 h). Grey bar indicates the dark phase.
To study ultradian pulsatility in more detail, the pulsar algorithm was used to identify pulse characteristics in individual animals (Table 2). The persistence of very high corticosterone concentrations in the 100% corticosterone group resulted in very high values for the different pulse parameters. To prevent these data from skewing the results and to compare the ultradian pattern of the 40% group to vehicle in more detail, pulse characteristics were statistically compared between vehicle and the 40% corticosterone group using independent Student’s t-tests. pulsar analysis revealed a decrease in mean corticosterone concentrations (P = 0.04) and pulse height (P = 0.04) in the 40% corticosterone-treated group compared to vehicle. Furthermore, a trend toward a significant decrease was observed on AUC (P = 0.06) and pulse amplitude (P = 0.05). No significant difference in the number of pulses and pulse frequency (P = 0.14) was observed, indicating very similar pulse characteristics of the 40% corticosterone group compared to vehicle.
Table 2.
Mean ± SEM of pulsar Parameters Measurements of Treated Rats During 13.00–05.00 h of the Sampling Period.
| Vehicle | 40% Corticosterone | 100% Corticosterone | |
|---|---|---|---|
| AUC | 3491 ± 1124 | 1747 ± 317 | 13016 ± 2858 |
| Mean corticosterone (ng/ml) | 47.2 ± 14.0 | 22.4 ± 4.4* | 240.8 ± 65.2 |
| Pulse number | 5.8 ± 0.6 | 3.7 ± 1.4 | 8.5 ± 1.4 |
| Pulse amplitude (ng/ml) | 74.7 ± 19.2 | 35.6 ± 11.1 | 165.5 ± 51.0 |
| Pulse height (ng/ml) | 91.3 ± 20.1 | 49.1 ± 11.3* | 337.0 ± 78.3 |
| Pulse frequency (pulse/h) | 0.59 ± 0.06 | 0.39 ± 0.13 | 0.92 ± 0.11 |
Pellets from rats previously treated for 7 days with vehicle (n = 4), 40% (n = 6) or 100% corticosterone pellets (n = 6) were removed in the morning of post–surgery day 8. pulsar analysis only demonstrates significantly lower mean corticosterone concentrations and pulse height (*P < 0.05) in animals treated with 40% corticosterone pellets compared to vehicle as indicated by independent Student’s t-tests. AUC, area under the curve.
Effect of previous constant corticosterone exposure on stress responsiveness
To study functional consequences of previous exposure to constant corticosterone concentrations, animals were exposed to noise stress (99 dB for 10 min) and blood samples were collected every 20 min using the ABS system. Figure 3 depicts the stress-induced increase in corticosterone concentrations in each treatment group. As expected, vehicle animals demonstrate an increase in corticosterone concentrations, with hormone peak concentrations of 103 ± 11.9 ng/ml, 20 min after onset of the stress (effect of time: P < 0.001). Interestingly, animals that were previously implanted with 40% corticosterone pellets did not differ in their stress response compared to vehicle animals and had similar corticosterone peak concentrations (82.6 ± 22.1 ng/ml). Noise stress did not affect plasma corticosterone concentrations in the 100% corticosterone pellet group. At the time of stress, basal corticosterone concentrations were increased compared to the other groups (P < 0.01).
Fig. 3.
Effect of previous constant corticosterone (Cort) exposure on stress-induced plasma corticosterone release. Values are represented as mean ± SEM of plasma corticosterone concentration measured in rats 1 day after removal of vehicle (n = 4), 40% (n = 6) or 100% corticosterone pellets (n = 5). Blood samples were automatically collected every 20 min. Rats were exposed to 10 min of noise stress (99 dB; hatched bar) at 06.00 h, which increased corticosterone levels in vehicle and 40% corticosterone animals [effect of time: F(7,96) = 15.7; P < 0.001]. No difference in stress-induced corticosterone levels was observed between vehicle and 40% corticosterone animals. Animals previously treated with 100% corticosterone showed no significant endocrine response to the stressor as basal concentrations were elevated (*P < 0.01; two-way repeated measures anova and Bonferroni’s post-hoc test).
Effect of previous constant corticosterone exposure on stress-induced behavioural responses
Behavioural responsiveness to noise stress was assessed in 10-min intervals before, during and after the stress. A complete analysis of the data is reported in Table 3. Before exposure to the stressor, there was no difference between the treatments groups in any of the behaviours analysed (Fig. 4 and Table 3).
During the stressor, significant effects and interactions of time and treatment were found by two-way repeated measures and one-way anova. Noise stress significantly induced changes in locomotion (Fig. 4a; P < 0.001), sitting (Fig 4b; P < 0.001) and risk assessment (Fig 4c; P < 0.001), respectively, indicating changes in the behavioural pattern over time. Analysis of the interaction effect showed that, in the 40% animals, the stressor induced significantly less locomotion (P < 0.001) and risk assessment (P = 0.04) compared to vehicle, indicating less total activity (P < 0.05); Table 3). Indeed, the duration of those behaviours after the onset of the stressor was shorter in 40% corticosterone animals compared to vehicle and 100% corticosterone animals because the percentage of time spent dropped almost to zero 2–3 min after onset of the stressor (Fig. 4a–c). By contrast, in the 100% group, there was an increase in risk assessment (P = 0.03), number of digging events (P < 0.01) and a trend of increased rearing events (P = 0.1). However, in these animals, there was no difference in the total activity compared to vehicle animals (P > 0.05; Table 3), indicating differences in the make up of the activity pattern compared to vehicle animals (Fig. 4 and Table 3). Furthermore, for both corticosterone-treated groups, there was a decrease in the percentage of time spent face and body grooming during the stressor (Table 3; P < 0.05).
After cessation of the stressor, both 40% and 100% corticosterone animals continued to show decreased face grooming (Table 3; P < 0.01). No statistical significant differences were found in any of the other behaviours analysed.
Discussion
In the present study, we demonstrated that constant exposure to exogenous corticosterone by means of subcutaneous pellet implantation in adrenally intact rats not only abolishes diurnal corticosterone rhythmicity, but also diminishes rapid ultradian fluctuations. Termination of the constant signal resulted in a rapid recovery of ultradian patterns in animals exposed to daily average concentrations (40% corticosterone), but not in animals exposed to high concentrations of corticosterone (100% corticosterone). Paradoxically, although the endocrine response to noise stress in the 40% corticosterone group also was not different compared to vehicle, these animals showed the most substantial changes in the behavioural response to stress.
Validation of model system
Plasma corticosterone concentrations were determined during time frames in which marked differences in diurnal and ultradian corticosterone levels have been well described in normal intact animals (5, 20, 21). Irrespective of limitations in the number of blood samples collected, our approach using high-frequency ABS led to the novel observation of disappearance of ultradian corticosterone pulses after constant glucocorticoid administration at the same time as maintaining basal concentrations at constant daily average (40% corticosterone) or pathological concentrations (100% corticosterone). The 40% corticosterone group is of particular interest given that these animals only differed in the pattern from vehicle (e.g. constant versus pulsatile) but not in the total amount of corticosterone exposure (e.g. AUC and mean corticosterone concentrations). Furtermore, this finding confirms the notion that experimentally-clamped levels of glucocorticoids can be achieved without adrenalectomy [(13, 14); provided that no stressors are applied]. We conclude that 40% corticosterone pellet implantation can be used to easily manipulate pulse characteristics in intact rats while maintaining physiological glucocorticoid concentrations, whereas 100% corticosterone rather resembles conditions of stress-related disease.
We and others have clamped circadian circulating corticosterone concentrations in this way previously (12–14). The HPA axis, via a constant negative-feedback signal, disrupts corticosterone rhythms by adjusting circadian and ultradian trough and peak concentrations, resulting in steady-state corticosterone concentrations. Indeed, episodic feedback signals are crucial for maintaining ultradian feedforward–feedback oscillitatory activity between the pituitary and adrenal gland (23). We have now demonstrated that the current approach is also suitable for studying this relationship in more detail.
Normalisation of basal HPA axis activity after corticosterone removal
Previous studies have shown that, for some measures of HPA axis reactivity, normalisation of circadian corticosterone in adrenalectomised animals is sufficient (24, 25). Even though the present study does not allow us to fully descriminate between ultradian and circadian corticosterone pulsatile patterns in relation to HPA axis reactivity, our study is the first to present data on basal ultradian corticosterone patterns in intact animals. pulsar analysis indicated rapid progressive normalisation of ultradian pulse characteristics within approximately 6 h of removal of the 40% corticosterone pellet. This is most likely the result of a delayed feedback mechanism suppressing pulse amplitude at earlier time points (23), and could also potentially affect adrenocorticotrophic hormone (ACTH). Limitations in the volume of blood that can be collected via ABS preclude conclusions on basal and stress-induced ACTH levels. However, this issue has been previously adressed in the literature because blunting circadian variation in intact animals by exogenous corticosterone administration attenuates ACTH release in response to stress (13). Although we have no information on the stress response, we observed supressed basal ACTH levels in morning and evening before and during corticosterone washout in a separate cohort of animals (data not shown).
The rapid decline of plasma hormone concentrations after 40% corticosterone pellet removal is in line with the normal corticosterone half-life of approximately 10 min (an important factor in allowing the existence of sharp ultradian peaks) (6). The results of the 100% corticosterone group are dramatically different. No recovery of corticosterone pulsatility was observed; moreover, a very slow decline in circulating hormone concentrations after removal of the pellet was noticed. A practical explanation for the lack of ultradian recovery could be the formation of a local corticosterone depot in this group of animals because the slow decline did not follow the half-life time of corticosterone. Even though the pellets were easily removed at the time of surgery, we noticed a thickening of the skin around the pellet, possibly indicating the formation of a depot due to the very high local steroid concentrations. This was not observed in the vehicle and 40% corticosterone groups. In previous studies, however, we did notice a ‘normal’ decline in corticosterone levels (15). Therefore, it is very likely that the issue in the present study is merely technical and may be attributed to the different sampling methods (tail vein sampling versus automated blood sampling) and/or animal supplier and surroundings (Leiden versus Bristol). The continued high concentrations prevent conclusions on the recovery of corticosterone pulses after washout of constant high concentrations of corticosterone. In the context of sustained stress, or treatment with (synthetic) glucocorticoids, prolonged exposure to high concentrations may actually occur. It would be interesting to explore further whether ultradian rhythmicity and behavioural responsiveness is eventually restored in the 100% corticosterone group after washout. The issue of normalisation after high corticosterone exposure therefore merits thorough investigation in future studies. However, for now, this group can still be used as a reference for the effects of the 40% pellet on stress responsiveness.
Dissocation in behavioural and neuroendocrine response to noise stress
In agreement with previous studies (6, 20, 26), noise stress evoked a transient increase in corticosterone release in vehicle-treated animals. Alterations in the basal pulse frequency and amplitude are known to be a major factor influencing reactivity to acute stressors (20, 27, 28). The normalisation of the HPA response to stress in the 40% corticosterone animals is in line with the return of basal pulsatile corticosterone secretion. Although adrenal weight takes longer than 24 h to recover (15), a finding in line with the decrease in mean corticosterone concentrations and pulse height, we conclude that the activity of the axis in the 40% corticosterone animals appears to be very close to normal at the moment we applied the noise stress. Thus, the half-life time of the suppression of HPA axis reactivity brought about by 40% corticosterone pellet implantation (13) is significantly less than 24 h. This is reminiscent of the acute effects of mineralocorticoid and glucocorticoid receptor (MR and GR) ligands on the axis, that last in the order of hours (29–32). The absence of a HPA response to stress in the 100% group may be attributed to any negative-feedback mechanism because of the high concentrations of circulating steroid at the time of the stressor.
Interestingly, during the stressor, a strong dissociation between hormonal and behavioural stress responsiveness was observed. Our data indicate longer-lasting changes induced by glucocorticoids in the brain even after normalisation of basal HPA axis activity. Others have also demonstrated behavioural changes in aggression and social deficits in animals chronically exposed to low levels of glucocorticoids (33). Using the same experimental paradigm of corticosterone pellet implantation as employed in the present, we recently showed that GR levels and molecular signalling in the brain remain significantly affected after removal of the corticosterone pellets (15). However, our results may also pertain to (m)any neurotransmitter system(s) such as the serotonergic system (34), although only few data are available. Flattened corticosterone secretion induced by implanted corticosterone pellets was previously found to decrease 5-HT1A receptor expression exclusively in the rat dentate gyrus (14) and somatodendritic 5-HT1A autoreceptors function in the raphe nucleus (35). Functionally, however, chronic excess of corticosterone obtained by daily injections of the steroid, attenuated 5-HT1A receptor-induced membrane hyperpolarisation in hippocampal CA1 neurones without affecting the 5-HT1A receptors (36). The persistence of these effects is not known.
With respect to the behavioural performance of high corticosterone animals, which in our hands was remarkably similar to vehicle rats, most available data point to substantial changes in information processing after chronic high corticosterone and stress (19, 37). Chronic stress modulates limbic regions, such as the hippocampus and amygdala, and influences learning and memory in both rodents and humans (38, 39), most likely as a result of changes in the neuroarchitecture and plasticity (19, 37, 40). Chronic stress and high glucocorticoid concentrations are known to potentiate emotionality and exploratory behaviour in rodents (38, 41). In the present study, the 40% corticosterone group showed a modest response to audiogenic stimulation in terms of attenuated behavioural activity (i.e. increased sitting) and risk assessment (i.e. reduced exploration). By contrast, the 100% corticosterone group resembles vehicle-treated animals more but showed higher emotionality to the stressor with increased activity (i.e. increased locomotion, risk assessment and rearing). The current design allows dissection of these different behavioural aspects of chronic corticosterone exposure and suggests that disrupted pulsatility (40%) is more linked to exploratory behaviour and risk assessment. How persistent these effects are however remains to be determined, although previous studies have suggested long-term effects of glucocorticoids on behaviour in rodents and humans (16, 17, 33). The duration of disturbed stress responsiveness likely depends on the duration of aberrant exposure. A parametric study of development and duration of changed responsiveness is needed before the validity of our model in relation to long-term changes in humans can be estimated.
Retrospectively, it would certainly have been interesting to study behavioural stress responsiveness after 7 days of pellet implantion. However, because we aimed to focus on longer-lasting glucocorticoid effects on the brain and possible restoration of ultradian corticosterone patterns, we chose to compare the experimental groups to vehicle. Whether the altered stress reactivity in the 40% group after pellet removal is in fact advantageous or detrimental is open to interpretation and may depend on the particular circumstances. The results obtained in the present study, however, suggest that the history of HPA axis pulsatility of the individual has consequences for the behavioural response to stress, emphasising the diversity in the modulatory actions of pulsatile glucocorticoid release.
Persistence of behavioural effects
In the context of differential responses to an acute stressor, either the history of corticosterone exposure may differ (42, 43) or rapid (non)genomic effects may play a role (44). Ultradian glucocorticoid pulses are translated into consecutive bursts of GR nuclear translocation, DNA binding of GR and transcriptional initiation, resulting in ‘gene-pulsing’ of native transcripts (45, 46). In addition to the classic intracellular receptors that mediate genomic responses, there is strong evidence for nongenomic (behavioural) actions of glucocorticoids (39, 47, 48), putatively via low affinity membrane-associated MR and GR (49, 50). In the 40% group, the profile of corticosterone secretion during the stressor did not differ from control animals, and an explanation involving nongenomic mechanisms therefore hinges on changed responsiveness of nongenomic effects.
Although the role or significance of glucocorticoid fluctuations is largely unknown, the evolutionary conservation of pulsatile endocrine systems suggests important biological and clinical consequences. In stress-related disease, there are persistent effects of changes in basal pulse characteristics (3, 51, 52). The results obtained in the present study show a striking parallel with data in humans. Emotional and cognitive effects are still highly prevalent in patients with long-term cured Cushing’s disease compared to matched controls, indicating possible irreversible effects of prolonged previous glucocorticoid excess (16, 53). Washout after corticosterone pellet implantation may therefore constitute a model to study these clinically relevant processes.
In conclusion, the data obtained in the present study suggest that HPA axis activity is remarkably sensitive to changes in the pattern of corticosterone exposure and adjusts in a reversible fashion to alterations in this pattern as long as there is no overt hypercorticism. However, the data also indicate that normalisation of HPA activity in response to an acute stressor does not necessarily indicate normal behavioural responses, which may need more time to adapt. We propose that it is the pulsatile pattern rather than the absolute concentrations of corticosterone exposure that determines subsequent responsiveness to stress, and this has obvious implications for understanding the pathogenesis of stress-related disease.
Acknowledgements
This research was supported by NWO Mozaïek grant 017.002.021, NWO-IRTG DN95-420 and the Royal Academy of Arts and Sciences (KNAW).
Footnotes
Disclosure
The authors have nothing to disclose.
References
- 1.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 2.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 3.Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 5.Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol. 1991;261:R1257–R1268. doi: 10.1152/ajpregu.1991.261.5.R1257. [DOI] [PubMed] [Google Scholar]
- 6.Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology. 1998;139:443–450. doi: 10.1210/endo.139.2.5721. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin Chim Acta. 2005;359:189–194. doi: 10.1016/j.cccn.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Lightman SL, Windle RJ, Julian MD, Harbuz MS, Shanks N, Wood SA, Kershaw YM, Ingram CD. Significance of pulsatility in the HPA axis. Novartis Found Symp. 2000;227:244–257. doi: 10.1002/0470870796.ch14. discussion 57–60. [DOI] [PubMed] [Google Scholar]
- 9.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 10.de Kloet ER, Sarabdjitsingh RA. Everything has rhythm: focus on glucocorticoid pulsatility. Endocrinology. 2008;149:3241–3243. doi: 10.1210/en.2008-0471. [DOI] [PubMed] [Google Scholar]
- 11.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583:255–262. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 12.Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am J Physiol. 1985;249:R527–R532. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- 13.Akana SF, Scribner KA, Bradbury MJ, Strack AM, Walker CD, Dallman MF. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology. 1992;131:585–594. doi: 10.1210/endo.131.2.1322275. [DOI] [PubMed] [Google Scholar]
- 14.Meijer OC, Van Oosten RV, De Kloet ER. Elevated basal trough levels of corticosterone suppress hippocampal 5-hydroxytryptamine(1A) receptor expression in adrenally intact rats: implication for the pathogenesis of depression. Neuroscience. 1997;80:419–426. doi: 10.1016/s0306-4522(97)00008-0. [DOI] [PubMed] [Google Scholar]
- 15.Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, de Kloet ER, Meijer OC. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology. 2010;151:1177–1186. doi: 10.1210/en.2009-1119. [DOI] [PubMed] [Google Scholar]
- 16.Tiemensma J, Kokshoorn NE, Biermasz NR, Keijser BJ, Wassenaar MJ, Middelkoop HA, Pereira AM, Romijn JA. J Clin Endocrinol Metab. 2010 Apr 6; doi: 10.1210/jc.2009-2032. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Ratka A, Sutanto W, De Kloet ER. Long-lasting glucocorticoid suppression of opioid-induced antinociception. Neuroendocrinology. 1988;48:439–444. doi: 10.1159/000125046. [DOI] [PubMed] [Google Scholar]
- 18.Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann NY Acad Sci. 2004;1018:141–150. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- 19.Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL. Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. J Neuroendocrinol. 2006;18:526–533. doi: 10.1111/j.1365-2826.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 21.Spiga F, Harrison LR, Wood SA, Atkinson HC, MacSweeney CP, Thomson F, Craighead M, Grassie M, Lightman SL. Effect of the glucocorticoid receptor antagonist Org 34850 on basal and stress-induced corticosterone secretion. J Neuroendocrinol. 2007;19:891–900. doi: 10.1111/j.1365-2826.2007.01605.x. [DOI] [PubMed] [Google Scholar]
- 22.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 23.Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010 doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- 25.Akana SF, Jacobson L, Cascio CS, Shinsako J, Dallman MF. Constant corticosterone replacement normalizes basal adrenocorticotropin (ACTH) but permits sustained ACTH hypersecretion after stress in adrenalectomized rats. Endocrinology. 1988;122:1337–1342. doi: 10.1210/endo-122-4-1337. [DOI] [PubMed] [Google Scholar]
- 26.Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- 27.Windle RJ, Wood SA, Lightman SL, Ingram CD. The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology. 1998;139:4044–4052. doi: 10.1210/endo.139.10.6238. [DOI] [PubMed] [Google Scholar]
- 28.Windle RJ, Wood SA, Kershaw YM, Lightman SL, Ingram CD, Harbuz MS. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J Neuroendocrinol. 2001;13:905–911. doi: 10.1046/j.1365-2826.2001.00715.x. [DOI] [PubMed] [Google Scholar]
- 29.Ratka A, Sutanto W, Bloemers M, de Kloet ER. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989;50:117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- 30.van Haarst AD, Oitzl MS, Workel JO, de Kloet ER. Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology. 1996;137:4935–4943. doi: 10.1210/endo.137.11.8895366. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson HC, Wood SA, Castrique ES, Kershaw YM, Wiles CC, Lightman SL. Corticosteroids mediate fast feedback of the rat hypothalamic-pituitary-adrenal axis via the mineralocorticoid receptor. Am J Physiol Endocrinol Metab. 2008;294:E1011–E1022. doi: 10.1152/ajpendo.00721.2007. [DOI] [PubMed] [Google Scholar]
- 32.Spiga F, Harrison LR, Wood SA, MacSweeney CP, Thomson FJ, Craighead M, Grassie M, Lightman SL. Effect of the glucocorticoid receptor antagonist Org 34850 on fast and delayed feedback of corticosterone release. J Endocrinol. 2008;196:323–330. doi: 10.1677/JOE-07-0503. [DOI] [PubMed] [Google Scholar]
- 33.Haller J, Halasz J, Mikics E, Kruk MR. Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. J Neuroendocrinol. 2004;16:550–557. doi: 10.1111/j.1365-2826.2004.01201.x. [DOI] [PubMed] [Google Scholar]
- 34.Bush VL, Middlemiss DN, Marsden CA, Fone KC. Implantation of a slow release corticosterone pellet induces long-term alterations in serotonergic neurochemistry in the rat brain. J Neuroendocrinol. 2003;15:607–613. doi: 10.1046/j.1365-2826.2003.01034.x. [DOI] [PubMed] [Google Scholar]
- 35.Leitch MM, Ingram CD, Young AH, McQuade R, Gartside SE. Flattening the corticosterone rhythm attenuates 5-HT1A autoreceptor function in the rat: relevance for depression. Neuropsychopharmacology. 2003;28:119–125. doi: 10.1038/sj.npp.1300016. [DOI] [PubMed] [Google Scholar]
- 36.Karten YJ, Nair SM, van Essen L, Sibug R, Joels M. Long-term exposure to high corticosterone levels attenuates serotonin responses in rat hippocampal CA1 neurons. Proc Natl Acad Sci USA. 1999;96:13456–13461. doi: 10.1073/pnas.96.23.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci. 2006;11:2746–2758. doi: 10.2741/2004. [DOI] [PubMed] [Google Scholar]
- 38.Schwabe L, Dalm S, Schachinger H, Oitzl MS. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiol Learn Mem. 2008;90:495–503. doi: 10.1016/j.nlm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Schwabe L, Schachinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as switch between memory systems. J Cogn Neurosci. 2010;22:1362–1372. doi: 10.1162/jocn.2009.21278. [DOI] [PubMed] [Google Scholar]
- 40.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tejani-Butt SM, Pare WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague–Dawley and Wistar Kyoto (WKY) rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- 42.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 43.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 44.Joels M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology. 2007;148:5470–5477. doi: 10.1210/en.2007-0585. [DOI] [PubMed] [Google Scholar]
- 46.Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11:1093–1102. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandi C, Venero C, Guaza C. Novelty-related rapid locomotor effects of corticosterone in rats. Eur J Neurosci. 1996;8:794–800. doi: 10.1111/j.1460-9568.1996.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 48.Mikics E, Kruk MR, Haller J. Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology. 2004;29:618–635. doi: 10.1016/S0306-4530(03)00090-8. [DOI] [PubMed] [Google Scholar]
- 49.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 52.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 53.Tiemensma J, Kokshoom NE, Biermasz NR, Keijser BJ, Wassenaar MJ, Middelkoop HA, Pereira AM, Romijn JA. Cognitive Impairments in Patients with Long-Term Cure of Cushing’s Disease. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-2032. doi: jc.2009-2032v1. [DOI] [PubMed] [Google Scholar]