Abstract
Ultrasonography has multiple advantages over traditional radiologic imaging modalities when used for interventional procedures. It allows improved visualization of the anatomy while avoiding ionizing radiation and risks associated with contrast use. It has proved superiority at accuracy of delivery and procedural effectiveness over blind procedures when used in association with interventional pain procedures. Although limited in its ability to see through bony structures, ultrasound has utility in visualizing soft tissues and vascular structures in anatomic regions of interest resulting in increased use for posterior neuraxial, periaxial, peripheral nerve and joint-related structures. Current evidence for use in these settings is presented here. In some cases, optimal utility may be improved by combining ultrasonography with other imaging modalities.
KEYWORDS : chronic pain management, interventional pain management, joint injections, knee injection, neuraxial injections, shoulder injections, ultrasound-guided interventional pain, ultrasound-guided neuraxial injections
Practice points.
Use of ultrasound in medicine has grown with smaller size equipment, increased portability and decreased cost.
Ultrasound has multiple advantages over radiographic imaging when used for interventional pain procedures.
Sonography is useful for medial branch blocks and intra-articular facet blocks in the cervical and lumbar spine but there is little evidence to support its use in the thoracic spine.
Ultrasonography is limited in utility for many types of axial injections because of bony and acoustic shadowing artifact but it does show some promise with transforaminal epidurals or selective nerve root blocks.
Ultrasound guidance is effective in preventing soft tissue and vascular injury in stellate ganglion blocks compared with blind techniques. Soft tissue and vascular anomalies can be identified that fluoroscopic technique will miss.
Ultrasound improves accuracy and efficacy in ilioinguinal, iliohypogastric and genitofemoral blocks for inguinal and testicular pain.
Lateral femoral cutaneous nerve injections are more accurate under ultrasound guidance, especially given the anatomic variation the nerve exhibits in cadaver dissections.
Injections into the knee, shoulder and hip joints are more successful when performed using ultrasound.
Advantages of ultrasound imaging
Interventional pain procedures were traditionally performed under fluoroscopic (FS), computed tomography (CT) or MRI guidance. The use of ultrasound (US) to perform injections for chronic pain has significantly increased in the past decade [1,2]. This increase is attributed to its multiple advantages over other imaging modalities including ease of performance, absence of ionizing radiation, better visualization of soft tissue (i.e., muscle, ligament) and blood vessels, real time visualization of needle advancement and, at times, the ability to observe the spread of injectate [3]. US also avoids the need for use of contrast agents which can be associated with allergic reactions and renal damage, is portable, and cost effective. There are no known contraindications to its use [3].
Some data suggest US guidance may be safer and more efficient than landmark based or FS-guided injections, especially in the cervical spine [1]. Despite this, there are limits to US imaging which include difficulty mastering scanning techniques (the quality of images obtained are provider dependent), narrow imaging window, inability to visualize structures deep to bone due to acoustic shadowing artifact, and difficulties obtaining quality images in the obese (Box 1) [1,2].
Box 1. Advantages and limits to ultrasound imaging.
Advantages
Ease of performance
No exposure to ionizing radiation
Visualization of soft tissues
Real-time needle advancement
Possibility of observing spread of injectate
Limits
Images obtained are operator dependent
Difficult skillset to master
Inability to visualize deep structures or difficulties in the obese
Narrow imaging window
Acoustic shadowing artifact limits imaging deep to bony structures
Organization of the review
In the following pages, we present some of the most common US-guided chronic pain procedures. After brief descriptions of US imaging, discussion will be organized according to anatomical regions starting with the neuraxis, moving to joints and finishing with some commonly performed peripheral blocks that have been facilitated with US (Box 2). This topic has been previously reviewed [4–10] and this review builds on those previous reviews, deferring in some cases to those reviews as sources for primary references. It is recognized that US images are best observed in real time as they represent ‘shadows’ of structures with limited resolution. In order to effectively utilize US and perform injections safely, it is imperative that the provider possess a detailed understanding of the anatomy in the area of interest [11,12]. It is assumed that the reader is generally familiar with the discussed procedures as well as the ‘knobology’ of US devices [13].
Box 2. Types of ultrasound-guided interventional pain injections.
Neuraxial
Intra-articular facet blocks or medial branch nerve blocks, epidural injections (selective nerve root block, interlaminar, transforaminal, caudal)
Joints
Knee, wrist, elbow, shoulder (glenohumeral or acromioclavicular), hip (intra-articular and greater trochanteric bursa), sacroiliac joint, intercostal nerve, lateral femoral cutaneous nerve, suprascapular nerve, ilioinguinal nerve, iliohypogastric nerve, genitofemoral nerve
Peripheral/other
Stellate ganglion bock, greater occipital nerve, branches of the brachial plexus (i.e., median, ulnar or radial nerves), branches of the lumbosacral plexus (i.e., obturator, peroneal nerves)
Bolded text is not discussed as part of this review.
• Brief description of US imaging
US devices send out pulses of vibrations at frequencies beyond the range of human hearing from a flat (linear) or curved (curvilinear) probe [14]. These pulses produce echoes from interfaces between tissues of differing density. These echoes can be used to construct 2D (planar) or 3D images of the echoing interface. Needle advancement is observed either ‘in-plane’ where the needle continually stays in view by maintaining an acute angle to the line of pulsations (Figure 1) or ‘out-of-plane’ where the needle tip enters the plane of imaging as it interacts with the target site and maintains a perpendicular angle to the probe (Figure 2). Differences in density between water, fat and calcified structures allow real-time imaging of anatomical structures. Resolution of adjacent images are a function of the wavelength of the US; at higher frequencies, greater image resolution is observed but at limited depths whereas at lower frequencies, depth is increased but at the cost of image resolution. Soft tissues may allow additional echoes to be perceived from areas distal to those tissues but dense tissues such as bone will create image ‘shadows’ that limit visualization of those distal structures. It is possible to observe motion of echoing interfaces (like blood cells in vessels) by sensing Doppler shifts in the frequency of the pulse echoes returning to the probe – objects moving away from the probe will echo a slightly lower frequency, objects moving towards the probe will echo a slightly higher frequency. This ability to detect motion is used to identify vascular structures and is one of the major advantages of using US.
Figure 1. . Example of positioning of probe and needle (short axis view) for in-plane needle approach while performing a median nerve block at the antecubital fossa.
Sterile sheath for ultrasound removed for illustrative purposes so markings are visible.
Figure 2. . Example of positioning of probe and needle (short axis view) for out-of-plane needle approach while performing a median nerve block at the antecubital fossa.
Sterile sheath for ultrasound removed for illustrative purposes so markings are visible.
In the past, US machines were predominantly used for diagnostic imaging. However, due to its real-time nature and utility in the identification and visualization of vascular structures, US became increasingly popular for use in acquiring central (and difficult peripheral) venous access [15,16]. Critical factors that led clinicians other than radiologists to use it for therapeutic interventions were its reliability, portability, and cost–effectiveness. A natural extension of vascular cannulation techniques was the injection of local anesthetics into perineural sites (typically neurovascular bundles) and other body spaces which could also be visualized [14]. There has been limited study of blocks commonly performed within pain clinics which will be listed in the following three sections.
Spinal injections
• Intra-articular facet injections & medial branch nerve blocks
The paired facet joints (zygapophyseal or z joints) consist of the articulation of the superior articular process of one vertebra to the inferior articular process of the vertebral body above it at the intersection of the lamina and pedicle [2,17]. Each joint is covered by a fibrous capsule and contains a synovial lining as well as cartilaginous surfaces [2,17]. Z joints, though small, help account for a great deal of the range of motion allowed by the vertebral column [1,17]. Anatomy of the z joints differ based on their location. Cervical facet joints are oriented parallel to the axial plane. In the thoracic spine, they become more steeply angulated. Lumbar z joints tend to have a more oblique orientation. Medial branches of the dorsal ramus of the spinal nerves innervate the facet joints [17].
The facet joints account for a large percentage of low back, mid back, or neck pain in the USA [17]. Facet pain is often dull and achy, nonradiating, nonradicular and usually accompanied by a negative straight leg test. Typically, the patient will describe pain that gradually worsens over the course of months and years [17]. On physical examination, patients may experience tenderness with palpation over the affected joint; this pain is exaggerated by spine extension, waxes and wanes with physical activity and is often worse at the end of the day [17]. Definitive diagnosis is made by performing an intra-articular injection of local anesthetic or by selectively anesthetizing the medial branch of the dorsal ramus of the spinal nerves corresponding to the level of the patient's pain [18]. In the past, diagnostic intra-articular injections and medial branch blocks were considered equivalent [17]. A recent publication suggests that medial branch nerve blocks are better prognostic indicators of success from radiofrequency ablation as compared with intra-articular facet injections [19]
US-guided facet injections are performed with the patient in a prone position and using a high-frequency linear probe (cervical or thoracic spine) or a low-frequency curvilinear probe (lumbar or thoracic spine) [2]. The spinous process at the level of interest is first identified with the probe in transverse orientation; the transducer is moved laterally and rotated ninety degrees to facilitate identification of the lamina, superior articular process, and inferior articular process at the level of interest (see Figures 3 & 4 for example in the cervical spine) [1,5]. By employing an in-plane approach, the provider can advance the needle into the joint space under direct visualization. Poor visibility of the facet joints with US (in obese or physically disabled patients) and acoustic shadowing artifact makes it impossible to locate the needle tip once it has entered the area of the joint capsule deep to bone [17,18].
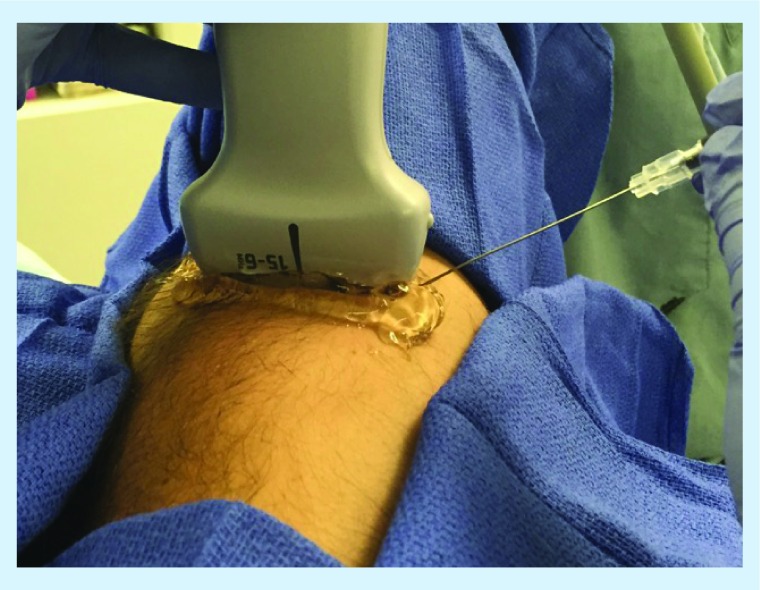
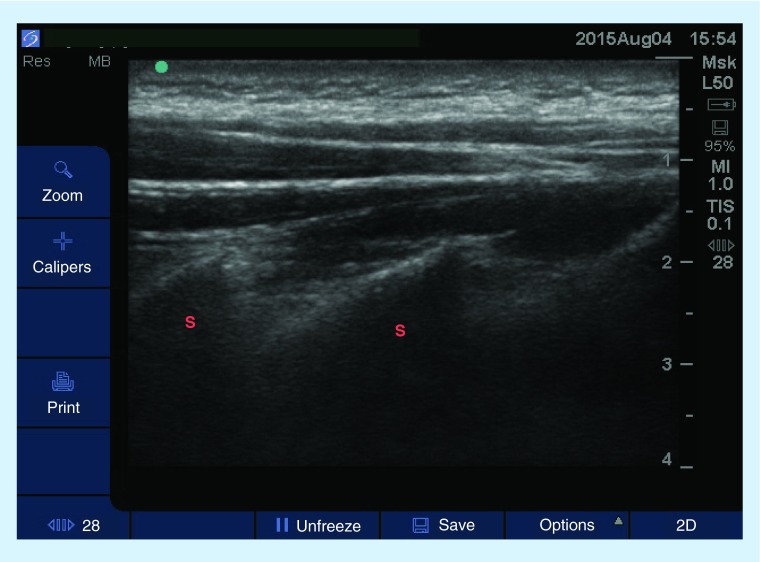
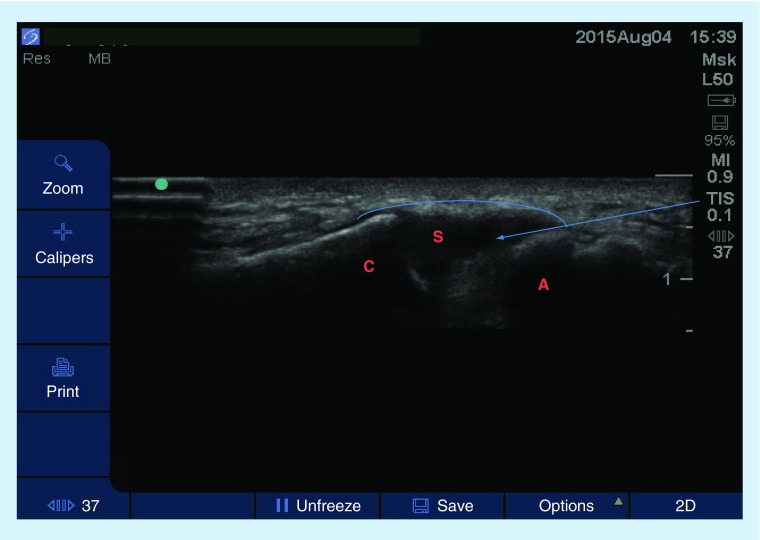
Figure 3. . Sagittal view (high-frequency linear probe) at the neck showing the cervical spinous processes.
The patient is in a prone position with neck slightly flexed.
S: Spinous processes.
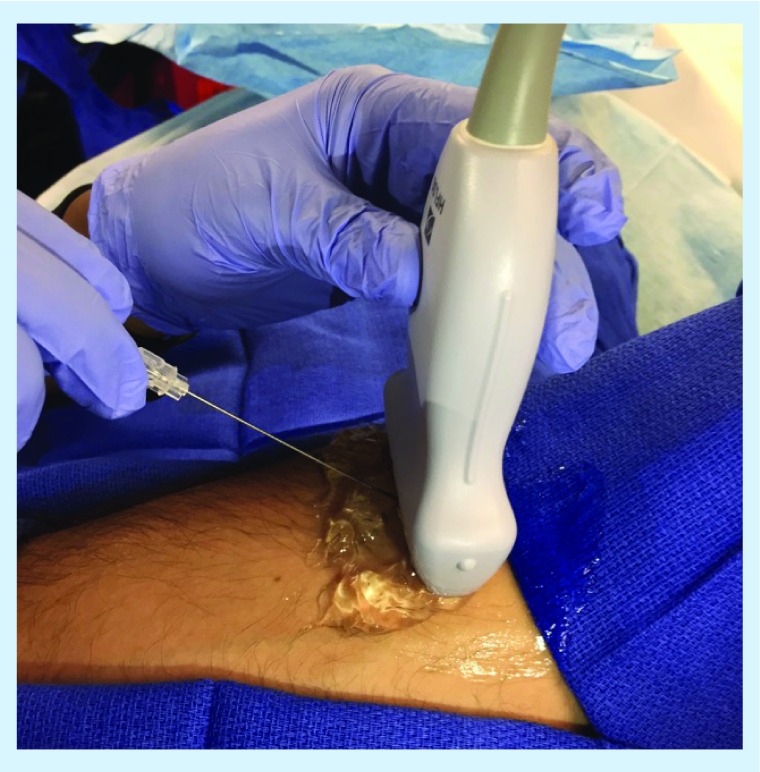
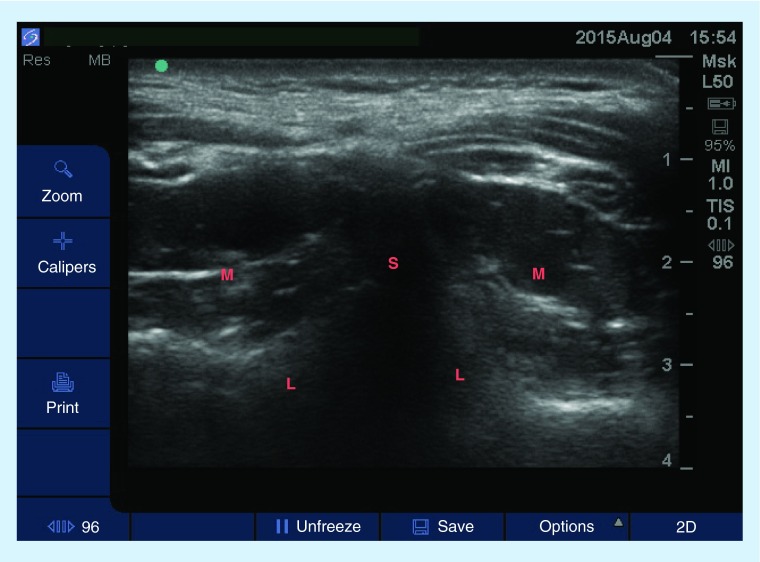
Figure 4. . Transverse sonogram at C7–T1 using a high-frequency linear probe showing the spinous process, lamina and cervical paraspinous muscles.
Image was obtained with the patient in prone position.
L: Lamina; M: Muscle; S: Spinous process.
Cervical facet joints
It has been well established that US guidance is not only feasible but also clinically useful in the performance of cervical facet joint or medial branch blocks [20–22]. Galliano et al. published a feasibility study using cadavers to assess US guidance for cervical facet injections. They noted successful placement of the needle (confirmed by CT) in 36 of 40 subjects [20]. In the cervical spine, two randomized controlled trials (RCTs) have been performed that compared US to CT or to FS-guidance for cervical facet blocks. Obernauer et al. [22] studied 40 patients and reported superior outcomes with US in terms of accuracy and reduced need for needle repositioning. One should note that there was no difference in pain scores between the two groups. Finlayson et al. demonstrated appropriate needle placement 80.9% of the time when placed using US, with injected contrast covering the desired area in 94.5% of patients [21].
Thoracic facet joints
Pain attributable to the thoracic facets is less common than in the cervical or lumbar spine. Thoracic facets are localized by first identifying T12; after, the vertebral bodies are counted moving cephalad until the joint of interest is identified [18]. Feasibility studies have shown some promise but currently, there are no prospective randomized studies that compare US-guided thoracic facet or medial branch blocks to traditional approaches such as CT or FS [18].
Lumbar facet joints
To visualize the facet joints at this level, a low-frequency curvilinear probe should be used to assure adequate depth of penetration of soft tissue. Direct visualization of the lumbar medial branch is not due to image degradation that is commonly seen with increasing depth. While there are no RCTs that compare FS to US in this part of the body, several series have demonstrated a high success rate while employing US for lumbar medial branch blocks or facet joint injections [23–26]. For example, Greher et al. [25] demonstrated accurate needle placement in 25 of 28 patients studied using US. Multiple studies have demonstrated similar efficacy (80% or higher) for intra-articular needle placement using US and confirming needle position with FS [3,5]. In all of the studies reviewed, participants reported a reduction in VAS scores of at least 50% within 30 min of injection. This finding is consistent with results achieved when FS is used [25]. An important factor to note related to these studies is that a vast majority of patients enrolled had body mass indices less than 40; in morbidly obese patients the success rates were significantly lower (60%) [1,5,25,27].
• Epidural steroid injections & nerve root injections
Translaminar epidural steroid injections (ESI), transforaminal ESIs and selective nerve root injections are utilized to treat radicular pain in the extremities secondary to degenerative disc disease, spinal stenosis, foraminal stenosis, disc herniation and failed back surgery syndrome [2,5,28]. Interlaminar ESIs are typically performed blindly using a loss of resistance technique (i.e., for obstetric/postoperative pain) or with FS combined with the loss of resistance technique (in the chronic pain setting). Limited visualization of deeper structures reduces the value of US in this application [1,2]. Practically speaking, the limitations of this technology is not conducive to the performance of intrathecal or interlaminar epidural injections. As such, its role for epidural injections in the clinical setting has been limited to assisting anesthesiologists in the morbidly obese or patients with abnormal spinal anatomy [29,30]. US may have a role in selective nerve root injections which are used to better isolate and treat the inflamed nerve when pathology is present at multiple levels to help plan future surgical intervention [28]. There is also a role in a similar procedure, the transforaminal ESI, in which medication is deposited adjacent to the nerve responsible for the patient's radicular symptoms [28]. For ease of discussion, the terms selective nerve root block, periradicular and transforaminal ESI are going to be used interchangeably and are not meant to reflect the relative risk of performing each individual injection.
Cervical epidural steroid injections
The principal advantage of US for cervical nerve root injections is to prevent damage to critical vascular structures in that region. FS techniques can reliably identify intravascular injection after it has occurred but with US, a practitioner can avoid blood vessels altogether [31,32]. In the cervical spine, radicular and medullary arteries may travel near the spinal nerves and are at risk of penetration during cervical nerve root injections under FS [28]. Devastating results from such an insult include spinal cord infarct or injury to the vertebral artery [5].
To perform these procedures, the patient is placed in the lateral decubitus (unilateral) or prone (bilateral) position and a linear probe is used to identify the ‘camel hump’ (anterior and posterior tubercles of the cervical transverse processes) [5]. Once the appropriate spinal level is identified, the needle can be guided to the nerve root of interest, using an in-plane approach, and medication is injected [5]. Color Doppler mode has a bigger role in cervical spine injections due to the variable course of the deep cervical artery as well as variable anastomoses between the vertebral and cervical arteries [5,22]. The Doppler mode should also be utilized to identify arteries that may lie in the path of the needle or adjacent to the structure of interest so that vascular puncture is avoided. Feasibility and 1-year retrospective studies [31,33] all suggest significant utility of US-guidance for cervical nerve root injections. A recent RCT [22] evaluating the feasibility, accuracy and procedural time of US- verus CT-guided injections demonstrated that US-guided approaches produced similar results in terms of accuracy and reduction in visual analog scores. In addition, US significantly reduced procedure time. Jee et al. [34] conducted a randomized, blinded controlled study of 120 patients with similar results. The majority of the studies were designed to assess feasibility of injection in the middle to lower cervical spine only [5,22,34].
Thoracic epidural steroid injections
At this time, no practical means of performing US-guided transforaminal ESI, periradicular or selective nerve root blocks in the thoracic spine has been described.
Lumbar epidural steroid injections
FS- or CT-guidance remains the gold standard in the lumbar spine because of the difficulty in detecting intravascular injection, visualizing spread of injectate or accurately identifying the lumbar nerve roots with US [5]. A few techniques have been described for injections targeting the lumbar nerve roots involving the transverse process, intertransverse ligaments, lamina and posterior vertebral body. Initial feasibility studies reported success rates nearing 60% [35] but later studies have demonstrated successful injection in approximately 90% of patients [35].
Gofeld and colleagues [36] published a feasibility study designed to evaluate and validate US-guided transforaminal ESIs in the lumbar spine. Using cadavers, the authors targeted the most medial border of the vertebral body for injection of contrast followed by fluoroscopy to determine contrast spread. They reported intraformainal contrast spread in >90% of cases, the remainder being extraforaminal (nerve root) [36]. Loizides et al.'s technique was to identify the transverse process at the level of interest and find the intertransverse ligament as the spinal nerve is thought to be anterior to this structure [37]. Thereafter, medication is deposited deep to this ligament [37,38].
One RCT comparing US- to CT-guidance for lumbar periradicular injections in a group of 40 patients was published in 2013 [37]. The authors assessed and compared accuracy, pain relief and patient comfort. Their results did not show a significant difference in any of the above factors or in degree of pain relief [37]. A second prospective RCT [39] used US to perform lumbar transforaminal ESIs in 80 patients with low back and radicular pain, confirming needle placement with FS. This was notable because studies prior to this had utilized only cadavers [39]. Their results showed reduced procedure time in the US group but without significant difference in pain relief [39].
Caudal epidural steroid injection
The caudal approach to the epidural space is often used when the interlaminar approach is contraindicated [5,40]. Imaging is critical for this procedure because of documented failure rates as high as 56% when blind injection techniques are used [40,41]. The caudal epidural space is entered via the sacral hiatus (formed by incomplete fusion of the S4 and S5 vertebrae posteriorly) [5].
To perform the injection, the patient is placed in prone position and a linear US probe is employed to identify the sacral cornua, sacral hiatus and the sacrococcygeal ligament. At first the needle is inserted out of plane through the sacrococcygeal ligament; then the probe is rotated longitudinally/in plane to show the needle entering the epidural space. Using this method, Chen et al. [40] demonstrated accurate placement of the needle 100% of the time. Their results have been confirmed in independent studies by Blanchais [41] and Klocke [42]. An added advantage of using US is potentially reducing the risk of intravascular injection, avoiding exposure to ionizing radiation in patients of reproductive age (especially considering proximity of site of injection to reproductive organs), and helping identify patients with significantly narrowed or closed sacral hiatus [40,42–43]. Despite these advantages and fairly straightforward technique, US guidance for caudal injections remains unpopular because of the acoustic shadowing artifact that prevents the practitioner from recognizing inaccurate intravascular or intrathecal needle placement prior to injection [5,40,42].
Joint injections
• Knee joint
The knee joint is the largest joint in the body and consists of three primary components (tibiofemoral, patellofemoral and superior tibiofibular) whose stability is maintained by four ligaments (anterior and posterior cruciate ligaments; medial and lateral collateral ligaments) [8,44]. Osteoarthritis in the knee is twice as common in men as women [44]. Knee injections are performed to deliver steroid/local anesthetic mixtures, viscosupplementation, platelet rich plasma and stem cells [8,44].
There are at least six landmark-based (superolateral, superomedial, medial mid patellar, lateral midpatellar, anteromedial and the anterolateral) and two US-guided (anteromedial and suprapatellar) approaches to knee joint injections [8,44]. The suprapatellar approach is preferred for US-guided injections and has the highest accuracy [8,44]. The patient is positioned supine with the knee flexed 25 degrees. A high-frequency linear US probe is then placed parallel to the quadriceps femoris muscle. The distal femur, patellar, suprapatellar fat pad, prefemoral fat pad, and suprapatellar recess are identified. The transducer is rotated 90 degrees until the quadriceps tendon, the distal femur, the suprapatellar fat pad and bursa are identified. Then the needle is guided in an in-plane approach through the joint space and into the suprapatellar recess and the medication of choice injected (Figure 5) [8,44].
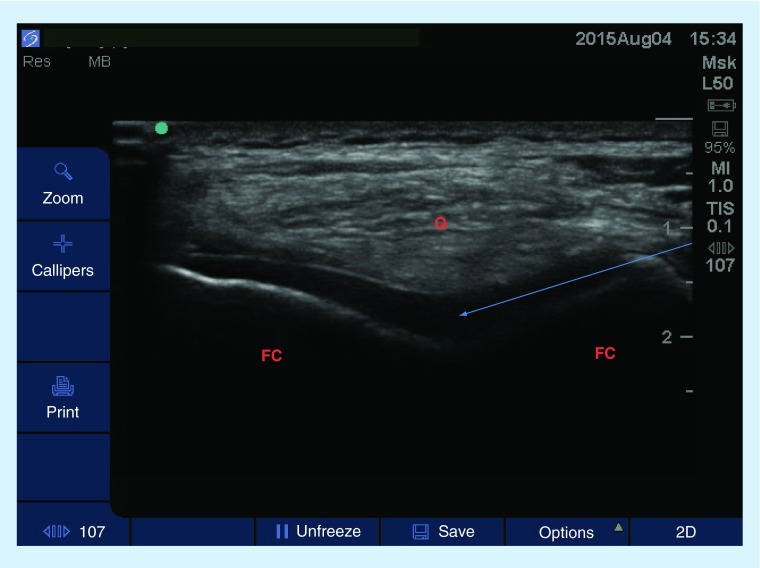
Figure 5. . Transverse sonogram of the knee joint.
A high-frequency linear probe was used and patient was placed in a seated position, knee flexed approximately 20 degrees. Arrow represents the projected path of needle.
FC: Femoral condyle; Q: Quadriceps tendon.
Overall, the accuracy of landmark-based guided injections is about 70–79%. Therefore, approximately 20% of patients are at high risk of obtaining inadequate analgesia from their injections or sustaining damage to ligaments and tendons due to misplacement of the needle. FS is employed when patient anatomy, postsurgical changes, obesity, or joint effusions cause difficulty in accessing the joint space [8]. Multiple consecutive studies have demonstrated increased reliability, better clinical outcomes, higher accuracy rate (˜95%) of US-guided as compared with blind injections [8]. A recent review by Berkoff et al. [45] compared outcomes and accuracy of US- versus landmark-based procedures. The authors noted that US guidance increased accuracy rates from 77.8 to 95.8% [45]. Additionally, with US, patients reported decreased procedural pain, better reduction in pain scores and better function postinjection as compared with landmark-based techniques [45]. Results from Sibbitt et al.'s [46] RCT of 94 osteoarthritic knees comparing US to landmark-based techniques corroborated the aforementioned reports, indicating that US guidance led to greater patient comfort and increased duration of pain relief in the short term (2 months) [46].
• Hip joint
The hip joint is a ball and socket joint formed at the junction of the acetabulum and the femoral head. Hip US is used to detect fluid or hematoma, diagnose bursitis, or assess for other pathology; it is also used to perform therapeutic injections for hip joint pain secondary to arthritis [47].
US-guided intra-articular hip injection is performed with the patient in a supine position, hip slightly abducted and the knee minimally flexed. A low-frequency, curvilinear probe is preferred and is placed in a sagittal orientation, medial to the ASIS. After the femoral head and neck are identified, the probe is rotated to achieve a long-axis view of the femoral head, neck and a portion of the acetabulum/labrum [44,47]. At this point, the provider should identify the femoral vessels using Color Doppler. Then a needle can be guided from lateral to medial into the joint space until the anterior recess is reached and the medication of choice is injected (Figure 6) [47]. The anterior or lateral approach may be taken; however, the lateral approach is preferred due to its higher success rate and to avoid the femoral neurovascular bundle [40,44,47].
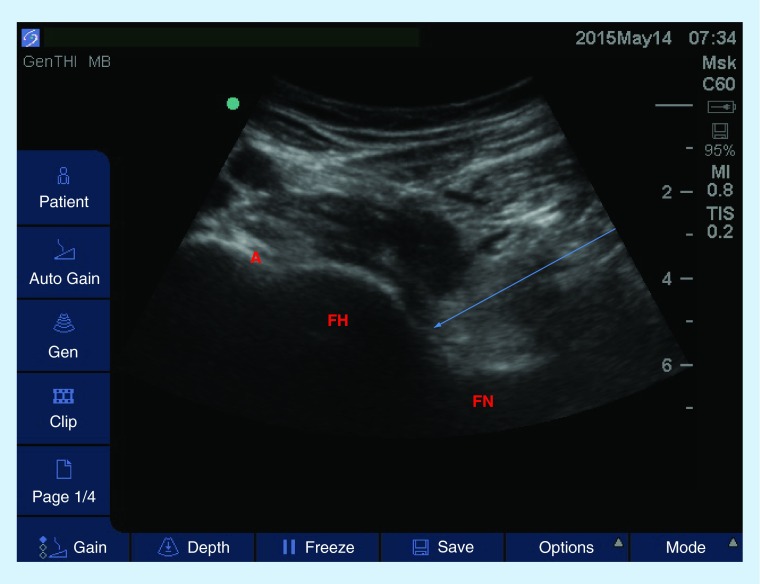
Figure 6. . Longitudinal view of the hip joint using a low-frequency curvilinear probe.
The patient is placed in a supine position, knee slightly flexed and ipsilateral hip externally rotated. Arrow represents the projected path of needle.
A: Acetabulum; FH: Femoral head; FN: Femoral neck.
Blind hip injections have yielded accuracy rates ranging from 52 to 80% [7,48]. As such, FS has become the gold standard for intra-articular hip injections [47,48]. Use of US is increasing in popularity due to factors previously discussed and superior success rates (>95% accuracy) [44,47–48]. Byrd et al.'s [49] study comparing patient experience with US-or FS-guided intra-articular hip injections indicated patient satisfaction was higher and pain score was lower with US technique when compared with FS.
Greater trochanteric bursa
The greater trochanteric bursa (GTB) consists of three bursae between the gluteal tendons: the subgluteus medius bursa, the subgluteus maximus and the subgluteus minimus [44,47]. Greater trochanteric bursitis (also known as greater trochanteric pain syndrome) presents as pain over the lateral portion of the hip or the GTB, sometimes radiating to the knee. This condition presents in patients of all ages and is exacerbated by ambulatory activities [44,47].
Trochanteric bursa injection is achieved by placing the patient in a lateral recumbent position, affected side up [47]. The US probe is used to identify the femoral cortex and the greater trochanter, the bursa usually appears as a hypoechoic line above the greater trochanter (Figure 7). The majority of GTB pain is thought to be secondary to disruption of the gluteus medius tendon. Therefore, the goal of the injection is to place the needle tip between the iliotibial band and the gluteus medius tendon [7]. Clinical outcomes of FS-guided GTB injections did not significantly improve on blinded techniques [7,44]. To our knowledge, there are no data to suggest that the US-guided approach is superior to the blind or FS-guided technique.
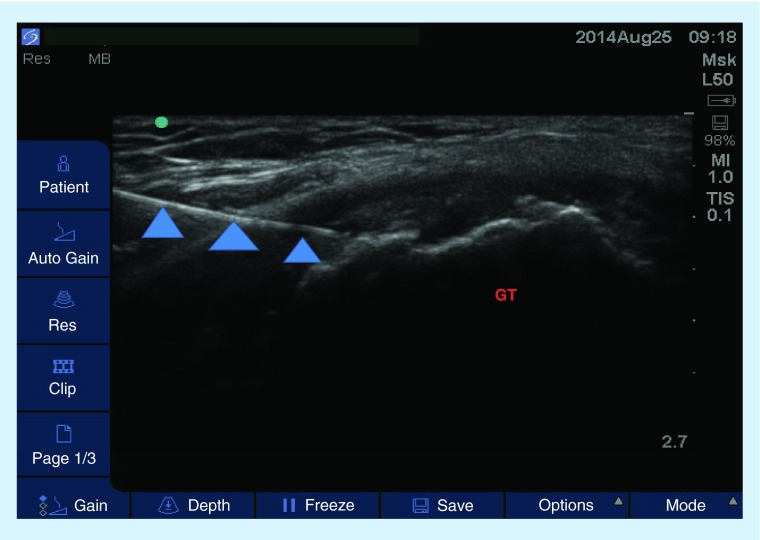
Figure 7. . Transverse view of a greater trochanteric bursa injection.
Image was obtained with a high-frequency linear probe and the patient was in a lateral recumbent position. Arrowheads represent the needle.
GT: Greater trochanter.
• Shoulder joint
The glenohumeral joint (GHJ) is the multiaxial ball and socket joint of the shoulder and is made up of the humeral head and the glenoid cavity of the scapula [6,44,50]. This joint has a wide range of motion and is inherently unstable because only a third of the humeral head lies in the glenoid cavity [44,50]. The remainder of the joint is supported by the supraspinatus, infraspinatus, and teres minor, and subscapularis muscles [50]. Multiple tendons and muscles play a role in supporting the shoulder joint, making shoulder pain a difficult condition to diagnose and treat [50]. Conditions that can cause chronic shoulder pain include, but are not limited to: rotator cuff syndromes, adhesive capsulitis, shoulder instability, arthritis, bursitis, tendonitis, impingement syndromes, avascular necrosis, trauma, and degenerative joint disease [44,50].
The GHJ can be injected from an anterior or posterior approach. While the approaches do not differ in terms of efficacy, the posterior approach is preferred because the needle does not traverse the structures that stabilize the shoulder joint in order to reach the target. To inject from a posterior approach, the US probe is placed on the shoulder in a coronal plane, visualizing the posteromedial aspect of the humeral head and the posterolateral portion of the glenoid cavity. The ipsilateral hand should be on the contralateral shoulder. The needle is advanced in-plane until the joint capsule is punctured (Figure 8) [48].
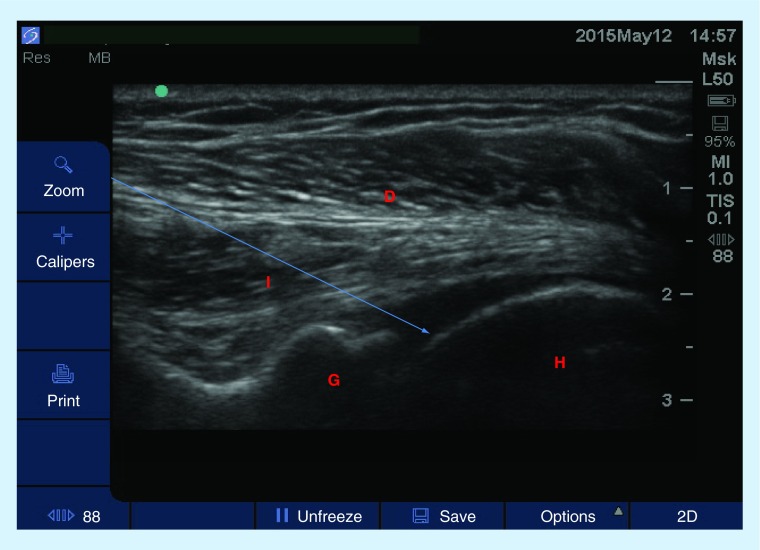
Figure 8. . Transverse view of the glenohumeral joint with a high-frequency linear probe.
Patient is in a seated position, ipsilateral arm placed on contralateral waist. Arrow represents the projected path of needle.
D: Deltoid muscle; I: Infraspinatus muscle; G: Glenoid; H: Humerus.
Though some studies have suggested that shoulder injections are only as effective as systemic steroid injections [51–53], US-guided injections are more accurate than blind injections (30 vs up to 90%), depending on approach. US-guided shoulder injections appear to have a higher percentage of success, more effective analgesia and better functional recovery 6 weeks after injection [54,55].
• Acromioclavicular joint
The acromioclavicular (AC) joint is a synovial, diarthrodial joint between the lateral end of the clavicle and the medial aspect of the acromion. AC joint injections are used to treat osteoarthritis or osteolysis of the distal clavicle. In this superficial area, success rate as low as 40% have been demonstrated with blinded approaches while FS yields accuracy rates near 100% [44]. For this injection, a high-frequency probe is placed over the joint and the capsule, acromium and distal clavicle are identified (Figure 9). Then, the needle is passed in plane until the joint capsule is pierced and medication is injected [44].
Figure 9. . Transverse sonogram of the acromioclavicular joint using a high-frequency linear probe.
The patient was in a seated position, the ipsilateral hand was placed on the contralateral shoulder. Arrow represents the projected path of needle. The arch represents the joint capsule.
A: Acromion; C: Clavicle; S: Joint space.
• Sacroiliac joint injections
The sacroiliac (SI) joints are paired joints located at the junction of the sacrum and ilium [56]. The majority of the SI joint space is fibro cartilaginous with a small synovial portion in the posterior and inferior most portion of the joint space where it typically lays obliquely, 30 or so degrees from the sagittal plane [56,57]. SI joint pain is located in the lower back or mid buttocks area and may radiate down the posterior aspect of the thigh to the knee or calf region [56]. The etiology of SI joint pain is typically unknown but in some cases can be attributed to Reiter's syndrome, ankylosing spondylitis or inflammatory bowel disease [56]. Clinically it can be very difficult to distinguish SI joint pain from facet or myofascial lumbar spine pain [56]. In most cases, diagnostic injections of local anesthetics and corticosteroids can assist the provider.
Current standard is to perform SI joint injections fluoroscopically due to its complex anatomy and the low accuracy (less than 20%) of blind injections [57]. The SIJs are easily visualized sonographically and the injection is typically performed with the patient in prone position. A curvilinear probe is placed over the mid to lower portion of the sacrum at the sacral hiatus. The joint space is tracked caudally to identify the most inferior edge. An in-plane or out-of-plane technique can be used to complete the injection [5].
Feasibility studies have demonstrated success rates ranging from 40 to 90% for US-guided SI joint injections which are comparable but not superior to FS guidance [57]. Periarticular SI joint injections are equally efficacious when compared with intra-articular injections as demonstrated by Hartung et al. [57]. They were able to show that despite placing the needle intra-articularly <50% of the time, there were no significant differences in clinical outcome in their cohort. Similarly, Jee et al. [58] published a prospective, randomized single-blinded study that evaluated the efficacy of US- versus FS-guided intra-articular injections into the SI joint over a period of 12 weeks in 120 patients. In the US group, intra-articular needle placement was successful in 87.3% of patients as compared with 98.2% in the FS group [58]. Both groups demonstrated significant reduction in pain scores and decrease in disability as measured by the Oswestry disability index. There was also no significant difference between the US-guided and FS groups in terms of pain relief experienced by patients and overall satisfaction [58]. Particular utility may be associated with use of US for SI joint injections in children [59].
Peripheral nerve & other injections
• Stellate ganglion block
The stellate ganglion is formed by the fusion of the inferior cervical and superior thoracic sympathetic ganglia. It supplies sympathetic innervation to the upper extremities and part of the head and neck. Stellate ganglion blocks (SGB) have been performed for multiple indications including complex regional pain syndrome of the upper extremity, phantom limb pain, cancer pain, herpetic neuralgia and orofacial pain. Landmark-based and FS techniques have been limited by inefficacy as well as increased risk of soft tissue injury [60].
The stellate ganglion is classically described as located at the C7–T1 level of the neck. In blind or FS techniques, the needle is advanced to Chassaignac's tubercle (transverse process of C6), withdrawn and directed inferomedially to the body of C6; medication is injected after the needle is withdrawn 1–2 mm [60]. This allows relative safety from vertebral artery injury. Alternately, anesthetic can spread to the vagus nerve or carotid baroreceptors, leading to hemodynamic compromise. Injury to the esophagus, thyroid gland, thyroidal arteries and vertebral artery is a legitimate risk with both techniques [61,62]. US-guided SGB uses a medial or lateral approach [63]. The medial technique increases the chance of trauma to the esophagus and thyroidal arteries; therefore, we prefer a lateral in-plane approach which allows the needle to pass lateral and posterior to the carotid artery and anterior to Chassaignac's tubercle (Figure 10). The injectate is directed between the prevertebral fascia and the longus colli muscle [64]. Four milliliters of injectate may be adequate when directed with US [65].
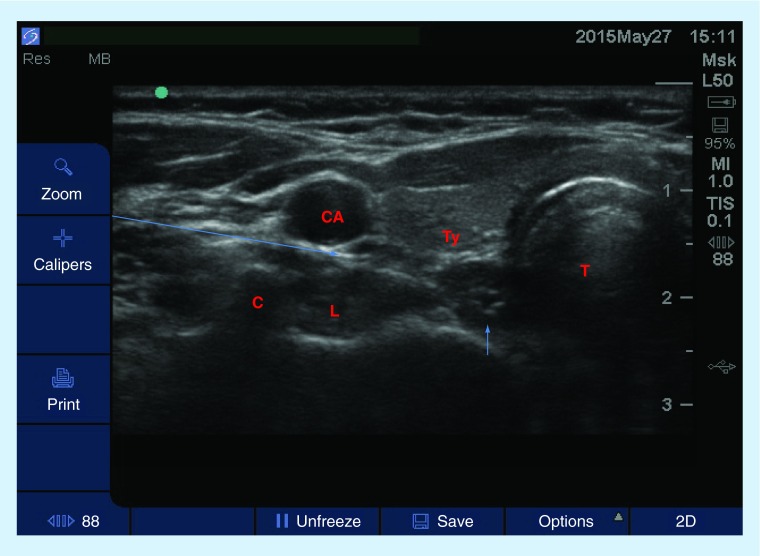
Figure 10. . Transverse (short axis) view of the neck for the performance of a stellate ganglion block.
A high-frequency linear probe was used to obtain this image and the patient was in a supine position, neck extended. Thearrow represents the projected path of needle. The up arrow represents the esophagus.
C: Chassignac's tubercle; CA: Carotid artery; L: longus colli mx; T: Trachea, Ty: Thyroid.
US-guided SGBs are more accurate (as determined by resultant Horner's syndrome) as compared with the landmark technique (100 vs 80–90%) [63]. Sonography is associated with fewer complications such as organ injury or hematoma [61]. In the original description of US-guided SGB, Kapral [66] reported no hematomas in the US group, while three of 12 of the landmark technique patients had a hematoma.
• Genitofemoral, Ilioinguinal, Iliohypogastric nerves
The ilioinguinal (IL) and iliohypogastric (IH) nerves emerge from the lumbar plexus and course laterally out of the quadratus lumborum and above the anterior superior iliac spine (ASIS). From there, they run adjacent and parallel to each other, going from deep inguinal canal to the superficial inguinal canal in the fascial plane between the internal oblique and the transversus abdominis [64]. They innervate the lateral lower abdominal wall. The genitofemoral (GF) nerve travels along or within the spermatic cord in males, and along the inguinal canal and the round ligament in females [67]. The IL, IH and GF nerves are routinely blocked for postorchiectomy pain or chronic pain related to urethral sling, appendectomy, nephrectomy and ureterectomy [64,68]. Injections of these nerves may be diagnostic or therapeutic. Differentiating which nerve is the cause of the pain is difficult because of spread of the injectate and close relationship of the nerves [68,69].
To perform the IH and IL block, the patient is placed supine and a high-frequency linear probe is placed perpendicular to the inguinal ligament, lateral to the ASIS. The abdominal muscle layers are identified under US after which the nerve is identified in the fascial plane between the internal oblique and transversus abdominus muscles [64]. The IL and IH nerves are usually found in close association and within 1.5 cm of the iliac crest but sometimes a stimulator needle is used with paresthesia in the groin as an endpoint to increase accuracy [64,67,69].
There is some controversy of the effectiveness of the IL and IH blocks for postherniorrhaphy pain. A double blinded, randomized, crossover trial showed no efficacy when compared with placebo [70]. The authors hypothesized that the GF nerve may have a greater role in lower abdominal and pelvic pain than realized [70]. The GF nerve block is easy to perform in males under US visualization. The probe should be placed 3 cm lateral to the pubic symphysis, perpendicular to the inguinal ligament with a long axis view of the femoral artery. From there the artery is traced cephalad until the spermatic cord is visualized in cross-section. Note is made to avoid the testicular artery as the medication is delivered within the spermatic cord and in the inguinal canal outside of the spermatic cord [64]. Epinephrine should never be used in this block due to its effects on the testicular artery [67]. In women, injection of medication within the inguinal canal around the round ligament is sufficient [67].
• Suprascapular nerve
The suprascapular nerve (SSN) is a branch of the superior trunk of the brachial plexus. It emerges through the suprascapular notch and lies in the basin of the suprascapular fossa, before entering the infrascapular fossa through the spinoglenoid notch. The SSN innervates 70% of the shoulder joint and has been blocked with good success for patients with acute and chronic shoulder pain related to arthritis, bursitis, trauma and tendonitis [71]. Multiple techniques have been used, including anatomic or landmark, FS, CT-guidance, EMG or some combination of the above [6].
A high-frequency linear probe is placed above and parallel to the scapular spine in an almost coronal plane with the coracoid process lateral to the probe and the US beam directed anterocaudally. The trapezius and suprascapular muscle layers should be visualized and the needle passed in-plane from medial to lateral. The suprascapular notch is not a reliable landmark to approach the nerve because it is missing in up to 8% of cadavers. As such, the suprascapular fossa has been proposed as the most reliable location to inject medication [6]. In cadaver studies, this technique resulted in successful spread of dye to the nerve in 19 of 20 injections [3]. The US-guided SSN block is effective in achieving longer lasting analgesia compared with a landmark-based approach. There have been no reported complications with the US technique, though the anatomic technique has had case reports of complications [3].
• Lateral femoral cutaneous nerve
The lateral femoral cutaneous nerve (LFCN) is a small nerve branch from the lumbar 2 and 3 nerves that supplies sensory innervation for the anterolateral portion of the thigh [71,72]. Pain in this nerve distribution is called ‘meralgia paresthetica’ [72]. The nerve emerges from the psoas, travels laterally and caudally along the iliacus and emerges from under the inguinal ligament medial to the ASIS between the fascia lata and fascia iliaca [64]. There is considerable variability in its course and it may travel superficial or deep to the inguinal ligament. Below the inguinal ligament, it can be found superficial to the sartorious muscle but in 22% of cases may travel within or lateral to the sartorius muscle [64].
Chronic pain associated with LFCN is common in obese and pregnant patients whose abdominal tissue folds over the inguinal canal and awkwardly compresses the small nerve as it passes under and through the inguinal canal [73]. Symptoms include numbness, paresthesia and aching pain in the distribution. Landmark techniques to treat this syndrome have poor success rates, while stimulation-guided injections have had improved success but are associated with significant discomfort to the patient [64].
Using a linear probe, it is possible to see the LFCN and deposit medication adjacent to it. Starting with the lateral edge of the probe on the ASIS and the probe oriented parallel to the inguinal ligament, the nerve can be seen in cross section as an oval between the fascia lata and fascia iliaca. If not visualized at this level, the operator may scan below the inguinal ligament where it can be found in the plane above the sartorious muscle. An in plane or out of plane may be used to place the injectate adjacent to the nerve [64,72].
Here US is clearly superior to landmark-based techniques. In a cadaver study landmark techniques had 0–15% accuracy rate as compared with >80% accuracy rate with US [74]. These results were confirmed in a volunteer study where a nerve stimulator was used [3]. In a series of 20 patients with meralgia paresthetica, all had resolution of their symptoms within 2 weeks of injection; however, four patients required repeat injections at 1 week [73]
• Other
US-guidance has also been described for injections of the ganglion of impar [75], pudendal nerves [76] and abdominal cutaneous nerves [77]. These studies demonstrated feasibility of the methods rather than superiority of the technique. In contrast, a meta-analysis by Dubreuil et al. [78] of ten studies described greater pain relief from US-guided wrist injections when compared with palpation-guided injections. Given the advantages of US-imaging in examining soft tissues, one might expect utility in association with the painful myofascial bands. Benefits have been reported [79,80] but there is ongoing debate as to the actual image characteristics to be targeted and the precise treatment modalities to be employed (injections vs ‘dry needling’).
Conclusion
Using US to perform interventional procedures for patients with chronic pain is in its initial stages. Over the past decade, pain practitioners have continued to fine-tune their practice and commonly incorporate US-guidance into the care of their patients. A significant barrier to widespread use of US is the paucity of RCTs to authenticate the higher efficacy of this modality as compared with traditional imaging techniques. Due to the benefit of using US to identify vascular structures it seems logical that it may have a unique role in cervical spine procedures such as SGBs. To our knowledge, there are no limitations on US use thus proceduralists should consider including dual modality imaging methods when performing higher risk procedures. As there is a significant learning curve associated with US-guided procedures, proper training is crucial to safe and successful practice.
• Future perspective
Rapid technological advances in ultrasonography have led to the creation of smaller and more portable high-resolution devices. Due to advantages in delineating anatomy and lack of ionizing radiation, US guidance for interventional pain procedures will undoubtedly increase in popularity. Coupled with other imaging modalities, it will also improve safety and accuracy of pain-related injections.
Footnotes
Financial & competing interests disclosure
TJ Ness receives support from NIH DK51413 and contract support from Medtronics, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Narouze SN. Ultrasound-guided interventional procedures in pain management. Reg. Anesth. Pain Med. 2010;35(2 Suppl.):s55–s58. doi: 10.1097/AAP.0b013e3181d24658. [DOI] [PubMed] [Google Scholar]
- 2.Narouze SN, Provenzano DA. Sonographically-guided cervical facet nerve and joint injections: why sonography? J. Ultrasound Med. 2013;32(11):1885–1896. doi: 10.7863/ultra.32.11.1885. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia A, Brull R. Is ultrasound guidance advantageous for interventional pain management? A systematic review of chronic pain outcomes. Anesth. Analg. 2013;117(1):236–251. doi: 10.1213/ANE.0b013e31828f5ee4. [DOI] [PubMed] [Google Scholar]; •• A comprehensive review of some of the more popular procedures and evidence that supports their performance under ultrasound (US; or not).
- 4.Peng PWH, Narouze S. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy, and procedures. Part I: nonaxial structures. Reg. Anesth. Pain Med. 2009;34(5):458–474. doi: 10.1097/AAP.0b013e3181aea16f. [DOI] [PubMed] [Google Scholar]; • Reviews nonaxial US-guided procedures.
- 5.Narouze SN, Peng PWH. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy, and procedures. Part II: axial structures. Reg. Anesth. Pain Med. 2010;35(4):386–396. doi: 10.1097/aap.0b013e3181e82f42. [DOI] [PubMed] [Google Scholar]
- 6.Peng PWH, Cheng P. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy, and procedures. Part III: shoulder. Reg. Anesth. Pain Med. 2011;36(6):592–605. doi: 10.1097/AAP.0b013e318231e068. [DOI] [PubMed] [Google Scholar]; • Comprehensive review of anatomy of this joint and description of various injections.
- 7.Peng PWH. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy and procedures. Part IV: hip. Reg. Anesth. Pain Med. 2013;38(4):264–273. doi: 10.1097/AAP.0b013e318291c8ed. [DOI] [PubMed] [Google Scholar]
- 8.Peng PWH, Shankar H. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy and procedures. Part V: knee Joint. Reg. Anesth. Pain Med. 2014;39(5):368–380. doi: 10.1097/AAP.0000000000000135. [DOI] [PubMed] [Google Scholar]; • Comprehensive review of anatomy of knee joint, the US examination of the joint as well as evidence or US-guided injection and how to perform US-guided injections.
- 9.Bookman JS, Pereira DS. Ultrasound guidance for intra-articular knee and shoulder injections: a review. Bull. Hosp. Joint Dis. 2014;72(4):266–270. [PubMed] [Google Scholar]
- 10.Huang Z, Du S, Qi Y, Chen G, Yan W. Effectiveness of ultrasound guidance on intra-articular and peri-articular joint injections. Am. J. Phys. Med. Rehab. 2015 doi: 10.1097/PHM.0000000000000260. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Sites BD, MacFarlane AJ, Sites VR, et al. Clinical sonopathology for the regional anesthesiologist. Part I: vascular and neural. Reg. Anesth. Pain Med. 2010;35(3):272–280. doi: 10.1097/AAP.0b013e3181ddd1f8. [DOI] [PubMed] [Google Scholar]; •• This is a good review of basic US physics for the interventionalist.
- 12.Sites BD, MacFarlane AJ, Sites VR, et al. Clinical sonopathology for the regional anesthesiologist. Part II: bone, viscera, subcutaneous tissue and foreign bodies. Reg. Anesth. Pain Med. 2010;35(3):281–299. doi: 10.1097/AAP.0b013e3181ddd21f. [DOI] [PubMed] [Google Scholar]
- 13.Brull R, Macfarlane AJ, Tse CC. Practical knobology for ultrasound-guided regional anesthesia. Reg. Anesth. Pain Med. 2010;35(2 Suppl.):S68–S73. doi: 10.1097/AAP.0b013e3181d245f9. [DOI] [PubMed] [Google Scholar]; • Excellent review of US physics and knobology. A good how-to for the beginner.
- 14.Terkawi AS, Karakitsos D, Elbarbary M, Blaivas M, Durieux ME. Ultrasound for the anesthesiologists: present and future. Sci. World J. 2013;2013:683685. doi: 10.1155/2013/683685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD006962.pub2. CD006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian and femoral vein catheterization. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD006962.pub2. CD011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathmell JP. Facet injection: intra-articular injection, medial branch block, and radiofrequency treatment. In: Rathmell JP, editor. Atlas of Image-Guided Spine Intervention in Regional Anesthesia and Pain Medicine. Lippincott, Williams, and Wilkins; Philadelphia, PA, USA: 2006. pp. 65–92. [Google Scholar]
- 18.Stuluc SM, Hurdle MJ, Brault JS, Porter CA. Ultrasound-guided thoracic facet injections: description of a technique. J. Ultrasound Med. 2011;30(3):357–362. doi: 10.7863/jum.2011.30.3.357. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SP, Moon JY, Brummett CM, White RL, Larkin TM. Medial branch blocks or intra-articular injections as a prognostic tool before lumbar facet radiofrequency denervation: a multicenter, case–control study. Reg. Anesth. Pain Med. 2015;40(4):376–383. doi: 10.1097/AAP.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 20.Galiano K, Obwegeser AA, Bodner G, et al. Ultrasound-guided facet joint injections in the middle to lower cervical spine: a CT controlled sonoanatomic study. Clin. J. Pain. 2006;22(6):538–543. doi: 10.1097/01.ajp.0000202977.98420.27. [DOI] [PubMed] [Google Scholar]
- 21.Finlayson RJ, Gupta G, Alhujairi M, Dugani S, Tran DQ. Cervical medial branch block: a novel technique using ultrasound guidance. Reg. Anesth. Pain Med. 2012;37(2):219–223. doi: 10.1097/AAP.0b013e3182374e24. [DOI] [PubMed] [Google Scholar]
- 22.Obernauer J, Galliano K, Gruber H, et al. Ultrasound-guided versus computed tomography-controlled facet joint injections in the middle and lower cervical spine: a prospective randomized clinical trial. Med. Ultrason. 2013;15(1):10–15. doi: 10.11152/mu.2013.2066.151.jo1ugc2. [DOI] [PubMed] [Google Scholar]; • One of the few randomized controlled trials on this subject. This one compared computed tomography versus US guidance in the performance of cervical facet injections.
- 23.Shim JK, Moon JC, Yoon KB, Kim WO, Yoon DM. Ultrasound-guided lumbar medial-branch block: a clinical study with fluoroscopy control. Reg. Anesth. Pain Med. 2006;31(5):451–454. doi: 10.1016/j.rapm.2006.06.246. [DOI] [PubMed] [Google Scholar]
- 24.Gofeld M, Bristow SJ, Chiu S. ultrasound-guided injection of lumbar zygapophyseal joints: an anatomic study with fluoroscopic validation. Reg. Anesth. Pain Med. 2012;37(2):228–231. doi: 10.1097/AAP.0b013e3182461144. [DOI] [PubMed] [Google Scholar]
- 25.Greher M, Sharbert G, Kamolz LP, et al. Ultrasound-guided lumbar facet nerve block: a sonoanatomic study of a new methodologic approach. Anesthesiology. 2004;100(5):1242–1248. doi: 10.1097/00000542-200405000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Jung H, Jeon S, Ahn S, Kim M, Choi Y. The validation of ultrasound-guided lumbar facet nerve blocks as confirmed by fluoroscopy. Asian Spine J. 2012;6(3):163–167. doi: 10.4184/asj.2012.6.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauch S, Kasuya Y, Turan A, Neamtu A, Vinayakan A, Sessler DI. Ultrasound-guided lumbar medial branch block in obese patients: a fluoroscopically confirmed clinical feasibility study. Reg. Anesth. Pain Med. 2009;34(4):340–342. doi: 10.1097/aap.0b013e3181ada563. [DOI] [PubMed] [Google Scholar]
- 28.Rathmell JP. Epidural steroid injection. In: Rathmell JP, editor. Atlas of Image-Guided Spine Intervention in Regional Anesthesia and Pain Medicine. Lippincott, Williams, and Wilkins; Philadelphia, PA, USA: 2006. pp. 53–64. [Google Scholar]
- 29.Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can. J. Anaesth. 2009;56(9):643–650. doi: 10.1007/s12630-009-9132-8. [DOI] [PubMed] [Google Scholar]
- 30.Perlas A. Evidence for the use of ultrasound in neuraxial blocks. Reg. Anesth. Pain Med. 2010;35(2 Suppl.):s43–s46. doi: 10.1097/AAP.0b013e3181d2462e. [DOI] [PubMed] [Google Scholar]; • This article discusses utility (or lack thereof) of US in neuraxial procedures.
- 31.Narouze SN, Bydyanathan A, Kapral L, Sessler DI, Mekhail N. Ultrasound-guided cervical selective nerve root block: a fluoroscopy-controlled feasibility study. Reg. Anesth. Pain Med. 2009;34(4):342–348. doi: 10.1097/AAP.0b013e3181ac7e5c. [DOI] [PubMed] [Google Scholar]
- 32.Narouze SN. Ultrasound-guided cervical spine injections: ultrasound ‘prevents’ whereas contrast fluoroscopy ‘detects’ intravascular injections. Reg. Anesth. Pain Med. 2012;37(2):127–130. doi: 10.1097/AAP.0b013e31823f3c80. [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Ahn JK, Sohn Y, et al. Treatment effects of ultrasound-guided selective nerve root block for lower cervical radicular pain: a retrospective study of 1-year follow-up. Ann. Rehabil. Med. 2013;37(5):658–667. doi: 10.5535/arm.2013.37.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jee H, Lee JH, Kim J, Park KD, Lee WY, Park Y. Ultrasound-guided selective nerve root block versus fluoroscopy-guided transforaminal block for the treatment of radicular pain in the lower cervical spine: a randomized, blinded, controlled study. Skeletal Radiol. 2013;42(1):69–78. doi: 10.1007/s00256-012-1434-1. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Choi D, Kim C, Kim J, Choi Y. Transverse process and needles of medial branch block to facet joint as landmarks of ultrasound-guided selective nerve root block. Clin. Orthop. Surg. 2013;5(1):44–48. doi: 10.4055/cios.2013.5.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gofeld M, Bristow SJ, Chiu SC, McQueen CK, Bollag L. Ultrasound-guided lumbar transforaminal injections. Spine (Phila. Pa 1976) 2012;37(9):808–812. doi: 10.1097/BRS.0b013e3182340096. [DOI] [PubMed] [Google Scholar]
- 37.Loizides A, Gruber H, Peer S, Galiano K, Bale R, Obernauer J. ultrasound-guided versus CT-controlled pararadicular injections in the lumbar spine: a prospective randomized clinical trial. AJNR Am. J. Neuroradiol. 2013;34(2):466–470. doi: 10.3174/ajnr.A3206. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Well-designed randomized controlled trial that evaluated US vs computed tomography for nerve root injections in the lumbar spine.
- 38.Loizides A, Peer S, Plaikner M, et al. Ultrasound-guided injections in the lumbar spine. Med. Ultrason. 2011;13(1):54–58. [PubMed] [Google Scholar]
- 39.Yang G, Liu J, Ma L, et al. Ultrasound-guided versus fluoroscopy-controlled lumbar transforaminal epidural injections: a prospective randomized clinical trial. Clin. J. Pain. 2015 doi: 10.1097/AJP.0000000000000237. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Chen CPC, Lew HL, Tsai WC, Hung YT, Hsu CC. Ultrasound-guided injection techniques for the low back and hip joint. Am. J. Phys. Med. Rehabil. 2011;90(10):860–867. doi: 10.1097/PHM.0b013e318228c084. [DOI] [PubMed] [Google Scholar]
- 41.Blanchais A, LeGoff B, Guillot P, Berthelot JM, Glemarec J, Maugars Y. Feasibility and safety of ultrasound-guided caudal epidural glucocorticoid injections. Joint Bone Spine. 2010;77(5):440–444. doi: 10.1016/j.jbspin.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Klocke R, Jenkinson T, Glew D. Sonographically guided caudal epidural steroid injections. J. Ultrasound Med. 2003;22(11):1229–1232. doi: 10.7863/jum.2003.22.11.1229. [DOI] [PubMed] [Google Scholar]
- 43.Park Y, Lee J-H, Park KD, Ahn JK, Park J, Jee H. Ultrasound-guided vs. fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single blind study. Am. J. Phys. Med. Rehabil. 2013;92(7):575–586. doi: 10.1097/PHM.0b013e318292356b. [DOI] [PubMed] [Google Scholar]
- 44.Narouze S, Raju SVY. Joint injections. In: Benzon HT, Raja SN, Liu SS, Fishman SM, Cohen SP, editors. Essentials of Pain Medicine (3rd Edition) Elsevier; Philadelphia, PA, USA: 2011. pp. 423–431. [Google Scholar]
- 45.Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin. Interv. Aging. 2012;7:89–95. doi: 10.2147/CIA.S29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sibbitt WL, Kettwich LG, Band PA, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand. J. Rheumatol. 2012;41(1):66–72. doi: 10.3109/03009742.2011.599071. [DOI] [PubMed] [Google Scholar]
- 47.Rowbotham EL, Grainger AJ. Ultrasound-guided intervention around the hip joint. AJR Am. J. Roentgenol. 2011;197(1):W122–127. doi: 10.2214/AJR.10.6344. [DOI] [PubMed] [Google Scholar]
- 48.Collins JM, Smithuis R, Rutten MJ. US-guided injection of the upper and lower extremity joints. Eur. J. Radiol. 2012;81(10):2759–2770. doi: 10.1016/j.ejrad.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 49.Byrd JW, Potts EA, Allison RJ, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42–46. doi: 10.1016/j.arthro.2013.09.083. [DOI] [PubMed] [Google Scholar]
- 50.Cheng P, Modir JG, Kim HJ, Narouze S. Ultrasound-guided shoulder joint injections. Techniques Reg. Anesth. Pain Management. 2009;13:184–190. [Google Scholar]
- 51.Ekeberg OM, Bautz-Holter E, Tveita EK, Juel NG, Kvalheim S, Brox JI. Subacromial ultrasound-guided or systemic steroid injection for rotator cuff disease: randomized double blind study. BMJ. 2009;338:a3112. doi: 10.1136/bmj.a3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bloom JE, Rischin A, Johnston RV, Buchbinder R. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858.CD009147.pub2. CD009147. [DOI] [PubMed] [Google Scholar]
- 53.Sage W, Pickup L, Smith TO, Denton ER, Toms AP. The clinical and functional outcomes of ultrasound-guided vs landmark-guided injections for adults with shoulder pathology – a systematic review and meta-analysis. Rheumatology (Oxford) 2013;52(4):743–751. doi: 10.1093/rheumatology/kes302. [DOI] [PubMed] [Google Scholar]
- 54.Zuffery P, Revaz S, Degailler X, Balague F, So A. A controlled trial of the benefits of ultrasound-guided steroid injection for shoulder pain. Joint Bone Spine. 2012;79(2):166–169. doi: 10.1016/j.jbspin.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Soh E, Li W, Ong KO, Chen W, Bautista D. Image-guided versus blind corticosteroid injections in adults with shoulder pain, a systematic review. BMC Musculoskeletal Disord. 2011;12:137. doi: 10.1186/1471-2474-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rathmell JP. Sacroiliac joint injection. In: Rathmell JP, editor. Atlas of Image-Guided Spine Intervention in Regional Anesthesia and Pain Medicine. Lippincott, Williams, and Wilkins; Philadelphia, PA, USA: 2006. pp. 93–100. [Google Scholar]
- 57.Hartung W, Ross CJ, Straub R, et al. Ultrasound-guided sacroiliac joint injection in patients with established sacroilitis: precise IA injection verified by MRI scanning does not predict clinical outcome. Rheumatology (Oxford) 2010;49(8):1479–1482. doi: 10.1093/rheumatology/kep424. [DOI] [PubMed] [Google Scholar]
- 58.Jee H, Lee JH, Park KD, Ahn J, Park Y. Ultrasound-guided versus fluoroscopic-guided sacroiliac joint intra-articular injections in the non-inflammatory sacroiliac joint dysfunction: a prospective, randomized, single-blinded study. Arch. Phys. Med. Rehabil. 2014;95(2):330–337. doi: 10.1016/j.apmr.2013.09.021. [DOI] [PubMed] [Google Scholar]; •• This study showed that intra-articular and periarticular sacroiliac joint injections using US had similar efficacy.
- 59.Klauser AS, Sailer-Hoeck M, Abdellah MM, et al. Feasibility of ultrasound-guided sacroiliac joint injections in children presenting with sacroilitis. Ultraschall. Med. 2015 doi: 10.1055/s-0034-1399145. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Gofeld M, Bhatia A, Abbas S, Ganapathy S, Johnson M. Development and validation of a new technique for ultrasound-guided stellate ganglion block. Reg. Anesth. Pain Med. 2009;34(5):475–479. doi: 10.1097/AAP.0b013e3181b494de. [DOI] [PubMed] [Google Scholar]
- 61.Narouze S, Vydyanathan A, Patel N. ultrasound-guided stellate ganglion block successfully prevented esophageal puncture. Pain Physician. 2007;10(6):747–752. [PubMed] [Google Scholar]
- 62.Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr. Pain Headache Rep. 2014;18(6):434. doi: 10.1007/s11916-014-0424-5. [DOI] [PubMed] [Google Scholar]; •• This article describes technique for and evidence supporting use of ultrasound for performance of stellate ganglion blocks.
- 63.Bhatia A, Flanner D, Pend PWH. Evaluation of sonoanatomy relevant to performing stellate ganglion blocks using anterior and lateral simulated approaches: an observational study. Can. J. Anesth. 2012;59(11):1040–1047. doi: 10.1007/s12630-012-9779-4. [DOI] [PubMed] [Google Scholar]
- 64.Soneji N, Peng PWH. Ultrasound-guided pain interventions – a review of techniques for peripheral nerves. Korean J. Pain. 2013;26(2):111–124. doi: 10.3344/kjp.2013.26.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung G, Kim BS, Shin KB, Park KB, Kim SY, Song SO. The optimal volume of 0.2% ropivacaine required for ultrasound-guided stellate ganglion block. Korean J. Anesth. 2011;60(3):179–184. doi: 10.4097/kjae.2011.60.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kapral S, Kraft P, Gosch M, Fleischmann D, Weinstabl C. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. A pilot study. Reg. Anesth. 1995;20(4):323–328. [PubMed] [Google Scholar]
- 67.Peng PWH, Tumber PS. Ultrasound-guided interventional procedures for patients with chronic pelvic pain – a description of techniques and review of literature. Pain Physician. 2008;11(2):215–224. [PubMed] [Google Scholar]
- 68.Shanthana H. Successful treatment of genitofemoral neuralgia using ultrasound-guided injection: a case report and short literature review. Case Rep. Anesthiol. 2014:371703. doi: 10.1155/2014/371703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmutz M, Schumacher PM, Luyet C, Curatolo M, Eichenberger U. Ilioinguinal and iliohypogastric nerves cannot be selectively blocked using ultrasound guidance: a volunteer study. Br. J. Anaesth. 2013;111(2):264–270. doi: 10.1093/bja/aet028. [DOI] [PubMed] [Google Scholar]
- 70.Bischoff JM, Koscielniak-Nielsen ZJ, Kehlet H, Werner MU. ultrasound-guided ilioinguinal/iliohypogastric nerve blocks for persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled, crossover trial. Anesth. Analg. 2012;114(6):1323–1329. doi: 10.1213/ANE.0b013e31824d6168. [DOI] [PubMed] [Google Scholar]
- 71.Harmon D, Hearty C. Ultrasound-guided suprascapular nerve block technique. Pain Physician. 2007;10(6):743–746. [PubMed] [Google Scholar]
- 72.Kim JE, Lee SG, Kim EJ, Min BW, Ban JS, Lee JH. Ultrasound-guided lateral femoral cutaneous nerve block in meralgia paresthetica. Korean J. Pain. 2011;24(2):115–118. doi: 10.3344/kjp.2011.24.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tagliafico A, Serafini G, Lacelli F, Perrone N, Valsania V, Martinoli C. Ultrasound-guided treatment of meralgia paresthetica (lateral femoral cutaneous neuropathy): technical description and results of treatment in 20 consecutive patients. J. Ultrasound Med. 2011;30(10):1341–1346. doi: 10.7863/jum.2011.30.10.1341. [DOI] [PubMed] [Google Scholar]
- 74.Ng I, Vaghadia H, Choi PT, Helmy N. ultrasound imaging accurately identifies the lateral femoral cutaneous nerve. Anesth. Analg. 2008;107(3):1070–1074. doi: 10.1213/ane.0b013e31817ef1e5. [DOI] [PubMed] [Google Scholar]
- 75.Lin CS, Cheng JK, Hsu YW, et al. Ultrasound-guided ganglion impar block: a technical report. Pain Med. 2010;11(3):390–394. doi: 10.1111/j.1526-4637.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 76.Bellingham GA, Bhatia A, Chan CW, Peng PW. Randomized controlled trial comparing pudendal nerve block under ultrasound and fluoroscopic guidance. Reg. Anesth. Pain Med. 2012;37(3):262–266. doi: 10.1097/AAP.0b013e318248c51d. [DOI] [PubMed] [Google Scholar]
- 77.Kankarajan S, High K, Nagaraja R. Chronic abdominal wall pain and ultrasound-guided abdominal cutaneous nerve infiltration: a case series. Pain Med. 2011;12(3):382–386. doi: 10.1111/j.1526-4637.2011.01056.x. [DOI] [PubMed] [Google Scholar]
- 78.Dubreuil M, Greger S, LaValley M, Cunnington J, Sibbitt WL, Jr, Kissin EY. Improvement in wrist pain with ultrasound-guided glucocorticoid injections: a meta-analysis of individual patient data. Semin. Arthritis Rheum. 2012;42(5):492–497. doi: 10.1016/j.semarthrit.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas K, Shankar H. Targeting myofascial taut bands by ultrasound. Curr. Pain Headache Rep. 2013;17(7):349. doi: 10.1007/s11916-013-0349-4. [DOI] [PubMed] [Google Scholar]
- 80.Bubnov RV. The use of trigger point ‘dry’ needling under ultrasound guidance for the treatment of myofascial pain (technological innovation and literature review) Lik. Sprava. 2010;5(6):56–64. [PubMed] [Google Scholar]