Abstract
Rationale
The touchscreen continuous trial-unique non-matching to location task (cTUNL) has been developed to optimise a battery of tasks under NEWMEDS (Novel Methods leading to New Medication in Depression and Schizophrenia, http://www.newmeds-europe.com). It offers novel task features of both a practical and a theoretical nature compared to existing touchscreen tasks for spatial working memory.
Objectives
To determine whether the cTUNL task is sufficiently sensitive to differentiate between dentate gyrus (DG) and CA3 hippocampal subregion contributions to performance.
Methods
The effect of DG and CA3 dysfunction on memory for locations in the cTUNL task was tested. Rats were assessed on versions of the task --2-choice and 3-choice – that differed in memory load. Performance was challenged using manipulations of delay and the spatial separation between target and sample locations.
Results
Dysfunction of the DG disrupts performance across both delay and spatial separations in 2-choice cTUNL when the delay is variable and unpredictable. Increasing the working memory load (3 stimuli) increases sensitivity to DG dysfunction, with deficits apparent at fixed, short delays. In contrast, CA3 dysfunction did not disrupt performance.
Conclusion
Acquisition of cTUNL was rapid, and the task was sensitive to manipulations of delays and separations. A 3-choice version of the task was found to be viable. Finally, both the 2- and 3-choice versions of the task were able to differentiate between limited dysfunction to different areas within the hippocampus. DG dysfunction affected performance when using unpredictable task parameters. CA3 dysfunction did not result in impairment, even at the longest delays tested.
Keywords: Spatial working memory, memory load, dentate gyrus, CA3, hippocampus, touchscreen, rat
1. Introduction
The NEWMEDS (Novel Methods leading to New Medication in Depression and Schizophrenia, http://www.newmeds-europe.com) initiative is a collaborative project between industry and academia with a focus on the development of novel methods to aid drug discovery relevant to schizophrenia. Within this consortium, one objective has been to develop, improve and optimise tests for use in the rodent touchscreen operant chamber. The touchscreen task presented here, continuous trial-unique non-matching to location (cTUNL), has been developed as part of efforts to optimise a NEWMEDS battery of tasks (Hvoslef-Eide et al. this issue-a), and offers novel task features of both a practical and a theoretical nature compared to existing touchscreen tasks for spatial working memory.
Translational preclinical tests of psychological constructs that are relevant to human disease and disorders are of great importance for the development of novel treatments, as extensively discussed elsewhere (Bussey et al. 2012; Horner et al. 2013; Hvoslef-Eide et al. this issue-a). The previously published touchscreen trial-unique non-matching to location (TUNL) task is sensitive to both prefrontal (McAllister et al. 2013) and hippocampal dysfunction in rats (Talpos et al. 2010) and mice (Kim et al. this issue). Additionally, the use of multiple locations allows for the assessment of spatial pattern separation ability. That is, the physical distance between the stimuli on the screen allows for variation of similarity, whilst minimising mediating strategies reported as problematic in traditional delayed non-matching to position tasks (Chudasama and Muir 1997; Dudchenko and Sarter 1992; Hearst 1962; Herremans et al. 1996).
cTUNL is a variation on TUNL whereby the sample phase of the delayed non-matching-to-position paradigm is built into the test phase (see Fig. 1). This is achieved by using the correct location (novel; S+) on the previous trial as the incorrect (familiar; S-) on the current trial, alongside a novel location as the S+. A novel conceptual task feature is the ability to manipulate the separation between stimulus locations during encoding of the to-be-remembered stimulus (referred to as separation during encoding) as well as during choice (referred to as separation during retrieval). This is a valuable task feature in the context of measuring pattern separation, as DG-focused manipulations can differentially affect pattern separation at encoding versus retrieval (Bekinschtein et al. 2013). A second novel task feature is the ability to increase the number of stimuli on the screen, requiring the animal to maintain more than two representations of locations active across a delay. Increasing the load on memory in this way is similar to touchscreen tasks used to assess spatial working memory in human patients, such as the CANTAB spatial working memory task (Barnett et al. 2010; Sahakian et al. 1988).
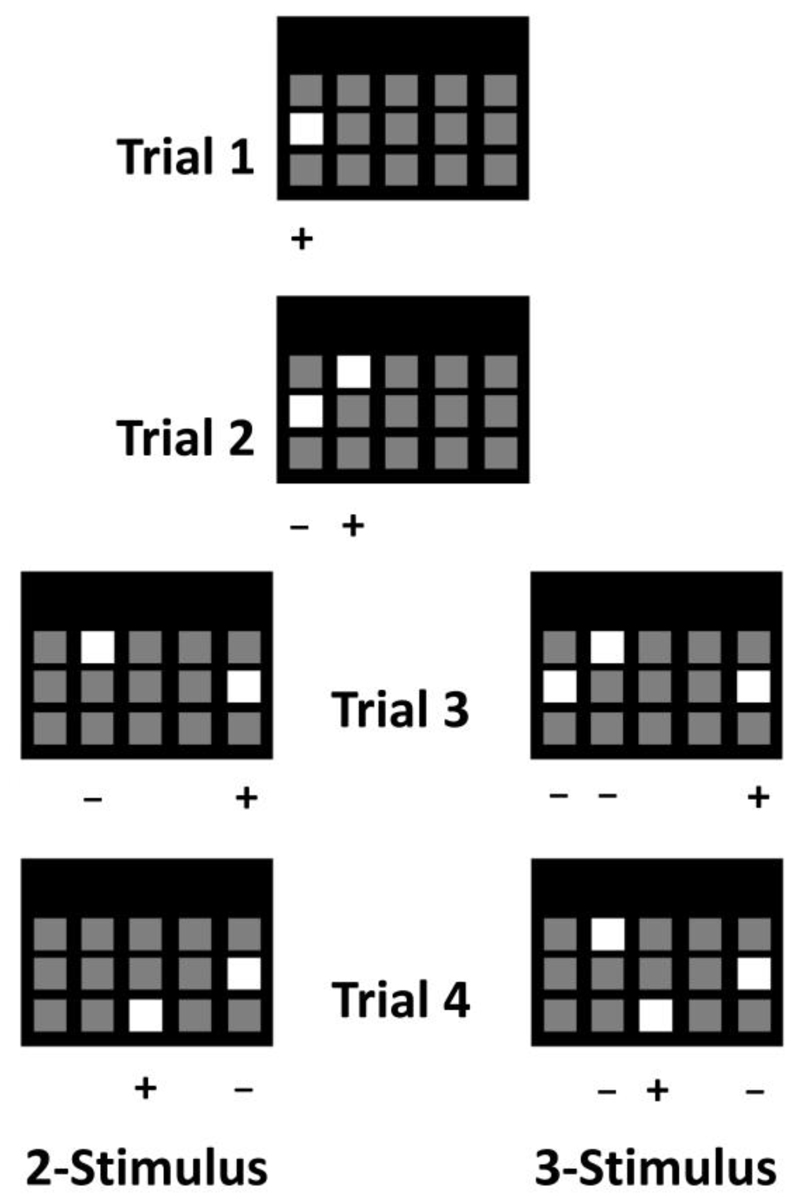
Fig. 1.
cTUNL trial types in chronological order. Trials 1 and 2 are representative examples of the start of a 98-trial session. Sessions start with a response to a single location (trial 1), which sets the S- of the next trial (trial 2). Example trials for 2-choice cTUNL are depicted on the left (trial 3 and 4) and example trials for 3-choice cTUNL on the right (trial 3 and 4). For both versions of the task, animals are required to non-match to their previous response. Different separations between stimuli on the screen are named 0 to 3, and are based on the number of empty squares between response locations. Trial 2 is an example of ‘0’ separation (at retrieval), trial 3 (left) is an example of ‘2’ separation (at retrieval). For trial 3, the separation at encoding is ‘0’.
In the present study we tested the ability of rats to acquire and perform the novel 2-choice and 3-choice cTUNL task. These two versions of the task were also assessed for sensitivity to dysfunction in limited areas within the hippocampus, namely the DG and CA3 subregions. Whilst dorsal hippocampal lesions have been shown to affect performance of both rats and mice in the TUNL task (Kim et al. this issue; Talpos et al. 2010), both preclinical disease models and treatment compounds are likely to have more subtle effects on the hippocampal system. Thus, it is important that the task is sensitive to relatively selective manipulations. The DG and the CA3 regions were chosen due to their proposed roles in pattern separation and working memory (and/or pattern completion) respectively.
2. Materials and Methods
2.1. Animals
A total of 72 male Lister hooded rats were obtained from Harlan (UK), weighing 225-250 grams at arrival. Rats were group housed (4 animals/cage) on a reversed light-dark cycle (lights on 7 pm - 7 am) under constant humidity conditions. A restricted diet was employed to maintain animals at no less than 85% of their free-feeding weight, with water available ad libitum. Rats were habituated to the facility and handled for one week prior to behavioural training. Data presented in this study were obtained in animals aged 3-6 months (Experiment 1) and 16-20 months (Experiment 2). This experiment was conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986.
2.2. Touchscreen operant chambers
Behavioural testing was conducted in 12 automated touchscreen-equipped operant chambers (Campden Instruments Ltd., Loughborough, U.K.) for rats as described elsewhere (Horner et al, 2013). In short, trapezoidal chambers (20 cm h x 33 cm l x 25 cm w at screen or 13 cm w at magazine) were situated within a ventilated sound and light-attenuating box. The walls were made from black Perspex, the floor was a perforated metal grid and the chamber was covered by a transparent lid. Chambers were equipped with 15 inch touch-sensitive LCD monitors on one side, and a food magazine connected to a pellet dispenser at the other side (pellets: 45mg 5-TUL AIN-76A dustless pellets (TestDiet, Indiana, USA)). The touchscreen was covered with a black Perspex mask (35.8 h x 28 w) with 15 response windows (3 rows of 5 locations; Oomen et al, 2013). Each response window was sized 3.3 x 3.3 cm, spaced 1.5 cm apart with the lower row located 1.5 cm from the floor. The food tray was fitted with a light bulb and a beam detector that registered nose-pokes into the pellet receptacle. Above the food magazine was a click and tone generator as well as a 3 W bulb house light. Front and rear infrared beams were installed to keep track of an animal’s position in the chamber. Behaviour was also monitored by means of an infrared camera positioned above the chamber.
2.3. cTUNL - task design
For an illustration of several consecutive trials in cTUNL and a detailed flow chart of the trial structure, see Fig. 1 and 2. Each cTUNL session is capped at 98 trials per session (maximum 1 hour) and starts with the presentation of a single lit location that the animal is required to touch (Fig 1, top). After this ”start-trial”, rats are (after a delay) presented with two stimuli on the screen on every subsequent trial in the case of 2-choice cTUNL (Fig 1, left): one lit square in the previously-touched location (S-), and one lit square in a novel location (S+). In order to receive a food reward, the rat is required to select the novel location. After an incorrect choice, the same locations are presented and the same non-matching rule applies until a correct response is made. Such repeated presentations are labelled correction trials. During the test, a new trial is signalled by a light in the reward tray, where rats have to make a nose poke to trigger the next stimulus presentation. A correct response is paired with a tone and reward delivery. An incorrect response is unrewarded and followed by illumination of the houselight and a time-out that matches the delay. Stimuli remain on the screen until animals make a response. The spatial separation between the target (S+) and sample (S-) was defined as the number of empty locations between stimuli. The minimum separation was 0 (adjacent S+ and S-), whilst the maximum separation was 3.
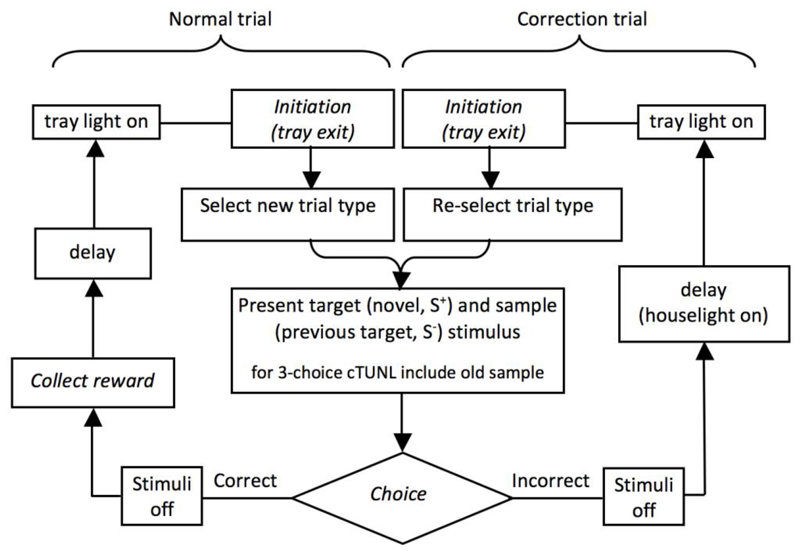
Fig. 2.
Flowchart of cTUNL trial structure. Animals start each trial by a nose poke into the reward tray (Initiation). After tray exit, two locations are presented on the screen (or three, in the case of 3-choice cTUNL). The animal is required to touch the novel location, thus ignoring the location it has touched on the previous trial. After a correct choice the animal receives a reward and the delay starts, at the end of which a new trial can be initiated. After an incorrect choice, the houselight is turned on during the delay, serving as a mild punishment. After the delay, the same two stimuli are re-selected in order to continue the non-matching rule. Labels in italic indicate steps in which the animal is required to perform an action. Not shown: the onset of each session, which consists of the presentation of a single target defining the S- on the subsequent trial (illustrated as trial 1 in Fig. 1).
In addition to the standard 2-choice version of cTUNL, we modified the task to increase working memory load in a 3-choice version of the task, in which the S+ of a given trial remains visible on the screen during the next trial (as in standard cTUNL) and during the subsequent trial (Fig 1, right). As a consequence, the animal must discriminate between 3 stimuli: a novel location (S+), the target of the previous trial (now sample; S-) and the sample of the previous trial (now old sample; S-), see Fig. 1. The spatial separations between the target, the sample and the old sample were defined as the number of empty locations between stimuli, which were then summed. Trials with separations 0, 1 or 2 were classified as small. Trials with separations 3 or 4 were classified as medium, whilst large separation trials were made up of 5 and 6 separation trials.
2.4. Experimental design
2.4.1. Training
For both experiments, rats were initially trained to touch the screen for a food reward in a so-called pretraining paradigm. Defined pretraining stages were implemented over the course of 7-14 sessions and were identical to TUNL pretraining, described elsewhere (Oomen et al. 2013). Subsequently, for cTUNL task acquisition, all rats were trained over the course of 14 sessions on 2-choice cTUNL in 98-trial sessions (maximum 1 hour), using a fixed inter-trial delay of 2s. After these 14 sessions (in order to prevent overtraining) animals that had reached a pre-set criterion of 65% correct for 2 consecutive sessions (excluding correction trials), were paused from daily training and given weekly reminder sessions, until all animals had reached the performance criterion.
2.4.2. Experiments
In Experiment 1, 36 naive rats were trained to the cTUNL criterion as described above. All manipulations with this cohort were carried out using the 2-choice version of cTUNL. Once proficient, rats were exposed to extended delay sessions in order to reduce the novelty of this manipulation post-surgery. This was done by giving 3 sessions in which the delay between trials was fixed at 1, 6 or 11 seconds (a total of 9 sessions). Delays are referred to as “fixed” when set to the same duration for every trial in a session. Half of the rats started with 1s delay sessions, whilst the other half started with 6s delay sessions. All then progressed to 11s delay sessions, before finishing with either 1s or 6s delay sessions (whichever the animal had not started with). This was followed by a single 2s delay session. Then, based on performance, animals were assigned (matched based on acquisition speed and accuracy) to a sham, DG or CA3 group and underwent surgery. After recovery, rats were reminded of the task by exposure to the same test-parameters as during acquisition (using a fixed 2s delay) for 2 sessions. This was followed by 18 sessions with fixed delays (either 1s, 6s or 11s) in each session, in order to assess the effect of DG and CA3 dysfunction on conditions of increased working memory load. Six sessions were given per delay condition, counterbalanced in the same way as pre-surgery. Finally, all animals were tested on 25 sessions in which three delays were randomly intermixed within a session. That is, every trial was assigned one of three possible delays (1s, 6s or 11s) and the same delay condition was presented on less than three consecutive trials. Delays are referred to as “variable” when different trials have different delays within the same session. Variable delay sessions can, in our experience, yield different results than fixed delay sessions on a number of manipulations in both rats and mice in various tasks (Oomen et al., 2013).
In Experiment 2, cTUNL acquisition and the surgery detailed in Experiment 1 were repeated using 36 rats. After post-surgery sessions (data not shown), rats were tested on a different working memory task for locations (self-ordered working memory; SOWM, (Mar et al. this issue)), before being assessed on a hand run spatial working memory task (data not shown). After a break from behavioural testing, this cohort was re-exposed to the 2-choice cTUNL task as used in Experiment 1 using 2s fixed delays. Animals were at this point 16 months of age, and group size was n=7 for all groups. After exposure to 2-choice cTUNL rats were transferred to 3-choice cTUNL, in order to assess the effect of increasing the number of stimuli on the screen on DG and CA3 dysfunction. The specific locations used for the acquisition training of 3-choice cTUNL contained trials with separations ranging from 1 to 6 (largest possible), but avoided separation 0 trials to make acquisition easier (referred to as larger separation schedule). After 50 sessions of acquisition training with this larger separation schedule (in which the delay was fixed at 2s throughout), rats were challenged with 6 sessions which included separation 0 trials (small separation probe). This was carried out to assess whether DG or CA3 dysfunction affected performance when pattern separation was taxed using very similar locations (i.e. small separations between stimuli). In a final probe, the effect of DG and CA3 dysfunction on working memory was assessed by using a longer fixed delay of 6s throughout the session (on the larger separation schedule) on a total of 6 sessions.
2.5. Surgeries
Surgical procedures were based on publications on dorsal DG and CA3 manipulations for permanent, fibre-sparing inactivation (Lee and Kesner 2003). For stereotactic surgery, rats were deeply anaesthetised with Isoflurane (5% for induction and 2.5% for maintenance). After a midline incision of the skin, placement in a stereotaxic frame ensured a flat skull surface before localised holes were drilled in the skull above the injection sites. The four coordinates for bilateral targeting of the DG were 2.7 and 3.7 mm posterior to bregma, ±1.6 and ± 2.5 mm lateral to midline, and 3.6 and 3.1 mm ventral to dura. For bilateral targeting of the CA3, the six coordinates were as follows: 2.5, 3.3, and 4.1 mm posterior to bregma, ±2.6, ±3.3 and ±4.2 mm lateral to midline, and 3.2, 3.2 and 3.1mm ventral from dura. A 5-ml Hamilton microsyringe, fitted in a microinjection pump, was connected to the stereotactic frame using H2O-filled polythene tubing and an injector (31 Gage) used to inject different neurotoxins dissolved in saline. Colchicine, an excitotoxic, fibre-sparing neurotoxin selective for dentate granule cells (2.5 mg/ml) was administered into two sites per hemisphere (0.2µl/site) to permanently inactivate the DG, whereas ibotenic acid, an excitotoxic, fibre-sparing neurotoxin with a preference for NMDA receptors (6mg/ml) was infused into three sites per hemisphere (0.2 µl/site, 0.3µl/site, 0.6µl/site for the 3 injection sites targeting CA3, starting at the most anterior coordinate). Sham surgery to complement the DG and CA3 groups was executed at the same coordinates (including lowering the needle to the same DV), yet no neurotoxin was delivered. Metacam (0.2 mg/kg) to manage post-operative pain and valium (5 mg/kg) to prevent seizures was administered. Animals recovered in a heated cage in a darkened room. During surgery, 6 out of 72 animals (Experiments 1 and 2) died due to respiratory failure, which resulted in an n=11 per group after surgery.
2.6. Histology
In order to examine the extent of DG- and CA3 inactivation, immunohistochemical staining of neuronal nuclei (NeuN) was performed. For this, rats were terminally anaesthetized with an overdose of sodium pentobarbital (Dolethal, Vetoquinol, UK) and perfused transcardially with 0.01 M PBS (pH 7.4) followed by paraformaldehyde (PFA) solution (4% PFA in PBS). Brains were removed and post-fixed for 18 hours in PFA. Coronal sections were made (60 µm) using a sliding microtome, for which brains were cryoprotected using 20% sucrose solution and rapidly frozen. Sections were collected in PB (0.01M, pH 7.4) and stained for NeuN using the primary antibody Mouse anti-NeuN (1:10.000; Vector) and secondary antibody biotinylated horse anti-mouse (1: 200; Vector). Chromogen development was performed using diaminobenzidine (DAB). The extent of tissue damage was microscopically assessed in every 6th section of the dorsal hippocampus and drawn onto atlas diagrams.
2.7. Analysis and Statistics
The main dependent variable was performance defined as percentage correct choices (correct trials/total trials*100) on unique trials only i.e., not including performance on correction trials. Since, with very minor exceptions, animals were able to finish all trials per session, total trials are not presented in the results section. Performance (percentage correct) was also broken down and analysed for subsets of trials based on specific separations at encoding and retrieval and varying delays. For this, behavioural log files of each session were extracted using ABET II Touch software (Lafayette Instruments) in which each correct or incorrect response of the animal was coupled to its delay-, separation at encoding-, and separation at retrieval condition. Responses were summed across sessions first and then used to calculate percentage correct. Other variables included latency to respond during correct and incorrect trials and latencies to collect rewards. The median values for these variables were determined during the final two sessions of variable delay (Experiment 1, 2-choice cTUNL) and the final two sessions of task acquisition (Experiment 2, 3-choice cTUNL) from which groups averages were calculated. For all latencies, values greater than 5 seconds were excluded from analysis. For statistical analysis, repeated measures ANOVA was used with separation, delay and session (when relevant) as within-subject factors, and lesion status as a between-subject factor. Separate ANOVAs were used for the analysis of performance as a function of encoding and retrieval respectively. Post-hoc analysis was carried out using Dunnett’s test comparing the DG and CA3 group to the sham group.
3. Results
3.1. Histology
For representative photomicrographs of all three treatment groups, see Fig. 3A-C. Animals from both experiments are represented in schematic drawings onto atlas sections (Fig. 3D, E). In the description below, all measures are taken from bregma. In Experiment 1 two DG animals were excluded from analysis. One rat showed little damage to the left DG and exhibited complete sparing of the right DG. Another rat exhibited extensive bilateral damage to the CA1 region. In the CA3 group, two animals were excluded: one rat showed only minor unilateral damage and one animal showed extensive bilateral damage to the DG and CA1 regions. In Experiment 2, one rat in the DG group and one rat in the CA3 group were excluded, both on the grounds of unilateral sparing.
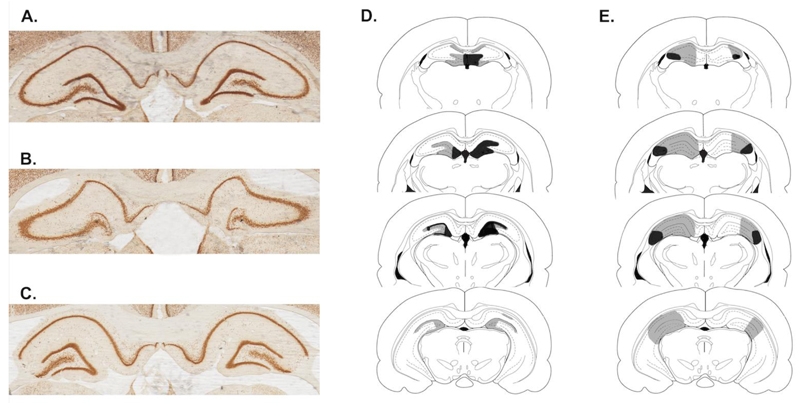
Fig. 3.
Histology results. (A) Example photo of a sham animal. (B) Representative example of DG damage after colchicine infusion. (C) Representative example of CA3 damage as a result of ibotenic acid infusion. Furthermore, schematic drawings of the largest (grey) and smallest (black) damage for DG animals (D) and CA3 animals (E) are shown.
All sham animals had intact hippocampi and all animals were included in analysis. In four animals, needle damage was visible in overlying cortical areas, and in the case of two sham animals this occurred in the overlying CA1 region as well. Cortical damage was restricted to -2.30mm and -2.56mm for two rats; in one animal, damage occurred between -2.56mm and -3.60mm. Finally, one sham rat had unilateral cortical damage at -4.30mm.
For the animals included in the DG group (Fig. 3D), damage was restricted to granule neurons with minimal effects in other hippocampal subregions. One DG rat had bilateral cortical damage at -2.56mm, and a second rat had unilateral damage at -3.60mm. In some DG animals, minor damage to the CA1 area was visible. Some sparing of granule cells could be observed in most DG-animals, as can be seen in the photo (Fig. 3B). The extent of DG damage along the anterior-posterior axis was restricted to the dorsal DG and was not visible in the ventral DG or in any section beyond -5.4 mm.
For rats that were included in analysis of performance of the CA3 group (Fig. 3E), damage to the CA3 subregion involved partial sparing of the proximal CA3 in most animals (Fig. 3C). Furthermore, no CA3 damage was visible beyond -5.2 mm. Occasionally, the CA2, CA1 and/or the dentate gyrus were affected. Specifically, two rats showed extensive damage unilaterally, encompassing all subregions. In addition, another rat had bilateral and unilateral cortical damage at 2.56mm and 3.60mm respectively, and finally in one rat bilateral cortical damage was visible at 3.60mm.
3.1. Experiment 1: cTUNL - 2 choice
3.1.1. Acquisition
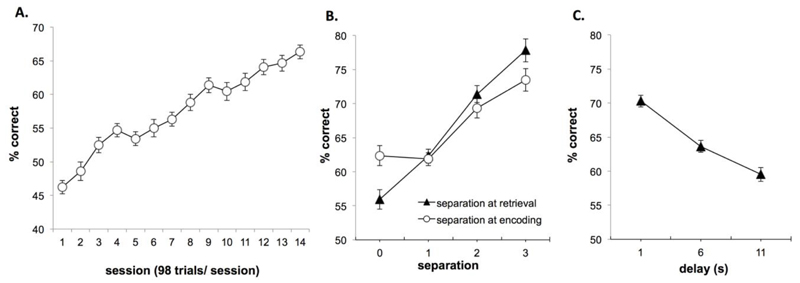
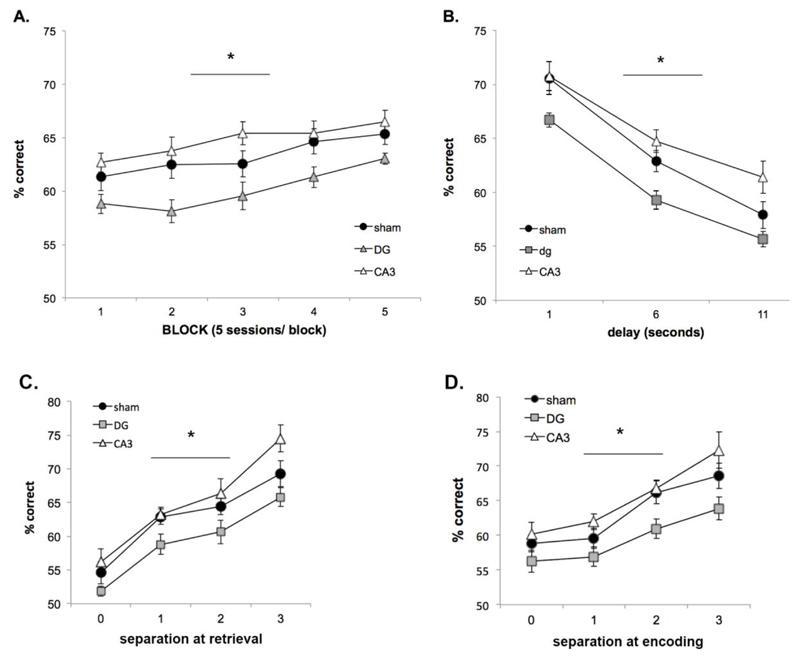
All 36 animals were able to acquire cTUNL and reached criterion (65% correct on two consecutive sessions) within 6 to 26 sessions, with an average of 16.7 ± 0.7 sessions. All animals were trained for 14 consecutive sessions, and improved significantly during these sessions (Fig. 4A) as revealed by a significant main effect of session (F(13,312) = 90.63; p = 0.0001). To avoid overtraining, animals were put on pause when they reached the pre-set criterion after session 14, with weekly reminder sessions. Analysis of the final 2 sessions of acquisition (Fig. 4B), revealed that performance was higher on trials with a larger distance between response locations at retrieval (effect of separation F(3,105) = 65.9; p = 0.0001). Post hoc analysis revealed that for separation at retrieval, performance on each distance differed significantly from each other (all p values lower than p = 0.001 observed when comparing separation 0 to separation 1). The distance between locations on the previous trial (i.e. during encoding) affected performance in a similar way (effect of separation F(3,105) = 47.5; p = 0.0001). Post hoc analysis revealed that separation 0 differed significantly from separation 2 and 3 (p = 0.002 and 0.0001 respectively) and separation 1 differed significantly from separation 2 and 3 (both p = 0.0001). In contrast, separation 0 and 1 (p=1.000), as well as separation 2 and 3 (p = 0.093) did not significantly differ from each other.
Fig. 4.
Performance on 2-choice cTUNL, pre-surgery (A) Acquisition of cTUNL (n=36) with a fixed delay (2s) between trials within each session. (B) Performance depends on separation at retrieval and separation at encoding (both p=0.0001), based on the final two sessions of acquisition. (C) Changes in inter-trial delay significantly affect performance (p=0.0001), based on fixed delay probes pre-surgery (sessions of fixed 1, 6 or 11s delay between trials). Error bars ±SEM.
3.1.2. Performance on 2-choice cTUNL is delay-dependent
Sessions with different, fixed delays were presented before surgery (1, 6 or 11s). Animals were all first exposed to sessions of 6s delay before the 11s condition, in order to increase difficulty in a step-wise fashion. On the first session with increased delay (6s) performance dropped to 50.7% ± 1.1, recovering to 63.6% ± 0.9 on session 3. Therefore, comparison of delays when analysing every third session shows that performance decreases with increasing delay (Fig. 4C); effect of delay F(2,68) = 129.2; p = 0.0001).
3.1.3. DG dysfunction impairs performance when the delay varies within the session, but not when it is fixed
After surgery, all rats were re-baselined on the cTUNL acquisition task (2s delay), followed by 18 sessions of fixed 1, 6 and 11s inter-trial delay (counterbalanced). Subsequently, animals were trained on 25 sessions of variable delay (1, 6, and 11s within session). Overall average performance on each condition is summarised in table 1. For both fixed- and variable delay sessions, as during pre-surgery testing, longer delays decrease performance level and smaller separations lower the percentage correct score (both at encoding and retrieval; e.g., main effect of separation when using a 2s fixed delay schedule: F(3,78) = 61.2, p = 0.0001). No interactions between separation and lesion (or separation, lesion and delay) were found on any of the post-surgery sessions.
Table 1. Average overall performance (% correct) on 2-choice cTUNL post-surgery.
| Delay | all | sham (n=11) | DG (n=11) | CA3 (n=11) |
|---|---|---|---|---|
| 2s. fixed delay (re-baseline) | 64.7 ± 0.9 | 67.1 ± 1.6 | 62.9 + 1.5 | 63.6 + 0.9 |
| 1s fixed delay | 71.3 ± 0.6 | 71.6± 0.8 | 70.6 ± 1.2 | 71.6 ± 1.2 |
| 6s fixed delay | 62.7 ±0.6 | 63.7 ± 0.9 | 61.5± 1.2 | 62.8 ± 1.3 |
| 11s fixed delay | 57.0 ± 0.7 | 55.9 ± 1.1 | 56.8± 1.3 | 58.7 ±1.4 |
| 1s variable delay | 69.4 ±0.8 | 70.6 ±1.5 | 66.7± 0.7* | 70.7 ± 1.5 |
| 6s variable delay | 62.3 ± 0.7 | 62.9 ±1.0 | 59.3± 0.9* | 64.7 ± 1.1 |
| 11s variable delay | 58.3 ± 0.8 | 57.9 ±1.3 | 55.6 ± 0.8* | 61.4 ± 1.6 |
Main effect of lesion, driven by a reduced performance of animals with DG damage, when compared to sham animals.
As can be seen from table 1, DG and CA3 damage did not affect performance on cTUNL during the fixed delay sessions (no main effect of group at encoding: F(2,26) = 0.72, p = 0.5 or retrieval: F(2,26) = 0.99, p = 0.38). However, during sessions of variable delay (Fig. 5), a significant main effect of lesion was found [both when analysing separation at encoding (F(2,26) = 6.26; p = 0.006; post-hoc comparison sham vs. DG p = 0.04; sham vs. CA3 p = 0.98) and when analysing separation at retrieval (F(2,26) = 6.09; p = 0.007; post-hoc comparison sham vs. DG p = 0.03; sham vs. CA3 p = 0.97)]. This group difference was consistently driven by impaired DG performance compared to shams. The impairment was stable over sessions (Fig. 5A). Delay-dependent performance (including all separations) and separation-dependent performance (6 s delay trials only) is depicted in Fig. 5 (B-D).
Fig. 5.
Effects of DG- and CA3 lesion on 2-choice cTUNL performance under conditions of variable delay (1, 6 or 11s between trials, within session). (A) Average performance on cTUNL variable delay across 20 sessions), depicted in blocks of 5 sessions (all groups n=11). A main effect of lesion was found across all sessions (p = 0.006), which was driven by a reduced performance of DG-lesioned animals. (B) Delay-dependent performance. DG- and CA3 lesioned animals were affected by changes in inter-trial delay in a similar manner as shamtreated animals (no interaction between lesion and delay, but a main effect of lesion). (C) Average performance depending on separation at retrieval reveals a main effect of lesion but no interaction with separation. (D) Average performance depending on separation at encoding reveals a main effect of lesion but no interaction with separation. Error bars ±SEM.
Analysis of latencies latencies during the final two variable delay sessions of 2-choice cTUNL reveals that DG- and CA3 dysfunction did not affect the time it takes animals to collect their reward after screen touch (average median values ± s.e.m.: sham = 2.15 ± 0.08; DG = 2.21 ± 0.12; CA3 = 2.31 ± 0.1; p = 0.56). As for the response latencies, DG animals were found to respond slower during trials in which they made an incorrect response when compared to control animals (sham = 2.82 ± 0.13; DG = 3.42 ± 0.14; CA3 = 3.03 ± 0.19; p = 0.03, post-hoc comparison sham vs DG, p=0.03 others n.s.). Response latencies during correct trials did not differ between groups (sham = 2.64 ± 0.11; DG = 3.07 ± 0.12; CA3 = 3.00 ± 0.2; p = 0.11).
3.2. Experiment 2: cTUNL - 3 choice
3.2.1. DG dysfunction impairs acquisition of the 3-choice version of cTUNL
DG, CA3 and sham rats were first trained on the 2-choice version of cTUNL before exposure to the 3-choice version of the task. As in Experiment 1, there was no impairment in DG or CA3 rats compared to sham animals in the 2-choice cTUNL version with a fixed 2s delay [average performance across all sessions: F(2,18) = 1.716, p = 0.208; average performance on the final session: F(2,18) = 1.745, p = 0.203)].
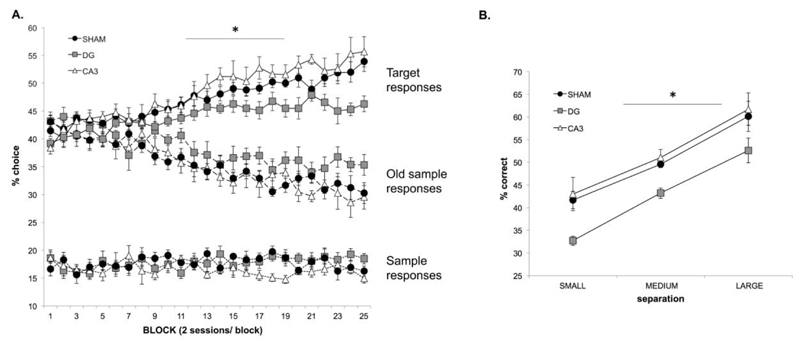
On 3-choice cTUNL, rats can select one of three options on every trial; the target (S+), the sample (S-, but correct on the previous trial), or the old sample (S-, but correct two trials previous). Fig. 6A depicts the acquisition curve over 50 sessions of 3-choice cTUNL (each point represents two sessions). Initially, rats were unable to discriminate between the target and the old sample, whilst all groups successfully avoided selecting the sample on more than 80% of trials. With increasing exposure to the task, all groups responded more frequently to the target stimulus (S+) than the sample, a distinction first evident in sham and CA3 animals around session block 8, and in DG animals around session block 10. Analysis of the % correct (target) responses across session blocks revealed a significant improvement across sessions (F(24,432) = 25.708, p = 0.0001). An interaction between block and lesion status (F(48,432)=1.771, p = 0.002) revealed that this improvement was driven by CA3 and sham animals when comparing performance on the first and the final session (sham rats; p = 0.14, CA3 rats; p = 0.0001, DG rats; p = 0.890).
Fig. 6.
3-choice cTUNL (A) Acquisition of 3-choice cTUNL for CA3 (n=7), DG (n=7) and sham (n=7) rats. The percentage choice for the target, sample and old sample are plotted for comparison across session blocks (2 sessions per block). (B) The effect of separation on the last 3 session blocks demonstrates an effect of DG dysfunction compared to sham rats regardless of separation. Error bars ±SEM.
The effect of separation was analysed when animals had successfully acquired the 3-choice version, and therefore focused on the last 3 sessions (i.e. last 3 session blocks, see Fig. 6B). When analysing the % correct responses, an expected effect of separation was observed whereby small separation trials had significantly lower % correct scores than medium separation trials, and medium separation trials had significantly lower % correct scores than large separation trials (F(2,36)=57.959, p = 0.0001). DG rats had significantly lower % correct scores than sham rats (F(2,18) =8.183, p =0.003; post hoc analysis DG vs. sham; p = 0.010, CA3 vs. sham; p = 0.769), but there was no interaction DG/CA3/sham status and separation (F(4,36) =0.112, p =0.977). Lower responding to the target stimulus in DG rats compared to sham rats is due to an increase in the responding to the old sample, as opposed to the sample stimulus, as can be seen in figure 6A.
Analysis of latencies latencies during the final two sessions of 3-choice cTUNL acquisition shows that DG- and CA3 dysfunction did not affect the time it takes animals to collect their reward after screen touch (average median values ± s.e.m.: sham = 2.83 ± 0.18; DG = 2.73 ± 0.08; CA3 = 3.04± 0.10; p = 0.39). As for the response latencies, no differences were found for incorrect response latencies (sham = 3.81 ± 0.14; DG = 3.69 ± 0.23; CA3 = 3.64 ± 0.34; p = 0.93) or correct response latencies (sham = 3.40 ± 0.14; DG = 3.34 ± 0.16; CA3 = 3.58 ± 0.35; p = 0.11).
3.2.2. DG dysfunction impairs probe biased towards smaller separations
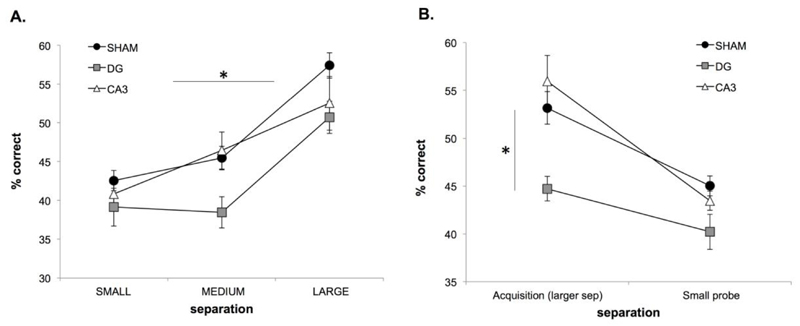
In order to assess whether DG or CA3 dysfunction affected performance when pattern separation was taxed using very similar locations (i.e. small separations between stimuli), 6 sessions of a schedule which included the smallest separation were administered. On this small separation-biased probe, DG rats performed significantly worse than sham rats regardless of separation (F(2,18) = 5.619, p = 0.013; post hoc analysis DG vs. sham; p = 0.008, CA3 vs. sham; p = 0.469). As with the acquisition schedule, performance in all groups was significantly lower when separations were smaller (F(2,36) = 29.169, p = 0.0001; see Fig. 7A), with no interaction between separation and DG/CA3/sham status (F(4,36) = 1.132, p = 0.357).
Fig. 7.
3-choice cTUNL. Performance of CA3 (n=7), DG (n=7) and sham (n=7) rats on the small separation biased probe (A) and on the last session block of the acquisition schedule (with varying small, medium and large separations) compared to the small separation biased probe (B). Error bars ± SEM.
In order to analyse the effect of altering the bias in terms of the number of small separations included in a session, the last session block of the acquisition data (mixed small, medium and large separations) was compared to the first session block of the small separation probe (biased towards smaller separation trials). As expected, performance was significantly lower when a higher number of small separation trials was included in the schedule (F(1,18) = 57.814, p = 0.0001; see Fig. 7B), but importantly, the performance of each group of rats was not reduced to chance level (33%), demonstrating that the smallest separations can be successfully utilised in 3-choice cTUNL. The performance of DG rats was significantly lower than that of sham rats (F(2,18) = 8.198, p = 0.003); post hoc analysis DG vs. sham; p = 0.007, CA3 vs. sham; p = 0.931), specifically on the last acquisition session block (F(2,18) = 4.372, p = 0.028; post hoc analysis DG vs. sham; p = 0.023, CA3 vs. sham; p = 0.703).
3.2.3. DG dysfunction impairs when shifting from short delay to long delay
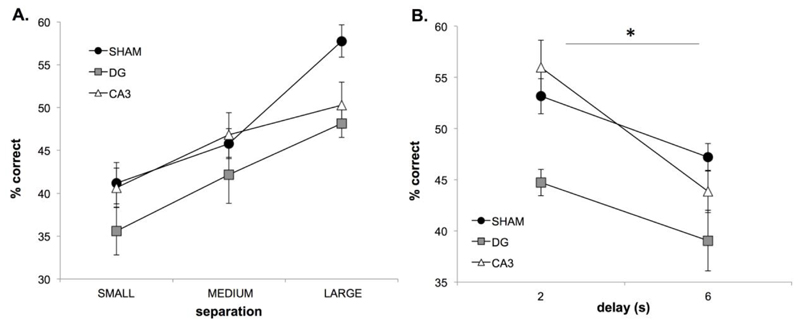
The effect of DG and CA3 dysfunction on working memory was assessed by using a longer fixed delay of 6s throughout the session (on the larger separation schedule). On 6 sessions of this delay probe, the groups did not significantly differ in performance (F(2,17)=2.918, p = 0.081). As with the acquisition schedule, performance in all groups was significantly lower when separations were smaller (F(2,34)=33.178, p = 0.0001; see Fig. 8A).
Fig. 8.
3-choice cTUNL. Performance of CA3 (n=7), DG (n=6) and sham (n=7) rats on the delay probe (A) and on the last two sessions of 2s delay (acquisition) and first two sessions of 6s delay (delay probe) (B). Error bars ± SEM.
Variable delays (i.e. different delays on different trials within a session) were not used with the 3-choice version of cTUNL given the complexity of analysing different delay effects associated with the sample and old sample. Instead, sessions of fixed delay (i.e. the same delay on every trial in a session) were used to tax working memory. In order to analyse the effect of increasing delay, the last session block of the acquisition data (2s fixed delay) was compared to the first session block of the delay probe (6s fixed delay; see Fig. 8B). As expected, performance was significantly lower when the delay was longer (F(1,17)=25.339, p = 0.0001) but importantly, the performance of the sham rats was not reduced to chance level (33%), demonstrating that 6s delays can be successfully utilised in 3-choice cTUNL. In addition, the performance of DG rats was significantly lower than that of sham rats (F(2,17)=7.810, p = 0.004; post hoc analysis DG vs. sham; p = 0.005, CA3 vs. sham; p = 0.990), which did not interact with delay (F(2,17)=1.659, p = 0.220).
Discussion
The present study introduces a novel test of spatial working memory and pattern separation, cTUNL. This task was developed under the NEWMEDS initiative as part of an expanding battery of touchscreen tests (Hvoslef-Eide et al. this issue-a). Acquisition of cTUNL is relatively rapid (when compared to other complex operant paradigms) and the task was sensitive to manipulation of delays and separations. A novel 3-choice version of the task was also found to be viable. Finally, both the 2- and the 3-choice version of the task were able to differentiate between limited dysfunction to different areas within the hippocampus, namely within the DG and CA3 subregions.
On the 2-choice version of cTUNL, DG dysfunction affected performance under conditions of variable delay. Additionally, animals without an intact DG were unable to perform at control levels a 3-choice version of cTUNL in which the sample of the previous trial (’old sample’) remains visible on the screen. The impairment was driven by a reduced ability to discriminate between the target and the old sample, as all groups were equally successful at avoiding the most recently visited location (sample). This indicates that the ability of rats to correctly classify a location as most recent decreases with time (since the last interaction with that location) more rapidly in rats with DG dysfunction compared to control rats. Both the 2-choice and 3-choice versions of cTUNL are sensitive to DG dysfunction consistently over more than 2 months of continuous testing and task exposure. This demonstrates a stability of the behavioural readout which could be particularly useful when implementing manipulations (e.g., pharmacological) aimed at rescuing such deficits. With the exception of a longer response time of DG-lesioned animals to incorrect trials in the 2-choice version of cTUNL, DG- and CA3 dysfunction did not affect reward collection latencies or response latencies. This indicates that, at least at the motivational level, no clear group differences exist. However it should be noted that the DG impairment on the 2-choice task was delay- and separation-independent, i.e., the impairment was present across all delays and separations. CA3 dysfunction, in contrast, had little effect. These findings are discussed further below.
The cTUNL task has a number of advantageous theoretical and practical task features. Firstly, because every trial is both a ‘sample’ and a ‘choice’ trial, unlike other matching-to-location tasks every response from the rat yields a data point; perhaps chiefly due to this feature, acquisition of the task appears to be faster than TUNL (Talpos et al. 2010), which unlike cTUNL has distinct sample and choice phases (Oomen et al, unpublished observation). This feature also eliminates the need for intermittent sample reward (as is sometimes necessary in other nonmatching-to-location tasks) in order to maintain motivation to respond. Secondly, the present study shows that 2- and 3-choice versions of the task are possible, and it is possible that rats would engage with an even higher number of stimuli. This feature brings the tasks closer than most rodent tests to the “n-back” task widely used in human studies (Dobbs and Rule 1989; Moore and Ross 1963). Thirdly, a novel aspect of the cTUNL task is that it can differentiate between the effect of separation manipulations at the encoding and retrieval stages of memory. Performance was found to depend on both separation at encoding and separation at retrieval. The influence of separation at retrieval on memory accuracy was most pronounced, as performance here was found to be different on each degree of separation. As mentioned earlier, dorsal DG (but not CA3) inactivation affected performance across all delays and separations, and therefore does not result in a differential effect on separation at encoding versus separation at retrieval. However, this does not exclude the possibility that other manipulations would affect this differently, and given the disruption of pattern separation ability in disorders such as Alzheimer’s disease (Mueller and Weiner 2009; Palop et al. 2003; Yassa et al. 2010), normal ageing (Stark et al. 2010; Yassa et al. 2011) and schizophrenia (Das et al. 2014), a translational preclinical task measuring pattern separation at both encoding and retrieval may prove to be a valuable tool. TUNL might be a better choice than cTUNL if one is interested in the dissociation of delay-dependent and interference-dependent performance as operationalized through delay and inter-trial interval manipulations respectively. A side-by-side comparison of cTUNL to TUNL is depicted in table 2.
Table 2. Comparison of cTUNL to TUNL.
| Parameter | Comparison |
|---|---|
| Sample phase and inter-trial interval (ITI) | In cTUNL, the absence of a separate sample phase and an inter-trial interval (ITI) allows for a higher number of trials per session. |
| Inter-trial interval (ITI) | In TUNL, ITI and delay can be varied independently — for example, ITI can be varied to modulate the level of interference between trials (shorter ITIs increases interference), which was shown to involve the prefrontal cortex (McAllister et al, 2013) — whereas in cTUNL they cannot, because the ITI is the delay. |
| 3-Choice task | In cTUNL, a 3-choice version has been developed, which can be used to increase interference by increasing the number of to-be-remembered stimuli. |
| Correction trials | In cTUNL, correction trials are required to maintain the nonmatching rule throughout a session. TUNL can be applied without correction trials, although implementation of such trials is recommended to avoid the formation of a location bias. |
| Separation at encoding and retrieval | In cTUNL performance can be assessed based on separation during retrieval (stimulus distance on the current trial) and based on separation during encoding (stimulus distance on the previous trial). |
| Underlying circuitry | Evidence for a role of the hippocampus (TUNL) or hippocampal DG (cTUNL) and the prefrontal cortex in performance in both TUNL and cTUNL has been found. |
Based on Talpos et al. 2010; McAllister et al. 2013; Oomen et al, 2013; Hvoslef-Eide et al. this issue; this paper.
In the current study, no separation-dependent effect of DG dysfunction was observed, despite the DG being extensively linked to pattern separation, whereby DG dysfunction can impair discrimination of similar, but not dissimilar stimuli (Clelland et al. 2009; Gilbert et al. 2001; Leutgeb et al. 2007; Sahay et al. 2011, but see Saxe et al. 2007). In other words, even the largest separation conditions were sensitive to DG dysfunction. However it should be noted that separation-dependent DG effects have been most often reported, not following complete lesions of DG, but following more subtle DG manipulations, in particular manipulations of adult hippocampal neurogenesis (Clelland et al. 2009; Sahay et al. 2011; Bekinschtein et al. 2014). Thus it is not totally surprising that, at least under some conditions, dysfunction of both immature and mature cells in the DG might lead to broader impairments than dysfunction of immature cells alone.
Interestingly, although DG dysfunction impaired performance in “variable/mixed” but not “fixed” delay conditions (possibly due to fixed delay allowing repeated behaviour patterns that facilitated performance, suggesting cTUNL is better run using mixed delays only), when tested on the 3-choice version of cTUNL, the performance deficit of DG rats was evident even at fixed, short delays. Thus, the DG may be particularly important when memory load is increased, perhaps increasing interference, an interpretation consistent with the proposed role for the DG in pattern separation (although an important caveat is that the two cohorts of animals had different training histories). This finding is in line with those from a study using a memory span task in rats in which hippocampal lesions impaired performance when the spatial span was larger than 1 (Dudchenko et al. 2000, although see Steele and Rawlins 1993 for a general impairment regardless of span length), but extends this literature by providing evidence that sensitivity to memory load can be observed following dysfunction of the DG alone.
The lack of an effect following permanent inactivation of the CA3 was unexpected given its proposed role in working memory in theoretical and computational work (Lisman 1999; Rolls and Kesner 2006). However it is worth noting that -- perhaps surprisingly given the popularity of this idea -- few empirical studies have found a causal relationship between selective CA3 dysfunction and impairments in working memory. Indeed, the only study reporting significant and stable deficits on a spatial working memory task following CA3 lesions used a match-to-location paradigm in which the delay was not actually manipulated (Gilbert and Kesner 2006), and without delay-dependence, the claim for a role in working memory must remain tentative. Instead, it seems the CA3 may play more of a role in supporting information over a short period of time when conditions are novel (Kesner 2007). Jarrard (1983) found no impairment of CA3 lesioned rats on the radial arm maze, whilst Handelmann and Olton (1981) found differing effects of CA3 lesions depending on the level of pre-operative training the rats received, indicating a degree of functional recovery if sufficiently familiar with the task requirements prior to CA3 damage (Handelmann and Olton 1981; Jarrard 1983). Direct administration of AP5 to the CA3 (Lee and Kesner 2002) or lesions of that structure (Lee and Kesner 2003) impaired spatial working memory when tested in a novel, but not a well-learned paradigm. Furthermore, CA3 lesions impaired performance in a familiar room, but only 1) transiently and 2) if no pre-operative training is provided (Lee and Kesner 2003). In mice, deletion of the NMDA receptor specifically in CA3 impairs a delayed matching-to-position Morris water maze task when the platform location is novel, but not when the location is familiar (Nakazawa et al. 2003).
Thus it may have been an important feature of the current study that novelty was minimised by extensive pre-exposure through pre-lesion acquisition and familiarisation of task conditions such as delays prior to surgery. CA3 dysfunction clearly does not impair cTUNL performance under these circumstances, consistent with an interpretation of CA3 function in terms of novelty. A more prosaic explanation is that the damage to CA3 was subtotal and not extensive enough to be effective; indeed as shown in Figure 3, the most medial part of CA3 was consistently spared (which was necessary to avoid damage to DG). However, in the studies cited above in which permanent CA3 dysfunction led to impairments in novel situations, the degree of CA3 sparing appears to be about the same as in the present study. In addition, the CA3 manipulation in these same animals did prove effective in another study whereby a transient impairment was observed, interestingly when a novel delay was introduced in a novel touchscreen self-ordered spatial working memory task (Mar et al. 2015, see this issue).
To summarise, the cTUNL task is rapidly acquired and has several advantages, including the ability to implement 2- and 3-choice versions of the task, both of which were sensitive to limited damage to the hippocampus (the DG subregion). In addition, cTUNL has been demonstrated to be sensitive to medial prefrontal cortex inactivation in a delay-dependent manner, as well as to adrenergic manipulations (Hvoslef-Eide et al. this issue-b). In addition, a pharmacological model of schizophrenia in which methylazoxymethanol acetate is administered at E-17 (MAM-E17), were impaired at both acquisition and performance of cTUNL (Howe et al. this issue). Thus cTUNL may prove highly useful for evaluating dysfunction in multiple brain regions and models of neuropsychiatric and neurodegenerative disease.
Acknowledgements
This work was supported by the Innovative Medicine Initiative Joint Undertaking under grant agreement no. 115008 of which resources are composed of EFPIA in-kind contribution and financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013). As part of this project, CAO was funded by Janssen Pharmaceuticals, Inc., of Johnson & Johnson. The authors thank Jytte van Huijstee and Siyu Jiang for valuable assistance with experiments.
Footnotes
Disclosures
TJB consults for Campden Instruments Ltd. LMS consults for Campden Instruments Ltd.
References
- Barnett JH, Robbins TW, Leeson VC, Sahakian BJ, Joyce EM, Blackwell AD. Assessing cognitive function in clinical trials of schizophrenia. Neurosci Biobehav Rev. 2010;34:1161–77. doi: 10.1016/j.neubiorev.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, Bussey TJ. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus. 2014 doi: 10.1002/hipo.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Jr, Gage FH, Saksida LM, Bussey TJ. BDNF in the Dentate Gyrus Is Required for Consolidation of “Pattern-Separated” Memories. Cell Reports. 2013;5:1–10. doi: 10.1016/j.celrep.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Ivleva EI, Wagner AD, Stark CE, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr Res. 2014;159:193–7. doi: 10.1016/j.schres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and aging. 1989;4:500–3. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Dudchenko P, Sarter M. Behavioral microanalysis of spatial delayed alternation performance: rehearsal through overt behavior, and effects of scopolamine and chlordiazepoxide. Psychopharmacology (Berl) 1992;107:263–70. doi: 10.1007/BF02245146. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci. 2000;20:2964–77. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav Brain Res. 2006;169:142–9. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–36. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Handelmann GE, Olton DS. Spatial memory following damage to hippocampal CA3 pyramidal cells with kainic acid: impairment and recovery with preoperative training. Brain Res. 1981;217:41–58. doi: 10.1016/0006-8993(81)90183-9. [DOI] [PubMed] [Google Scholar]
- Hearst E. Delayed alternation in the pigeon. Journal of the experimental analysis of behavior. 1962;5:225–8. doi: 10.1901/jeab.1962.5-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res. 1996;711:102–11. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SR, Alsio J, Oomen CA, Holmes A, Saksida LM, Bussey TJ. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–84. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Mar AC, Nilsson SRO, Heath CJ, Saksida LM, Robbins TW, Bussey TJ. The NEWMEDS rodent touchscreen test battery for cognition relevant to schizophrenia. Psychopharmacology (Berl) doi: 10.1007/s00213-015-4007-x. (this issue-a) [DOI] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Oomen CA, Fisher BM, Heath CJ, Robbins TW, Saksida LM, Bussey TJ. Facilitation of spatial working memory performance following intra-prefrontal cortical administration of the adrenergic alpha1 agonist phenylephrine. Psychopharmacology (Berl) doi: 10.1007/s00213-015-4038-3. (this issue-b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place and cue tasks. Behav Neurosci. 1983;97:873–89. doi: 10.1037//0735-7044.97.6.873. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–81. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kim CH, Romberg C, Hvoslef-Eide M, Oomen CA, Mar AC, Berthiaume AA, Bussey TJ, Saksida LM. Trial-unique, delayed nonmatching-to-location (TUNL) touchscreen testing for mice: Effects of dorsal hippocampal dysfunction. Psychopharmacology (Berl) doi: 10.1007/s00213-015-4017-8. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nat Neurosci. 2002;5:162–8. doi: 10.1038/nn790. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav Neurosci. 2003;117:1044–53. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–6. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–42. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Mar AC, Gamallo B, Oomen CA, Saksida LM, Bussey TJ. The self-ordered spatial working memory tasks is sensitive to lesions of the dentate gyrus and CA3. Psychopharmacology (Berl) (this issue) [Google Scholar]
- McAllister KAL, Saksida LM, Bussey TJ. Dissociation between memory retention across a delay and pattern separation following medial prefrontal cortex lesions in the touchscreen TUNL task. Neurobiology of Learning and Memory. 2013;101:120–126. doi: 10.1016/j.nlm.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ME, Ross BM. Context effects in running memory. Psychological Reports. 1963;12:451–465. [Google Scholar]
- Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19:558–64. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–15. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, Saksida LM. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc. 2013;8:2006–21. doi: 10.1038/nprot.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc Natl Acad Sci U S A. 2003;100:9572–7. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease. Brain : a journal of neurology. 1988;111(Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104:4642–6. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn Mem. 2010;17:284–8. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele K, Rawlins JN. The effects of hippocampectomy on performance by rats of a running recognition task using long lists of non-spatial items. Behav Brain Res. 1993;54:1–10. doi: 10.1016/0166-4328(93)90043-p. [DOI] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem. 2010;94:341–52. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci U S A. 2011;108:8873–8. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci U S A. 2010;107:12687–91. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]