Abstract
Rucaparib camsylate (CO-338; 8-fluoro-2-{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one ((1S,4R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)methanesulfonic acid salt) is a PARP1, 2 and 3 inhibitor. Phase I studies identified a recommended Phase II dose of 600 mg orally twice daily. ARIEL2 Part 1 established a tumor genomic profiling test for homologous recombination loss of heterozygosity quantification using a next-generation sequencing companion diagnostic (CDx). Rucaparib received US FDA Breakthrough Therapy designation for treatment of platinum-sensitive BRCA-mutated advanced ovarian cancer patients who received greater than two lines of platinum-based therapy. Comparable to rucaparib development, other PARP inhibitors, such as olaparib, niraparib, veliparib and talazoparib, are developing CDx tests for targeted therapy. PARP inhibitor clinical trials and CDx assays are discussed in this review, as are potential PARP inhibitor combination therapies and likely resistance mechanisms.
KEYWORDS : CO-338, companion diagnostic, ovarian cancer, PARP inhibitor, rucaparib
Ovarian cancer is the eighth most common type of cancer in women, and the fifth leading cause of cancer-related deaths. A lack of effective early stage disease detection results in 70% of patients with metastatic disease (stage II–IV) at the time of diagnosis [1,2]. Ovarian cancer has three major groups: epithelial (90%), germ cell (5%) and sex cord stromal cell (5%). Epithelial ovarian cancer (EOC), which will be used to refer to high-grade serous epithelial ovarian carcinoma, fallopian tubal and primary peritoneal carcinoma, is highly heterogeneous. EOC subtypes include high- and low-grade serous (75–80%), mucinous (3%), endometrioid (10%) and clear cell (10%). Somatic genomic studies by The Cancer Genome Atlas (TCGA) classify EOC molecular and clinical profiles to influence potential future treatment paths [3].
Advanced ovarian cancer is chemosensitive to frontline platinum/taxane-based therapy [4–6]. New frontline therapies are under investigation, as are maintenance therapies, with a focus on anti-angiogenesis inhibitors, such as bevacizumab and pazopanib [7,8]. Despite these efforts, recurrence occurs frequently; advanced-stage (stage II–IV) patients relapse (70%) within 5 years [9], establishing a need for treatment of recurrent cancer. Patients exhibiting recurrence usually die from emergent chemoresistant disease complications; intensive investigation into new agents and strategies is ongoing. Recently, bevacizumab was US FDA approved with chemotherapy in the setting of platinum-resistant disease [10]. Coleman et al. provide additional information about the contemporary management and future directions of ovarian cancer treatment [4].
The purpose of this drug review is to provide perspective, in the background of current therapies, about the various poly(ADP-ribose) polymerase inhibitors (PARPis) under clinical development for recurrent ovarian cancer patients. PARPi mechanism of action, rationale as drug candidates, patient tumor genomic profiling, accompanying companion diagnostics, efficacy, toxicity profile and potential resistance mechanisms to PARPi therapy is of primary concern. Additional information about new and potential therapies aimed at improving the recurrence rate is discussed.
PARP inhibitor & companion diagnostic development
• PARP biology
PARP1, 2 and 3 are integral in the DNA damage response system by activating response pathways and facilitating repair [11,12]. Single-strand breaks (SSBs) in DNA, occurring at a rate of 104/day, are predominantly repaired by utilization of PARP1 in base excision repair (BER), which accounts for 90% of cellular PARP activity [13]. PARP1 also functions in nonhomologous end-joining (NHEJ) regulation, chromatin remodeling and homologous recombination (HR) DNA repair pathways [14,15].
• PARP as a therapeutic target
Initial evaluation of PARP as a novel anticancer therapeutic target first appeared in 2005 for BRCA1-mutated cancer cells in vitro [16]. BRCA2-mutated tumor growth in vivo was diminished by PARPis, indicating a promising therapeutic target [17]. BRCA1 participates in a variety of cellular processes, including DNA replication, transcription regulation, cell cycle checkpoints, apoptosis and chromatin structuring. BRCA1 and BRCA2 are involved in HR repair. BRCA2 loads RAD51, an HR repair protein, to DNA double-strand breaks, or lesions [18]. Importantly, defects in BRCA2 regulation of RAD51 may explain increased cancerous phenotypes observed in BRCA-mutated cells. Approximately 10–15% of breast and ovarian cancers are due to gBRCA1 and gBRCA2 mutations [19,20]. However, why gBRCA mutations have tissue-specific propensity to develop in ovarian and breast tissue is still unclear, but increased relative DNA damage and subsequent dependency on lower fidelity HR is a likely causative factor. In each ovarian cancer subtype, BRCA1-mutations (BRCA1mut) or BRCA2-mutations (BRCA2mut) are evident in the following percentages: serous (10.4%, 6.2%), clear cell (6.3%, 0.0%), endometrioid (5.9%, 2.5%), carcinosarcoma (4.5%, 0.0%) and other (2.7%, 5.5%) [21,22]. Studies concerned with PARP inhibition in gBRCA-mutated tumors revealed a synthetic lethality mechanism of action.
• PARP inhibition & synthetic lethality
Synthetic lethality exists when two nonlethal defects combine and result in cell death [23]. PARPis are synthetically lethal in HR-deficient (HRD) cells, such as BRCA1-mutated tumors, due to unsalvageable DNA damage [16]. Recently, PARP inhibition was shown to prevent poly(ADP-ribos)ylation-dependent BRCA1 recruitment to damaged DNA [24]. Upon PARP inhibition, SSBs are converted to DSBs at replication forks, and HRD cells fail to repair DSBs, resulting in apoptosis [25]. PARPis trap PARP1 and PARP2 on DNA, forming toxic PARP–DNA complexes, termed ‘PARP trapping’ [26]. Interestingly, PARP inhibition shows greater toxicity than PARP genetic deletion, further supporting the PARP trapping mechanism. Due to the utility of PARP inhibition in HR pathways, synthetic lethality may be exploitable in sporadic tumors with pathologic features similar to BRCA-deficient tumors, such as BRCA-like tumors with HR defects [27].
• Potential homologous recombination pathway targets
In an attempt to identify which HR defects may induce synthetic lethality, HR pathway modulators were subsequently explored. Homozygous deletion of PTEN in 7% of EOC is proposed to downregulate RAD51, resulting in synthetic lethality upon PARP inhibition [28,29]. Amplification of the 11q13 locus resulted in the overexpression of EMSY, a suppressor of BRCA2 transcriptional activity, in 14% of EOCs; however, EMSY is still controversial due to poor outcomes associated with EMSY overexpression [30]. PARP sensitization occurs when BRCA1 levels are reduced after CDK12 inactivation [31]. Targeting HR-associated genes with miRNA is also of interest [32]. Recognizing potential HRD manifestations is vital to identifying likely patient responders to PARPi therapy.
• PARPs & alternative end-joining
PARP1 activity in the alternative end-joining (alt-EJ) DSB repair pathway is of interest, primarily due to increased alt-EJ repair protein expression, such as the PARP1-mediator Polθ, when other HR pathways are deficient [33,34]. Polθ inhibition exhibits synthetic lethality in HRD tumors due to Polθ-dependent alt-EJ repair with PARP1 and also prevention of RAD51 assembly on ssDNA. Recently, the function of PARP1 and Polθ in the error-prone alt-EJ pathway may explain a global mechanism of PARPi sensitivity in BRCAwt tumors. Targeting Polθ in HRD tumors may enhance selective toxicity. Therefore, cells with active alt-EJ pathways may indicate HRD, with insertion or deletion (indel) DNA signatures as HRD biomarkers [35].
• PARP inhibition & genotoxic agents
PARPis are also useful in combination with genotoxic agents. Temozolomide (TMZ) alkylates purine bases, which can be removed by robust BER activity. PARP is central to BER through nick sensing and DNA strand separation via electrostatic repulsion of ADP-ribose polymers. Thus, PARP-deficient cells are sensitized to genotoxic stress, as was evident in a clinical study of TMZ potentiation by PARPis, where cytotoxic methyl-purine adducts accumulated [36]. PARP inhibition shows preferential activity for agents that disrupt DNA replication relative to transcriptional processes. The cytotoxic effect of 5-fluorouracil (5-FU) is thought to be through incorporation in RNA, whereas 5-fluorodeoxyuridine (FdUrd) is cytotoxic by DNA replication disruption. BER was shown to be important in FdUrd-treated cells, but not 5-FU. Interestingly, PARP inhibition sensitizes ovarian cancer cells to FdUrd, but not 5-FU, which is reflective of the importance of BER disruption [37].
• PARPs & cell cycle checkpoint control
A majority of EOCs are dependent on S and G2 checkpoints due to loss of p53 functional control at G1 [38]. Targeting the S/G2 checkpoints with WEE1 and CHK1 inhibitors, such as AZD1775 and GDC-0425, leads to cellular death. WEE1 and CHK1 inhibition ultimately blocks functional ATR protein kinase activity, and disrupts downstream phosphorylation of HR proteins. The DNA damage-induced G2 checkpoint arrest does not occur in cells with inhibited ATR–CHK1–WEE1 pathway, resulting in mitotic catastrophe. Monotherapy with AZD1775 in BRCAmut tumors showed clinical efficacy [39]. Synthetic lethality is observed in HRD EOC when therapy is targeted at the ATR–CHK1–WEE1 in addition to chemotherapy; investigation of PARPi activity in these tumors is an intriguing prospect. Overall, a rush to determine HRD sporadic tumors sensitive to PARP inhibition is on the forefront of therapeutic goals.
• Combining PARP inhibition with companion diagnostics
The greatest impending impact on treatment options can be elucidated by the utilization of companion diagnostic (CDx) techniques based on the power of whole-genome analysis. Approximately 50% of all high-grade serous ovarian tumors are deficient in the Fanconi anemia-BRCA pathway, which depends on HR, thereby indicating a need to further explore BRCA-like HRD genomic scar identification [21,29,40–41]. PALB2 and BARD1, which are both associated with the Fanconi anemia-BRCA pathway, were recently implicated as frequently mutated genes in hereditary EOC [22]. Genomic scarring results from HRD of a variety of origins, including mutations, deletions, loss of heterozygosity (LOH), miRNA and DNA methylation, and can be detected by next-generation sequencing (NGS). With accurate evaluation of the specific HRD in a tumor, sensitivity to PARP inhibition is predictable. Effective use of PARPis with CDx techniques may provide personalized treatment options.
• PARP resistance in homologous recombination deficient tumors
Mechanisms of resistance in HRD EOCs primarily involve indirect or direct restoration of HR. In BRCAmut tumors, restoration of BRCA function is one of the primary resistance mechanisms [42]. Back mutation, reading frame restoration, loss of BRCA promoter methylation, stabilization of the BRCA1 C-terminal (BRCT) domain of BRCA1 by HSP90 under PARPi selection, decreased expression of PARP1, expression of ABC transporters like the P-glycoprotein (P-gp) efflux pump and reacquisition of DNA end resection capabilities – that is, by loss of 53BP1, are all potential resistance mechanisms, noninclusive. The first four mechanisms mentioned above can restore BRCAwt function. Loss of PARP1 expression, whether by promoter hypermethylation or increased protein turnover, decreases PARP-trapping [43]. P-gp-mediated resistance is usually due to gene upregulation by promoter fusion, resulting in PARPi efflux [44]. In BRCAmut cells, 53BP1 prevents the replication protein A (RPA) phosphorylation-based DNA damage repair pathway from restoring ssDNA lesions. However, if 53BP1 is also nonfunctional, RPA can load onto DNA and permit repair, bypassing the need for functional BRCA [45]. Therefore, BRCAmut cells without 53BP1 expression are PARPi resistant, and capable of error-free repair. These recent discoveries elucidate potential PARPi resistance mechanisms clinically.
• Expanding PARP inhibitor utility
Approximately 50% of ovarian cancers are HRD, which limits PARPi therapy to 50% of patients. However, combination of agents that inhibit HR may expand the use of PARPis to de novo or acquired HR-proficient tumors. To address de novo HR proficiency and PARPi resistance, several preclinical and early clinical trials will evaluate PARPi combined with inhibitors of: CDK1 to prevent phosphorylation of BRCA1 [46]; VEGFR or AKT to mediate BRCA downregulation [47,48]; HSP90 to prevent BRCA1-mutant stabilization [32]; PgP to decrease PARPi efflux [49]; and HDAC to downregulate HR genes [50]. Expanding the treatable patient population has associated risk. Proof of mechanistic principle while monitoring adverse events (AEs) in early clinical studies is vital to developing successful combination therapies.
Overview of the market
Several PARPis are under ovarian cancer therapeutic development. These include: olaparib (AZD2281, Lynparza®, AstraZeneca); niraparib (MK4827, Tesaro); veliparib (ABT-888, Abbvie); talazoparib (BMN-673, Medivation) and rucaparib (CO-338, Clovis Oncology). Veliparib, olaparib, rucaparib and niraparib induce PARP-trapping primarily by catalytic inhibition, without an allosteric mechanism [51]. In common, these PARPis are oral formulations, potentiate DNA alkylating agents clinically [36], and, except for talazoparib, have ongoing randomized controlled trials for maintenance treatment. Partnerships exist between biopharmaceutical and biotechnological companies to develop CDx tests to identify PARPi-responsive patients. PARPi efficacy in Phase II open-label clinical trials is compared (Table 1).
Table 1. . Summary table of main differences between PARP inhibitors investigated in open-label Phase II studies in patients with advanced ovarian cancer.
| Drug | Company | Molecular target | Trial, dose and delivery | BRCA status in Phase II study (n) | PFS (95% CI); months | CR (%) | PR (%) | SD (%) | Dose reduced due to AE (%) | AEs (all grades, ≥40% prevalence) | AEs (grade 3/4, ≥5% prevalence) | Regulatory status for EOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rucaparib (CO-338) |
Clovis Oncology |
PARP1–4, 10, 12, 15, 16 and TNKS1/2 |
NCT01891344, 600 mg b.i.d. (oral) |
BRCAmut, 40 BRCA-like, 82 Biomarker(-), 70 |
12.8 (9.0-NR) 5.7 (5.2–7.6) 5.3 (3.5–7.1) |
BRCAmut, 16 |
NA |
NA |
Single reduction, 25 Multiple reduction, 14 |
Nausea, fatigue, elevated ALT/AST |
Anemia, elevated ALT/AST, fatigue |
US FDA Breakthrough Therapy, 04/2015 |
| Olaparib, Lynparza® (AZD2281) |
Astra- Zeneca |
PARP1–4, 12, 15, 16 |
NCT01078662, 400 mg b.i.d. (oral) |
BRCA1mut, 148 BRCA2mut, 44 BRCA1/2mut, 1 |
7 |
3 |
28 |
33 |
NA |
Fatigue, nausea, (ALT/AST, NR) |
Fatigue, anemia, abdominal pain† |
FDA approved, fourth line, 12/2014 |
| Veliparib (ABT-888) |
Abbvie |
PARP1–4 |
NCT01540565, 400 mg b.i.d. (oral) |
BRCA1mut, 39 BRCA2mut, 11 |
8.11 |
4 |
22 |
48 |
≥1 reductions, 62 |
Leukopenia, anemia, nausea, vomiting, metabolism/nutrition, nervous system |
General and administration site, other investigations |
– |
| Niraparib (MK4827) | Tesaro | PARP1/2 | NCT02354586, 300 mg q.d. (oral) | BRCAmutBRCA-like | Final data collection in 2016 for primary outcome measures | – | ||||||
†Two cases of acute myeloid leukemia and one case of myelodysplasia syndrome observed.
AE: Adverse event; b.i.d.: Twice daily; CR: Complete response; EOC: High-grade serous ovarian, fallopian tubal, or primary peritoneal cancer; NA: Not available; NR: Not reached; PFS: Progression-free survival; PR: Partial response; q.d.: Once daily; SD: Stable disease; TNKS: Tankyrase.
• Companion diagnostics
CDx are vital to identifying PARPi responders. Myriad's BRACAnalysis CDx™ is the only FDA-approved test to determine olaparib treatment eligibility. Veliparib is also under development with BRACAnalysis CDx. Niraparib and talazoparib are under development with myChoice HRD™. However, the talazoparib/myChoice HRD partnership is not currently under development for ovarian cancer patients. Rucaparib uses Foundation Medicine's NGS-based CDx to identify tumors with a BRCA-like signature, but the specifics of this assay are yet to be revealed. Current CDx platforms are discussed (Table 2).
Table 2. . Companion diagnostics under development with PARP inhibitors for high-grade serous ovarian, fallopian tubal, or primary peritoneal cancer.
| Companion diagnostic | Company | PARPi | Indications | Genes assessed | Type(s) of analysis | Sample preparation | Result classification(s) relevant to PARPi therapy | Additional comments |
|---|---|---|---|---|---|---|---|---|
| BRACAnalysis CDx™ |
Myriad Genetics (UTAH) |
Olaparib Veliparib |
Ovarian, metastatic BC, (neo)adjuvant BC Ovarian, metastatic BC |
gBRCA1 gBRCA2 |
Sanger sequencing and multiplex PCR |
Whole blood |
BRCA1/2 status: deleterious, suspected deleterious, VUS, favor polymorphism, polymorphism |
Provides a thorough evaluation of BRCA1/2 |
| myChoice HRD™ |
Myriad Genetics (UTAH) |
Talazoparib Niraparib |
Pancreatic, metastatic BC Ovarian, metastatic BC |
gBRCA1/2 and non-gBRCA1/2 tumors with HRD |
LOH, TAI, LST, Sanger sequencing and multiplex PCR |
FFPE |
HRD score: HRD low = 0–41 HRD high = 42–100 |
Relatively inexpensive and expedient compared with standard genetic testing Indicator of genomic instability HRD score reliable at ≤65% contamination |
| Foundation Medicine's NGS-based CDx† | Foundation Medicine (MASS) | Rucaparib | Ovarian cancer | Tumor BRCA status and 28 HRD genes† | Base substitutions, MAF, indel, CNA, rearrangements | FFPE, core- and fine-needle biopsy | HRD LOH cutoff: High genomic LOH Low genomic LOH |
Accommodates: Low MAF Low tumor purity Small tissue samples |
†The specifics of Foundation Medicine's CDx for rucaparib are not yet available; however, tumor analysis is thought to be comparable to the methodology of FoundationOne™.
BC: Breast cancer; CDx: Companion diagnostic; CNA: Copy number alteration; EOC: High-grade serous ovarian, fallopian tubal, or primary peritoneal cancer; FFPE: Formalin-fixed, paraffin-embedded; HRD: Homologous recombination deficient; LOH: Loss of heterozygosity; LST: Large-scale state transitions; PARPi: PARP inhibitor; TAI: Telomeric allelic imbalance; VUS: Variant of uncertain significance.
• BRACAnalysis CDx
BRACAnalysis CDx comprises two in vitro assays for gBRCA1/2mut identification: BRACAnalysis CDx Sanger Sequencing for sequence variants, and BRACAnalysis CDx Large Rearrangement Test (BART®) for large rearrangements. PCR and subsequent Sanger sequencing evaluate exons and exon/intron boundaries of BRCA1/2 (17,337 bases total) for single nucleotide polymorphism (SNP), insertions ≤2 base pairs (bp) and deletions ≤5 bp. Sanger sequencing was compared with NGS for accuracy, which showed 100% agreement for negative (95% CI: 99.99–100%), positive (95% CI: 99.62–100%), and overall (95% CI: 99.99–100%) concordance in identifying 796 variant and 1,732,907 nonvariant bases [52]. BART® utilizes multiplex PCR to assess single- and multi-exon deletions/duplications, flanking introns, the Portuguese founder mutation and proximal promoter sequences, with full sequence determination as follows: BRCA1, 5400 bp of 22 exons, and approximately 750 adjacent intronic bp; BRCA2, 10,200 bp of 26 exons, and approximately 900 adjacent intronic bp. BART was compared with microarray for accuracy, which showed 97.3% negative agreement (95% CI: 90.6–99.7%), 84.6% positive agreement (95% CI: 65.1–95.6%) and 94% overall agreement (95% CI: 87.4–97.8%). Novel deleterious missense mutation discovery increases with time. Therefore, variants are classified into one of five categories (Table 2); the ‘polymorphism’ category addresses SNPs not considered detrimental. About 1–2% of all variants identified by BRACAnalysis CDx require confirmatory analysis by alternate primer sequencing or PCR analysis. As a bridging study, available archival specimens (n = 61) from the Study 42 population [53] were subjected to BRACAnalysis CDx and compared with local BRCA test results, which showed a 0.13 difference in objective response rate (ORR).
Limitations to the BRACAnalysis CDx include an inability to detect deletions >5 bp, insertions >2 bp, some RNA transcript processing errors, and cannot differentiate between gene duplication and triplication [54]. Patients previously diagnosed with a hematologic malignancy should forego BRACAnalysis CDx, as false-positive results could be generated. False-negative results are of concern for polymorphisms at primer sites, leading to unequal allele amplification. Fortunately, Myriad provides a more comprehensive NGS panel, called myRisk, for patients initially screened with BRACAnalysis CDx.
• myChoice HRD
Myriad's myChoice HRD is an enhancement of BRACAnalysis CDx, as it assesses LOH beyond BRCA. While 14% of ovarian cancer patients test positive by BRACAnalysis CDx, 48% test positive with myChoice HRD [55]. myChoice HRD is an NGS-based assay to assess BRCA1/2 sequences and genomic scarring (HRD Score), which is a sum of three components: loss of heterozygosity (LOH), telomeric allelic imbalance (TAI) and large-scale state transitions (LST).
LOH regions are ≥15 Mb, but shorter than chromosomal length; HRD is detected regardless of etiology/mechanism and is highly correlated with defects (e.g., promoter methylation) in BRCA1/2, PTEN, FANCM and RAD51C [56]. TAI defines regions with allelic imbalance that do not cross the centromere, but extend to the subtelomere [57]. An inverse proportion existed between BRCA1 levels and the number of TAI regions in BRCA1/2wt serous ovarian cancers, suggesting a high TAI score indicates DNA repair defects. LST assesses chromosomal breaks in adjacent regions ≥10 Mb after filtering all variation ≤3Mb [58]. All BRCA1/2mut tumors had high LST scores, and BRCA1 inactivation was evident in 80% of near-diploid tumors. Regardless of BRCA status, high LST scores were associated with interchromosomal translocations as detected by complete genome sequencing. High LST scores are thought to indicate HRD better than BRCA status, and may be due to defects in HR pathway gene products (e.g., PALB2/FANCN, RAD51, among others). Conveniently, the LST signature is inexpensive, relatively expedient and a more global measure of genomic instability. Tumors are scored (0–100), with a cutoff of 42. Scores ≥42 are considered to have high HRD, which encompasses 95% of BRCAmut tumors [59]. Recently, a retrospective analysis of ovarian cancer cohorts that compared dichotomized (high/low) individual components (LST, TAI and LOH) to the combined three biomarker HRD showed excellent significance for the combined HRD score in regard to progression-free survival (PFS; p = 2 × 10-6) and OS (p = 1 × 10-8), but no significance was established for any of the individual components [60]. A patient-derived xenograft ovarian cancer model showed 50% of BRCAmut tumors responded to niraparib, 50% of BRCAwt HRD+ tumors responded and all sensitive models had an HRD score ≥42 [61]. In NOVA Phase III tumor samples (n = 174), myChoice HRD identified 100% (68/68) of gBRCAmut tumors, and 57% (61/106) of gBRCAwt patients with HR deficiencies that would benefit from niraparib therapy.
• FoundationOneTM
Foundation Medicine applies massively parallel DNA sequencing to accurately detect genomic alterations in therapeutically relevant cancer genes. Unlike BRACAnalysis CDx, FoundationOneTM utilizes archival formalin-fixed, paraffin-embedded (FFPE) solid tumor samples, and is highly tissue sparing. While simultaneously accounting for the degree of stromal admixture, the NGS-based test analyzes 315 cancer-related genes (≥4557 exons) and ≥47 introns of 28 genes by whole-genome shotgun library construction and hybridization capture with biotinylated DNA oligonucleotides for base substitutions using a Bayesian method, indels (1–40 bp) using the deBruijn approach, copy number alterations (CNAs), rearrangements and homozygous deletions. In regard to potential PARPi targets, the current 315-gene list includes BRCA1/2, PALB2, FANCM, BARD1, CHK1, ATM, RAD51C, RAD51B and BLM. In regard to heredity EOC genes, PALB2 and BARD1 were recently reported as genes of future investigation, based on mutant frequency [22]. In a validation study [62], 3.06 alterations per sample (2221 specimens) were reported overall, and 1.57 alterations per sample (1579 unique alterations) were identified in tumors with clinically actionable treatment option(s). Specificity exceeded 99% for all genomic alteration testing, with sensitivity as follows: base substitutions (>99% when mutant allele frequency [MAF] ≥5%), indels (>97% when MAF ≥10%), CNAs (>95%) and rearrangements (>90%). Clinically relevant and pertinent negative alterations, FDA-approved therapies, and potential clinical trials are integrated in FoundationOne reports. Variants of unknown significance (VUS), equivocal and subclonal designations are also given. VUS are included for variants with currently inadequate scientific literature characterizations. Equivocal labels ambiguous evidence of homozygous loss or amplification. Subclonal denotes when tumor DNA contains <10% of a certain alteration. Clinically, FoundationOne is advantageous; it identifies large indels, does not require a matched normal sample, consumes a small tumor fraction (≥40 μM tissue, >20% malignant origin), is amenable to core- and needle biopsies, has a 14-day turn-around, exhibits a 97% concordance between replicates, has high sensitivity and specificity, detects mutations at low MAF, identifies actionable alterations (76% of patients) and may reveal additional treatment options to consider. Specific for the ARIEL2 and Foundation Medicine utilizes a modified NGS-based CDx to develop an HRD LOH cutoff to identify EOC patient tumors with a BRCA-like signature. The specifics of this customized assay are not currently available publicly, but are thought to be similar to the analytical capacity of FoundationOne.
• Olaparib
Olaparib is an oral small molecule inhibitor of PARP1/2/3, and received US FDA accelerated approval in December 2014 as fourth line and beyond monotherapy for deleterious gBRCAmut advanced ovarian cancer patients. The BRACAnalysis CDx was FDA-approved alongside olaparib in December 2014, and can only be marketed in the USA with this CDx. However, the European Commission (EC) approved olaparib for maintenance therapy use in platinum-sensitive, relapsed BRCA-mutated high-grade serous epithelial ovarian cancer. The EC and US FDA approvals are based on Phase II clinical trial evaluations [53,63]. Olaparib extended PFS versus placebo from 4.8 to 8.4 months without significant change in overall survival (OS). Retrospective tumor BRCAmut evaluation revealed a PFS of 11.2 months for those treated with olaparib, versus 4.3 months for placebo (HR: 0.18; 95% CI: 0.10–0.31; p < 1 × 10-4). Common AEs were mild-to-moderate fatigue, anemia, nausea and vomiting. FDA approval for maintenance therapy was withheld due to a concern for a lack of randomization in the gBRCA-mutated subgroup, a lack of significant increase in OS and a concern for cases of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). These diseases are of concern among patients carrying gBRCAmut treated with PARPi, but are also seen as a result of DNA damaging chemotherapeutics, particularly alkylators, which are commonly used in the treatment of EOC patients. Nevertheless, accelerated approval was granted based on impressive single-agent response rates (31%; 95% CI: 25–38%) in heavily pretreated patients and the lack of therapeutic options available.
Olaparib utility was expanded by Kaufmann et al. to tumors of the breast, pancreas and prostate, and was effective therapy in these BRCA1/2mut tumors as well [53]. Additional Phase III data elaborating PFS and OS benefits in the maintenance setting will be conducted in SOLO trials, which investigates efficacy following frontline chemotherapy (primary maintenance, SOLO1: NCT01844986), and in platinum-sensitive, relapsed high-grade serous ovarian cancer (switch maintenance, SOLO2: NCT01874353). A recent randomized Phase II study of olaparib monotherapy compared with olaparib and cediranib combination showed an increase in median PFS from 9.0 to 17.7 months (HR: 0.42; 95% CI: 0.23–0.76) [64]. This PARPi and VEGFR inhibitor combination study prompted two additional Phase III studies. EOC patients with platinum-sensitive and recurrent disease will be evaluated in NCT02446600 (experimental arms: platinum-based chemotherapy vs olaparib vs olaparib and cediranib), and platinum-resistant or -refractory tumors will be evaluated in NCT02502266 (experimental arms: physician's choice chemotherapy vs olaparib or cediranib vs olaparib and cediranib).
• Veliparib
Veliparib is an oral small molecule PARP1/2 inhibitor. Phase I data demonstrated a comparable safety profile to other PARPis [65]. Combination of oral cyclophosphamide and veliparib did not improve PFS or ORR compared with cyclophosphamide alone in BRCA-mutant ovarian cancer [66], but this may be attributable to the low dose of veliparib (60 mg q.d.), which was below the 250–400 mg b.i.d. doses used in other trials. A Phase II study showed effective veliparib monotherapy against platinum-resistant BRCA-mutated ovarian cancer [67]. Veliparib (400 mg b.i.d., 28-day cycle) response in platinum-resistant and platinum-sensitive patients was 20 and 35%, with a median PFS of 8.2 months. Only one grade 4 (G4) event (thrombocytopenia) was found, and G3 AEs were fatigue (6%), nausea (4%), leuokopenia (2%), neutropenia (2%), dehydration (2%) and ALT (2%). Veliparib efficacy against platinum-resistant disease warrants further investigation. Evaluation of veliparib combination with carboplatin/paclitaxel and as maintenance therapy in patients with previously untreated stage III/IV EOC in a double-blind, randomized Phase III study is currently recruiting participants (n = 1100; NCT02470585). Veliparib switch maintenance trials are under planning [68].
• Niraparib
Another oral small molecule PARP1/2 inhibitor, niraparib, showed antitumor activity in gBRCA mut tumors in Phase I/II studies [69,70], and also in tumor models with loss of BRCA and PTEN function. Niraparib fourth-line monotherapy (300 mg q.d.) is under investigation for recurrent ovarian cancer patients with HRD or gBRCAmut tumors in a multicenter, open-label, single-arm Phase II study (n < 225; NCT02354586). Niraparib is under development with myChoice HRD, which measures HRD (i.e., LOH, TAI and LST). Combination niraparib-bevacizumab is under investigation in Phase I/II trials (AVANOVA: NCT01244789), with patient assessment based on myChoice HRD scores. Common AEs include fatigue, anemia, nausea, vomiting and anorexia [69]. Phase I dose-limiting toxicities (DLTs) include G3 fatigue (dosage: 30 mg) and pneumonitis (60 mg), and G4 thrombocytopenia (400 mg). A Phase III switch maintenance therapy trial against platinum-sensitive ovarian cancer is ongoing (NOVA: NCT01847274).
• Talazoparib
All worldwide rights to talazoparib were acquired by Medivation from BioMarin Pharmaceutical in August 2015. Selective against BRCA1/2 and PTEN mutants, talazoparib is a potent PARP1/2 inhibitor (PARP1 IC50: 0.57 nM), which demonstrated greater stereospecific PARP-DNA-trapping ability than other PARPis [71], and also potentiated cytotoxic effects of TMZ, SN-38 and carboplatin [72]. Combination of talazoparib with a DNA methyltransferase inhibitor in vitro decreased ovarian cancer cell line clonogenic survival, regardless of BRCA status [73]. In a single-arm, open-label Phase I study to evaluate safety, PK and preliminary efficacy of talazoparib in patients with advanced or recurrent solid tumors, the recommended Phase II dose (RP2D) was established (1 mg/day) for single agent therapy (NCT01286987). Patients with gBRCAmut ovarian tumors had RECIST (response evaluation criteria in solid tumor), CA-125 and clinical benefit responses of 44, 70 and 82%. Fatigue, nausea and alopecia were observed in 30% of patients, as were myelosuppression-related dose reductions (15%) and G3/4 anemia (13%), thrombocytopenia (14%) and neutropenia (6%). Ongoing and future talazoparib open-label, single-arm studies include: a Phase 0 study of the effects of talazoparib on DNA copy number, RNA expression and protein levels (NCT02316834); a Phase I study of the utility and tolerability of talazoparib to treat advanced or metastatic nonresectable stage III/IV ovarian cancer and liver or kidney disease (NCT02567396); a Phase I/II study in BRCAmut advanced solid tumors at 1 mg/day (28-day cycle), with tumor biopsies for DNA damage response markers prior to treatment, during cycle 1, and if disease progresses (NCT01989546); and a Phase II evaluation of talazoparib in patients with metastatic gBRCAmut ovarian cancer previously treated with a PARPi (NCT02326844).
Chemistry
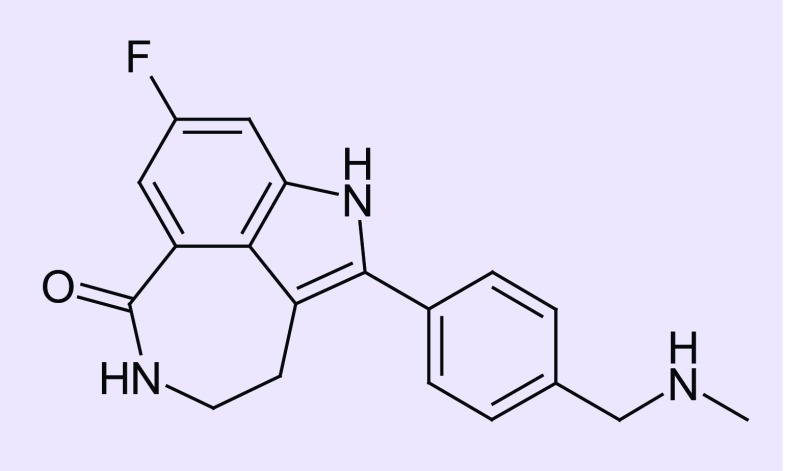
Rucaparib, a potent PARP1/2/3 inhibitor, refers to the free base (formerly known as PF01367338 and AG014447). Rucaparib camsylate (CO-338; 8-fluoro-2-{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one ((1S,4R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)methanesulfonic acid salt) (Figure 1) is formulated into oral tablets.
Figure 1. . Chemical structure of rucaparib (CO338; 8-fluoro-2-{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one).
Pharmacokinetic & pharmacodynamic
Rucaparib was granted FDA Breakthrough Therapy designation in April 2015, and is under investigation as monotherapy and in combination with cytotoxic/anti-angiogenic agents for solid tumors with BRCA mutation(s) or a BRCA-like phenotype. A breakthrough designation was granted based on interim ORRs from two ongoing Phase II trials. Using Foundation Medicine's NGS-based CDx platform in ARIEL2 Part 1, patients with platinum-sensitive tumors that received one prior platinum-based therapy were evaluated. Tumors were classified into three subgroups based on tumor HRD status: BRCAmut (n = 23; 65% ORR), BRCAwt/LOHhigh (n = 25; 40% ORR) and BRCAwt/LOHlow (n = 13; 8.0% ORR) [74]. For comparison, olaparib received FDA accelerated approval in 2014 for the BRCAmut patient population, yet this study observed only a 34% ORR in BRCAmut patients.
PARP1 and PARP2 inhibition constants (Ki) are 1.4 and 0.17 nM [75]. Rucaparib, veliparib and olaparib PARP catalytic domain binding capacities were compared [76]: all stabilized PARP1, 2, 3 and 4. However, veliparib did not stabilize PARP12, 15 or 16, and only rucaparib stabilized PARP10. Unlike olaparib and veliparib, rucaparib distinguishably stabilized TNKS1 and TNKS2 catalytic domains. Rucaparib has enhanced PARP enzyme inhibition compared with olaparib. An enzymatic screen showed rucaparib and olaparib inhibited PARP5A (TNKS1) at 796 nM and 1.90 μM, and inhibited PARP5B (TNKS2) at 486 nM and 7.40 μM [75]. A tankyrase inhibition cellular assay showed 50% inhibition by rucaparib at 2.07 μM, whereas olaparib showed no detectable tankyrase inhibition. Rucaparib's ability to bind and inhibit TNKS1 and TNKS2 in addition to PARP1–4, 12, 15 and 16 is unique, although the clinical relevance of such distinction remains to be understood. Promiscuous activity could harbor the potential for enhanced therapeutic significance, yet may result in a surplus of side effects.
Studies in vivo were consistent with the synthetic lethality concept. Xenograft BRCA1-mutated tumors were 15-fold more sensitive to rucaparib compared with BRCAwt. Rucaparib cytotoxicity of epigenetically silenced BRCAwt cells was comparable to BRCA1/2mut [77]. Rucaparib potentiates type-I topoisomerase inhibitors (e.g., topotecan) and DNA alkylating agents (e.g., N-methyl-N’-nitro-N-nitrosoguanidine and TMZ) [78]. Xenograft mice data showed HRD cell lines were rucaparib-sensitive, and an additive or synergistic efficacy existed when combined with PI3K pathway inhibitors [79].
Vasoactivity of rucaparib may account for emesis and GI toxicity via inhibition of myosin light chain kinase (MLCK). However, this same vasoactivity may account for rucaparib's newly hypothesized function as a chemosensitizer through MLCK inhibition [80].
• Companion diagnostic development
Clinical data show gBRCAmut tumors respond to rucaparib therapy [81,82], as do tumors with other HR defects [75]. To address the issue of identifying PARPi therapy beyond gBRCAmut, a collaboration with Foundation Medicine to develop a biomarker assay for BRCA-like tumors based on genomic scarring is underway [56]. Quantification of genome-wide loss-of-heterozygosity (GLOH) by NGS identifies base substitutions, insertions/deletions (indels) and homozygous deletions in BRCA1/2 and 28 other HR-pathway genes through pretreatment screening biopsies and FFPE tumor specimens [83,84]. However, this assay does not assess all DNA repair genes that respond to rucaparib [85]. A GLOH cutoff was established for ARIEL2 Part 1 (NCT01891344) using public data. TCGA data analysis was used to establish a GLOH cutoff for tumors with a BRCA-like signature. Using this cutoff, median OS was increased in high GLOH (LOHhigh) tumors versus low GLOH (LOHlow) tumors (56.4 vs 38.2 months). Initial ARIEL2 clinical data showed that in 54% of BRCAwt patients, LOHhigh tumors were detected (p < 1 × 10-4), and response rates were 36% (LOHhigh) and 16% (LOHlow) (p = 0.0072). Therefore, prospective identification of rucaparib-responsive BRCAwt ovarian tumors was accomplished with this GLOH assay for BRCA-like tumors. This CDx is prospectively incorporated in the ongoing ARIEL2 Part 2 study and the maintenance study (ARIEL3, NCT01968213).
Clinical efficacy
Initial clinical safety and tolerability were established in Phase I studies of advanced solid tumor patients treated with rucaparib/AG-014699 and TMZ [86]. Rucaparib clinical trial data are in Table 3.
Table 3. . Rucaparib evaluation in epithelial ovarian cancer clinical trials.
| Study, Phase | Study title (abbreviated) | Study design | HR status classification | Study arms | Primary objective | Results |
|---|---|---|---|---|---|---|
| A4991002, Phase I |
Study of rucaparib and TMZ in patients with AST or malignant melanoma |
AST or malignant melanoma. Open-label, dose-escalation |
Not evaluated |
Part 1: Rucaparib: escalating doses (1–18 mg/m2) iv. on days 1–5 every 4 weeks TMZ: 100 mg/m2 p.o. on days 1–5 every 4 weeks Part 2: Rucaparib: 12 or 18 mg/m2/day iv. on days 1–5 every 4 weeks TMZ: 135, 170 or 200 mg/m2 p.o. on days 1–5 every 4 weeks |
Safety, efficacy, pharmacokinetic and pharmacodynamic results of rucaparib and TMZ combination |
PARP inhibitory dose: 12 mg/m2 (74–97% inhibition) Recommended dose of 12 mg/m2 rucaparib, 200 mg/m2 TMZ. Mean tumor PARP inhibition at 5 h, 92% (46–97%). No attributable toxicity for rucaparib alone |
|
NCT01482715, CO-338-010, Phase I/II |
Study of oral rucaparib in patients with a solid tumor (Phase I) or with gBRCA mutation ovarian cancer (Phase II) |
AST or EOC associated with gBRCA-mut. Open-label, dose-escalation (Phase I), Simon 2-stage (Phase II) |
Part 2A/2B/3: BRCA-mut |
Part 1: Rucaparib: escalating p.o. doses (40 mg q.d. to 840 mg b.i.d.) on days 1–21 of every 21-day cycle Part 2A/2B/3: RP2D (600 mg b.i.d.) of oral rucaparib established in part 1 on days 1–21 of every 21-day cycle |
Part 1: Incidence of grade 3/4 AE and clinical laboratory abnormalities Part 2A/2B: ORR per RECIST criteria Part 2B: overall survival Part 1/3: PK and steady-state profile for single higher dose strength tablets |
Phase I: RP2D determined (600 mg b.i.d.). gBRCA-mut, 80% ORR (3/4 EOC and 1/1 BC). G2/3 myelosuppression in 50% of patients (dose-dependent). G1/2 fatigue, nausea, neutropenia, anemia in ≥25% patients Phase II: Platinum-sensitive, BRCA-mut EOC (n = 35): 74% ORR, 77% DCR. No treatment discontinuations |
|
NCT01891344 CO-338-017, Phase II |
Study of rucaparib in platinum-sensitive EOC (ARIEL2) |
Platinum-sensitive, relapsed EOC Open-label |
BRCA-mut BRCA-like Biomarker(-) |
Prospectively and molecularly defined HRD signature. Rucaparib RP2D (600 mg p.o. b.i.d.) established in Study CO-338-010 |
Part 1: Disease progression by RECIST criteria in patients who received ≥1 prior patient-based regimen Part 2: ORR by RECIST criteria in patients who received ≥3 prior chemotherapy regimens |
Part 1: Median PFS (95% CI); ORR by RECIST (%) BRCA-mut: 12.8 (9.0–NR); 75 BRCA-like: 5.7 (5.2–7.6); 36 Biomarker neg.: 5.3 (3.5–7.1); 16 Part 2: NA |
|
NCT00664781, Phase II |
Rucaparib in treating patients with locally advanced or metastatic breast cancer or advanced ovarian cancer |
BC, BRCA-mut BC, EOC. Open-label, dose-escalation |
BRCA1-mut BRCA2-mut |
Rucaparib p.o. q.d. for 7, 14 or 21 days every 3 weeks, 12 cycles until absence of disease progression or unacceptable toxicity |
Antitumor activity by RECIST criteria and safety profile |
NA |
| NCT01968213, CO-338-014, Phase III | Rucaparib as switch maintenance following platinum-based chemotherapy in patients with relapsed, platinum-sensitive EOC (ARIEL3) | Platinum-sensitive, relapsed EOC. Double-blind, randomized, placebo controlled |
BRCA-mut BRCA-like Biomarker(-) |
Arm A: Rucaparib 600 mg p.o. b.i.d., 28-day cycle Arm B: Placebo p.o. b.i.d., 28-day cycle |
Antitumor activity by RECIST criteria | NA |
AE: Adverse event; AST: Advanced solid tumor; BC: Breast cancer; b.i.d.: Twice daily; BRCA-mut: BRCA1/2-mutated; DCR: Disease control rate, sum of complete response, partial response and stable disease for 24 weeks; EOC: High-grade serous ovarian, fallopian tube or peritoneal ovarian cancer; HR: Homologous recombination; iv.: Intravenous; NA: Not available; NR: Not reached; ORR: Objective response rate; PFS: Progression-free survival; q.d.: Once daily; RECIST: Response Evaluation Criteria in Solid Tumor; RP2D: Recommended Phase II dose; TMZ: Temozolomide.
• Study CO-338-010 (Phase I/II)
In a Phase I, open-label, multicenter, 3+3 dose-escalation (40 mg q.d. to 840 mg b.i.d.) study to determine the maximum tolerated dose, RP2D, and efficacy of rucaparib monotherapy in patients with ovarian (n = 20), breast (n = 27) or pancreatic (n = 9) cancer with gBRCAmut tumors, an 80% response rate (3/4 ovarian cancer and 1/1 breast cancer patients) by RECIST and CA-125 levels was observed at 600 mg b.i.d. doses. No G4 events were treatment-associated, and dose-dependent G2/3 myelosuppression occurred in 50%, which was manageable with dose reduction. Treatment-related AEs with ≥10% patient involvement included: G1/2 fatigue (30%), nausea (30%), vomiting (23%), diarrhea (13%), anorexia (11%); and G2/3 anemia (29%/29%), thrombocytopenia (0/14%) and neutropenia (29%/0) [81]. With acceptable tolerance and encouraging clinical benefit, the RP2D was determined (600 mg b.i.d.) in fasted and fed states, with maximum serum concentrations 4 h after administration. All responders harbored BRCA1/2mut; responses were evident in platinum-sensitive and -resistant tumors.
A Simon two-stage design was incorporated in a Phase II, open-label safety and efficacy evaluation for relapsed, platinum-sensitive ovarian cancers with gBRCAmut. RECIST v1.1 and CA-125 levels assessed the ORR primary endpoint. Secondary endpoints included AEs, laboratory and electrocardiogram abnormalities and response duration. Overall response in relation to BRCA1/2 status was an exploratory endpoint. All ovarian cancer patients enrolled (n = 35) were platinum-sensitive and BRCA-mutated, with a prominent ORR (74%) and disease control rate (DCR; sum of complete response, partial response and stable disease after 24 weeks) of 77%, regardless of prior treatment number [87]. No treatment discontinuations existed at the data cutoff, with G3/4 AEs managed by dose reduction. Fatigue (64%), nausea (58%), anemia (50%) and elevated ALT/AST (42%) were most common, without liver dysfunction evidence. Primary endpoints were met, as were exploratory endpoints.
Patient enrollment is open for Study CO-338-010 extensions to evaluate EOC patients with ≥3 prior chemotherapy treatments, rucaparib efficacy to treat any advanced solid tumor, inclusive of lymphoma, which is BRCAmut and the pharmacokinetics of a higher dose strength tablet in fed versus fasted states while maintaining 600 mg b.i.d. dosages.
• ARIEL2
ARIEL2, a novel international Phase II study to prospectively identify HRD tumors using Foundation Medicine's NGS-based CDx, will evaluate PFS, ORR, safety and pharmacokinetics in platinum-sensitive ovarian cancer patients with ≥1 chemotherapy regimen (Part 1; enrollment complete) or ≥3 prior chemotherapy regimens (Part 2; currently enrolling) based on the following tumor molecular subgroups; BRCAmut, BRCA-like and biomarker negative. The genomic scarring molecular signature established in Part 1 will be prospectively applied to Part 2 and ARIEL3.
Tumor HRD status in Part 1 (n = 204) was: BRCAmut (20%); BRCA-like, defined as BRCAwt/LOHhigh (40%); biomarker-negative, defined as BRCAwt/LOHlow (34%); and unclassified (6%). HRD status is pending for Part 2 (n = 300). Treatment-related AEs accounted for few Part 1 discontinuations (6%; n = 10) due to anemia and fatigue. Rucaparib is well tolerated, and AEs were comparable between BRCAwt and BRCAmut, with a predominance of G1/2 nausea, asthenia/fatigue and elevated ALT/AST without alkaline phosphatase or bilirubin elevation. G3/4 AEs present in ≥5% of patients in Part 1 were: anemia (19%); elevated ALT/AST (11%); asthenia/fatigue (7%) and neutropenia (8%). Approximately 90% of patients experienced G1/2 creatinine increases without elevated BUN (see the ‘Safety & tolerability’ section for an explanation). Single and multiple dose reduction schedules will be elaborated in the future.
In ARIEL2 Part 1, rucaparib efficacy in patients with platinum-sensitive tumors (n = 205) was evaluated [88]. ORRs by RECIST criteria were: BRCAmut (75%), BRCA-like (36%) and biomarker negative (16%). Median duration of responses (months; 95% CI) were: BRCAmut (9.5; 7.4–12.9), BRCA-like (8.2; 5.6–10.8) and biomarker negative (5.5; 2.1–7.4). Out of the 152 BRCAwt patients, four had RAD51C alterations (germline truncation, somatic homozygous deletion and two germline splice), all of which were LOHhigh. Partial responses were evident in the RAD51C truncation and homozygous deletion tumors, and one partial response and one stable disease outcome existed in the two splice-based mutations. Median PFS results (months; 95% CI) were: BRCAmut (12.8; 9.0–NR), BRCA-like (5.7; 5.2–7.6) and biomarker negative (5.3; 3.5–7.1). Subgroup efficacy data were compared (HR; 95% CI; p-value): BRCAmut versus biomarker negative tumors (0.22; 0.12–0.40; p < 1 × 10-4) and BRCA-like versus biomarker negative tumors (0.67; 0.45–0.99; p = 0.0445). Preliminary Part 1 data suggest a robust ability of comprehensive genomic analysis to identify rucaparib-sensitive ovarian cancer patients. Completed Part 2 data are not yet available.
• ARIEL3
ARIEL3 (NCT01968213) will evaluate rucaparib switch maintenance after response to platinum-based therapy in a Phase III, double-blinded, randomized study of EOC patients to serve as a confirmatory study for NDA approval. RECIST v1.1 will evaluate investigator-assessed PFS as the primary end point, with secondary endpoints of OS, safety and pharmacokinetics. Patients will be stratified into three groups by the NGS-based HRD signature assay, with PFS analyzed according to LOH status. Enrollment (approximately 540 patients) is on target to be completed in 2Q 2016.
Safety & tolerability
The synthetic lethality mechanism of action may protect against severe PARPi toxicity. Noncancerous cells in BRCAwt patients are capable of homologous recombination, and are less likely to be susceptible to rucaparib-induced AEs. In line with other PARPi side effects, AEs were primarily GI related, and were manageable with dose modification and concomitant treatment.
G3/4 events were primarily laboratory abnormalities (anemia, neutropenia and elevated ALT/AST), which subsided upon supportive care and treatment modifications. A lack of alkaline phosphatase and bilirubin increase is a favorable observation in regard to hepatic toxicity. Creatinine elevation did stall some treatment deliveries. Elevation of serum creatinine, a surrogate marker, is due to transporter inhibition by rucaparib and olaparib, with elevation resolving upon treatment interruption. Rucaparib is likely to inhibit uptake and efflux transporters, as olaparib inhibits OCT1, OCT2, MATE1 and MATE2-K [89].
Other rucaparib safety parameters are noteworthy. Myelosuppression is of concern for all PARPis, as demonstrated by olaparib clinically [53]. However, no instances of MDS or AML have been reported for rucaparib to date. The transporters ABCG2 and ABCB1/P-gp/MDR1 efficiently efflux rucaparib in vitro [90]. A consequence of MDR1 efflux susceptibility is that rucaparib delivery to the central nervous system is limited [91].
Regulatory affairs
Rucaparib received US FDA Breakthrough Therapy designation in April 2015.
Conclusion
Rucaparib is a potent inhibitor of PARP1/2/3 with synthetic lethality in BRCA-mutated and BRCA-like tumors. Although not the most biochemically potent PARPi available, the therapeutic window is sufficiently ample to allow targeted therapy with minimal toxicity to nontumor cells. Out of all PARPis under development, rucaparib shows unique promiscuous binding to tankyrase, which may enhance its clinical efficacy over its competition. Preclinical studies showed antitumor activity in a variety of solid tumors, which was confirmed in clinical trials. In humans, a favorable toxicity profile was observed, and primarily limited to fatigue, asthenia and GI side effects, which were relieved with supportive care and dosage modification. Rucaparib's robust activity in ARIEL2 Part 1 is an exciting prospect for subsequent ARIEL studies.
Foundation Medicine's NGS-based CDx and Myriad's BRACAnalysis CDx are limited, as defects are restricted to known genetic aberrations; contrarily, myChoice HRD provides a sense of genomic instability regardless of etiology. However, in regard to identifying the most deleterious mutations with excellent sensitivity and specificity in the current time, FoundationOne assesses samples superiorly. The ability of the NGS-based CDx to prospectively identify BRCAwt patients with high GLOH offers further utility for rucaparib beyond BRCAmut patients, and provides an additional line of treatment – as monotherapy, switch maintenance and/or in combination with other chemotherapeutics – for advanced, recurrent ovarian cancer patients. With an effective CDx, dependence on BRCA status becomes less important, and identification of patients likely to benefit from PARPi therapy increases.
Further evaluation of rucaparib in platinum-resistant cancers is warranted, as veliparib recently demonstrated this efficacy clinically. Rucaparib has yet to be explored in combination therapy with agents that convert de novo and acquired HR proficient tumors into HRD tumors. Rucaparib combinations with inhibitors of CDK1, VEGFR3, HSP90 and HDAC may sensitize HR proficient tumors to PARPi. The appreciable activity and response rate, in conjunction with a selective HRD CDx and low toxicity profile, establishes rucaparib as a formidable drug candidate, and potentially the most anticipated PARPi under development in clinical trials.
EXECUTIVE SUMMARY.
Rucaparib camsylate (CO-338; 8-fluoro-2-{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one ((1S,4R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)methanesulfonic acid salt), developed by Clovis Oncology, is a potent oral inhibitor of poly(ADP-ribose) polymerase 1, 2 and 3 (PARP1, PARP2, PARP3). Rucaparib has a synthetically lethal mechanism of action in homologous recombination deficient (HRD) tumors.
Rucaparib is under development for treatment of recurrent ovarian cancers with BRCA mutation(s) or a BRCA-like phenotype using a next-generation sequencing (NGS)-based companion diagnostic (CDx) that quantifies tumor genomic loss of heterozygosity (LOH).
Overview of the market
Several PARP inhibitors, such as olaparib (AZD2281, Lynparza®, AstraZeneca), niraparib (MK4827, Tesaro), and veliparib (ABT-888, Abbvie), have shown therapeutic efficacy in ovarian cancer Phase II clinical trials. Rucaparib, along with other PARPis, exhibits synthetic lethality and PARP trapping primarily by catalytic inhibition, and are all sensitizers to DNA alkylating agents.
Talazoparib (BMN673, Medivation), a highly potent PARPi with favorable selectivity of HRD tumor cells in vitro, is currently under investigation in clinical trials as monotherapy and in combination studies.
Olaparib and Myriad Genetics’ BRACAnalysis CDxTM tests for germline BRCA mutants have been approved by the US FDA for fourth-line treatment of advanced ovarian cancer, and by the EC for maintenance treatment of platinum-sensitive, relapsed BRCA-mutated ovarian cancer. Niraparib and talazoparib are under development with Myriad Genetics’ myChoice HRDTM test for HRD by measuring LOH, telomeric allelic imbalance and large-scale state transitions.
Differing from other PARPis, rucaparib has been shown to clinically inhibit progression of BRCAwt patients, and has increased vasoactivity by myosin light chain kinase inhibition.
In regard to PARPi resistance, the effects of P-gp or HSP90 inhibitors, and also the restoration of 53BP1 expression to prevent RPA loading and subsequent HR of ssDNA breaks, are of interest.
Sensitizing de novo and acquired HR proficient tumors to PARPi by inhibiting BRCA1 phosphorylation with CDK1 inhibitors, angiogenesis with VEGFR blockers, BRCA1/2 expression with PI3K or AKT inhibitors and HR-associated gene expression with HDAC inhibitors may expand PARPi utility.
Pharmacodynamics & pharmacokinetics
PARP1 and PARP2 enzymatic IC50 for rucaparib (0.8 and 0.5 nM) are more favorable than olaparib (1.1 and 0.9 nM), with talazoparib showing the highest potency (PARP1 IC50 = 0.59 nM). The recommended Phase II dose is 600 mg orally twice daily rucaparib.
The most common adverse events of rucaparib are nausea, asthenia/fatigue, anemia and ALT/AST increase without elevation of alkaline phosphatase or bilirubin. Myelosuppression is an important consideration for PARPi usage, as illustrated by MDS and AML incidents following olaparib treatment.
Associated creatinine elevation is likely due to uptake and efflux transporter inhibition.
Clinical efficacy
A Phase II study of rucaparib in 205 patients with EOC (ARIEL2 Part 1) evaluated the clinical benefit of prospective comprehensive tumor genomic profiling based on NGS for BRCAmut, BRCA-like and biomarker-negative subgroups showed favorable PFS (9.4 vs 7.1 vs 3.7 months) and ORR by RECIST and CA-125 (75, 36 and 15%).
BRCA-like tumors were BRCAwt with high genomic LOH, with most responses occurring in RAD51C defective tumors. The hazard ratio for PFS in BRCA-like versus biomarker-negative subgroups is 0.67 [0.45–0.99], thereby demonstrating prospective identification of BRCAwt patients responsive to rucaparib.
Advantages in overall survival, safety and pharmacokinetics of rucaparib as fourth line treatment of platinum-sensitive ovarian cancer will be evaluated in a Phase II expansion study, in addition to a Phase III study of rucaparib efficacy as switch maintenance therapy.
Regulatory affairs
Rucaparib is the only PARP inhibitor to receive US FDA Breakthrough Therapy designation for third-line treatment of platinum-sensitive BRCA-mutated advanced ovarian cancer.
Footnotes
Financial & competing interests disclosure
RL Coleman is an investigator on the ARIEL 2, Principal Investigator for the ARIEL 3 clinical trial and a member of the scientific advisory board for these trials. He is also co-PI on ongoing clinical trials with olaparib, veliparib and talazoparib in gynecologic tumors. He also serves on the scientific advisory board for GOG-3005 (Phase III trial of veliparib in EOC). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Centers for Disease Control and Prevention: Gynecologic Cancers: Ovarian Cancer. www.cdc.gov
- 2.Saber MM, Zeeneldin AA, Mosaad MEG, et al. Treatment outcomes of female germ cell tumors: The Egyptian National Cancer Institute experience. J. Egypt Natl Canc. Inst. 2014;26(2):103–108. doi: 10.1016/j.jnci.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Kuo KT, Guan B, Feng Y, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009;69(9):2036–2042. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2013;10(4):211–224. doi: 10.1038/nrclinonc.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 6.Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J. Natl Cancer Inst. 2004;96(22):1682–1691. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 7.Aravantinos G, Pectasides D. Bevacizumab in combination with chemotherapy for the treatment of advanced ovarian cancer: a systematic review. J. Ovarian Res. 2014;7(57) doi: 10.1186/1757-2215-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 2014;32(30):3374–3382. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 9.Herzog TJ, Pothuri B. Ovarian Cancer: a focus on management of recurrent disease. Nat. Clin. Pract. Oncol. 2006;3(11):604–611. doi: 10.1038/ncponc0637. [DOI] [PubMed] [Google Scholar]
- 10.Stockler MR, Hilper F, Friedlander M, et al. Patient-reported outcome results from the open-label Phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J. Clin. Oncol. 2013;51:4240. doi: 10.1200/JCO.2013.51.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YT, Nam EJ, Yoon BS, et al. Germline mutations of BRCA1 and BRCA2 in Korean sporadic ovarian carcinoma. Gynecol. Oncol. 2005;99(3):585–590. doi: 10.1016/j.ygyno.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012;26(5):417–432. doi: 10.1101/gad.183509.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott CL, Swisher EM, Kaufmann SH. Poly(ADP-ribose) polymerase inhibitors: recent advances and future development. J. Clin. Oncol. 2015;58:8848. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochegger H, Dejsuphong D, Fukushima T, et al. PARP-1 protects homologous recombination from interference by Ku and Ligase IV in vertebrate cells. EMBO J. 2006;25(6):1305–1314. doi: 10.1038/sj.emboj.7601015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc. Natl Acad. Sci. USA. 2011;108(8):3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 17.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 18.Powell SN, Willers H, Xia F. BRCA2 keeps Rad51 in line. High-fidelity homologous recombination prevents breast and ovarian cancer? Mol. Cell. 2002;10(6):1262–1263. doi: 10.1016/s1097-2765(02)00789-x. [DOI] [PubMed] [Google Scholar]
- 19.Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2 . Genet. Med. 2010;12(5):245–259. doi: 10.1097/GIM.0b013e3181d38f2f. [DOI] [PubMed] [Google Scholar]
- 20.Welcsh PL, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum. Mol. Genet. 2001;10(7):705–713. doi: 10.1093/hmg/10.7.705. [DOI] [PubMed] [Google Scholar]
- 21.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2015;10:5495. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartwell LH, Szankasi P, Roberts CJ, Murray AW, Friend SH. Integrating genetic approaches into the discovery of anticancer drugs. Science. 1997;278(5340):1064–1068. doi: 10.1126/science.278.5340.1064. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Yu X. The role of poly(ADP-ribosyl)ation in DNA damage response and cancer chemotherapy. Oncogene. 34(26):3349–3356. doi: 10.1038/onc.2014.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Lorenzo SB, Patel AG, Hurley RM, Kaufmann SH. The elephant and the blind men: making sense of PARP inhibitors in homologous recombination deficient tumor cells. Front. Oncol. 2013;3:228. doi: 10.3389/fonc.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:81–89-8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 28.Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 2009;1(6–7):315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spellman PT. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown LA, Kalloger SE, Miller MA, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer. 2008;47(6):481–489. doi: 10.1002/gcc.20549. [DOI] [PubMed] [Google Scholar]
- 31.Bajrami I, Frankum JR, Konde A, et al. Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity. Cancer Res. 2014;74(1):287–297. doi: 10.1158/0008-5472.CAN-13-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YE, Batteli C, Watson J, et al. Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget. 2014;5(9):2678–2687. doi: 10.18632/oncotarget.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceccaldi R, Liu JC, Amunugama R, et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature. 2015;12(518):258–262. doi: 10.1038/nature14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mateos-Gomez PA, Gong F, Nair N, et al. Mammalian polymerase theta promotes alternatie NHEJ and suppresses recombination. Nature. 2015;518:254–257. doi: 10.1038/nature14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horton TM, Jenkins G, Pati D, et al. Poly(ADP-ribose) polymerase inhibitor ABT-888 potentiates the cytotoxic activity of temozolomide in leukemia cells: influence of mismatch repair status and O6-methylguanine-DNA methyltransferase activity. Mol. Cancer Ther. 2009;8(8):2232–2242. doi: 10.1158/1535-7163.MCT-09-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huehls AM, Wagner JM, Huntoon CJ, et al. Poly(ADP-ribose) polymerase inhibition synergizes with 5-fluorodeoxyuridine but not 5-fluorouracil in ovarian cancer cells. Cancer Res. 2011;71(14):4944–4954. doi: 10.1158/0008-5472.CAN-11-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evers B, Helleday T, Jonkers J. Targeting homolgous recombination repair defects in cancer. Trends Pharmacol. Sci. 2010;31:372–380. doi: 10.1016/j.tips.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Do KT, Wilsker D, Balasubramanian P, et al. Phase I trial of AZD1775 (MK1775), a wee1 kinase inhibitor, in patients with refractory solid tumors. J. Clin. Oncol. 2014;60:4009. doi: 10.1200/JCO.2014.60.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Andrea AD. Susceptibility pathways in Fanconi's anemia and breast cancer. N. Engl. J. Med. 2010;362:1909–1919. doi: 10.1056/NEJMra0809889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science. 2011;331:1553–1558. doi: 10.1126/science.1204040. [DOI] [PubMed] [Google Scholar]
- 42.Bouwman P, Jonkers J. Molecular pathways: how can BRCA-mutated tumors become resistant to PARP inhibitors? Clin. Cancer Res. 2014;20:540–547. doi: 10.1158/1078-0432.CCR-13-0225. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Han EK, Anderson M, et al. Acquired resistance to combination treatment with temozolomide and ABT-888 is mediated by both base excision repair and homologous recombination DNA repair pathways. Mol. Cancer Res. 2009;7:1686–1692. doi: 10.1158/1541-7786.MCR-09-0299. [DOI] [PubMed] [Google Scholar]
- 44.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 45.Bunting SF, Callen E, Wong N, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141:243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson N, Li YC, Walton ZE, et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat. Med. 2011;17:875–882. doi: 10.1038/nm.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim JJ, Yang K, Taylor-Harding B, et al. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2 . Neoplasia. 2014;16:343–353e1-2. doi: 10.1016/j.neo.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juvekar A, Burga LN, Hu H, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2:1048–1063. doi: 10.1158/2159-8290.CD-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl Acad. Sci. USA. 2008;105(44):17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konstantinopoulous PA, Wilson AJ, Saskowski J, et al. Suberoylanilide hydroxamic acid (SAHA) enhances olaparib activity by targeting homologous recombination DNA repair in ovarian cancer. Gynecol. Oncol. 2014;133:599–606. doi: 10.1016/j.ygyno.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopkins TA, Shi Y, Rodriguez LE, et al. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol. Cancer Res. 2015 doi: 10.1158/1541-7786.MCR-15-0191-T. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 52.www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=p140020 FDA: Medical Devices: Products and Medical Procedures: Device Approvals, Denials and Clearances: BRACAnalysis CDx – P140020: Summary of Safety and Efficacy Data.
- 53.Kaufman B, Shapira-Frommer R, Schutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2014;56:2728. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical trial data leading up to olaparib's US FDA accelerated approval are presented. Germline BRCA mutants were highly sensitive to PARPi.
- 54.Gunderson CC, Moore KN. BRACAnalysis CDx as a companion diagnostic tool for Lynparza. Expert Rev. Mol. Diagn. 2015;15(9):1111–1116. doi: 10.1586/14737159.2015.1078238. [DOI] [PubMed] [Google Scholar]; •• Development and regulatory issues concerning olaparib's US FDA-approved CDx, BRACAnalysis®. Components of the genome variant detection methods are discussed.
- 55.Burke PM. New Mexico Biotechnology and Biomedical Association. 11 August2015. BRACAnalysis CDx™, Tumor BRACAnalysis CDx™, myChoice HRD™: a portfolio of companion diagnostic products to optimize therapeutic selection for cancer patients.https://nmbio.org/wp-content/uploads/NMBio-Myriad-Presentation-August-2015.pdf [Google Scholar]
- 56.Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer. 2012;107(10):1776–1782. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Establishment of an homologous recombination deficiency (HRD) score by measuring genomic loss of heterozygosity. HRD score and BRCA1, BRCA2 and RAD51C mutations were highly correlated. Loss of heterozygosityis included in some PARP inhibitor companion diagnostic tests.
- 57.Birkbak NJ, Wang ZC, Kim JY, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitity to DNA damaging agents. Cancer Discov. 2012;2(4):366–375. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Popova T, Manié E, Rieunier G, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinoma with BRCA1/2 inactivation. J. Cancer Res. 2012;72:5454. doi: 10.1158/0008-5472.CAN-12-1470. [DOI] [PubMed] [Google Scholar]
- 59.Mills GM, Timms KM, Reid J, et al. Homologous recombination deficiency (HRD) score shows superior association with outcome compared with its individual score components (LOH, TAI, and LST scores) in platinum treated serous ovarian cancer. SGO. 2016 Abstract #6286. [Google Scholar]
- 60.The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcoxen K, Becker M, Neff C, et al. Homologous recombination deficiency (HRD) score enriches for niraparib sensitive high grade ovarian tumors. J. Clin. Oncol. 2015;33(Suppl.) Abstract 5532. [Google Scholar]
- 62.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013;31(11):1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Validation of a massively parallel DNA sequencing cancer genome profiling test that assesses analytical sensitivity, specificity, accuracy and precision. This is the basis for Foundation Medicine's next-generation sequencing-based assay.
- 63.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 64.Liu JF, Barry WT, Birrer M, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomized Phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huggins-Puhalla SL, Beumer JH, Appleman LJ, et al. A Phase I study of chronically dosed, single-agent veliparib (ABT-888) in patients (pts) with either BRCA1/2 mutated cancer (BRCA+), platinum-refractory ovarian cancer, or basal-like breast cancer (BRCA-wt) J. Clin. Oncol. 2012;30(Suppl) Abstract 3054. [Google Scholar]
- 66.Kummar S, Oza AM, Fleming GF, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin. Cancer Res. 2015;21(7):1574–1582. doi: 10.1158/1078-0432.CCR-14-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coleman RL, Sill MW, Bell-McGuinn K, et al. A Phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation – an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015;137(3):386–391. doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khalique S, Hook JM, Ledermann JA. Maintenance therapy in ovarian cancer. Curr. Opin. Oncol. 2014;265(5):521–528. doi: 10.1097/CCO.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 69.Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a Phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 70.Michie CO, Sandhu SK, Schelman WR, et al. Final results of the Phase I trial of niraparib (MK4827), a poly(ADP)ribose polymerase (PARP) inhibitor incorporating proof of concept biomarker studies and expansion cohorts involving BRCA1/2 mutation carriers, sporadic ovarian, and castration resistant prostate cancer (CRPC) J. Clin. Oncol. 2013;31 Abstract 2513. [Google Scholar]
- 71.Murai J, Huang SYN, Renaud A, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014;13(2):433–443. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen Y, Rehman FL, Feng Y, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin. Cancer Res. 2013;19(18):5003–5015. doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pulliam N, Taverna P, Lyons J, Nephew KP. Novel combination therapy of DNMT inhibitor SGI-110 and PARP inhibitor BMN-673 (talazoparib) for BRCA-proficient ovarian cancer. Cancer Res. 2015;106 Abstract 2943. [Google Scholar]
- 74.Swisher ES, Oza A, Coleman RL, et al. Tumor BRCA mutation or high genomic LOH identify ovarian cancer patients likely to respond to rucaparib: interim results for ARIEL2 clinical trial. Gynecol. Oncol. 2015;138:1–4. [Google Scholar]
- 75.McNeish IA, Oza AM, Coleman RL, et al. Results of ARIEL2: a Phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis. J. Clin. Oncol. 2015;33(Suppl.) Abstract 5508. [Google Scholar]
- 76.Wahlberg E, Karlberg T, Kouznetsova E, et al. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nature Biotechnol. 2012;10:1038. doi: 10.1038/nbt.2121. [DOI] [PubMed] [Google Scholar]; • PARP catalytic binding domain capacity of rucaparib, veliparib and olaparib are compared. Rucaparib was shown to have the most promiscuous binding profile, and may account for enhanced activity.
- 77.Drew Y, Mulligan EA, Vong WT, et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2 . J. Natl Cancer Inst. 2011;103:334–346. doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- 78.O'Sullivan CC, Moon DH, Kohn EC, Lee JM. Beyond breast and ovarian cancers: PARP inhibitors for BRCA mutation-associated and BRCA-like solid tumors. Front. Oncol. 2014;4:42. doi: 10.3389/fonc.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robillard L, Harding TC, Walter AO. Preclinical efficacy of the PARP inhibitor rucaparib (AG014699/PF-01367338) as a monotherapy and in combination with PI3K inhibition. Cancer Res. 2013;73(Suppl. 8) Abstract 3349. [Google Scholar]
- 80.McCrudden CM, O'Rourke MG, Cherry KE, et al. Vasoactivity of rucaparib, a PARP-1 inhibitor, is a complex process that involves myosin light chain kinase, P2 receptors, and PARP itself. PLoS ONE. 2015;10(2):e0118187. doi: 10.1371/journal.pone.0118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kristeleit RS, Burris HA, LoRusso P, et al. Phase I/II study of oral rucaparib: final Phase 1 results. J. Clin. Oncol. 2014;32(Suppl. 5) Abstract 2573. [Google Scholar]
- 82.Frommer RS, Oza AM, Domchek SM, et al. A Phase II open-label, multicenter study of single-agent rucaparib in the treatment of patients with relapsed ovarian cancer and deleterious BRCA mutation. J. Clin. Oncol. 2015;33(Suppl.) Abstract 5513. [Google Scholar]
- 83.Yelensky R, Wang K, Dogan S, et al. Next-generation sequencing of FFPE solid tumor specimens for clinical use. J. Clin. Oncol. 2012;30(Suppl.) Abstract 10524. [Google Scholar]
- 84.Sun J, McNeish I, Coleman L, et al. A novel companion diagnostic predicts response to the PARP inhibitor rucaparib in ovarian cancer. Cancer Res. 2015;106 Abstract 4670. [Google Scholar]
- 85.Swisher E, Scott C, Lin K, et al. AACR Ovarian Meeting 2015. Orlando, FL, USA: 7–20 October 2015. Identification of germline and somatic alterations in homologous recombination pathway genes in high-grade ovarian carcinomas and response to the PARP inhibitor rucaparib in ARIEL2. Presented at. Abstract A14. [Google Scholar]
- 86.Plummer R, Jones C, Middleton M, et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin. Cancer Res. 2008;14:7917. doi: 10.1158/1078-0432.CCR-08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shapira-Frommer R, Oza AM, Domchek SM, et al. A Phase II open-label, multicenter study of single-agent rucaparib in the treatment of patients with relapsed ovarian cancer and a deleterious BRCA mutation. J. Clin. Oncol. 2015;33 Abstract 5513. [Google Scholar]
- 88.Kristeleit R, Swisher ES, Oza AM, et al. European Cancer Congress. Vienna, Austria: 25–29 September 2015. Final results of ARIEL2 (part 1): a Phase 2 trial to prospectively identify ovarian cancer (OC) responders to rucaparib using tumor genetic analysis. Presented at. [Google Scholar]; •• Recent report of ARIEL2 Part 1 efficacy data, which utilized a companion diagnostic to stratify tumors by homologous recombination-deficient status. Data are highly encouraging for future rucaparib trials.
- 89.CHMP assessment report. European Medicines Agency. 2014. p. 789139.www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003726/WC500180154.pdf [Google Scholar]
- 90.Durmus S, Sparidans RW, van Esch A, et al. Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) restrict oral availability and brain accumulation of the PARP inhibitor rucaparib (AG-014699) Pharm. Res. 2015;32(1):37–46. doi: 10.1007/s11095-014-1442-z. [DOI] [PubMed] [Google Scholar]
- 91.Parrish KE, Cen L, Murray J, et al. Efficacy of PARP inhibitor rucaparib in orthotopic glioblastoma xenografts is limited by ineffective drug penetration into the central nervous system. Mol. Cancer Ther. 2015;14(12):2735–2743. doi: 10.1158/1535-7163.MCT-15-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]