Abstract
Novel effective immunotherapies are needed for patients with multiple myeloma (MM), since disease recurrence remains a major obstacle. B-cell maturation antigen (BCMA), a cell surface protein universally expressed on malignant plasma cells , has emerged as a very selective antigen to be targeted in novel treatments for MM. We here first review BCMA-related biology, and then highlight the recent clinical development of a novel afucosylated anti-BCMA monoclonal antibody conjugated with monomethyl auristatin F via noncleavable linker (GSK2857916). Chimeric antigen receptor-expressing T cells targeting BCMA may also induce specific and durable anti-MM responses by patients’ own effector cells. Clinical trials testing these two approaches (NCT02064387, NCT02215967) are currently ongoing in relapsed and refractory MM patients.
Keywords: : ADC, antibody drug conjugate, B-cell maturation antigen, BCMA, bone marrow, BM, CAR-expressing T cells, CAR-T, chimeric antigen receptor, Fc-engineered therapeutic antibody, microenvironment, MM, targeted immunotherapy, multiple myeloma
Multiple myeloma (MM) is the second most prevalent hematopoietic malignancy characterized by the abnormal accumulation of immunoglobulin-producing plasma cells (PCs) in the bone marrow (BM) and the development of osteolytic bone lesions. Over the past two decades, advances in the treatment of MM using conventional and novel therapeutics, alone or in combination with transplantation, have led to improved response rates and prolonged survival [1]. For example, the proteasome inhibitor bortezomib [2,3] has significantly improved outcome in both relapsed and newly diagnosed MM [4,5]. Second generation proteasome inhibitor carfilzomib has achieved impressive responses even in bortezomib-resistant MM. However, disease recurrence remains a major obstacle, and significant adverse effects are inevitable. Thus, there is still a large unmet need for more effective and less toxic treatments to improve patient outcome in MM.
Accumulated studies have characterized the natural history of MM from a premalignant precursor condition (Monoclonal gammopathy of undetermined significance, MGUS) to smoldering MM (SMM) to active MM and ultimately to end-stage plasma cell leukemia (PCL) [6]. Multiple genetic and oncogenic events, including deregulation of cyclin D1/D2, c-MYC [7], IRF4 [7] and c-MAF [8], as well as mutations of CDKN2C, FAM46C, K-/N-RAS and TP53 have been associated with these stages [6]. Besides abnormalities in critical growth and survival pathways within MM cells, the BM microenvironment is required for MM maintenance, progression, and development of drug resistance [9]. Following binding of MM cells in the BM, signaling cascades can be activated both by adhesion to the BM as well as accessory growth factors/ligands secreted by BM accessory cells, that is, bone marrow stromal cells (BMSCs), osteoclasts (OCs), osteoblasts (OBs), endothelial cells (ECs), T cells, dendritic cells (DCs), plasmacytoid DCs (pDCs), myeloid derived suppressor cells (MDSCs) and mesenchymal cells (MSCs) [10]. These BM accessory cells play a critical role in the MM niche to both promote disease and escape from immune surveillance. These accessory cells produce growth and antiapoptotic factors and cytokines for MM cells, for example, IL-6, IGF-1, SDF-1α, B-cell activation factor (BAFF), a proliferation-inducing ligand (APRIL), while expressing M-CSF, RANK ligand (RANKL), MIP1α, TGFβ, which cells also act in the BM milieu, that is, stimulation of OC differentiation leading to severe bone lysis. Elevated VEGF levels in MM patients enhance EC function and increase angiogenesis. Important cell growth and survival signaling cascades including ERK1/2, STAT3, AKT/PI3K and NF-κB, are constitutively activated via increased binding of receptors on MM cells and ligands on non-MM cells, leading to further induction of downstream target genes (i.e., NF-κB target genes) during disease progression. Therefore, novel targeted therapies may not only directly inhibit MM cell growth and survival, but also abrogate MM-promoting factors in the BM milieu.
Specifically, the ideal antigens for effective immunotherapies would be protein receptors highly expressed on tumor cell membrane during all stages of MM development. B-cell maturation antigen (BCMA), as the TNF receptor superfamily 17 (TNFRSF17), is an excellent candidate due to its selective expression in PCs at high level.
BCMA is an ideal antigen for targeted immunotherapy for MM
BCMA/TNFRSF17/CD269, closely related to BAFF receptor (BAFF-R) and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), plays a central role in regulating B-cell maturation and differentiation into PC. These three functionally related receptors are type III transmembrane proteins lacking a signal-peptide and containing cystein-rich extracellular domains (Figure 1). They promote B-cell survival at distinct stages of development by engaging APRIL and/or BAFF [11]. BCMA is expressed exclusively in B-cell lineage cells, particularly in the interfollicular region of the germinal center [12] as well as on plasmablasts and differentiated PCs [13,14]. It is selectively induced during PC differentiation, associated with loss of BAFF-R [15]. BCMA may enhance humoral immunity by stimulating the survival of normal PCs and plasmablasts [13,15]; however, it is absent on naïve and most memory B cells. Thus, BCMA does not appear to be critical for overall B-cell homeostasis, but is required for optimal survival of long-lived PCs in the BM [14,16].
Figure 1. . B-cell maturation antigen-induced signaling in the pathophysiology of MM B-cell maturation antigen belongs to the TNFR superfamily and is closely related to BAFF receptor and calcium modulator and cyclophilin ligand interactor (TACI).
Specifically, downregulation of BAFF-R on plasma cell (PC) is coincident with the upregulation of BCMA, which can bind BAFF and a proliferation-inducing ligand (APRIL) at low (μM) and high (nM) affinity, respectively. Both ligands are synthesized as membrane-bound proteins that can be released as soluble cytokines by furin protease cleavage and form soluble trimers. APRIL, a more specific growth and survival factor for PC, binds to sulfated side chains of HSPG (such as syndecan-1/CD138) at a site distinct from its binding site to bind to TACI and BCMA. Constitutively activated APRIL/BCMA signaling cascade leads to increased numbers of hyperactive malignant PC, and therefore represents a very promising target for novel immunotherapies in MM.
BAFF-R: BAFF receptor; BCMA: B-cell maturation antigen; HSPG: Heparan sulfate proteoglycan; MM: Multiple myeloma; TNFSF: TNFR superfamily.
In MM, BCMA is widely expressed on malignant PCs at elevated levels [17,18]. Using chromatin immunoprecipitation in the KMS12 MM cell line, BCMA is co-immunoprecipitated with interferon regulatory factor 4 (IRF-4), a master transcription factor mediating myeloma cell survival, suggesting its potential in MM oncogenesis [7]. BCMA, but not TACI or BAFF-R, is unequivocally expressed at high levels in all MM cell lines and MM patient cells [18,19]. When compared with BCMA, TACI is expressed at lower levels and reduced frequency on patient MM cells. Since TACI expression is linked to the status of differentiation and propensity for adherence of MM cells in the BM [20], TACIhigh MM cells show a more promising response than TACIlow group to atacicept, a TACI-Fc therapeutic [21]. In contrast and as on normal PCs, BAFF-R is hardly detected in malignant PCs in MM [18]. Nonetheless, the use of BAFF-R-Fc has minimal effect on survival of MM cells, suggesting a stronger dependency on either APRIL alone or APRIL plus BAFF for in vivo MM cell survival [21].
Most recently, gene and protein expression profiling confirm that BCMA is the most selectively expressed cell surface receptor on MM cell lines and patient MM cells [22–25]. BCMA expression is increased with progression from normal to MGUS to SMM to active MM. Since BCMA protein is undetectable on normal human tissues except for PCs, it has a very restricted expression pattern [22]. The other cell type with detectable BCMA mRNA and protein are pDCs (CD138-/BDCA-4+), which reside in the BM proximate to MM cells to promote their growth, survival and drug resistance [26]. Its level is significantly lower (more than tenfold difference) on pDC versus CD138+ PC derived from the same patient [24]. Thus, BCMA might be functional in pDC, further promoting MM cell survival and development of drug resistance. Importantly, donor derived anti-BCMA mAbs are identified in MM patients in remission after allogeneic transplant with graft-versus-MM response following donor lymphocyte infusion [27], further suggesting BCMA as a promising immunotherapeutic target in MM.
APRIL and BAFF are significantly increased in the circulation of patients in MM versus normal donors [20], suggesting that they may constitutively stimulate MM cells via induction of downstream targets, that is, antiapoptotic molecules MCL1, BCL2 [18,20,28,29]. BCMA has significantly higher affinity for APRIL than for BAFF (Kd range nM vs μM) [30]. Unlike BAFF, which also binds BAFF-R besides BCMA and TACI, APRIL binds to BCMA and TACI associated with heparan sulfate proteoglycan (HSPG), that is, CD138/syndecan-1 (Figure 1), suggesting a more PC-specific role of APRIL than BAFF [31]. In addition, serum BCMA is higher in MM patients versus healthy donors, suggesting a new biomarker for monitoring disease status and overall survival of MM patients [32]. All these results indicate an important role for the BCMA/APRIL signaling cascade in the pathophysiology of MM.
Approaches for targeting BCMA by cancer immunotherapies in MM
Since BCMA is a cell surface receptor on malignant PCs and induces signaling cascades upon binding to APRIL and BAFF, it is a bonafide PC target for drug candidates in MM and other BCMA-expressing B-cell malignancies, including Waldenström's macroglobulinemia (WM), Burkitt lymphoma, Diffuse Large B-Cell Lymphoma. Current clinical development of targeting BCMA includes mAb-based and effector T cell-based immunotherapies.
Rationale of targeting BCMA with mAb in MM
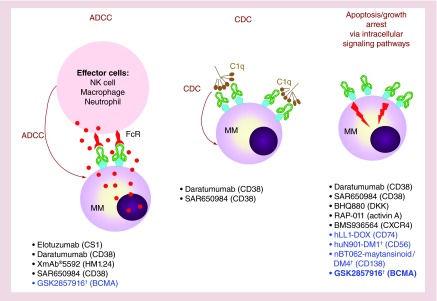
In brief, therapeutic mAbs function by blocking receptor-growth factor interactions, directly triggering growth arrest and apoptosis, or inducing elimination of mAb-coated target cells via activation of host defense mechanisms regulated by various Fcγ receptor (FcγR)-expressing effector cells (Figure 2). They have the ability to further activate immune effectors as well. Unlike currently available anti-MM drugs, therapeutic mAbs have longer half-lives, which can enhance their mechanisms of action at all stages of treatment. These additional features have indeed distinguished mAbs from small compounds as the drugs of choice in recent novel treatment strategies for a variety of diseases and cancers.
Figure 2. . Mechanisms of action of monoclonal antibody-based targeted therapies against multiple myeloma.
Therapeutic IgG monoclonal antibodies (mAbs) are designed to mediate ADCC or CDC, and can also directly inhibit growth or trigger apoptotic signaling pathways. Examples listed are mAbs tested in recent clinical trials in MM. The majority of MM target receptors/cell surface proteins on MM cells or extracellular factors (i.e., DKK1, activin A) in the BM microenvironment. Antibody drug conjugates are designed to directly kill MM cells to increase effectiveness of naked mAb. These include mAbs targeting CD56, CD138, CD74 and BCMA (the focus of this paper). In preclinical studies, only BCMA antibody drug conjugates can both interfere with ligand binding and elicit ADCC to lyse MM cells as well as directly induce apoptosis due to potent drug conjugates released inside the tumor cells following Ab–antigen binding.
†Ab–drug conjugate.
Ab: Antibody; ADCC: Antibody-dependent cellular cytotoxicity; CDC: Complement-mediated cytotoxicity; MM: Multiple myeloma; NK: Natural killer.
Promising clinical activities with reversible safety and tolerability profiles have recently been reported in completed and ongoing trials combining with lenalidomide/len and dexamethasone/dex with elotuzumab (elo) targeting CS1 (SLAMF7) [33], as well as daratuzumab (dara) and SAR650984 (SAR) targeting CD38 [34–36]. Dara and SAR show clinical activity as monotherapy. However, CS1 and CD38 are also expressed in other normal hematopoietic cells including activated NK cells, key mediators of antibody-dependent cellular cytotoxicity (ADCC). In addition, endothelial cells as well as activated lymphocytes and CD34+ hematopoietic progenitor cells also express CD38, which may limit clinical utilities of anti-CD38 mAbs. Blocking mAbs targeting BCMA, selectively and abundantly expressed on patient MM cells may have a very favorable therapeutic index.
One way to improve efficacy of naked therapeutic IgG mAbs and simultaneously circumvent defective immune cell function in advanced cancer patients is to develop antibody–drug conjugates (ADC). They conscript antibody specificity for anticancer drug delivery to achieve much greater potency than conventional cancer chemotherapeutics, while reducing the risk of toxic side effects. Thus far, Brentuximab vedotin (CD30) and Trastuzumab-DM1 (HER2) are two ADCs approved by the US FDA for treatment of relapsed Hodgkin's lymphoma and metastatic HER-2-positive breast cancer, respectively. Since the immune system in the majority of relapsed MM patients is impaired by the disease and/or cancer treatment, it is critical to develop ADCs targeting more specific antigens to direct and indirectly kill MM cells. To date, three ADC molecules with classical or novel drug payloads can directly kill MM cells, but do not induce effector-mediated activity in preclinical studies: CD56-maytansinoid (DM1) (Lorvotuzumab/IMGN901) [37,38], CD138-DM1/DM4 (BT062)[39,40] and CD74-doxorubicin (IMMU-110)[41]. A Phase II study combining BT062 (CD138-DM4) with len and dex is ongoing, demonstrating good tolerability and encouraging efficacy in Phase I clinical trial [40]. In order to develop more effective mAb-based therapy for MM, a novel ADC targeting BCMA was recently developed to very specifically kill MM cells, while sparing toxic side effects on surrounding normal cells [24].
Characteristics & mechanisms of actions (MOAs) of GSK2857916
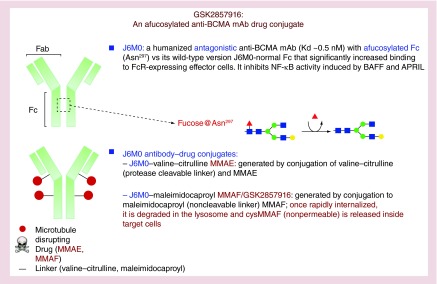
An anti-BCMA murine clone 118G03 was first generated and selected for its high affinity to human BCMA based upon competing binding of APRIL and BAFF to BCMA [24]. It specifically binds to BCMA on the cell membrane of all MM cell lines and pDC derived from MM patients. This murine mAb was subsequently chimerized (CA8) and humanized to derive J6M0, which maintains high affinity to BCMA (Kd of ∼0.5 nM) (Figure 3). J6M0 was Fc-engineered by Potelligent™ Technology, as was used for FDA-approved obinutuzumab (CD20), to completely eliminate fucose from the carbohydrate structure of an IgG1 antibody, thereby significantly improving its binding to FcR-expressing effector cells by up to 100-fold as compared with J6M0 with normal Fc. Although mutations in Fc residues (S293D:A330L:I332E) can also enhance mAb binding to effector cells [42,43], afucosylation was chosen for Fc engineering in J6M0. Importantly, minimal change is seen in selectivity, pharmacokinetics and immunogenicity of afucosylated Fc versus mutated Fc. Defucosylation of Fc glycans can also avoid the inhibitory effects of both plasma IgG on the binding of FcγRIIIa on NK cells and of their fucosylated counterparts on binding to the antigen on target cells.
Figure 3. . Development of a novel anti-B-cell maturation antigen antibody–drug conjugate GSK2857916.
BCMA: B-cell maturation antigen; mAb: Monoclonal antibody; MMAE: Monomethyl aurastatin E; MMAF: Monomethyl aurastatin F.
As expected and like J6M0 with normal Fc, afucosylated J6M0 binds to all MM cell lines and MM patient cells in flow cytometry analysis and immunohistochemistry (IHC) studies [24]. J6M0 showed more intensive binding on CD138+ versus pDC cells, indicating a correlation of BCMA mRNA with its protein level on the cell membrane. J6M0, as effectively as its normal-Fc homolog and murine chimeric CA8, neutralizes binding of both BCMA ligands, and subsequently blocks the induction of NF-κB signaling cascades. EC50 of J6M0 to inhibit NF-κB-p65 phosphorylation (ser536) is 0.91 and 2.43 μg/ml for BAFF and APRIL, respectively (Figure 3).
Since J6M0, either with normal or afucosylated Fc, did not directly induce MM cell death, it was next linked to potent anticancer drug auristatins to become J6M0 ADCs. Two J6M0 ADCs were made to effectively and directly induce killing of MM cells. Specifically, afucosylated J6M0 was next conjugated with a new class of antimicrotubulin drug aurastatin in order to directly induce killing of MM cells following binding and internalization of J6M0 [24]. J6M0 was linked to either valine-citrulline (vc; protease cleavable linker)-monomethyl aurastatin E (MMAE) or maleimidocaproyl (mc; noncleavable)-monomethyl aurastatin F (MMAF) [24,44]. Both J6M0 ADCs were released inside target MM cells following J6M0 binding via lysosome-dependent mechanisms as shown in Brentuximab vedotin which contain aurastatin but targeting CD30 [45]. J6M0-mc-MMAF, more potently (∼fivefold) and specifically than J6M0-vc-MMAE, blocked MM target cells without adverse effects on surrounding BCMA-negative normal cells (NK, monocytes, PBMCs or BMSCs). This indicates that mc-MMAF is more stable than vc-MMAE, and that MMAF is less membrane permeable than MMAE after being released from lysosomes. J6M0-mc-MMAF therefore minimizes the leakage of potent auristatin into culture supernatants, thereby reducing off-target effects on BCMA-negative surrounding cells. As a novel potent microtubule disrupting drug, MMAE and MMAF released inside MM cell from these ADCs potently induces G2/M arrest, followed by caspase3/7-dependent apoptosis (Figure 4.) J6M0-mcMMAF (GSK2857916) significantly blocks colony formation of MM cells even with soluble BCMA detected in MM cell cultures. The extent of toxicity correlates with BCMA levels in MM cells in both short-term (4-d) viability and long-term (3-week) colony formation assays, indicating that GSK2857916 effectively induces specific killing of MM cells even in the presence of soluble BCMA.
Figure 4. . GSK2857916 induces direct and indirect killing of multiple myeloma cells in the bone marrow microenvironment.
GSK2857916 specifically binds to BCMA on the MM cell membrane, and MMAF is then released inside MM cells by lysosome to induce G2-M growth arrest, followed by caspase 3/7-dependent apoptosis. It inhibits binding of APRIL and/or BAFF to BCMA, thereby blocking NF-κB signaling cascades critical for MM cell growth and survival. It has been Fc-engineered to enhance its binding to effector cells (i.e., NK cells, monocytes, macrophages), leading to significantly increased antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). ADCC and ADCP is induced via binding of FcγRs on NK cells and macrophages (myeloid effector cells) by tumor cell-bound GSK2857916. These three key anti-MM activities of GSK2857916 are further enhanced by lenalidomide or pomalidomide, triggering both direct and indirect killing of MM cells. Dexamethasone (Dex)/predonisolone, melphalan (Mel), or bortezomib (BTZ) also augment direct toxicity induced by GSK2857916.
BCMA: B-cell maturation antigen; MM: Multiple myeloma.
Afucosylated Fc of GSK2857916 consistently enhances potency (>1 log) and efficacy of effector cell-mediated ADCC, when compared with its homolog with normal Fc [24]. Importantly, enhanced maximal lysis and lower half maximal effective concentrations are evident even in autologous settings of patient MM cells. Both GSK2857916 and its naked mAb induce comparably higher NK-mediated ADCC against MM patient cells than its naked mAb with normal Fc, regardless of disease status of MM patients. Moreover, it induces dose-dependent MM1S cell lysis in the presence of its own cell culture supernatant containing soluble BCMA. The question of whether the efficacy of GSK2857916 may potentially be affected by a soluble BCMA sink can only be learned from the ongoing (NCT02215967) clinical trial. Of note, reduced ADCC has also been seen in similar in vitro assays using elotuzumab or daratumumab/SAR650984, which has demonstrated promising clinical responses when combined with len [46,47]. In a similar fashion, len enhances GSK2857916-induced ADCC against MM cells even in the presence of BCMA or soluble BCMA.
GSK2857916 rapidly eradicates MM cells in subcutaneous and disseminated mouse models with mice remaining tumor-free up to 3.5 months [24]. Naked J6M0 significantly inhibits MM cell growth and extends survival of mice, although less effectively than GSK2857916. In addition, Fc-bearing macrophages are significantly recruited to tumor tissues from both GSK2857916- and naked J6M0-treated SCID-beige mice with defective NK cells. In vitro phagocytosis assays using cultured human macrophages further confirm antibody-dependent phagocytosis (ADCP) as an additional Fc-dependent effector mechanism of GSK2857916 and its naked homolog to eliminate MM cells (Figure 4).
Therefore, GSK2857916 is the first therapeutic ADC with three distinct MOAs (apoptosis, ADCC, ADCP) to effectively eradicate MM cells in the BM microenvironment, while sparing essential normal cells in preclinical studies (Figure 4). A clinical trial in refractory/relapsed MM is ongoing (NCT02215967).
Combination of GSK2857916 with common anti-MM treatments
GSK2857916 can be combined with reagents commonly used in current treatment of MM, since combined treatments further enhance cytotoxicity of GSK2857916 and overcome potential inhibition by the presence of soluble BCMA in patient sera. In preclinical studies, direct cytotoxicity induced by GSK2857916 is enhanced by combined treatment with bortezomib, dex, or melphalan even in drug-resistant MM cells ([24] and unpublished data). GSK2857916-induced ADCC is increased when it is combined with Len or Pom even in the presence of BMSCs. In the absence of effector cells, Len further blocks GSK2857916-inhibited cell viability in dex- and len-resistant MM cells. Len or Pom might also activate function of FcR-bearing macrophages, thereby resulting in higher ADPC conferred by GSK2857916.
Anti-BCMA CAR-transduced T cell-based immunotherapy
In order to improve cytotoxicity against resistant cancer cells, major efforts and progress has been made with recombinant chimeric antigen receptor (CAR)-expressing T effector cells over the past decades [48–50]. CAR-modified T effector cells express fusion proteins of antigen receptors against tumor-associated surface antigens (TAAs) and T cell activation domains, thus redirecting the effector T cells and enhancing tumor-specific immunosurveillance. CAR consists of a single-chain antibody variable fragment (scFv; extracellular domain) linked through a CD8 hinge to transmembrane cytoplasmic signaling regions derived from CD3ζ (endodomain), with or without additional signaling domains derived from co-stimulatory molecules, that is, 4–1BB, CD28 and CD27. The scFv (an antigen recognition moiety) binds to a TAA on cancer cells and triggers effector cell activation upon target engagement in a non-MHC-restricted manner, which is a distinct advantage over T cell receptors (TCRs). Thus, they can be used in all individuals independent of their HLA type, unlike TCRs, in antigen-specific adoptive T cell therapy. Very encouragingly, the first clinical trial using anti-CD19 CAR-transduced T cells has showed long-term remissions in patients with difficult-to-treat B-cell malignancies [51], based upon preclinical studies demonstrating cure of leukemia and lymphoma by these engineered T cells in mice [52]. Such early impressive success on CAR-redirected autologous T cells has spurred much interest in broadening this novel technology to other hematologic malignancies and solid tumors. Given the extent of the known surface antigens, the relative ease of sampling tumor and the predisposition of T cell homing to BM, it is rational to develop CAR-transduced T cells for MM. Since CD19 is rarely expressed in patient MM cells, clinical use of anti-CD19 CAR-transduced T cells could be limited in MM. CS1 (SLAMF7), CD138 and CD38 are candidate TAAs for CAR-expressing effector cells, as recently reported in preclinical studies of CAR CD138 NK cells [53] and CAR CS1 NK [54] and T cells [55,56]. However, their expression profiles are not as restricted as BCMA on PCs versus other normal cells. Although testing is ongoing to determine the exact number of molecules that CAR T cell recognizes leading to a clinical response, CAR T cells can detect and target cells expressing even low levels of cognate antigen, thereby representing potential emerging toxicities of these CAR T cells. Thus, an additional advantage of BCMA for CAR-engineered T cells to treat MM is to reduce the emerging off tumor/on target toxicity by these powerful effector cells.
The first anti-BCMA CAR-transduced cytotoxic T cell (BCMA CAR T) was recently made by manipulating T cells to express a second-generation BCMA-specific CAR incorporating CD28-CD3ζ signaling moieties [22]. These BCMA CAR T cells include an additional CD28 co-stimulation domain linked to CD3 to increase cytokine production and enhance ability of adoptively transferred T cells to mediate tumor regression. Inclusion of co-stimulation domain, either from CD28 or 4–1BB, in addition to CD3ζ activation domain, is critical for CAR T cells to elicit clinical activity. Indeed, these performance-enhancing T cells specifically recognized BCMA on MM cells and proliferated when stimulated with BCMA-expressing target cells. Both CD4+ and CD8+ T cells showed BCMA-specific activation, with higher percentage of CD4+ T cells secreting IL-2 than CD8+ T cells. BCMA CAR T cells further produced IFNγ and TNF when stimulated with MM patient cells, and killed them in vitro. Moreover, in a xenograft mouse model of human MM, BCMA-redirected T cells completely eradicated human MM tumors in all mice and significantly prolonged mouse survival. As in the case for mAb-based therapies, a potential disadvantage of using CARs is that they detect native antigen; thus the interaction of T cells expressing CARs with MM cells may be blocked by soluble antigen released or shed from tumors into the surrounding tissue and circulation. However, BCMA protein in culture media did not block recognition of BCMA+ MM cells by BCMA CAR T cells in vitro, as evidenced by IFNγ induction. In addition, BCMA CAR T cells still abolish MM cell growth in mice with detectable soluble human BMCA before treatment. Human BCMA protein is undetected in the serum of a mouse that did not have a tumor after administration of BCMA CAR T cells. Nevertheless, follow-up of the mice was short in this report (up to 30 days), and the question remains as to whether the mice were actually cured by the BCMA CAR-transduced T cells.
Most recently, CS1 CAR T cell trial is initiated following preclinical results showing that T cells modified by CAR to target CS1 improve eradication of MM cells [56]. When compared with anti-CS1 CAR-transduced T cells which do not appear to mediate T cell intrinsic suicide in preclinical studies [56], anti-BCMA CAR-transduced T cells may be superior due to the lack of BCMA expression on T cells. Therefore, they may not impair CAR T cell expansion and the potential side effects due to rapid death of normal cells with low level of target expression. An ongoing clinical trial (NCT02215967) will determine whether soluble BCMA will inactivate the BCMA CAR T cells in patients.
Complementing BCMA targeting by blocking BAFF & APRIL
Targeting BCMA could be complemented by blocking binding of its ligands APRIL and BAFF since subsets of patient MM cells also express TACI, which also bind to APRIL and BAFF. However, TACI expression pattern is not as restricted as BCMA due to its presence in normal T and B cells [57]. Subsets of patient MM cells express higher levels of TACI when compared with normal PC and the levels of TACI are linked to the dependence of MM cells on BM microenvironment [20]. A neutralizing BAFF mAb (LY2127355) has been tested in combination with bortezomib in MM patients, with good tolerability and safe profile [58]. Atacicept, a fusion protein that binds to and neutralizes BAFF and APRIL, is designed to block activation of B cells by TACI [21,59]. It inhibits proliferation and induces apoptosis of patient MM cells [19,21]. It shows clinical and biological activity consistent with its mechanism of action when tested in MM and WM [60]. The first therapeutic APRIL neutralizing mAb was recently evaluated in preclinical studies in MM [61]. It specifically blocks APRIL-induced signaling cascade via BCMA, and further inhibits growth and survival of MM cells in the BM milieu in vitro and in vivo. Anti-APRIL mAb may be combined with anti-BCMA ADC or naked mAb to further enhance cytotoxicities of either agent alone and overcome drug resistance in the BM microenvironment.
Conclusion & future perspective
BCMA is very appealing target for mAb-based and CAR-modified effector cell-based immunotherapies for MM. To date, the use of mAb-based therapy in MM remains relatively nascent, while cellular immunotherapies have not shown significant clinical activities. GSK2857916, the first afucosylated auristatin-based next generation anti-BCMA ADC, has selective preclinical activities in MM. It potently induces apoptosis, ADCC and ADCP, as well as quickly eradicates MM in vivo in a model of disseminated MM in the BM of SCID-beige mice. It blocks BCMA signaling cascades constitutively triggered by APRIL and BAFF, suggesting its ability to overcome MM BM microenvironment-induced drug resistance in relapse/refractory MM. Importantly, the management of potential ADC off-target toxicities and the selection of appropriate dosing regimen should be carefully planned in order to bring highest clinical benefit of this ADC.
The second approach is to use an even more powerful immunotherapy by generating autologous BCMA CAR T cells which, at low doses, redirect T cells expressing BCMA to induce binding and killing MM cells. This new strategy has demonstrated promising preclinical activities and moved to clinical trial. Unlike many cellular-based therapies that attempt to increase persistence of the transferred cells in vivo, lack of BCMA expression on non-PCs may allow for expansion of BCMA CAR T effector cells in vivo and potential generation of memory CAR T cells to further harness immune function towards a cure of the disease. Moreover, it is likely that activities of BCMA CAR T cells can be modulated by IMiDs Len/Pom. Lack of target BCMA expression on the majority of normal cells may reduce toxicities and improve tolerability. Lessons learned from successful CD19 CAR T directed therapy of aggressive hematologic malignancies will provide very useful information to inform the design of clinical trials to test whether BCMA CAR T cells efficaciously eliminate MM cells, with sustained responses.
Since antigen binding moiety is different between GSK2857916 and BCMA CAR T cells sequential treatments with these approaches may provide even more robust anti-MM activity in future clinical trials. For example, BCMA CAR T cells may achieve long-lasting responses after treatments with GSK2857916 or when patients become refractory to the ADC. One potential clinical problem would be whether previous treatment with GSK2857916, although not binding the same target epitope as the BCMA CAR T cells, will select for escape variants that may or may not be recognized by BCMA CAR T cells; or vice versa. Like GSK2857916, activities of BCMA CAR T cells may be inhibited by soluble BCMA if the binding epitope is preserved in the soluble form compared with the membrane-bound form.
Other promising BCMA-based immunotherapies are simultaneously binding MM cells and cytotoxic T cells using mAb-like molecules, including bispecific T cell engager (BiTEs)[62,63]. BiTE Abs are designed to utilize the binding properties of variable domains of two mAbs to leverage endogenous cytotoxic potential of T cells to target the killing of cancer cells. Like CAR T cells, remarkably low doses of CD19-BiTE induces antitumor activities against CD19+ aggressive B-cell malignancies, and the first such BiTE (Blinatumomab) was recently approved by FDA [64]. Like CAR T cells, BiTEs have the added potential to overcome mutations in signaling pathways that classically lead to resistance. Compared with the CAR T cell approach, BiTE may provide a similar degree of anti-tumor effect with less cytokine release syndrome (CRS) and macrophage activation syndrome (MAS), which are emerging toxicities using both T cell-based immunotherapies. However, CRS and MAS could be blocked by anti-cytokine therapy such as anti-IL-6 mAb tocilizumab or general immune suppression with cortocorsteroids. On the other hand, BiTEs, as opposed to CAR T cells, may not stimulate persistent immunity against tumor cells, since CAR T cells can expand in vivo, with memory CAR T cells detected following months of administration. Besides the manipulation of T cells, blocking the function of inhibitory killer cell immunoglobulin-like receptors (KIRs) [65–68] or CD200R [69–72] may enhance NK cell function, thereby leading to enhanced ADCC to lyse MM cells by anti-BCMA mAbs. Specifically, the anti-KIR (KIR2DL1/L2/L3 and KIR2DS1/S2) antagonist antibody IPH2101, combined with lenalidomide was completed in a Phase I trial of patients with MM experiencing a first or second relapse [66]. Although no objective responses were seen, IPH2101 is safe and tolerable at doses that achieve full inhibitory KIR saturation [66]. The following Phase II clinical trial was recently completed in SMM patients where immune cells are still intact [65]. Encouragingly, results from this trial showed that NK cells elicit a tumor specific cytotoxic response, without harming normal cells [65]. This early clinical activity suggests that a more efficacious treatment strategy of manipulating the immune system with blockers of inhibitory NK cell function might improve anti-MM responses induced by anti-BCMA mAbs. On the other hand, since subsets of MM patients express CD200 which imparts an immunoregulatory signal through CD200R leading to the suppression of NK or T-cell-mediated immune responses, blocking interaction of CD200 and CD200R may alleviate inhibitory effector cell function in these patients [72]. This concept is further supported by the finding that increased CD200 is associated with regulatory T cell expansion and disease progression in subsets of MM patients [71].
Modulating the amplitude of immune responses against tumor cells may also be achieved by incorporation of immune check-point blockade, that is, anti-PD1/PDL1, CTLA-4 mAbs [73]. Antagonistic mAbs alleviating check-point blockades of T cells have recently been FDA approved to treat metastatic and resistant melanoma, and clinical trials are ongoing in many cancers [74]. Another strategy uses check-point PD-1 blockade to enhance activated T-cell responses after DC/tumor fusion vaccination in MM [75]. Finally, integration of BCMA-based immunotherapies with immune checkpoint inhibitors may enhance activity and function of T effectors and allow for sustained responses.
This is an exciting time, since new immune-based treatment strategies for MM may improve long-term responses and quality of life in patients. Novel next-generation immunotherapies combining mAbs, checkpoint blockades, vaccines and cellular therapies may allow for sustained autologous anti-MM immunity and potential cure.
Executive summary.
Multiple myeloma (MM) has been incurable due both to its highly heterogeneous molecular abnormalities as well as the support from the MM bone marrow microenvironment. A highly complex network is driven by malignant plasma cells (PCs), which progressively promote functional impairment of the host immune system and immunotherapies, as well as development of drug resistance. There is an urgent need to develop effective immunotherapies targeting specific molecules on MM cells to overcome both intrinsic and tumor microenvironment-mediated drug resistance.
MAb-based immunotherapy has started to show clinical activity even as monotherapy. With identification of selective target antigens on the MM cell membrane, more efficacious mAb-based therapeutic strategies can be developed by linking naked mAb with potent chemoreagents which are otherwise too toxic to be used in clinics.
Multiple gene expression profiling studies at mRNA and protein levels confirm that B-cell maturation antigen (BCMA) is an excellent MM target antigen due to its universal and increased expression on PCs, but not other normal essential tissues. Plasmacytoid dendritic cells, which protect MM cells in the BM and play a role in the development of drug resistance, have detectable BCMA, although at much lower level than PCs from the same patient. Furthermore, excess APRIL and BAFF constitutively activate growth and survival signaling cascades of MM cells in the BM microenvironment. These results underscore BCMA overexpression in the pathophysiology of MM.
A novel antibody–drug conjugate (ADC) targeting BCMA with afucosylated Fc linked to monomethyl aurastatin F (GSK2857916) has been chosen for clinical application. The naked form of this ADC has augmented effector-mediated cytotoxicity (antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis) via enhanced binding to multiple FcR-expressing effector cells (NK, monocytes, macrophages). In addition, this ADC specifically induces killing of MM cells and decreases colony formation, whereas surrounding BCMA-negative BM accessory cells remain unharmed. Importantly, it rapidly and completely eliminates tumor burden in vivo and prolong survival in two murine models.
BCMA is also an appropriate target antigen for treating MM with CAR-expressing T cells. T cells transduced with lentiviral vectors encoding BCMA-specific CARs induce BCMA-specific functions including cytokine production, proliferation and cytotoxicity. They also quickly eradicate MM cells in mice, leading to prolonged survival.
Both mAb-base and CAR T cell-based approaches targeting BCMA show promising anti-MM activities in preclinical studies, and derived clinical trials are ongoing.
Footnotes
Financial & competing interests disclosure
Y-T Tai is a consultant for Onyx. KC Anderson serves on advisory boards to Onyx, Celgene, Gilead, Bristol-Myers Squibb and Sanofi-Aventis and is a scientific founder of Acetylon and Oncopep. Funding: NIH grants RO1050947, PO1-CA078378 and DF/HCC SPORE in Multiple Myeloma P50CA100707; KC Anderson is an American Cancer Society Clinical Research Professor. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Dimopoulos MA, Richardson PG, Moreau P, Anderson KC. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat. Rev. Clin. Oncol. 2015;12(1):42–54. doi: 10.1038/nrclinonc.2014.200. [DOI] [PubMed] [Google Scholar]
- 2.Richardson PG, Mitsiades C. Bortezomib: proteasome inhibition as an effective anticancer therapy. Future Oncol. 2005;1(2):161–171. doi: 10.1517/14796694.1.2.161. [DOI] [PubMed] [Google Scholar]
- 3.Teicher BA, Anderson KC. CCR 20th anniversary commentary: In the beginning, there was PS-341. Clin. Cancer Res. 2015;21(5):939–941. doi: 10.1158/1078-0432.CCR-14-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimopoulos MA, Terpos E. Hematology: first-line bortezomib benefits patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2009;6(12):683–685. doi: 10.1038/nrclinonc.2009.171. [DOI] [PubMed] [Google Scholar]
- 5.Musto P, D'Auria F, Pietrantuono G, et al. First-line treatment of multiple myeloma in elderly patients: the GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto) multiple myeloma working party perspective. Curr. Drug Targets. 2009;10(10):906–922. doi: 10.2174/138945009789577936. [DOI] [PubMed] [Google Scholar]
- 6.Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J. Clin. Invest. 2012;122(10):3456–3463. doi: 10.1172/JCI61188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature. 2008;454(7201):226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The first report indicating that B-cell maturation antigen (BCMA) is critical for long-lived plasma cells but not essential for B-cell homeostasis in vivo.
- 8.Hurt EM, Wiestner A, Rosenwald A, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5(2):191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 9.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat. Rev. Cancer. 2007;7(8):585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]; • Indication that BCMA plays a minor role in overall B-cell homeostasis in vivo.
- 10.Tai YT, Anderson KC. Principles of pathway directed therapy. In: Schey SA, Yong KL, Marcus R, Anderson KC, editors. Myeloma: Pathology, Diagnosis & Management. Cambridge University Press; NY, USA: 2014. pp. 110–120. [Google Scholar]
- 11.Elgueta R, de Vries VC, Noelle RJ. The immortality of humoral immunity. Immunol. Rev. 2010;236:139–150. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiu A, Xu W, He B, et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2007;109(2):729–739. doi: 10.1182/blood-2006-04-015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 2003;112(2):286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199(1):91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darce JR, Arendt BK, Chang SK, Jelinek DF. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J. Immunol. 2007;178(9):5612–5622. doi: 10.4049/jimmunol.178.9.5612. [DOI] [PubMed] [Google Scholar]
- 16.Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol. Cell. Biol. 2001;21(12):4067–4074. doi: 10.1128/MCB.21.12.4067-4074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claudio JO, Masih-Khan E, Tang H, et al. A molecular compendium of genes expressed in multiple myeloma. Blood. 2002;100(6):2175–2186. doi: 10.1182/blood-2002-01-0008. [DOI] [PubMed] [Google Scholar]
- 18.Tai YT, Li XF, Breitkreutz I, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66(13):6675–6682. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- 19.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103(8):3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Identification that BCMA signaling is induced upon binding of its ligands in myeloma cells, which play an important role in myeloma cell growth and survival.
- 20.Moreaux J, Cremer FW, Reme T, et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood. 2005;106(3):1021–1030. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Underscores this pathway in multiple myeloma (MM) pathophysiology.
- 21.Yaccoby S, Pennisi A, Li X, et al. Atacicept (TACI-Ig) inhibits growth of TACI(high) primary myeloma cells in SCID-hu mice and in coculture with osteoclasts. Leukemia. 2008;22(2):406–413. doi: 10.1038/sj.leu.2405048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013;19(8):2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Confirmation of BCMA as a promising target antigen for the development of cytotoxic T cells engineered by chimeric antigen receptor to potently target the destruction of myeloma cells.
- 23.Maus MV, June CH. Zoom zoom: racing CARs for Multiple Myeloma. Clin. Cancer Res. 2013;19(8):1917–1919. doi: 10.1158/1078-0432.CCR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai YT, Mayes PA, Acharya C, et al. Novel anti-B-cell maturation antigen antibody–drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123(20):3128–3138. doi: 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Validation of BCMA as a specific MM target antigen for the development of a novel next generation of anti-BCMA monoclonal antibody and its derived antibody–drug conjugate with multiple mechanisms of action to kill myeloma cells.
- 25.Frigyesi I, Adolfsson J, Ali M, et al. Robust isolation of malignant plasma cells in multiple myeloma. Blood. 2014;123(9):1336–1340. doi: 10.1182/blood-2013-09-529800. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16(4):309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellucci R, Alyea EP, Chiaretti S, et al. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood. 2005;105(10):3945–3950. doi: 10.1182/blood-2004-11-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Existence of anti-BCMA monoclonal antibody in patients in remission following donor lymphocyte infusion, suggesting BCMA as a viable target for the development of immunotherapy for MM.
- 28.Neri P, Kumar S, Fulciniti MT, et al. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin. Cancer Res. 2007;13(19):5903–5909. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- 29.Tai YT, Acharya C, Zhong MY, et al. Constitutive B-Cell maturation antigen (BCMA) activation in human multiple myeloma cells promotes myeloma cell growth and survival in the bone marrow microenvironment via upregulated MCL-1 and NFκB signalling. ASH Annual Meeting Abstracts. 2013:681. [Google Scholar]
- 30.Rennert P, Schneider P, Cachero TG, et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 2000;192(11):1677–1684. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Indication of BCMA blockage could produce antitumor activity induced by APRIL.
- 31.Moreaux J, Sprynski AC, Dillon SR, et al. APRIL and TACI interact with syndecan-1 on the surface of multiple myeloma cells to form an essential survival loop. Eur. J. Haematol. 2009;83(2):119–129. doi: 10.1111/j.1600-0609.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez E, Li M, Kitto A, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012;158(6):727–738. doi: 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- 33.Lonial S, Vij R, Harousseau J-L, et al. Phase 1/2 study of elotuzumab in combination with lenalidomide and low dose dexamethasone in relapsed or refractory multiple myeloma: interim results. Blood. 2009;114:432. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 34.Laubach JP, Voorhees PM, Hassoun H, Jakubowiak A, Lonial S, Richardson PG. Current strategies for treatment of relapsed/refractory multiple myeloma. Expert Rev. Hematol. 2014;7(1):97–111. doi: 10.1586/17474086.2014.882764. [DOI] [PubMed] [Google Scholar]
- 35.Laubach JP, Tai YT, Richardson PG, Anderson KC. Daratumumab granted breakthrough drug status. Expert Opin. Investig. Drugs. 2014;23(4):445–452. doi: 10.1517/13543784.2014.889681. [DOI] [PubMed] [Google Scholar]
- 36.Deckert J, Wetzel MC, Bartle LM, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin. Cancer Res. 2014;20(17):4574–4583. doi: 10.1158/1078-0432.CCR-14-0695. [DOI] [PubMed] [Google Scholar]
- 37.Lutz RJ, Whiteman KR. Antibody-maytansinoid conjugates for the treatment of myeloma. mAbs. 2009;1(6):548–551. doi: 10.4161/mabs.1.6.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berdeja JG. Lorvotuzumab mertansine: antibody–drug-conjugate for CD56+ multiple myeloma. Front. Biosci. (Landmark Ed.) 2014;19:163–170. doi: 10.2741/4202. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda H, Hideshima T, Fulciniti M, et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo . Clin. Cancer Res. 2009;15(12):4028–4037. doi: 10.1158/1078-0432.CCR-08-2867. [DOI] [PubMed] [Google Scholar]
- 40.Kelly KR, Chanan-Khan A, Heffner LT, et al. Indatuximab Ravtansine (BT062) in combination with lenalidomide and low dose dexamethasone in patients with relapsed and/or refractory multiple myeloma: clinical activity in patients already exposed to lenalidomide and bortezomib. Blood. 2014;122 Abstract 4736. [Google Scholar]
- 41.Sapra P, Stein R, Pickett J, et al. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin. Cancer Res. 2005;11(14):5257–5264. doi: 10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
- 42.Tai YT, Horton HM, Kong SY, et al. Potent in vitro and in vivo activity of an Fc-engineered humanized anti-HM1.24 antibody against multiple myeloma via augmented effector function. Blood. 2012;119(9):2074–2082. doi: 10.1182/blood-2011-06-364521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan MC, Hering M, Peckham D, et al. Antibody targeting of B-cell maturation antigen on malignant plasma cells. Mol. Cancer Ther. 2007;6(11):3009–3018. doi: 10.1158/1535-7163.MCT-07-0464. [DOI] [PubMed] [Google Scholar]
- 44.Alley SC, Okeley NM, Senter PD. Antibody–drug conjugates: targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010;14(4):529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland MS, Sanderson RJ, Gordon KA, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J. Biol. Chem. 2006;281(15):10540–10547. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- 46.Tai YT, Dillon M, Song W, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112(4):1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 2011;186(3):1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 48.Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol. Rev. 2015;263(1):68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 49.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci. Transl. Med. 2015;7(280):280ps287. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 51.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang H, Zhang W, Shang P, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol. Oncol. 2014;8(2):297–310. doi: 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu J, Deng Y, Benson DM, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maus MV, June CH. CARTs on the road for myeloma. Clin. Cancer Res. 2014;20(15):3899–3901. doi: 10.1158/1078-0432.CCR-14-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu J, He S, Deng Y, et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clin. Cancer Res. 2014;20(15):3989–4000. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 58.Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin. Cancer Res. 2011;17(6):1278–1286. doi: 10.1158/1078-0432.CCR-10-1804. [DOI] [PubMed] [Google Scholar]
- 59.Gatto B. Atacicept, a homodimeric fusion protein for the potential treatment of diseases triggered by plasma cells. Curr. Opin. Investig. Drugs. 2008;9(11):1216–1227. [PubMed] [Google Scholar]
- 60.Rossi JF, Moreaux J, Hose D, et al. Atacicept in relapsed/refractory multiple myeloma or active Waldenstrom's macroglobulinemia: a Phase I study. Br. J. Cancer. 2009;101(7):1051–1058. doi: 10.1038/sj.bjc.6605241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tai YT, Acharya C, An G, et al. A novel anti-a proliferation-inducing ligand hAPRIL.01A monoclonal antibody targets multiple myeloma cells in the bone marrow microenvironment. ASH Annual Meeting Abstracts. 2014:2098. [Google Scholar]
- 62.Oak E, Bartlett NL. Blinatumomab for the treatment of B-cell lymphoma. Expert Opin. Investig. Drugs. 2015;24(5):715–724. doi: 10.1517/13543784.2015.1021415. [DOI] [PubMed] [Google Scholar]
- 63.Ramadoss NS, Schulman AD, Choi SH, et al. An anti-B cell maturation antigen bispecific antibody for multiple myeloma. J. Am. Chem. Soc. 2015;137(16):5288–5291. doi: 10.1021/jacs.5b01876. [DOI] [PubMed] [Google Scholar]
- 64.Sanford M. Blinatumomab: first global approval. Drugs. 2015;75(3):321–327. doi: 10.1007/s40265-015-0356-3. [DOI] [PubMed] [Google Scholar]
- 65.Korde N, Carlsten M, Lee MJ, et al. A Phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99(6):e81–83. doi: 10.3324/haematol.2013.103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benson DM, Hofmeister CC, Padmanabhan S, et al. A Phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120(22):4324–4333. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benson DM, Bakan CE, Zhang S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118(24):6387–6391. doi: 10.1182/blood-2011-06-360255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alici E. IPH-2101, a fully human anti-NK-cell inhibitory receptor mAb for the potential treatment of hematological cancers. Curr. Opin. Mol. Ther. 2010;12(6):724–733. [PubMed] [Google Scholar]
- 69.Flores-Montero J, de Tute R, Paiva B, et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin. Cytom. 2015 doi: 10.1002/cyto.b.21265. [DOI] [PubMed] [Google Scholar]
- 70.Coles SJ, Wang EC, Man S, et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25(5):792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aref S, Azmy E, El-Gilany AH. Upregulation of CD200 is associated with regulatory T cell expansion and disease progression in multiple myeloma. Hematol. Oncol. 2015 doi: 10.1002/hon.2206. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 72.Moreaux J, Hose D, Reme T, et al. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108(13):4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- 73.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 74.Azijli K, Stelloo E, Peters GJ. AJ VDE. New developments in the treatment of metastatic melanoma: immune checkpoint inhibitors and targeted therapies. Anticancer Res. 2014;34(4):1493–1505. [PubMed] [Google Scholar]
- 75.Rosenblatt J, Glotzbecker B, Mills H, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J. Immunother. 2011;34(5):409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]