Abstract
Background
In addition to their respiratory symptoms, patients with COPD experience multiple, co-occurring symptoms.
Objectives
The aims of this study were to identify subgroups of COPD patients based on their distinct experiences with 14 symptoms and to determine how these subgroups differed in demographic and clinical characteristics and disease-specific quality of life.
Patients and methods
Patients with moderate, severe, and very severe COPD (n=267) completed a number of self-report questionnaires. Latent class analysis was used to identify subgroups of patients with distinct symptom experiences based on the occurrence of self-reported symptoms using the Memorial Symptom Assessment Scale.
Results
Based on the probability of occurrence of a number of physical and psychological symptoms, three subgroups of patients (ie, latent classes) were identified and named “high”, “intermediate”, and “low”. Across the three latent classes, the pairwise comparisons for the classification of airflow limitation in COPD were not significantly different, which suggests that measurements of respiratory function are not associated with COPD patients’ symptom burden and their specific needs for symptom management. While patients in both the “high” and “intermediate” classes had high occurrence rates for respiratory symptoms, patients in the “high” class had the highest occurrence rates for psychological symptoms. Compared with the “intermediate” class, patients in the “high” class were younger, more likely to be women, had significantly more acute exacerbations in the past year, and reported significantly worse disease-specific quality of life scores.
Conclusion
These findings suggest that subgroups of COPD patients with distinct symptom experiences can be identified. Patients with a higher symptom burden warrant more detailed assessments and may have therapeutic needs that would not be identified using current classifications based only on respiratory function.
Keywords: symptom experience, latent class analysis, COPD, quality of life
Introduction
COPD is the third leading cause of death worldwide1 and the second leading cause of disability-adjusted life-years lost.2 According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), COPD is defined by a nonfully reversible airway obstruction expressed as a ratio of the postbronchodilator forced expiratory volume in one second (FEV1) to the forced vital capacity of <0.7.3 The severity stages of COPD and associated treatments are defined primarily by the patient’s FEV1 as a percentage of the predicted value. However, recent work suggests that FEV1 is a poor predictor of prognosis and of COPD patients’ symptom burden.4 As a result, in 2011, the GOLD task force introduced a new model for defining the severity of COPD for an individual patient.3 While the spirometric definition for each stage remains relatively unchanged, increased emphasis was placed on the COPD patients’ experiences with specific respiratory symptoms and the number of acute exacerbations they had in the past year.3 However, although these respiratory symptoms are important, patients with COPD may experience a wider array of nonrespiratory symptoms.5,6 To better understand the heterogeneity among patients with COPD, new research has focused on the identification of COPD phenotypes.7 The intention of this phenotyping is to identify high-risk patients as well as specific characteristics that predict responses to treatment.8 Most of this clinical phenotyping relied on observational characteristics.9 However, predefined signs and symptoms of respiratory function (eg, dyspnea, cough, and sputum production)10–14 may not provide a complete picture of the patients’ experiences with their disease. Therefore, some studies have included an evaluation of other symptoms (eg, anxiety, depression, and fatigue)15,16 and quality of life (QOL) outcomes.11,13,14 Of note, in a recent study,17 some COPD patients reported up to 14 co-occurring symptoms. These findings suggest that the symptom experience of COPD patients is extremely complex.18 Based on these initial attempts to identify clinical phenotypes for COPD, recent work used newer analytic techniques (eg, cluster analysis and latent class analysis [LCA]) that may allow for the identification of subgroups of patients based on their distinct symptom experiences. For example, in one study of patients with severe COPD that used cluster analysis,16 patients with high levels of symptoms had poorer functional status and worse exercise capacity than those with low levels of symptoms. This approach allowed for the identification of a specific subgroup of patients who needed more aggressive management although all the patients in this study had severe COPD based on the classification system of airflow limitation in COPD.3 Additional phenotyping studies are needed with more heterogeneous samples of patients with COPD.19 By including COPD patients with a broader spectrum of disease and using a comprehensive and multidimensional symptom assessment instrument (ie, Memorial Symptom Assessment Scale, MSAS20), the aims of this study were to identify subgroups of COPD patients using LCA based on their distinct symptom experiences and to determine how these subgroups differed in demographic and clinical characteristics and disease-specific QOL outcomes.
Patients and methods
Design, sample, and data collection
In this cross-sectional study, patients were included if they were >18 years of age; were diagnosed with moderate, severe, or very severe COPD according to the classification of airflow limitation in COPD;3 were able to read and understand Norwegian; and had no cognitive impairments. Patients receiving ongoing treatment for pulmonary infection, disease exacerbation, or cancer were excluded from this study. Patients were recruited from three outpatient clinics and one referral hospital. The study was approved by the Regional Committees for Medical and Health Research Ethics, the Norwegian Directorate of Health, and the privacy ombudsman at Oslo University Hospital. Written informed consent for participation in the study was obtained from all patients.
Demographic and clinical characteristics
At enrollment, patients were asked to complete the study questionnaires and provide information on age, sex, education, marital status, and living arrangements. In addition, patients were asked to rate the severity of their dyspnea using the modified Medical Research Council Dyspnea scale21,22 and complete information on comorbidities using the Self-Administered Comorbidity Questionnaire (SCQ-19).23 The SCQ-19 includes 16 common medical conditions and three optional conditions. For this study, the 19 medical conditions were summed (range 0–19) to obtain the total number of comorbidities.
Research nurses at the different clinics completed information on pulmonary function, body mass index, number of years smoking, and number of years since diagnosis of COPD and reviewed patients’ medical records for disease and treatment information. Spirometry was performed according to the guidelines of the European Respiratory Society.24 Classification of severity of airflow limitation based on postbronchodilator FEV1 was defined as mild (FEV1 ≥80% predicted), moderate (FEV1 50%–79% predicted), severe (FEV1 30%–49% predicted), or very severe (FEV1 <30% predicted) COPD.3 Only patients with moderate, severe, and very severe COPD were included in this study. Partial pressure of oxygen in the arterial blood and performance in the 6-minute walk test,25 as well as the occurrence of chronic bronchitis, emphysema, and number of acute exacerbations (ie, number of prednisolone courses) during the last 12 months, were used as supplementary measures of lung function.
Chronic bronchitis was defined according to the conventional definition (ie, daily productive cough over >3 months in at least two consecutive years).3 Presence of emphysema was based on the clinician’s assessment at enrollment, usually based on chest configuration, chest X-ray, and diffusion capacity of carbon monoxide, and if available based on chest computerized tomography scans.
Memorial Symptom Assessment Scale
The MSAS,20 which consists of a list of 32 common symptoms, is a self-report questionnaire used to assess the multiple dimensions of a patient’s symptom experience during the past week. Patients were asked to indicate whether or not they had each symptom (ie, occurrence). If they experienced the symptom, they were asked to rate its frequency, severity, and distress. In this study, the symptom of “hair loss” was replaced with “weight gain” because hair loss is not a symptom associated with COPD, whereas weight gain is an important symptom to assess in these patients.26
From the 32 items on the MSAS, three subscale scores were calculated: the psychological subscale (PSYCH), the physical subscale (PHYS), and the global distress index (GDI).20 The validity and reliability of the MSAS are well established.20,27 The MSAS has good psychometric properties in patients with COPD.5,28 In this study, the Cronbach’s alphas were 0.9 for PSYCH, 0.8 for PHYS, 0.9 for GDI, and 0.9 for the MSAS total score.
St George’s Respiratory Questionnaire
The St George’s Respiratory Questionnaire (SGRQ)29 was used to evaluate disease-specific QOL. The SGRQ components (ie, symptoms, activity, and impact) and SGRQ total score can range from 0 to 100, with higher scores indicating worse QOL.30,31 A change of 4 in the total score is considered a clinically meaningful change.32,33 In the current study, the Cronbach’s alphas were 0.69 for the symptom component, 0.88 for the activity component, 0.77 for the impact component, and 0.90 for the total score.
Statistical analyses
LCA was used to identify subgroups of patients (ie, latent classes) with distinct symptom experiences based on symptom occurrence rates from the MSAS.34,35 The symptom dimension of occurrence was chosen because it is easy to use, easier to reproduce in clinical practice, and is a prerequisite to the evaluation of the other dimensions. LCA identifies latent classes based on an observed pattern.36,37 To have a sufficient number of patients with each symptom to perform the LCA, the 14 symptoms that occurred in ≥40% of the patients were used to identify the latent classes.
The final number of latent classes was determined by evaluating the Bayesian Information Criterion, the Vuong–Lo–Mendell–Rubin likelihood ratio test (VLMR) for the K versus K-1 model, and entropy. The model that fits the data best has the lowest Bayesian Information Criterion and a VLMR that shows that the selected solution is better than a solution with one fewer classes.38 In addition, well-fitting models produce entropy values of ≥0.80.39 As is common for this type of analysis, the log-likelihood and Akaike Information Criterion are reported for the interested reader. The LCA was performed using Mplus, Version 7.36,37,40 Estimation was carried out using robust maximum-likelihood and the expectation-maximization algorithm.34
After the latent class solution that best fit the data was identified, differences among the latent classes in demographic and clinical characteristics, MSAS subscale and total scores, and QOL outcomes were evaluated using analysis of variance, Kruskal–Wallis analyses, and chi-square analyses using SPSS Version 20 (IBM Corporation, Armonk, NY, USA) and Stata/SE Version 14.1 (StataCorp LP, College Station, TX, USA). A P-value of <0.05 was considered statistically significant. Post hoc contrasts were done using the Bonferroni procedure and evaluated using a corrected P-value of 0.017 (0.05/3 pairwise comparisons).
Results
Patient recruitment and enrollment
A total of 363 patients were asked to participate. Sixteen patients did not meet the inclusion criteria and 55 declined participation. Of the 292 patients enrolled, eight patients withdrew from the study and 17 patients did not return the questionnaires. The final sample consisted of 267 patients (response rate 76.9%).
Latent class analysis
Fourteen of the 32 symptoms from the MSAS that occurred in ≥40% of the patients were used in the LCA. These symptoms were shortness of breath (SOB), lack of energy, feeling drowsy, dry mouth, cough, worrying, pain, feeling bloated, difficulty sleeping, feeling sad, problems with sexual interest or activity, feeling nervous, feeling irritable, and difficulty concentrating.
The fit indices for the candidate models are shown in Table 1. The three-class solution was selected because the VLMR likelihood ratio test for the K versus K-1 model was significant for the three-class solution, indicating that three classes fit the data better than two classes. In addition, the VLMR was not significant for the four-class solution, indicating that too many classes were extracted. Finally, the four-class solution resulted in many estimation warnings because thresholds had to be fixed at extreme values for seven items due to small class sizes. These findings indicated that the results of the four-class solution were unlikely to generalize to other samples. Thus, using LCA, three subgroups, hereafter termed classes of patients, were identified based on their distinct experiences with the 14 MSAS symptoms (Figure 1).
Table 1.
Latent class fit indices for two- through four-class solutions using symptom occurrence rates for 14 symptoms from the Memorial Symptom Assessment Scale
| Model | LL | AIC | BIC | VLMR | Entropy |
|---|---|---|---|---|---|
| Two-class | −2,051.58 | 4,161.17 | 4,265.20 | 693.53** | 0.89 |
| Three-classa | −1,988.86 | 4,065.72 | 4,223.56 | 125.45* | 0.91 |
| Four-class | −1,937.50 | 3,992.99 | 4,204.64 | 102.73ns | 0.86 |
Notes:
P<0.01;
P<0.001.
The three-class solution was selected because its VLMR was significant indicating that three classes fit the data better than two classes, and the VLMR was not significant for the four-class solution, indicating that too many classes had been extracted. In addition, the four-class solution resulted in many estimation warnings, because thresholds had to be fixed at extreme values for seven items due to small class sizes. This result indicates that the results for the four-class solution are unlikely to generalize to other samples. VLMR, Vuong–Lo–Mendell–Rubin likelihood ratio test for the K versus K-1 model.
Abbreviations: AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion; LL, log-likelihood; ns, not significant.
Figure 1.
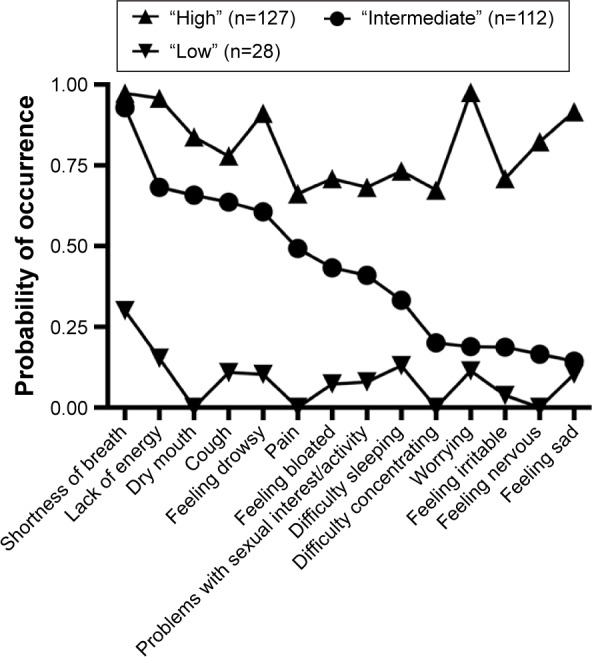
The probability of occurrence for the 14 symptoms in the three latent classes.
The largest class of patients (n=127; 47.6%), with the highest symptom occurrence rates for both physical (ie, SOB, lack of energy, dry mouth, cough, feeling drowsy, pain, feeling bloated, and problems with sexual interest/activity) and psychological (ie, worrying, feeling sad, feeling nervous, feeling irritable, difficulty concentrating, and difficulty sleeping) symptoms, was named the “high” class. The probability of occurrence for the MSAS symptoms for this class ranged from 0.66 (ie, pain) to 0.98 (ie, worrying).
The second largest class (n=112; 41.9%) was named the “intermediate” class. The probability of occurrence for the MSAS symptoms ranged from 0.14 to 0.93. Apart from SOB (0.93), these patients reported moderate occurrence rates for most of the physical symptoms (ie, lack of energy [0.68] and dry mouth [0.67]), but relatively low occurrence rates for the psychological symptoms (ie, feeling sad [0.14], feeling nervous [0.17], and feeling irritable [0.19]).
The third class (n=28; 10.5%) was named the “low” class. The probability of occurrence for the MSAS symptoms for this class ranged from 0.00 (ie, dry mouth, feeling nervous, difficulty concentrating, and pain) to 0.30 (ie, SOB). These patients reported low occurrence rates for both the physical and the psychological symptoms.
Differences in demographic and clinical characteristics among the three latent classes
In pairwise comparisons, compared with the “low” class, patients in the “high” class had significantly lower absolute values of FEV1 but not FEV1% of reference values (Table 2), and they reported a lower level of education. In addition, patients in the “high” class were more likely to be female compared with patients in the “low” and “intermediate” classes. When compared with the “intermediate” class, patients in the “high” class were significantly younger, had smoked for a significantly shorter amount of time, and had a significantly higher number of acute exacerbations during the last 12 months.
Table 2.
Differences in demographic and clinical characteristics among the three latent classes
| Characteristic | Mean (SD)
|
Statistics and post hoc contrast | ||
|---|---|---|---|---|
| Low (0), n=28 (10.5%) | Intermediate (1), n=112 (41.9%) | High (2), n=127 (47.6%) | ||
| Age (years) | 63.3 (8.1) | 65.3 (9.3) | 61.2 (8.6) | F=6.392, P=0.002 2<1 |
| Number of years with COPD | 5.0 (5.2) | 8.0 (6.7) | 7.9 (5.9) | F=2.506, P=0.084 |
| Number of years smoking | 38.8 (10.2) | 42.3 (10.9) | 37.9 (11.5) | F=4.76, P=0.011 2<1 |
| Number of comorbidities | 1.1 (1.1) | 2.4 (1.8) | 2.6 (1.7) | F=8.391, P<0.001 0<1 and 2 |
| BMI (kg/m2) | 24.0 (5.3) | 24.1 (4.7) | 23.8 (4.4) | F=0.092, P=0.912 |
| FEV1 (liters) | 1.3 (0.7) | 1.1 (0.6) | 0.9 (0.5) | KW, P=0.003 2<0 |
| FEV1 % predicted | 45.3 (20.8) | 40.6 (19.5) | 35.9 (18.4) | KW, P=0.04a |
| FEV1/FVC | 0.5 (0.1) | 0.4 (0.1) | 0.4 (0.1) | F=1.082, P=0.34 |
| PaO2 (kPa) | 9.8 (1.2) | 9.4 (1.5) | 9.3 (1.4) | F=1.346, P=0.262 |
| 6MWT (meters) | 460.2 (125.4) | 370.9 (134.8) | 364.86 (123.9) | F=5.985, P=0.003 1 and 2<0 |
| mMRC dyspnea scale (0–4) | 1.5 (1.3) | 2.4 (1.3) | 2.7 (1.3) | KW, P<0.001 0<1 and 2 |
| Acute exacerbations in last 12 months | 1.1 (2.6) | 1.1 (1.9) | 1.9 (2.7) | KW, P=0.017 1<2 |
| Sex (female) | 32.1 (9) | 44.6 (50) | 64.6 (82) | χ2=14.841, P=0.001 0 and 1<2 |
| GOLD classification | ||||
| Moderate | 46.4 (13) | 32.1 (36) | 26.8 (34) | KW, P=0.032a |
| Severe | 17.9 (5) | 29.5 (33) | 18.1 (23) | |
| Very severe | 35.7 (10) | 38.4 (43) | 55.1 (70) | |
| Education | ||||
| Primary | 18.5 (5) | 31.5 (34) | 45.2 (57) | KW, P=0.019 0>2 |
| Secondary | 66.7 (18) | 56.5 (61) | 45.2 (57) | |
| University/college | 14.8 (4) | 12.0 (13) | 9.5 (12) | |
| Living alone (% yes) | 32.1 (9) | 30.4 (34) | 37.1 (46) | χ2=1.231, P=0.54 |
| Other clinical characteristics (% yes) | ||||
| Emphysema | 50.0 (14) | 76.1 (83) | 75.4 (92) | χ2=8.417, P=0.015 0<1 and 2 |
| Chronic bronchitis | 3.7 (1) | 24.3 (26) | 30.3 (36) | χ2=8.330, P=0.016 0<1 and 2 |
| Heart disease | 16.7 (4) | 21.6 (22) | 15.7 (18) | χ2=1.313, P=0.519 |
| ≥2 acute exacerbations in last 12 months | 22.2 (6) | 24.3 (26) | 36.9 (45) | χ2=5.179, P=0.075 |
| Currently smoking | 34.6 (9) | 19.8 (22) | 26.0 (33) | χ2=2.915, P=0.233 |
| Oxygen therapy | 10.7 (3) | 27.7 (31) | 37.0 (47) | χ2=8.150, P=0.017 0<2 |
| Beta 2 agonists (short- and long-acting) | 40.0 (10) | 72.2 (78) | 76.0 (95) | χ2=13.245, P=0.001 0<1 and 2 |
| Anticholinergics | 68.0 (17) | 83.9 (94) | 87.9 (109) | χ2=6.244, P=0.044 0<2 |
| Combination therapy | 66.7 (16) | 74.5 (82) | 78.2 (97) | χ2=1.567, P=0.457 |
| Inhaled steroids | 19.0 (4) | 22.9 (24) | 25.9 (29) | χ2=0.578, P=0.749 |
| Oral prednisolone | 11.5 (3) | 13.6 (15) | 21.0 (26) | χ2=0.824, P=0.244 |
Note:
Indicates significant differences among the latent classes, but no significant pairwise group differences.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; KW, Kruskal–Wallis test; mMRC, modified Medical Research Council Dyspnea Scale; PaO2, partial pressure of oxygen in arterial blood; 6MWT, six-minute walk test; SD, standard deviation.
Compared with the “low” class, patients in the “intermediate” and “high” classes had a significantly higher number of comorbidities, walked for a significantly shorter distance, reported significantly more dyspnea, and had a significantly higher percentage of patients who had a diagnosis of emphysema or chronic bronchitis.
Differences among the latent classes in treatment for COPD are shown in Table 2. Of the total sample, only eleven patients used noninvasive ventilator support (ie, five patients in the “intermediate” class and six patients in the “high” class).
Differences in MSAS and SGRQ scores among the three latent classes
As shown in Table 3, significant differences were found among the latent classes in the mean number of symptoms reported (ie, “high” =17.6±5.4> “intermediate” =9.1±3.7> “low” =1.9±2.1, P<0.001). For the GDI and PHYS sub-scale scores, as well as the MSAS total score, the differences among the three latent classes had the same pattern (ie, “high” > “intermediate” > “low”). For the PSYCH subscale score, patients in the “high” class had significantly higher scores compared with patients in the “low” and the “intermediate” classes.
Table 3.
Differences among the three latent classes in MSAS and SGRQs
| Symptom and QOL scales | Mean (SD)
|
Statistics | ||
|---|---|---|---|---|
| Low (0), n=28 (10.5%) | Intermediate (1), n=112 (41.9%) | High (2), n=127 (47.6%) | ||
| Number of MSAS symptoms (0–32) | 1.9 (2.1) | 9.1 (3.7) | 17.6 (5.4) | F=194.944, P<0.001 0<1<2 |
| MSAS scores | ||||
| Global distress index | 0.1 (0.1) | 0.6 (0.4) | 1.6 (0.6) | F=191.995, P<0.001 0<1<2 |
| PHYS subscale score | 0.1 (0.1) | 0.7 (0.4) | 1.2 (0.5) | F=79.851, P<0.001 0<1<2 |
| PSYCH subscale score | 0.1 (0.3) | 0.4 (0.4) | 1.7 (0.7) | F=238.633, P<0.001 2>0 and 1 |
| MSAS total score | 0.1 (0.1) | 0.6 (0.3) | 1.2 (0.4) | F=142.740, P<0.001 0<1<2 |
| SGRQ scores | ||||
| Symptom component | 40.7 (22.0) | 59.3 (19.3) | 68.0 (18.0) | F=24.147, P<0.001 0<1<2 |
| Activity component | 52.2 (28.8) | 68.3 (23.1) | 76.1 (18.6) | F=12.192, P<0.001 0<1<2 |
| Impact component | 24.3 (17.4) | 40.8 (18.7) | 52.7 (18.1) | F=28.050, P<0.001 0<1<2 |
| SGRQ total score | 36.8 (18.6) | 52.5 (17.2) | 62.7 (15.7) | F=25.259, P<0.001 0<1<2 |
Abbreviations: MSAS, Memorial Symptom Assessment Scale; PSYCH, psychological; PHYS, physical; QOL, quality of life; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire.
For the SGRQ symptom, activity, and impact component scores, as well as the SGRQ total score, the significant differences among the three latent classes were in the expected direction (ie, “high” > “intermediate” > “low”).
Rank order of the probability of occurrence of symptoms for the three latent classes
Table 4 provides a summary of the rank order of the probability of occurrence of the MSAS symptoms for each of the latent classes. Except for worrying, which had the highest probability of occurrence in the “high” class, SOB and lack of energy were the most common symptoms in all three classes.
Table 4.
Rank order of the probability of occurrence of the 14 MSAS symptoms for each of the three latent classes
| Rank | Low, n=28 (10.5%) | Prob | Intermediate, n=112 (41.9%) | Prob | High, n=127 (47.6%) | Prob |
|---|---|---|---|---|---|---|
| 1 | Shortness of breath | 0.300 | Shortness of breath | 0.929 | Worrying | 0.976 |
| 2 | Lack of energy | 0.153 | Lack of energy | 0.682 | Shortness of breath | 0.974 |
| 3 | Difficulty sleeping | 0.130 | Dry mouth | 0.658 | Lack of energy | 0.958 |
| 4 | Worrying | 0.114 | Cough | 0.636 | Feeling sad | 0.916 |
| 5 | Cough | 0.109 | Feeling drowsy | 0.606 | Feeling drowsy | 0.911 |
| 6 | Feeling sad | 0.104 | Pain | 0.493 | Dry mouth | 0.838 |
| 7 | Feeling drowsy | 0.103 | Feeling bloated | 0.433 | Feeling nervous | 0.824 |
| 8 | Problems with sexual interest/activity | 0.080 | Problems with sexual interest/activity | 0.410 | Cough | 0.779 |
| 9 | Feeling bloated | 0.073 | Difficulty sleeping | 0.332 | Difficulty sleeping | 0.733 |
| 10 | Feeling irritable | 0.038 | Difficulty concentrating | 0.200 | Feeling irritable | 0.709 |
| 11 | Dry mouth | 0.000 | Worrying | 0.189 | Feeling bloated | 0.709 |
| 12 | Feeling nervous | 0.000 | Feeling irritable | 0.187 | Problems with sexual interest/activity | 0.682 |
| 13 | Difficulty concentrating | 0.000 | Feeling nervous | 0.165 | Difficulty concentrating | 0.674 |
| 14 | Pain | 0.000 | Feeling sad | 0.144 | Pain | 0.662 |
Abbreviations: MSAS, Memorial Symptom Assessment Scale; Prob, probability of occurrence.
Discussion
Because of the significant heterogeneity in the clinical manifestations of the disease, patients with COPD require a broad, individualized assessment of their symptom experience. Using LCA, we identified three classes of patients with COPD who had distinct symptom experiences. Although significant differences in the severity stages of COPD as well as in FEV1% were found among the latent classes, all the pairwise comparisons were not significant. These findings are interesting as they suggest that more attention should be paid on the assessment of the patient’s individual symptom experience as lung function measurements alone may not identify patients who are at greater risk of experiencing a higher symptom burden.
Patients in the “high” class had high occurrence rates for both physical and psychological symptoms and reported an average of 17.6±5.4 symptoms. This number of symptoms was significantly higher than the number reported by the patients in the other two classes, and higher than the eight to 14 symptoms reported in previous studies of patients with moderate28 to very severe5,17 COPD. In addition, this class had the highest scores on all the subscales of the MSAS and SGRQ. These findings are consistent with previous reports in COPD41 and oncology42 patients that showed that although symptom burden was associated with some clinical characteristics, patients with a higher symptom burden experience significant decrements in disease-specific QOL. Of note, the significant relationship between symptom burden and disease-specific QOL in this and other studies confirms that the identification of subgroups of patients using LCA provides clinically meaningful information about patients’ distinct symptom experiences.
Interestingly, 65% of the patients in the “high” class were women, compared with 45% and 32% in the “intermediate” and “low” classes, respectively. In addition, patients in the “high” class were younger and had a significantly shorter smoking history compared with those in the “intermediate” class. These findings related to age and sex are consistent with a previous report in cancer patients.43 Although inhaled noxious substances may be more harmful for women than men44 and younger women may be at higher risk for an early onset of more severe COPD,45 the reasons for the associations between younger age and female sex and a higher symptom burden are not completely clear. These associations suggest that particular attention should be given to the symptoms reported by younger women with COPD. However, these findings warrant confirmation in future studies.
Moreover, worrying has previously been shown to have a significant impact on the mental health of individuals with COPD. While COPD patients with depression and anxiety are at increased risk for mortality,46 female patients with COPD are particularly vulnerable to psychological impairment.47 Notably, in our study, worrying was the most common symptom in the “high” class. Our findings suggest the existence of a subgroup of COPD patients who are particularly troubled by psychological symptoms and highlight the need to identify these patients and treat both their respiratory and psychological symptoms.
Furthermore, the probability of occurrence of the psychological symptoms in the “high” class ranged from 0.67 to 0.98, whereas in the “intermediate” class the probability of occurrence for the same symptoms ranged from 0.14 to 0.33. In the “low” class, only three of the psychological symptoms were reported, and occurrence rates ranged from 0.04 to 0.11. These findings are consistent with the observation that >30% of COPD patients experience symptoms of depression and anxiety.48 Our observations suggest that treatment options as well as self-management strategies that address worrying and mental health should be considered in these patients.
Compared with patients in the “high” class, patients in the “intermediate” class were older, had a longer smoking history, and had fewer acute exacerbations in the last 12 months. Interestingly, the “intermediate” class showed some similarities with a previously described clinical phenotype of COPD characterized by older age, less severe airflow obstruction, and higher body mass index.13 In addition, compared with the rank order of the probability rates in the “high” class, the occurrence rates for the psychological symptoms in the “intermediate” class were relatively low. This finding is consistent with population-based studies,49–51 as well as with a previous study of oncology patients,42 that found that older patients tend to report lower occurrence rates for depression and anxiety.
Based on the higher occurrence rates for chronic bronchitis and emphysema, as well as the relatively higher probability of associated respiratory symptoms and relatively low rates of psychological symptoms, the “intermediate” class of COPD patients identified in this study is the class that best corresponds to the “classic” clinical picture of patients with COPD.
In the “low” class, a striking discrepancy was found between the relatively low symptom burden reported and the objective measures of poor pulmonary function. Despite the relatively small number of patients, it is noteworthy that almost 36% of the patients in the “low” class had very severe COPD based on the classification of airflow limitation in COPD. This observation suggests that in some patients measurements of lung function are a poor predictor of symptom burden.
Our findings suggest that the symptom burden of COPD patients is highly heterogeneous, and that a thorough symptom assessment is needed to optimize patients’ treatment regimens. However, some limitations warrant consideration. In this study, 46% of the 267 patients had very severe COPD. These patients were recruited primarily from a tertiary referral hospital. Therefore, the number and size of the classes found in this study may not generalize to the general COPD population. An additional limitation may be that the MSAS did not include a number of disease specific symptoms (eg, wheezing, chest pain, and chest pressure). Although previous work recommended the addition of these symptoms,5 we were able, using the original version of MSAS,20 to identify these classes of COPD patients with distinct symptom experiences.
Recently, the GOLD classification of COPD was revised to better assess the patient’s symptoms by including not only measurements of lung function but also certain respiratory symptoms. The COPD assessment test (CAT) and the Clinical COPD Questionnaire (CCQ) were recommended for a more comprehensive assessment of symptoms.3 Although our study was not intended to design a new symptom index or scoring system, our observations do suggest that an assessment of psychological symptoms such as worrying, feeling sad, or feeling nervous may be useful in identifying COPD patients with specific care needs.
Conclusion
We identified a subgroup of patients with COPD who have a particularly high burden of both respiratory and psychological symptoms. This group was associated with younger age, higher number of female patients, and a higher frequency of acute exacerbations. Findings from our study highlight the need to perform more comprehensive assessments of both physical and psychological symptoms in order to better identify the individual needs of COPD patients. Future studies need to determine if symptom burden changes over time and whether latent class membership remains consistent or changes as a patient’s disease progresses.
Acknowledgments
We are grateful to all the patients and clinicians who contributed to this study, especially the research nurses Gunilla Solbakk, Mari-Ann Øvreseth, and Britt Drægni. This study was funded by the South-Eastern Norway Regional Health Authority (2009055).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJL, Lopez A. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 3.Committee GS, editor. Global strategy for the diagnosis, management and prevention of COPD (Updated 2015) Global Initiative for Chronic Obstructive Lung Disease, Inc; 2015. [Accessed April 7, 2015]. serial on the Internet. Available from: http://www.goldcopd.org. [Google Scholar]
- 4.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 5.Blinderman CD, Homel P, Billings JA, Tennstedt S, Portenoy RK. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manage. 2009;38(1):115–123. doi: 10.1016/j.jpainsymman.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Borge CR, Wahl AK, Moum T. Association of breathlessness with multiple symptoms in chronic obstructive pulmonary disease. J Adv Nurs. 2010;66(12):2688–2700. doi: 10.1111/j.1365-2648.2010.05447.x. [DOI] [PubMed] [Google Scholar]
- 7.Burgel PR, Paillasseur JL, Roche N. Identification of clinical phenotypes using cluster analyses in COPD patients with multiple comorbidities. Biomed Res Int. 2014;2014:9. doi: 10.1155/2014/420134. Article ID 420134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez Campos J, Bustamante V, Muñoz X, Barreiro E. Moving towards patient-centered medicine for COPD management: multidimensional approaches versus phenotype-based medicine – a critical view. COPD. 2014;11(5):591–602. doi: 10.3109/15412555.2014.898035. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35(1):1–6. doi: 10.1016/j.ccm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Gagnon P, Casaburi R, Saey D, et al. Cluster Analysis in patients with GOLD 1 chronic obstructive pulmonary disease. PLoS One. 2015;10(4):e0123626. doi: 10.1371/journal.pone.0123626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Aymerich J, Gómez FP, Benet M, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax. 2011;66(5):430–437. doi: 10.1136/thx.2010.154484. [DOI] [PubMed] [Google Scholar]
- 12.Chen C-Z, Wang L-Y, Ou C-Y, Lee C-H, Lin C-C, Hsiue T-R. Using cluster analysis to identify phenotypes and validation of mortality in men with COPD. Lung. 2014;192(6):889–896. doi: 10.1007/s00408-014-9646-x. [DOI] [PubMed] [Google Scholar]
- 13.Burgel P-R, Paillasseur J-L, Peene B, et al. Two distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortality. PLoS One. 2012;7(12):e51048. doi: 10.1371/journal.pone.0051048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo KW, Ra SW, Chae EJ, et al. Three phenotypes of obstructive lung disease in the elderly. Int J Tuberc Lung Dis. 2010;14(11):1481–1488. [PubMed] [Google Scholar]
- 15.Rennard SI, Locantore N, Delafont B, et al. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc. 2015;12(3):303–312. doi: 10.1513/AnnalsATS.201403-125OC. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Meldrum C, Larson J. Subgroup analysis of symptoms and their effect on functioning, exercise capacity, and physical activity in patients with severe chronic obstructive pulmonary disease. Heart Lung. 2013;42(6):465–472. doi: 10.1016/j.hrtlng.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bausewein C, Booth S, Gysels M, Kuhnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13(9):1109–1118. doi: 10.1089/jpm.2010.0068. [DOI] [PubMed] [Google Scholar]
- 18.Joshi M, Joshi A, Bartter T. Symptom burden in chronic obstructive pulmonary disease and cancer. Curr Opin Pulm Med. 2012;18(2):97–103. doi: 10.1097/MCP.0b013e32834fa84c. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Larson J. Symptom cluster, healthcare use and mortality in patients with severe chronic obstructive pulmonary disease. J Clin Nurs. 2014;23(17–18):2658–2671. doi: 10.1111/jocn.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 21.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 22.Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 24.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 25.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratoires ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell DE, Ciavaglia CE, Neder JA. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann Am Thorac Soc. 2014;11(4):635–644. doi: 10.1513/AnnalsATS.201312-438FR. [DOI] [PubMed] [Google Scholar]
- 27.Chang VT, Hwang SS, Thaler HT, Kasimis BS, Portenoy RK. Memorial symptom assessment scale. Expert Rev Pharmacoecon Outcomes Res. 2004;4(2):171–178. doi: 10.1586/14737167.4.2.171. [DOI] [PubMed] [Google Scholar]
- 28.Eckerblad J, Todt K, Jakobsson P, et al. Symptom burden in stable COPD patients with moderate or severe airflow limitation. Heart Lung. 2014;43(4):351–357. doi: 10.1016/j.hrtlng.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):s25–s31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 30.Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):680–685. doi: 10.1164/rccm.2112043. [DOI] [PubMed] [Google Scholar]
- 31.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 32.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 33.Ståhl E, Lindberg A, Jansson SA, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;3:56. doi: 10.1186/1477-7525-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55(2):463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 35.Vermunt J, Magdison J. Applied Latent Class Analysis. New York, NY: Cambridge University Press; 2002. [Google Scholar]
- 36.Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: with Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 37.Nylund K, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: what does latent class analysis say? Child Dev. 2007;78(6):1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 38.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a monte carlo simulation study. Struct Equ Modeling. 2007;14(4):535–569. [Google Scholar]
- 39.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. J Classif. 1996;13(2):195–212. [Google Scholar]
- 40.Muthén LK, Muthén BO. Mplus User’s Guide. 7th ed. Los Angeles, CA: Muthén & Muthén; pp. 1998–2015. [Google Scholar]
- 41.Bentsen SB, Miaskowski C, Rustoen T. Demographic and clinical characteristics associated with quality of life in patients with chronic obstructive pulmonary disease. Qual Life Res. 2014;23(3):991–998. doi: 10.1007/s11136-013-0515-5. [DOI] [PubMed] [Google Scholar]
- 42.Miaskowski C, Dunn L, Ritchie C, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage. 2015;50(1):28–37. doi: 10.1016/j.jpainsymman.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miaskowski C, Cooper B, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer. 2014;120(15):2371–2378. doi: 10.1002/cncr.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sørheim I-C, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–485. doi: 10.1136/thx.2009.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011;184(4):414–420. doi: 10.1164/rccm.201011-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression-Important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615–1631. doi: 10.3978/j.issn.2072-1439.2014.09.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Marco F, Verga M, Reggente M, et al. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respir Med. 2006;100(10):1767–1774. doi: 10.1016/j.rmed.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 48.Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289–1306. doi: 10.2147/COPD.S72073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter AJ, Scott KM, Ferrari AJ, Norman RE, Vos T, Whiteford HA. Challenging the myth of an “epidemic” of common mental disorders: trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress Anxiety. 2014;31(6):506–516. doi: 10.1002/da.22230. [DOI] [PubMed] [Google Scholar]
- 50.Christensen H, Jorm AF, Mackinnon AJ, et al. Age differences in depression and anxiety symptoms: a structural equation modelling analysis of data from a general population sample. Psychol Med. 1999;29(2):325–339. doi: 10.1017/s0033291798008150. [DOI] [PubMed] [Google Scholar]
- 51.Hinz A, Kittel J, Karoff M, Daig I. Anxiety and depression in cardiac patients: age differences and comparisons with the general population. Psychopathology. 2011;44(5):289–295. doi: 10.1159/000322796. [DOI] [PubMed] [Google Scholar]


