Abstract
Arsenic trioxide has in vitro and in vivo radiosensitizing properties. We hypothesized that Arsenic trioxide would enhance the efficacy of the targeted radiotherapeutic agent 131I-metaiodobenzylguanidine (131I-MIBG), and tested the combination in a phase II clinical trial.
Methods
Patients with recurrent or refractory stage 4 neuroblastoma or metastatic paraganglioma/pheochromocytoma (MP) were treated on an institutional review board-approved protocol (Clinicaltrials.gov identifier NCT00107289). Planned treatment was 131I-MIBG (444 or 666MBq/kg) intravenously on day 1 plus arsenic trioxide (0.15 or 0.25mg/m2) intravenously on days 6–10 and 13–17. Toxicity was evaluated using National Cancer Institute Common Toxicity Criteria version 3.0. Response was assessed by International NB response criteria or (for MP) by changes in 123I-MIBG or PET scans.
Results
Twenty-one patients were treated: 19 with neuroblastoma and 2 with MP. Fourteen patients received 131I-MIBG and AT both at maximal dosages, 2 patients received a 444MBq/kg dose of 131I-MIBG plus a 0.15mg/kg/dose dose of arsenic trioxide; and 3 patients received a 666MBq/kg dose of 131I-MIBG plus a 0.15mg/kg/dose dose of arsenic trioxide. One did not receive arsenic trioxide because of transient central line-induced cardiac arrhythmia and another received only 6 of 10 planned doses of arsenic trioxide because of grade 3 diarrhea and vomiting with concurrent grade 3 hypokalemia and hyponatremia. Nineteen patients experienced myelosuppression higher than grade 2, most frequently, thrombocytopenia (n=18), though none required autologous stem cell rescue. 12 of 13 evaluable patients experienced hyperamylasemia higher than grade 2 from transient sialoadenitis. By International Neuroblastoma Response Criteria, 12 NB patients had no response and 7 had progressive disease, including 6 of 8 entering the study with progressive disease. Objective improvements in semiquantitative 131I-MIBG scores were observed in 6 patients. No response was seen in MP. Seventeen of 19 neuroblastoma patients continued on further chemotherapy or immunotherapy. Mean 5-year overall survival (±SD) for neuroblastoma was 37±11%. Mean blood absorbed dose of 131I-MIBG to blood was 0.134 cGy/mCi 131I-MIBG, well below myeloablative levels in all patients.
Conclusion
131I-MIBG plus arsenic trioxide was well tolerated with an adverse event profile similar to that of 131I-MIBG therapy alone. The addition of arsenic trioxide to 131I-MIBG did not significantly improve response rates when compared to historical data with 131I-MIBG alone.
Keywords: Radiosensitization, neuroblastoma, malignant pheochromocytoma/paraganglioma, MIBG therapy
INTRODUCTION
Meta-iodobenzylguanidine (MIBG) is a guanethidine analog that is taken up via the noradrenaline transporter (NET) by neuroendocrine malignancies arising from sympathetic neuronal precursors (1). These neoplasms include neuroblastoma (NB), the commonest solid tumor of childhood which is often metastatic at diagnosis, and malignant paraganglioma/pheochromocytoma (MP). Recent therapeutic advances have led to modest improvements in the outcome of patients with high-risk NB; however approximately half of the patients with stage 4 NB and a much higher proportion of patients with relapsed or chemo-refractory disease succumb to it (2). 123I-MIBG scans are the “gold standard” for staging of NB with >90% of patients having MIBG-avid disease (3). 131I-MIBG (MIBG therapy) has undergone several therapeutic trials for NB over more than three decades (4). A dose of 666MBq/kg is generally accepted as the maximum dose per administration (5). The consensus is that treatment, although active against resistant NB especially for disease palliation (6), is not curative. Reported response rates are ≤30% even when relatively non-stringent criteria were used to evaluate disease response (4). Similarly, responses in patients with MP are uncommon though 131I-MIBG therapy is associated with symptomatic relief and hormonal responses.(7)
The causes of these suboptimal responses have not been well-characterized and are likely multifactorial.(8) Strategies to enhance the clinical response to 131I-MIBG therapy have included increasing the amount (i.e. administered activity) and number of 131I-MIBG doses(5, 9), combining it with myeloablative chemotherapy and autologous stem cell transplant (ASCT)(10), and adding radiation-sensitizing agents.(11, 12) Preclinical mechanistic justifications for the clinical use of radiosensitizers in combination with 131I-MIBG therapy include enhancement of NET expression or activity, e.g. by topoisomerase inhibitors and a synergistic effect on inhibition of radiation-induced DNA-repair e.g. vorinostat.(13, 14)
Arsenic trioxide (AT) is an apoptotic agent acting via cytotoxic pathways distinct from conventional chemotherapeutic agents and suppresses growth of NB xenografts.(15, 16) Its radiation-sensitizing effects have been demonstrated in preclinical models of several tumors including fibrosarcoma(17) and glioma.(18, 19) The combination of 89Sr and AT showed beneficial cell killing in MCF7 cells.(20) In other preclinical experiments AT synergized with the radioiodinated anti-GD2 monoclonal antibody 131I-3F8 against neuroblastoma xenografts (Modak et al., unpublished data, 2003). Clinical evidence suggesting a radiosensitizing effect for AT is derived from reports of responses to concurrent AT and external beam radiotherapy in chemo-radioresistant cutaneous breast cancer(21) and extramedullary acute promyelocytic leukemia (APML)(22), and from a report of severe radiation recall in a patient previously treated with AT.(23) AT has been established as a highly effective agent for the treatment of APML in adults and children and the combination of AT and all-trans retinoic acid may reduce the need for subsequent anthracycline therapy.(24) In children with acute leukemia and infiltrating astrocytoma treated with AT in phase I studies, AT was well tolerated with adverse events similar to those encountered in adults: vomiting, diarrhea and QTc prolongation (25, 26) with a recommended dose of 0.15mg/kg/day. However, in children with NB and other solid tumors treated with AT as a single agent, dose-limiting toxicity was not encountered even at 0.25mg/kg/day when serum electrolytes were optimally maintained (clinicaltrials.gov identifier NCT00024258). On the basis of these preclinical and clinical data, and the non-overlapping toxicities of the two agents, we hypothesized that AT would act as a radiosensitizer for 131I-MIBG therapy and conducted a pilot phase II study (NCT00107289) in patients with relapsed or refractory NB and MP, the results of which we report below.
MATERIALS AND METHODS
Patient Selection
Patients > 1 year of age with high-risk NB (stage 4 disease diagnosed at >18 months of age or MYCN-amplified stage ≥3 tumor at any age), and a history of progressive disease (PD) or chemoresistance; and patients with MP <21 years of age were eligible. The presence of MIBG-avid evaluable or measurable disease ≥3 weeks after completion of systemic therapy was required for protocol entry. Other salient eligibility criteria included: availability of ≥2 X106 CD34+ cells/kg autologous hematopoietic stem cells cryopreserved for re-infusion, ability and willingness to comply with radiation safety procedures and lack of life-threatening infections or >grade 2 non-hematological toxicity (including renal, cardiac, hepatic, pulmonary, gastrointestinal and neurologic) according to the National Cancer Institute’s Common Toxicity Criteria version 3.0 (CTC v3.0).
Study Design
The protocol was approved by the institutional review board (IRB) of Memorial Sloan Kettering Cancer Center (MSKCC). Written informed consent was obtained from patients or their guardians. Since this was the first study of this novel combination, it was decided to proceed with maximal doses only if no serious adverse events (SAEs) were encountered in a pilot group of 5 patients who were treated with reduced doses of the therapeutic agents. Treatment consisted of a single dose of intravenous (IV) 131I-MIBG (444 [12mCi/kg] or 666MBq/kg [18mCi/kg]) administered over one hour on day 0 followed by IV AT as a single daily dose of 0.15mg/kg or 0.25mg/kg on days 5 through 9, and 12 through 16 administered over two hours. 131I-MIBG was radiolabeled at Nuclear Diagnostic Products, Rockaway, NJ and had a confirmed radiochemical purity of ≥99% within 4 hours prior to injection. Toxicities were graded with CTC v3.0 and responses for NB patients assessed 4–6 weeks after 131I-MIBG therapy, with International NB Response Criteria (INRC)(27) modified to include 123I-MIBG scans. Responses were classified as: complete remission (CR) - no evidence of disease including in bone marrow (BM); very good partial response - primary mass reduced >90%, no evidence of distant disease and normal catecholamines; partial remission (PR) - >50% decrease in measurable disease, number of positive bone sites decreased by 50%, and ≤1 positive BM site; mixed response (MR) - no new lesions, >50% decrease of any measurable lesion with <50% decrease in any other; no response (NR) - <50% decrease but <25% increase in any existing lesion; and PD - new lesion or >25% increase in an existing lesion. Patients with NR or MR were categorized as having stable disease (SD). For patients with MP, RECIST criteria were used if there was measurable disease. For both groups of patients objective changes in 123I-MIBG scans were recorded using the semiquantitative Curie score.(28, 29) Relative 123I-MIBG score (RMS) was calculated by dividing Curie score after treatment by prior Curie score. Dosimetry and pharmacokinetic data were obtained using serial whole body imaging and peripheral venous blood specimens; typically, up to 10 blood samples were obtained from <1 hour to 10 days post-131I-MIBG injection. The activity concentrations in weighed aliquots of blood were measured in a scintillation well counter calibrated for 131I, fit to a bi-exponential time-activity concentration function, and analytically integrated to yield the area under the curve. The resulting cumulated activity concentration was then multiplied by the equilibrium dose constant for the 131I beta-rays, Δβ = 0.405 g-cGy/37MBq, to yield the mean absorbed dose to blood. Implicit in the foregoing absorbed-dose calculation were the standard assumptions that the 131I beta-rays are completely absorbed in blood and the contribution of the highly penetrating gamma-rays was negligible.
Safety Precautions
Thyroid protection included oral administration of saturated solution of potassium iodide drops 5–7 drops (250–330mg) thrice daily and liothyronine (25–75 μg once daily) starting about 7 days prior to 131I-MIBG and continued for 14 and 42 days respectively after 131I-MIBG infusion. 131I-MIBG was administered in-patient only after demonstration of thyroid suppression as documented by sub-normal TSH levels. Urinary bladder protection was provided by maintenance of a Foley catheter for ≥72 hours after 131I-MIBG therapy. Radiation safety precautions were followed to minimize exposure to family, public and staff including use of isolation rooms and rolling lead shields. Patients were discharged only after the whole-body dose rate had fallen to <7cGy/h at a distance of 1m. AT was administered only if absolute QT interval was >500msec. During AT therapy, serum potassium and magnesium levels were maintained >4mEq/dL and >1.8mg/dL respectively. Filgrastim was given to maintain absolute neutrophil count (ANC) >500/μL. Platelet and red blood cell transfusions were administered as clinically indicated. Patients were required to receive autologous stem cell rescue (ASCR) if ANC remained <500/μL for >14 days despite filgrastim, if they required platelet transfusions > twice weekly for 4 weeks after 131I-MIBG therapy or if they experienced life-threatening infection in the setting of neutropenia.
Biostatistical Considerations
Since this was the first study of the novel combination of 131I-MIBG therapy and AT, we instituted early stopping rules for unacceptable toxicity: if ≥4 of the first 10 patients or 6 patients at any time experienced a ≥grade 3 non-hematologic toxic event or if ≥3 patients experienced graft failure after ASCR. The primary endpoint was efficacy of the combination as assessed by response. We proposed declaring treatment effective if probability of response was ≥0.4. A Simon two-stage design that differentiated between response rates of 0.4 and 0.65 was used with plans to treat 13 evaluable NB patients at maximal doses of 131I-MIBG and AT in the first phase and to add an additional 15 NB patients only if >5 responses (CR+PR) were observed in the first cohort. The efficacy rule did not apply to patients with MP. Survival analysis was performed using Kaplan Maier and log-rank tests (SPSS)
RESULTS
Patient Demographics
Twenty-one patients (12 male and 9 female with a median age of 7.8 y and range of 2–30.4 y) were treated from 2005 to 2008: 19 with NB including one treated “as per” protocol after compassionate approval from MSKCC IRB and 2 with MP (Table 1). Two patients received 444MBq/kg 131I-MIBG plus 0.15mg/kg/dose of AT and three received 666MBq/kg 131I-MIBG plus 0.15mg/kg/dose of AT. Fourteen patients (including one treated “as per” study) received MIBG plus AT at maximal doses of 666MBq/kg plus 0.25mg/kg/day respectively.
Table 1.
Patient demographics, responses and survival
| Doses administered | Responses | Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt No. |
Age at Rx (years) |
MYCN status |
No. of prior relapses |
Pre- study status |
Pre-study MIBG score |
131I-MIBG (actual) (MBq/kg) |
AT (mg/kg/day) |
INRC | Relative MIBG score |
PFS (months) |
OS (months) |
| Metastatic pheochromocytoma/paraganglioma | |||||||||||
| 1 | 9.8 | N/A | 1 | SD | 7 | 656.6 | 0.15 | N/A | 1.00 | 63.1‡ | 63.1‡ |
| 2 | 13.3 | N/A | 1 | SD | 9 | 668.4 | 0.15 | N/A | 1.00 | 100.2‡ | 100.2‡ |
| Neuroblastoma | |||||||||||
| 3 | 9.2 | NA | 1 | SD | 13 | 444 | 0.15 | NR | 0.85 | 100.6‡ | 100.6‡ |
| 4 | 7.7 | NA | 0 | SD | 11 | 462.5 | 0.15 | NR | 1.00 | 79.2 | 99.0‡ |
| 5 | 9.9 | NA | 1 | SD | 8 | 673.2 | 0.15 | NR | 0.88 | 94.1‡ | 94.1‡ |
| 6 | 6.0 | NA | 2 | PD | 6 | 669.7 | 0* | NR | 1.00 | 6.5 | 15.5 |
| 7 | 2.0 | A | 0 | SD | 2† | 647.5 | 0.25 | NR | 1.00 | 91.8‡ | 91.8‡ |
| 8 | 2.8 | NA | 1 | SD | 20 | 677.1 | 0.25 | NR | 1.00 | 83.3‡ | 83.3‡ |
| 9 | 3.3 | A | 2 | PD | 2† | 662.3 | 0.25 | PD | 1.00 | 0.9 | 3.7 |
| 10 | 4.7 | NA | 0 | SD | 14 | 632.7 | 0.25 | NR | 1.00 | 71.8‡ | 71.8‡ |
| 11 | 5.8 | NA | 5 | PD | 2† | 662.3 | 0.25 | PD | 1.00 | 0.7 | 4.8 |
| 12 | 6.1 | A | 2 | PD | 21 | 691.9 | 0.25 | PD | 0.90 | 2.8 | 4.0 |
| 13 | 6.1 | NA | 4 | PD | 2† | 677.1 | 0.25 | PD | 1.00 | 1.4 | 8.7 |
| 14 | 6.2 | A | 3 | SD | 5 | 662.3 | 0.25 | PD | 1.00 | 6.5 | 14.4 |
| 15 | 7.8 | NA | 4 | SD | 13 | 636.4 | 0.25 | NR | 1.00 | 3.7 | 14.7 |
| 16 | 8.3 | NA | 3 | SD | 21 | 658.6 | 0.25 | NR | 0.95 | 3.6 | 8.8 |
| 17 | 8.4 | NA | 0 | SD | 9 | 684.5 | 0.25 | NR | 0.88 | 83.3‡ | 83.3‡ |
| 18 | 8.4 | NA | 1 | PD | 10 | 654.9 | 0.25 | PD | 1.30 | 1.2 | 27.2 |
| 19 | 8.7 | NA | 3 | PD | 6 | 688.2 | 0.25 | PD | 1.50 | 1.2 | 9.8 |
| 20 | 15.2 | NA | 1 | SD | 9 | 647.5 | 0.25 | NR | 1.00 | 6.2 | 23.7 |
| 21 | 30.4 | NA | 2 | PD | 8 | 658.6 | 0.25§ | NR | 0.63 | 11.5 | 61.6 |
Abbreviations and Legend: A: Amplified; AT: Arsenic trioxide; INRC: International Neuroblastoma Response Criteria, N/A: not applicable; NA: Not amplified, No.: number, NR: No response; OS: overall survival; PFS: progression-free survival; PD: progressive disease; Pt: patient; Rx: treatment; SD: stable disease.
AT not administered due to central-line induced arrhythmia;
MIBG-avid soft tissue disease only;
continue to be progression-free;
6/10 planned AT doses administered due to diarrhea and electrolyte imbalance
NB patients were heavily prior treated, with a median of 3 previous therapeutic regimens; 15 patients were treated after prior relapse (median: 2 prior relapses) and 4 had chemorefractory metastatic disease. Fourteen had stage 4 disease at diagnosis, while one (patient #8; Table 1) was diagnosed with stage 2B NB and then relapsed with metastatic disease prior to study entry. Both MP patients had germline mutations in the succinyl dehydrogenase B (SDHB) gene, had multiple skeletal metastatic sites (123I-MIBG Curie scores of 7 and 9, respectively) and had recurrent disease 2 and 7 months after alkylator-based chemotherapy. Most patients had relatively high disease burden at entry with median (±standard deviation [SD]) Curie score of 9 ±5.9 (range 2–21).
Toxicities
Treatment was well tolerated in general without mortality or severe morbidity (Table 2). None of the five patients treated with reduced doses of the treatment agents experienced severe adverse events and a further 14 patients received both agents at maximal doses. One patient (#6, Table 1) did not receive any AT because of the development of a central line-related arrhythmia prior to commencing AT. A second patient developed AT-related grade 3 vomiting and diarrhea with accompanying grade 3 hypokalemia and hyponatremia, and received only 6 of 10 planned doses of AT. Other expected >grade 2 toxicities were those that have previously been described with 131I-MIBG therapy(9, 30) and included neutropenia (n=16 patients), thrombocytopenia (n=18), lymphopenia (n=18), anemia (n=12) and hyperamylasemia due to transient sialoadenitis (n=12/13 patients evaluated). No patient experienced febrile neutropenia or serious infection. All patients with thrombocytopenia received platelet transfusions. As defined in protocol criteria, ASCR was not required for any patient. TSH levels assessed 3–6 months after therapy were normal (n=12).
Table 2.
Toxicities related to therapy
| Toxicity | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Symptomatology | ||||
| Diarrhea | 5 | 4 | 1 | 0 |
| Vomiting | 8 | 7 | 1 | 0 |
| Dry skin | 2 | 1 | 0 | 0 |
| Headache | 0 | 1 | 0 | 0 |
| Anorexia | 1 | 0 | 0 | 0 |
| Pain (cheek) | 2 | 0 | 0 | 0 |
| Dry mouth | 1 | 0 | 0 | 0 |
| Fatigue | 1 | 0 | 0 | 0 |
| Laboratory values | ||||
| Anemia | 0 | 7 | 12 | 0 |
| Neutropenia | 0 | 3 | 8 | 8 |
| Thrombocytopenia | 1 | 2 | 3 | 15 |
| Lymphopenia | 0 | 0 | 3 | 15 |
| Hypokalemia | 6 | 2 | 1 | 0 |
| Hyponatremia | 7 | 0 | 1 | 0 |
| Hypermagnesemia | 2 | 0 | 0 | 0 |
| Elevated serum creatinine | 1 | 0 | 0 | 0 |
| Elevated serum AST | 7 | 0 | 0 | 0 |
| Elevated serum ALT | 9 | 0 | 0 | 0 |
| Hyperbilirubinemia | 1 | 0 | 0 | 0 |
| Hyperamylasemia* | 0 | 0 | 12 | 0 |
Serum amylase levels were measured in 13 patients
Abbreviations: AST: aspartate transaminase ALT: alanine transaminase
Pharmacokinetics, dosimetry and targeting
123I-MIBG in blood exhibited biphasic kinetics (evaluated in 16 patients), with an initial rapid component (87% of the blood activity on average) having a mean±SD biologic half-life of 1.37±1.41 hours and a second slower component (13% of the blood activity on average) having a biologic half-life of 28.1+5.62 hours. A typical blood time-activity curve is shown in Figure 1. The blood absorbed dose averaged 0.134+0.0928 cGy/mCi with maximum value of 0.354cGy/mCi. The total blood absorbed dose was 46.0+25.0 cGy. Posttreatment 131I-MIBG gamma camera scanning was performed on 20 patients. All sites of disease detected by pre-therapy 123I-123I-MIBG scans showed targeting with therapeutic 131I-MIBG. In addition, a significantly higher number of skeletal lesions were detected on post-131I-MIBG therapy scans in all (NB and MP) patients with skeletal disease (n=17), with 3.3±1.6 (range: 2–7) additional lesions noted (p<0.05 by t-test for pre-therapy vs post-therapy 123I-MIBG scores). However, no extra lesions were detected in patients who had only soft tissue disease (n=3).
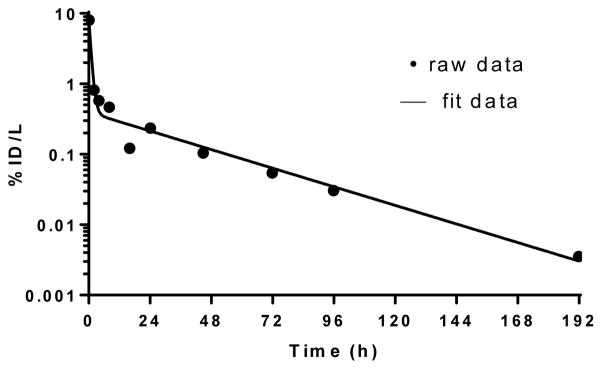
Figure 1.
Typical 131I-MIBG blood time-activity concentration curve. The data points represents the measured %injected dose/Liter (%ID/L) values and the curve the best-fit function. Blood clearance was rapid, with 94% clearing with a biological half-life of 1.88 hours and the 6% clearing with a biological half-life of 23.9 hours.
Disease responses
NB
Overall, there were no major responses (CR+PR) observed in NB patients (Table 1). Twelve patients had NR by INRC and seven had PD. Of eight patients with PD at study entry, six had further PD whereas 10/11 patients with stable non-progressing disease immediately prior to protocol therapy had NR to treatment. There was no reduction in measurable soft tissue disease (n=9) or in BM NB assessed by histology (n=6). 15/19 patients had 123I-MIBG-avid skeletal metastases and 4/19 had only 123I-MIBG-avid soft-tissue disease prior to therapy. Of 15 patients with skeletal disease, nine had no change on 123I-MIBG scanning (RMS of 1); six had objective responses on 123I-MIBG scanning but RMS was >0.5 in all, thus not meeting criteria for PR by extended INRC (Table 3). Three of the four patients with only soft-tissue disease prior to therapy had PD. Urine catecholamine levels were reduced in 4/7 patients with elevated levels.
Table 3.
Objective response rates* in patients with neuroblastoma
| Site of disease | Number of patients | Objective Responses | Objective Response Rate |
|---|---|---|---|
| Soft tissue | 9 | 0 | 0% |
| Bone Marrow (by histology) | 6 | 0 | 0% |
| Skeleton (by MIBG scan) | 15 | 6 | 40% |
There were no major responses as assessed by International Neuroblastoma Response Criteria.
MP
Both patients with MP had extensive skeletal disease (123I-MIBG scores of 7 and 9, respectively) and one had measurable soft-tissue disease; neither had an objective response to 131I-MIBG plus AT therapy. Urine normetanephrines decreased 50% in one patient, while the other did not secrete metanephrines.
The study was terminated after the first phase since it did not meet response criteria for continuation.
Post-protocol therapy and survival
Seventeen NB patients went on to receive additional therapy for their residual/PD after coming off study. Although ASCR was not indicated for any patient on study, 10 patients underwent ASCR after high-dose myelosuppressive (but non-myeloablative) chemotherapy after completing required protocol observations, and all patients engrafted after ASCR. Other post-protocol therapies included immunotherapy with the anti-GD2 monoclonal antibody 3F8 (n=7). One patient had early PD and did not receive any further therapy while another did not receive any further therapy but is a long-term survivor >91 months after therapy despite having persistent 123I-MIBG-avid pancreatic disease. All patients were followed for late toxicities, none of which were observed. The contribution of 131I-MIBG plus AT in prolonging survival could not be evaluated. Five-year overall survival (OS) for NB patients is 37±11% with seven long-term survivors, all except one (#17, Table 1) with residual disease. Patient #17 achieved CR after further therapies after 131I-MIBG and remains a long-term disease-free survivor. Median OS was 23.7±9.1months and median time to PD was 6.5±2 months. However, patients with PD at study entry had a significantly worse survival (p<0.001 for OS and PFS) compared to those with stable disease pre-therapy, with a median OS and PFS of 9.8±1 and 1.4±0.3 months respectively. Both patients with MP survive with residual but progression-free disease at 63.1 and 100.2 months after therapy. One was treated with a second dose of high-dose 131I-MIBG therapy without AT, while the other did not receive any post-protocol therapy.
DISCUSSION
The combination of high-dose 131I-MIBG therapy and AT, even at maximal doses, was well tolerated without any significant unexpected toxicity. As anticipated with 131I-MIBG doses of ≥444MBq/kg >grade 3 myelosuppression requiring blood product support was encountered, though no patient developed serious infections or required inpatient admission after discharge after 131I-MIBG infusion. AT did not add significant toxicity except for one patient. There was no effect of 131I-MIBG or AT dose on response or toxicity with the only non-hematologic >grade 2 toxicity observed in the patient who received 6/10 doses of 0.25mg/kg/day AT. Post-therapy scans showed excellent targeting of 131I-MIBG therapy to lesions and similar to previous reports,(31) revealing additional lesions not detected on pre-therapy 123I-MIBG scans. However, response rates for resistant NB were not improved when compared to historical data with single-agent high-dose 131I-MIBG, suggesting that AT did not have a radiosensitizing or other beneficial effect for 131I-MIBG therapy. Objective responses were not seen in the two MP patients.
Similar observations have been made for other radiosensitizers, though toxicity has generally been worse than that encountered with 131I-MIBG plus AT. Specifically, the addition of irinotecan and vincristine to 131I-MIBG therapy yielded response rates of 25% but with an increase in toxicity while the combination with vorinostat was associated with a response rate of 12–17%.(12, 32) The combination of 131I-MIBG therapy and myeloablative chemotherapy followed by ASCT has been investigated by several groups. 131I-MIBG therapy followed by myeloablative chemotherapy with carboplatin, etoposide plus melphalan,(10) or busulfan plus melphalan(33) was associated with significant toxicity, especially hepatic sinusoidal obstructive syndrome without improvement in responses in chemoresistant NB(34). The contribution of 131I-MIBG therapy to responses could not be defined and the use of 131I-MIBG therapy followed by myeloablative chemotherapy for patients with NB remains to be optimized. Small, non-randomized studies have reported better response rates when 131I-MIBG therapy has been used in the upfront treatment of NB, though these included children with low-stage and intermediate-risk disease and imaging did not consistently include sensitive 123I-MIBG scans.(35, 36) Possible reasons for the low major response rate observed in NB patients in our study could include (a) high disease burden at time of treatment, median pre-therapy 123I-MIBG score of 9) and (b) high proportion (74%) of patients treated after multiple relapses with a median of three relapses prior to treatment with 131I-MIBG plus AT. Furthermore, outcomes were even poorer in patients commencing therapy with PD immediately prior to study entry (further PD in 75%) suggesting that MIBG therapy be initiated only after disease has been stabilized with other modalities such as salvage chemotherapy.
Patients with refractory and relapsed metastatic NB are considered to have a very poor prognosis.(37, 38) Although we observed few objective responses to therapy, a higher than expected number of NB patients treated on our study (37%: 7/19) are long-term survivors, albeit all except one with residual MIBG-avid disease. Survivors include 3 patients treated at first relapse. Since 17/19 patients received further therapies including 3F8, dissecting out the possible role of 131I-MIBG+AT therapy to their improved survival is impossible.
The natural history of MP is often marked by prolonged remissions even without any anti-cancer therapy(39). Therefore, the contribution of 131I-MIBG plus AT to the long-term progression-free status of the two treated patients is unclear. Nevertheless, for both patients current progression free intervals are significantly (10x) higher than time to relapse from prior therapy.
Extensive blood time-activity data were successfully collected and analyzed in a total of 16 patients, with uniformly excellent fitting of bi-exponential functions to these data. The total blood absorbed doses based on this analysis averaged only 46 cGy, well below myeloablative doses. This is consistent with the very rapid initial clearance of 131I-MIBG, with 87% of the blood-borne activity being cleared with a biologic half-time of 1.37 hours on average. It is somewhat unexpected that these dose estimates would result in Grade 3 or 4 BM toxicity. However, all patients were heavily prior-treated with chemotherapy. An additional contribution to toxicity could be BM involvement with NB. Similar severe myelosuppression has been reported in all other studies using 131I-MIBG doses ≥444MBq/kg.
CONCLUSION
High-dose 131I-MIBG plus the radiosensitizer AT was well tolerated in heavily prior-treated patients who had a substantial disease burden and had received heavy prior treatment. Anti-NB activity was modest and approximated that observed in similar patient populations treated with high-dose 131I-MIBG with or without other radiosensitizers.
Acknowledgments
We thank Rodney Prosser and team at Nuclear Diagnostic Products for assisting with radiolabeling protocols and methods, Dr Peter Smith-Jones for quality control, Samantha Leyco for data management and Joe Olechnowicz for editorial input. We also thank radiopharmacy, radiation safety, Amabella Lindo, Louise Harris and the radiopharmacy, radiation safety, and the pediatric nursing teams at MSKCC team for dispensing and facilitating the administration of 131I-MIBG.
Footnotes
Disclosure: The authors report no potential conflict of interest relevant to this article.
References
- 1.Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Beierwaltes WH. Radiolabeled adrenergi neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med. 1980;21:349–353. [PubMed] [Google Scholar]
- 2.Modak S, Cheung NK. Neuroblastoma: Therapeutic strategies for a clinical enigma. Cancer Treat Rev. 2010;36:307–317. doi: 10.1016/j.ctrv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Kushner BH. Neuroblastoma: a disease requiring a multitude of imaging studies. J Nucl Med. 2004;45:1172–1188. [PubMed] [Google Scholar]
- 4.Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer. 2014;50:801–815. doi: 10.1016/j.ejca.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Matthay KK, Quach A, Huberty J, et al. Iodine-131--metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: a new approaches to neuroblastoma therapy phase I study. J Clin Oncol. 2009;27:1020–1025. doi: 10.1200/JCO.2007.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang TI, Brophy P, Hickeson M, et al. Targeted radiotherapy with submyeloablative doses of 131I-MIBG is effective for disease palliation in highly refractory neuroblastoma. J Pediatr Hematol Oncol. 2003;25:769–773. doi: 10.1097/00043426-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 7.van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EP. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf) 2014;80:487–501. doi: 10.1111/cen.12341. [DOI] [PubMed] [Google Scholar]
- 8.Streby KA, Shah N, Ranalli MA, Kunkler A, Cripe TP. Nothing but NET: A review of norepinephrine transporter expression and efficacy of I-mIBG therapy. Pediatr Blood Cancer. 2014 doi: 10.1002/pbc.25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthay K, DeSantes K, Hasegawa B, et al. Phase I Dose Escalation of 131 I-Metaiodobenzylguanidine with Autologous Bone Marrow Support in Refractory Neuoblastoma. J Clin Oncol. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- 10.Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to Neuroblastoma Therapy Consortium Study. J Clin Oncol. 2006;24:500–506. doi: 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- 11.Mastrangelo S, Tornesello A, Diociaiuti L, et al. Treatment of advanced neuroblastoma: feasibility and therapeutic potential of a novel approach combining 131-I-MIBG and multiple drug chemotherapy. Br J Cancer. 2001;84:460–464. doi: 10.1054/bjoc.2000.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuBois SG, Chesler L, Groshen S, et al. Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: a new approaches to neuroblastoma therapy trial. Clin Cancer Res. 2012;18:2679–2686. doi: 10.1158/1078-0432.CCR-11-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCluskey AG, Boyd M, Pimlott SL, Babich JW, Gaze MN, Mairs RJ. Experimental treatment of neuroblastoma using [131I]meta-iodobenzylguanidine and topotecan in combination. Br J Radiol. 2008;81(Spec No 1):S28–35. doi: 10.1259/bjr/27723093. [DOI] [PubMed] [Google Scholar]
- 14.More SS, Itsara M, Yang X, et al. Vorinostat increases expression of functional norepinephrine transporter in neuroblastoma in vitro and in vivo model systems. Clin Cancer Res. 2011;17:2339–2349. doi: 10.1158/1078-0432.CCR-10-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson J, Ora I, Porn-Ares I, Pahlman S. Arsenic trioxide-induced death of neuroblastoma cells involves activation of Bax and does not require p53. Clin Cancer Res. 2004;10:3179–3188. doi: 10.1158/1078-0432.ccr-03-0309. [DOI] [PubMed] [Google Scholar]
- 16.Gesundheit B, Malach L, Or R, Hahn T. Neuroblastoma cell death is induced by inorganic arsenic trioxide (As(2)O(3)) and inhibited by a normal human bone marrow cell-derived factor. Cancer Microenviron. 2008;1:153–157. doi: 10.1007/s12307-008-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu HW, Lin JH, Chen YA, Ho SY, Wang YJ. Combination treatment with arsenic trioxide and irradiation enhances cell-killing effects in human fibrosarcoma cells in vitro and in vivo through induction of both autophagy and apoptosis. Autophagy. 2010;6:353–365. doi: 10.4161/auto.6.3.11229. [DOI] [PubMed] [Google Scholar]
- 18.Ning S, Knox SJ. Optimization of combination therapy of arsenic trioxide and fractionated radiotherapy for malignant glioma. Int J Radiat Oncol Biol Phys. 2006;65:493–498. doi: 10.1016/j.ijrobp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Ning S, Knox SJ. Increased cure rate of glioblastoma using concurrent therapy with high dose radiation and arsenic trioxide. Int J Radiat Oncol Biol Phys. 2003;57:S257. doi: 10.1016/j.ijrobp.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Tao X, Ma F, Qiu J, Wu C, Wang M. Radiosensitizing effects of arsenic trioxide on MCF-7 human breast cancer cells exposed to 89 strontium chloride. Oncol Rep. 2012;28:1894–1902. doi: 10.3892/or.2012.1979. [DOI] [PubMed] [Google Scholar]
- 21.Lai YL, Chang HH, Huang MJ, et al. Combined effect of topical arsenic trioxide and radiation therapy on skin-infiltrating lesions of breast cancer-a pilot study. Anticancer Drugs. 2003;14:825–828. doi: 10.1097/00001813-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Kai T, Kimura H, Shiga Y, Ogawa K, Sato H, Maruyama Y. Recurrent extramedullary relapse of acute promyelocytic leukemia after allogeneic stem cell transplantation: successful treatment by arsenic trioxide in combination with local radiotherapy. Int J Hematol. 2006;83:337–340. doi: 10.1532/IJH97.05167. [DOI] [PubMed] [Google Scholar]
- 23.Keung YK, Lyerly ES, Powell BL. Radiation recall phenomenon associated with arsenic trioxide. Leukemia. 2003;17:1417–1418. doi: 10.1038/sj.leu.2402992. [DOI] [PubMed] [Google Scholar]
- 24.Abla O, Ribeiro RC. How I treat children and adolescents with acute promyelocytic leukaemia. Br J Haematol. 2014;164:24–38. doi: 10.1111/bjh.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox E, Razzouk BI, Widemann BC, et al. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008;111:566–573. doi: 10.1182/blood-2007-08-107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen KJ, Gibbs IC, Fisher PG, Hayashi RJ, Macy ME, Gore L. A phase I trial of arsenic trioxide chemoradiotherapy for infiltrating astrocytomas of childhood. Neuro Oncol. 2013;15:783–787. doi: 10.1093/neuonc/not021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 28.Messina JA, Cheng SC, Franc BL, et al. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer. 2006;47:865–874. doi: 10.1002/pbc.20777. [DOI] [PubMed] [Google Scholar]
- 29.Naranjo A, Parisi MT, Shulkin BL, et al. Comparison of (1)(2)(3)I-metaiodobenzylguanidine (MIBG) and (1)(3)(1)I-MIBG semi-quantitative scores in predicting survival in patients with stage 4 neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;56:1041–1045. doi: 10.1002/pbc.22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modak S, Pandit-Taskar N, Kushner BH, et al. Transient sialoadenitis: a complication of 131I-metaiodobenzylguanidine therapy. Pediatr Blood Cancer. 2008;50:1271–1273. doi: 10.1002/pbc.21391. [DOI] [PubMed] [Google Scholar]
- 31.Hickeson MP, Charron M, Maris JM, et al. Biodistribution of post-therapeutic versus diagnostic (131)I-MIBG scans in children with neuroblastoma. Pediatr Blood Cancer. 2004;42:268–274. doi: 10.1002/pbc.10454. [DOI] [PubMed] [Google Scholar]
- 32.DuBois SG, Groshen S, Park JR, et al. Phase I Study of Vorinostat as a Radiation Sensitizer with 131I-Metaiodobenzylguanidine (131I-MIBG) for Patients with Relapsed or Refractory Neuroblastoma. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.French S, DuBois SG, Horn B, et al. 131I-MIBG followed by consolidation with busulfan, melphalan and autologous stem cell transplantation for refractory neuroblastoma. Pediatr Blood Cancer. 2013;60:879–884. doi: 10.1002/pbc.24351. [DOI] [PubMed] [Google Scholar]
- 34.Yanik GA, Villablanca JG, Maris JM, et al. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II study. Biol Blood Marrow Transplant. 2015;21:673–681. doi: 10.1016/j.bbmt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Mastrangelo S, Rufini V, Ruggiero A, Di Giannatale A, Riccardi R. Treatment of advanced neuroblastoma in children over 1 year of age: the critical role of (1)(3)(1)I-metaiodobenzylguanidine combined with chemotherapy in a rapid induction regimen. Pediatr Blood Cancer. 2011;56:1032–1040. doi: 10.1002/pbc.22986. [DOI] [PubMed] [Google Scholar]
- 36.de Kraker J, Hoefnagel KA, Verschuur AC, van Eck B, van Santen HM, Caron HN. Iodine-131-metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. Eur J Cancer. 2008;44:551–556. doi: 10.1016/j.ejca.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Cole KA, Maris JM. New strategies in refractory and recurrent neuroblastoma: translational opportunities to impact patient outcome. Clin Cancer Res. 2012;18:2423–2428. doi: 10.1158/1078-0432.CCR-11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 39.Fliedner SM, Lehnert H, Pacak K. Metastatic paraganglioma. Semin Oncol. 2010;37:627–637. doi: 10.1053/j.seminoncol.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]