Abstract
The current therapy for depression is less than ideal with remission rates of only 25–35% and a slow onset of action with other associated side effects. The persistence of anhedonia originating from depressed dopaminergic activity is one of the most treatment-resistant symptoms of depression. Therefore, it has been hypothesized that triple reuptake inhibitors (TRIs) with potency to block dopamine reuptake in addition to serotonin and norepinephrine transporters should produce higher efficacy. The current review comprehensively describes the development of TRIs and discusses the importance of evaluation of in vivo transporter occupancy of TRIs, which should correlate with efficacy in humans.
Background
Depression is a debilitating illness characterized by symptoms like anhedonia, depressed mood leading to suicidal thoughts, impaired cognitive functions, slowing of speech and other actions. Other symptoms include insomnia, loss of appetite and psychomotor agitation. Major depressive disorder (MDD) is a severe form of depression characterized by multiple episodes of depressed mood that must persist for at least 2 weeks accompanied by at least four other symptoms [1,2]. MDD can be differentiated as either melancholic depression or atypical depression based on the classification system set by the diagnostic and statistical manual of mental disorders IV (DSM-IV) [2,3]. While patients with melancholic depression manifest above symptoms, atypical depression patients have contrasting characteristics like increase in appetite, weight gain, hypersomnia and improved mood in response to positive events. Other depression types include dysthemic disorder, adjustment order, double depression, seasonal affective disorder and minor depression. MDD is however the most prevalent form of depression affecting 15–20% of population in the USA [4]. It is estimated that approximately 20% of all individuals suffer from a major mood disorder at least once in their lifetime. According to the WHO by 2020, MDD would be the second-most leading cause of disability worldwide, affecting 121 million people, thus making it a global health problem [5]. Currently, there are six major classes of antidepressant drugs, targeting mainly monoamine serotonin and norepinephrine transporters (NET), available. Although a plethora of antidepressants are in market, it is believed that approximately 30–40% of patients do not respond to the therapy, thus reflecting an unmet need to develop novel therapeutics to combat MDD.
In this review, we provide a functional and structural overview of the monoamine transporters (MAT) and summarize major classes of antidepressant drugs with an emphasis on triple reuptake inhibitors (TRIs) as the next-generation antidepressants. In the first part, we have detailed the design and development of novel TRIs undergoing preclinical development in our laboratory. Next, we have detailed literature drug candidates that have transcended to preclinical and clinical evaluation.
The monoamine deficiency hypothesis
The modulation of serotonergic and noradrenergic systems laid the foundation of the ‘monoamine deficiency’ hypothesis of depression that presumes a deficit of monoamine neurotransmitters in the synaptic cleft. The hypothesis has been extended by studies that found reduced levels of secondary messenger system, cAMP and phosphatidylinositol, for serotonergic and noradrenergic neurotransmission in depressed patients [1,6–8]. Thus, impaired monoaminergic transmission resulting from either a deficit of monoamine neurotransmitters in the synaptic cleft and/or disturbed receptor signaling are some of the key contributors that lead to depression [1,9,10]. While alterations in serotonin (5-HT) transmission correlated with aggressive, impulsive and anxious behavior; abnormalities in norepinephrine (NE) are related to motor activity, attention, memory and arousal [11–14]. The role of dopamine was not clearly understood and initially gained less interest in the pathophysiology of depression. Major drug developmental efforts have thus been directed toward inhibiting serotonin transporter (SERT) and NET proteins to increase the 5-HT and NE levels in the brain synapses. Studies have shown that other neurotransmitters like glutamate and gamma-aminobutyric acid (GABA) might also be involved in the neurobiology of mood disorder [15,16]. Other theories include abnormal circadian rhythms [17], lower brain cholesterol levels [18], impaired cholinergic-muscarinic functions [19], dysregulated endogenous opioid functions [20] and low cerebrospinal transthyretin levels [21]. However, the ‘monoamine deficiency’ is by far the most accepted and validated hypothesis and considered the gold standard in the treatment of depression. Although, in some recent findings, ketamine which is an antagonist at N-methyl-d-aspartate (NMDA) receptor, produced promising antidepressant effects in clinical trials in treatment-resistant depressed population [22,23]. More studies are underway to understand the effect of this drug in greater detail.
Structural & functional understanding of the MAT
MAT are integral membrane proteins which perform the transport of the monoamines: serotonin, NE and dopamine. The MAT – SERT, NET and dopamine transporter (DAT) – are important pharmacological targets of many pshychostimulant and antidepressant drugs. MAT are members of Na-dependent neurotransmitter symporters (NSS) that use cotransport of Na+ as a driving force for transport. The NSS family, referred to as the Na- and Cl-dependent solute carrier 6 (SLC6) families in the human genome organization, also include transporters for the amino acid neurotransmitters GABA and glycine [24]. The SLC6 family is the largest members of the SLC superfamily with 20 genes encoding a group of structurally similar transporter proteins with 20–30% sequence similarity between bacterial and their mammalian counterparts. First structural information on the SLC6 transporters became available in early 1900s with the cloning of the gene encoding the rat GABA transporter type 1 (GAT1) followed by cloning of the other family members [25–28]. It revealed 40% sequence identity between their primary structures suggesting that these transporters share a similar general structure. The amino acids range from 599 for GAT1 to nearly 700 for the two glycine transporters and are arranged as 12 transmembranes (TMs) helices. Significant efforts were made in the following studies to unearth the functional roles of these transporters in ion transport and elucidate residues implicated in substrate specificity and inhibitor binding. Heterologous expression system and mutagenesis experiments mainly the substituted cysteine assessment method (SCAM) were performed to identify the structural and functional role of individual residues of the transporters. It was shown that tyrosine in the TM3 of GAT1 was implicated in GABA binding and transport. Moreover, a tyrosine residue was found to be highly conserved across the SLC6 family implying its common role in the recognition of all substrates of the family [29]. Furthermore, an aspartate in TM1 (Asp79 in human DAT) is conserved among the monoamine proteins indicating its common role in monoamine substrate recognition [30]. This aspartate is substituted with glycine in other transporter proteins for amino acid transport. In another study, SCAM analysis on SERT revealed that Ile172 is involved in substrate recognition [31]. Although these studies provided a wealth of information, absence of a tertiary structure has presented challenge in structure-based drug design and further our understanding of the SLC6 transporter proteins at a molecular level.
In a significant breakthrough in 2005, the first x-ray crystal structure of the membrane protein, the leucine transporter (LeuT), from the bacterium Aquifex aeolicus in complex with Leu was determined to a 1.65-Å resolution [32]. Later, structures of LeuT in complex with different substrates and inhibitors were also published [33–36]. Crystal structure of LeuT mutant in the outward- and inward-open states in the absence of substrate has also been reported [37]. LeuT shared 20, 21 and 24% sequence identity with DAT, SERT and NET, respectively, with several structurally conserved domains including TMs 1, 3, 6 and 8 accounting high sequence similarity (55–67%) near the substrate-binding site [38]. Furthermore, functional similarities to the SLC6 transporter proteins suggest a common mechanism for transport making LeuT a surrogate model for studying the functional properties and pharmacology of human membrane proteins.
Traditional antidepressants
The history of antidepressants dates back to nearly seven decades when the earliest antidepressant agents were discovered serendipitously in 1950s [39]. Iproniazid, a monoamine oxidase inhibitor (MAOI), originally discovered for tuberculosis in 1951 was observed to produce euphoria like effect. Its antidepressant property was later presented at a regional meeting of the American Psychiatric Association in 1957. In the same year, the therapeutic effect of imipramine, the first tricyclic antidepressants (TCA), which was discovered based on the antipsychotic agent chlorpromazine, was published in the Swiss Medical Journal [39]. MAOIs like phenylzine, isocarboxazid and tranylcypromine were among the earliest class of antidepressants used [40]. They block the degradation of monoamines by nonselectively and irreversibly inhibiting enzymes MAO-A and MAO-B. Although MAOIs are effective in treating major depression disorder, they also inhibit the catabolism of dietary amines and can precipitate hypertensive crisis the ‘cheese effect’ when taken with foods rich in tyramine [41]. Moreover, their liability to produce severe drug–drug interactions further limits their clinical use as the last resort. Moclobimide and transdermal selegiline were later developed as reversible inhibitors of MAO-A and MAO-B, respectively, to address some limitations associated with the use of MAOIs as antidepressant agents. Another structurally different class, belonging to TCAs, increased synaptic monoamine levels by preventing the reuptake of 5-HT and NE into the nerve terminals. Although for several years TCAs stood as a classical therapeutic class for treating depression; they were hampered by their nonspecific interactions in the CNS including antagonism of adrenergic, histamine and cholinergic receptors [42]. TCAs can also cause fatal side effects including hypertension and arrhythmias by blocking L-type calcium and sodium channels. Despite these limitations, the first-generation antidepressants laid the genesis of the ‘monoamine deficiency’ hypothesis, which has been the mainstay in the treatment of depression.
Second-generation antidepressants
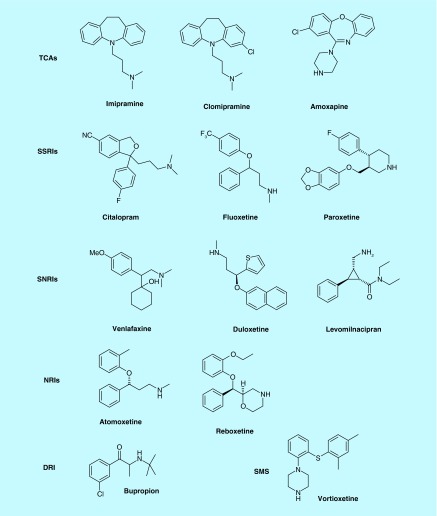
Over the past few decades, selective serotonin reuptake inhibitors (SSRIs) and dual serotonin/norepinephrine reuptake inhibitors (SNRIs) were developed (Figure 1) and hailed as second-generation antidepressants [43–47]. Prozac (fluoxetine) developed by the Eli Lilly company was the first SSRI approved in 1988, which grossed US$22 billion worldwide before its patent expired in 2001 [48]. Following fluoxetine, several SSRIs including citalopram, escaitalopram, sertaline and paroxetine also went on to attain the status of blockbuster drugs. Concurrently, research by the Danish University Antidepressant Group (DUAG) showed that clomipramine, a TCA that inhibited both serotonin and NE was much more effective than citalopram and paroxetine and fueled the concept, and controversy, of dual reuptake inhibitors for the management of MDD [49]. Later, in support of dual inhibition strategy, it was shown that venlafaxine, an SNRI, exhibited greater response and remission rates than SSRIs [50,51]. Inhibition of SERT is a common feature that TCAs, SSRIs and SNRIs possess to elicit an antidepressant response; however, selective NE reuptake inhibitors (NRIs): reboxetine, atomoxetine and viloxazine have also been developed for depression and are prescribed for attention-deficit/hyperactivity disorder (ADHD) [52–54]. SSRIs and SNRIs by virtue of their selective interactions at the SERT and NET exhibited an improved adverse effect profile; however, they were not without shortcomings [55,56]. These agents have failed to improve the efficacy and exhibit delayed onset, typically 4–6 weeks, of antidepressant response [57]. Moreover, because of relapse and unwanted sexual dysfunction side effects associated with SSRIs, it is imperative to discover newer targets for the treatment of depression [58,59]. Table 1 enlists monoamine inhibition data of approved MAT inhibitors including vortioxetine and livomilnacipran that have recently been approved for the treatment of depression.
Figure 1. . Representative structures of inhibitors from different classes of antidepressants.
DRI: Dopamine reuptake inhibitor; NRI: Norepinephrine reuptake inhibitor; SMS: Serotonin modulator and stimulator; SNRI: Serotonin/norepinephrine reuptake inhibitor; SSRI: Selective serotonin reuptake inhibitor; TCA: Tricyclic antidepressant.
Table 1. . List of monoamine transporter inhibitors as antidepressant agents.
| Inhibitor class | Inhibitor | Year approved | SERT (Ki, nM)† | NET (Ki, nM)† | DAT (Ki, nM)† |
|---|---|---|---|---|---|
| TCAs | Imipramine | 1951 | 1 | 37 | 8500 |
| Trimipramine | 1966 | 149 | 2450 | 1690 | |
| Amitriptyline | 1961 | 4 | 35 | 3780 | |
| Opipramol | 1961 | N/A‡ | N/A | N/A | |
| Desipramine | 1964 | 18 | 1 | 3190 | |
| Nortriptyline | 1963 | 18 | 4 | 1140 | |
| Protriptyline | 1966 | 20 | 1 | 2100 | |
| Dosulepin | 1969 | 9 | 46 | 5310 | |
| Butriptyline | 1974 | 1360 | 5100 | 3940 | |
| Clomipramine | 1970 | 0.3 | 38 | 2190 | |
| Doxepin | 1969 | 68 | 30 | 12,100 | |
| Maprotiline | 1974 | 5800 | 11 | 1000 | |
| Amoxapine | 1980 | 58 | 16 | 4310 | |
| Lofepramine | 1980 | 70 | 5 | 18,000 | |
| |
Amineptine |
1983 |
N/A |
N/A |
N/A |
| SSRI | Zimelidine | 1982 | 152 | 9400 | 11,700 |
| Fluoxetine | 1987 | 1 | 240 | 3600 | |
| Paroxetine | 1992 | 0.1 | 40 | 490 | |
| Fluvoxamine | 1994 | 2 | 1300 | 9200 | |
| Citalopram | 1989 | 1 | 4070 | 28,100 | |
| Sertraline | 1991 | 0.3 | 420 | 25 | |
| |
Escitalopram |
2001 |
1 |
>10000 |
>10,000 |
| SNRI | Sibutramine | 1997 | N/A | N/A | N/A |
| Venlafaxine | 1993 | 9 | 1060 | 9300 | |
| Milnacipran | 2009 | 420 | 77 | 6100 | |
| Duloxetine | 2004 | 1 | 8 | 240 | |
| Desvenlafaxine | 2008 | 40 | 558 | >100,000 | |
| |
Levomilnacipran§ |
2013 |
11 |
92 |
>10,000 |
| NRI | Viloxazine | 1977 | 17,300 | 155 | >100,000 |
| Mirtazapine | 1994 | >100,000 | 4600 | >100,000 | |
| Atomoxetine | 2002 | 152 | 5 | 658 | |
| |
Reboxetine |
1999 |
242 |
3 |
>100,000 |
| DRI |
Bupropion |
1985 |
9100 |
52,000 |
520 |
| NRI/DRI |
Nomifensine |
1977 |
1010 |
16 |
562 |
| SMS¶ | Trazodone | 1981 | 160 | 8500 | 7400 |
| Nefazodone | 1994 | 200 | 360 | 360 | |
| Vilazodone# | 2011 | 0.1 | 56 | 37 | |
| Vortioxetine†† | 2013 | 1.6 | 113 | >1000 |
‡N/A, no data available for human-cloned receptors.
§Activity values taken from [62].
¶Serotonin modulator and stimulator.
#Activity values taken from [63].
††Activity values taken from [64].
DAT: Dopamine transporter; DRI: Dopamine reuptake inhibitor; NET: Norepinephrine transporter; NRI: Norepinephrine reuptake inhibitor; SERT: Serotonin transporter; SNRI: Serotonin/norepinephrine reuptake inhibitor; SRS: Serotonin modulator and stimulator; SSRI: Selective serotonin reuptake inhibitor; TCA: Tricyclic antidepressant.
Triple reuptake inhibition: a panacea for depression?
To address the failure in full symptom remission following an antidepressant therapy, various augmentation approaches to SERT inhibition have been pursued. Triple reuptake inhibitors (TRIs) have emerged as a promising class that target SERT, NET and DAT at the same time. The concept of triple uptake inhibition has gained credence based on the evidence from the preclinical and clinical studies that dopamine plays an important role in the pathophysiology and treatment of depression. Dopaminergic neurons innervate brain in cortex, limbic and the pituitary gland the regions that are linked with cognition, motivation and reward [65–68]. Role of dopamine is suggested by the fact that depression amounts to 50% in patients with Parkinson’s disease (PD), a disease characterized by loss of dopaminergic neurons and reduction in DA transmission [69]. Biochemical evidences result from observations that concentration of homovanillic acid, a major metabolite of dopamine, is diminished in the cerebrospinal fluid of depressed patients [70]. It is reasoned that mesolimbic dopamine is associated with motivation and reward-related behavior and, therefore, including the dopamine activity should improve anhedonia that is the central component in depression [71,72]. Furthermore, it has been demonstrated that antidepressants could sensitize mesolimbic postsynaptic dopamine receptors, thereby implicating dopaminergic component in the therapy. This is validated by the findings that adjunct administration of a DAT blocker bupropion potentiates the action of both SSRI and SNRI in rat forced-swim tests (FST) and, furthermore, produced greater symptomatic improvement than when either drug is administered alone [73–76]. Moreover, piribedil a D2/D3 agonist and Pramipexole, a D3-preferring agonist, was found to be effective in both unipolar and bipolar depression [77,78]. Nomifensine, a DNRI, possesses significant dopaminergic activity and was formally approved as antidepressant [79]. Thus, an attractive strategy entails inhibiting the reuptake of all three monoamines: serotonin, noradrenaline and dopamine and developing TRIs for the treatment of depression [80,81]. Furthermore, it has been shown that treatment with bupropion improves SSRI-induced sexual dysfunction side effects [82,83]. A new era in the antidepressant therapy was ushered on the premise that a broader spectrum inhibition of the three major MAT would lead to enhanced efficacy and faster antidepressant response. Moreover, TRIs should be able to address broad range of depressive symptoms including anhedonia and also exhibit reduced side effects, which are associated with currently available antidepressants [84].
Our progress to the development of novel tris as potential antidepressant agents revisited
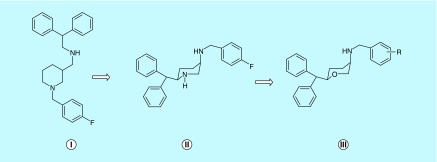
The research on TRIs in our laboratory stemmed from our interest in developing a pharmacotherapy for cocaine addiction. Initial attempts were directed in exploring the structural determinants in GBR 12909, a known DAT inhibitor, which conferred high and selective affinity for the DAT. The foremost study demonstrated that piperazine ring of GBR 12909 could be replaced by a piperidine ring with retention of activity [85]. Our next effort led to the identification of highly selective and more potent DAT inhibitors than the parent molecule GBR12909 with a promise as cocaine antagonists [86–88]. In subsequent efforts, with an aim to elucidate the bioactive conformation of our lead compounds, medicinal chemistry approach came to forefront in the design of conformationally rigid piperidine analogs (Figure 2), which emerged as more potent and selective at DAT than GBR12909 [89–91]. Further efforts led to the bioisosteric substitution of the piperidine ring with a pyran moiety resulting in the discovery of the cis-3,6-disubstituted pyran template, which became an established pharmacophore moiety of our lead TRIs, representing a unique structural class not known to any existing MAT [92]. It was observed that the cis-pyran analogs, like their piperidine counterparts, were more active than the trans-isomer. Although the pyran-based compounds did not significantly interfere with activity at the DAT in some cases, they were in general more active at the NET and SERT compared with the piperidine counterpart [92].
Figure 2. . Structural transformation in the design of pyran-based triple reuptake inhibitors.
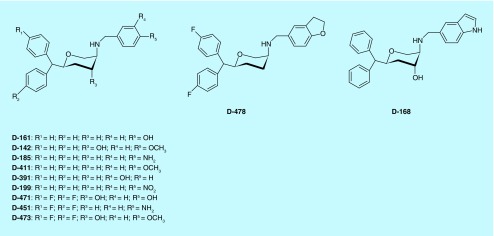
In a related field, neurotransporter blockers like SNRI and SSRI were widely used for the treatment of depression; albeit, applications of a broad spectrum approach to an effective antidepressant therapy were still nascent. In 2003, pioneering work from DOV Pharmaceuticals showed a TRI exhibiting an antidepressant effect [93,94]. Concurrently, research on neurotransporter inhibitors in our laboratory have started to shed more light on molecular determinants important for developing a TRI [95]. In 2005, we reported the asymmetric synthesis and characterization of novel tri-substituted pyran derivatives (2S,4R,5R) as potent TRIs [96]. The synthesized compounds were evaluated for their affinities for the DAT, SERT and NET by measuring their potencies in inhibiting the uptake of [3H]DA, [3H]5-HT and [3H]-NET. Compounds were also tested for their binding affinity at the DAT by their inhibition of [3H]-WIN 35,428. In general, tri-substituted pyran derivatives exhibited high affinity for SERT. Moreover, as expected, both regiospecificity and stereo selectivity played important roles as only derivatives with (-) 2S,4R,5R absolute configuration demonstrated appreciable activities particularly for SERT and NET. Furthermore, a cis-relationship between the benzhydryl moiety and the amino group was maintained for the SERT and NET interactions. Thus, starting from DAT selective inhibitors our endeavors have resulted in the discovery of several derivatives, including D142, which exhibited triple uptake inhibition profile with (Ki) values of 29.3, 14.7 and 59.3 nM (Table 2) for NET, SERT and DAT, respectively. Affinity of D142 for SERT was comparable to fluoxetine, a well-known SSRI. Furthermore, SAR studies led to the development of several tri-substituted (2S,4R,5R) pyran derivatives including D161 and D185 that exhibited potent triple uptake inhibitory activities [97]. D-161, a TRI, was most potent for NET followed by its affinity for SERT and DAT (Table 2). In the monoamine uptake assays, the potency of D-161 for NET and SERT was comparable to desipramine and fluoxetine or imipramine, respectively, indicating potent transporter blockade.
Table 2. . Binding affinity at dopamine transporter, serotonin transporter and norepinephrine transporter in rat brain.
| Compound | [3H]DA† uptake | [3H]5-HT† uptake Ki (nM) | [3H]DA† uptake |
|---|---|---|---|
| D-185‡ |
62.4 ± 5.6 |
16.1 ± 1.6 |
12.6 ± 3.7 |
| D-411§ |
15.9 ± 1.7 |
12.9 ± 1.3 |
29.3 ± 4.8 |
| D-161‡ |
42.0 ± 3.3 |
29.1 ± 3.5 |
30.5 ± 7.8 |
| D-142§ |
37.4 ± 3.9 |
14.7 ± 2.1 |
29.3 ± 7.9 |
| D-199¶ |
34.6 ± 6.5 |
19.9 ± 1.5 |
54.3 ± 9.8 |
| D-391§ |
31.3 ± 10.6 |
40.1 ± 4.9 |
38.5 ± 6.0 |
| D-471‡ |
38.4 ± 2.6 |
58.4 ± 4.0 |
4.20 ± 2.3 |
| D-478‡ |
11.2 ± 2.8 |
3.0 ± 0.3 |
6.1 ± 2.4 |
| D-451‡ |
32.5 ± 4.3 |
69.1 ± 19.8 |
8.4 ± 1.7 |
| D-168‡ |
85.2 ± 8.2 |
25.0 ± 8.4 |
25.5 ± 9.6 |
| D-473# |
70.4 ± 16.7 |
9.18 ± 1.69 |
39.7 ± 7.6 |
| Fluoxetine |
1092 ± 98 |
12.2 ± 2.4 |
158 ± 58 |
| Reboxetine | >10,000 | 503 ± 61 | 0.69 ± 0.21 |
Empowered by molecules (Figure 3) with three different biological profiles belonging to SNRI, NRI and TRI classes of drugs, the TRIs were then evaluated for their potential antidepressant effect in standard in vivo animal models. D-161 demonstrated potent in vivo activity in reducing immobility in preclinical antidepressant rat FST, and mouse tail suspension test [101]. In the FST, compared with vehicle control, D-161 at 10 and 20 mg/kg doses caused significant reduction of immobility. The result from the tail suspension test (TST) indicates that D-161 could reduce immobility dose-dependently with the highest dose (20 mg/kg) producing maximum reduction of immobility. At a dose of 15 mg/kg, the effect of D-161 in reduction of immobility was comparable to reference imipramine. Moreover, the results suggest that reduction of immobility did not result from activation of motor system as D-161 failed to increase motor activity when tested in the same dose range under the same experimental conditions. D-142 has emerged as another lead compound from our laboratory exhibiting potent antidepressant activity. In the rat FST, the dose range tested (2.5, 5 and 10 mg/kg), D-142 was much more efficacious than the reference imipramine. In the mouse TST, D-142 reduced immobility in a dose (2.5, 5 and 10 mg/kg)-dependent manner, suggesting potent antidepressant effect. In locomotor activity tests, compound D-142 did not exhibit any stimulation in the same dose ranges. Our continued efforts [98,99] have resulted in the discovery of several TRIs, which are listed in Table 2. In a recent report, we have described the development of an orally active TRI, D-473, which demonstrated in vivo efficacy in FST and showed elevated levels of all three monoamines in microdialysis study [100]. We have also developed a pharmacophore model for TRIs and have also embarked on a novel tetrahydrofuran template which demonstrated balanced, albeit moderate, activities at all three neurotransporters [102].
Figure 3. . Structures of lead pyran-based triple reuptake inhibitors.
TRIs in preclinical & investigational stage
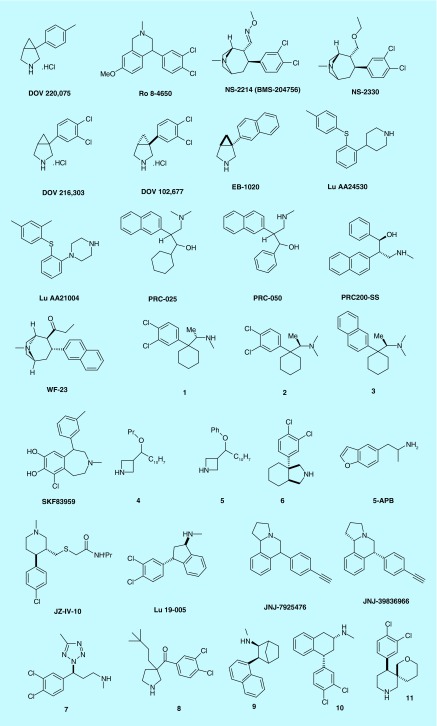
The structural differences between the MAT represent an enormous challenge to synthesize small molecules which can inhibit all three transporters appreciably while having improved bioavailability as well as broad safety profile. Moreover, until recently, no clinical data are available to pinpoint the optimal selectivity ratio of TRIs for all three transporters. However, it has been hypothesized that varying potency ratios can render different clinical outcomes which may be helpful for specific symptom-related treatments. For example, a specific potency ratio could be useful to treat pain, whereas another specific potency ratio may be useful to treat attention-deficit hypertension disorder. Here, we summarize TRIs that have been pursued to preclinical and investigational stage (Figure 4 and Table 3). DOV 220,075 or 1-(4-methylphenyl)-3-azabicyclo [3.1.0] hexane (Bicifadine) was developed as analgesic and for the treatment of pain by the DOV Pharmaceuticals [103]. It has stronger affinity toward SERT and NET compared with DAT (Table 3). Phase III clinical trial for this compound for pain indication has been completed. The half-life and T max of Bicafidine were found to be 1.6 and 1.06 h, respectively [104].
Figure 4. . Example of triple reuptake inhibitors in preclinical and investigational stage.
Table 3. . Physicochemical properties, binding affinities and functional potencies of investigational triple reuptake inhibitors.
| Compound | Mol wt. | CLogP | LE | Potency [3H]DA:[3H]NA:[3H]5-HT IC50 (nM) | Affinity DAT:NET:SERT Ki (nM/l) | Ref. |
|---|---|---|---|---|---|---|
| DOV-220,075 |
209.72 |
2.2 |
27.4 |
910:55:117 |
5.2:5.0:2.4 (μM) |
[103] |
| RO-84650 |
322.23 |
5.0 |
15.3 |
16.8:15.7:51 |
10.9:8.8:10.3 |
[105,106] |
| NS-2214 |
327.25 |
4 |
54 |
03:13:13 |
0.79:3.13:18.0 (EC50) |
[107,108] |
| NS-2330 |
314.25 |
3.6 |
10.9 |
01:07.7 |
N/A |
[80] |
| DOV-216,303 |
228.12 |
3 |
22.5 |
78:21:14 |
210:260:100 |
[109] |
| DOV-102,677 |
228.12 |
3 |
22.5 |
129:103:133 |
220:1000:740 |
[110] |
| EB-1020 |
209.29 |
2.9 |
30.5 |
0.2773495 |
N/A |
[111] |
| Lu-AA24530 |
283.43 |
4.5 |
22.6 |
X:23:8 |
DA<NE<SE |
[112] |
| Lu-AA21004 |
298.45 |
4.9 |
27.3 |
890:140:5.4 |
N/A:113:1.6 |
[113] |
| PRC-025 |
311.46 |
4.9 |
14.3 |
53:10:06 |
53:10:06 |
[114] |
| PRC-050 |
291.3 |
4.2 |
13.6 |
53:10:06 |
53:10:06 |
[114] |
| PRC200-SS |
291.3 |
4.2 |
13.6 |
18:0.6:2 (Kd) |
61:1.5:2.1 |
[115] |
| WF-23 |
307.43 |
4.2 |
17.9 |
0.65:0.32:0.47 |
0.12:0.39:2.9 |
[116] |
| 1 |
286.24 |
5.3 |
13.9 |
30:57:81 |
N/A |
[117] |
| 2 |
300.27 |
5.8 |
14.8 |
36:10:12 |
N/A |
[117] |
| 3 |
281.44 |
5.7 |
21.6 |
28:21:<1 |
N/A |
[117] |
|
SKF83959 |
317.81 |
4 |
6.7 |
1375:381:242 |
9010:600:1430 |
[118] |
| 4 |
255.35 |
2.9 |
17.4 |
12:06.8 |
N/A |
[119] |
| 5 |
289.37 |
4.6 |
21.3 |
35:8.3:3.1 |
N/A |
[119] |
| 6 |
270.2 |
4.7 |
17.9 |
140:150:53 (pIC50) |
N/A |
[120] |
| 5-APB |
175.23 |
2.3 |
16.2 |
6.3:6.3:5.8 (pKi) |
265:180:811 |
[121] |
| JZ-IV-10 |
354.94 |
3.1 |
13.2 |
|
1.0:0.8:1.1 |
[122] |
| Lu 19–005 |
292.2 |
4.8 |
21 |
11.9:8.8:5.1 |
1.7:5.8:0.42 |
[105,123] |
| JNJ-39836966 |
273.37 |
4.4 |
37.1 |
|
1.6:4.1:0.3 |
[124] |
| 7 |
300.19 |
2.7 |
|
19:08.8 |
N/A |
[125] |
| 8 |
328.28 |
5.6 |
7.3 |
N/A |
8.6:8.2:8.2 (pKi) |
[126] |
| 9 |
279.42 |
5.5 |
25.3 |
86:28:9 |
41:99:3.6 |
[127] |
| 10 |
306.23 |
4.9 |
19.7 |
72:73:107 |
N/A |
[128] |
| 11 |
300.22 |
3.6 |
10.6 |
8.0:8.9:8.6 |
7.0:8.4:9.5 |
[129] |
| DOV-21,947 |
228.12 |
3 |
22.5 |
96:23:12 |
213:262:99 |
[93] |
| SEP-225289 | 251.79 | 4.9 | 15.9 | 8 ± 7:17 ± 3:10 ± 8 | 49:20:15 | [130] |
DA:NA:5-HT: Dopamine:norepinephrine:serotonin; IC50: Concentration required for 50% inhibition in vitro; Ki: Binding affinity of the inhibitor; LE: Ligand efficiency; N/A: Not available.
Ro 8–4650 or diclofensine is a tetrahydroisoquinoline-based TRI that showed somewhat stronger potency for DA and NA compared with 5-HT (Table 3). In clinical trials, the compound showed efficacy to be an antidepressant with relatively less side effects [131]; however, due to its potential drug abuse issue, the compound was withdrawn from further clinical trials.
NS-2214 or BMS-204756 (brasofensine) is a phenyltropane-based TRI [107] and developed by the Bristol–Meyers Squibb company and later codeveloped by the NeuroSearch company. Due to its strong potency toward DAT over SERT and NET, it was a potential candidate to treat L-DOPA-induced dyskinesia in PD [132]. It was able to increase locomotor activity in MPTP-treated marmosets dose dependently. The compound is found to be metabolically more stable in human than rat [133]. Although it had shown good tolerability in Phase II clinical trial, further development was halted due to cis–trans isomerization of the aldoxime. Maintaining the same pharmacophore, GSK developed NS-2359 which is an analog of NS-2214. Primarily, it was being developed for ADHD [134]; however, further development was halted due to disappointing results from Phase II study [135]. NS-2330, the ethoxy analog of NS-2214, was initially developed for AD and PD [136]. Corresponding N-desmethyl compound (NS-2360) is the only metabolite and has slightly longer half-life (374 h) compared with the parent compound. Due to its comparatively low potency for DAT and weight loss side effect, later it was dropped from further development for PD.
Another azabicyclohexanes-based TRI, DOV 216,303 was also developed by the DOV Pharmaceuticals. It was able to inhibit the transport of all three monoamines in recombinant human MAT in vitro with desired potency [109]. In in vivo study, DOV 216,303 was able to reduce observed immobility both in the FST as well as in the tail suspension test. In clinical trial, DOV 216,303 was able to improve the Hamilton Depression Rating Scale (HAM-D) scores significantly when citalopram was used as a control [109]. The concern related to addiction of TRIs was alleviated in a recent study indicating that DOV 216,313 has much less addictive-like properties compared with cocaine [137].
DOV 102,677 is the (-)-isomer of DOV 216,303 and according to prediction, it showed nearly five- to eight-fold less potency at all three transporters; however, it has a ‘balanced’ potency, in other words, almost equal potency at all three transporters over the racemate and the (+)-isomer (Table 3) [110].
EB-1020 is being developed by the Euthymics company. It is a norepineprine and dopamine-preferring TRI which modulates reuptake of NE, DA and 5-HT in a 1:6:14 ratio. It is being developed as a drug to treat attention-deficit hypertension disorder [111]. It has been hypothesized that it may exhibit potential less risk of drug abuse liability. In TST, a lowest dose of 20 mg/kg was able to produce significant inhibition of immobility. In microdialysis experiment, EB-1020 was able to increase the extracellular concentration of NE significantly and DA to a lesser extent. In locomotor activity, only at a higher dose of 30 mg/kg it produced slight activity. EB-1020 also has strong ability to cross the blood–brain barrier (BBB), which is evident from a preferential blood to plasma ratio of 8 [111].
Lu AA24530 or tedatioxetine, a serotonin and NE-preferring TRI which is being developed by the Lundbeck and Takeda company [112]. According to the Montgomery–Asberg depression rating scale (MADRS) for primary efficacy end point study, the compound showed significant improvement compared with placebo. It was able to increase acetylcholine (Ach), NE, 5-HT and DA in brain. In addition, Lu AA24530 showed affinity to 5HT2C, 5HT2A and alpha 1A receptors [138]. Corresponding piperazine analog Lu AA21004 or Vortioxetine has been recently clinically approved for the treatment of MDD. It is a serotonin and NE-preferring TRI [113,139]. Typical side effects are dizziness, vomiting, headache, nausea, diarrhea and lower sexual side effects [140].
Racemic analogs of venlafaxine, PRC-025 and PRC-050, are serotonin- and NE-preferring TRIs developed by the Mayo Foundation [141]. Both compounds were able to reduce total immobility time in the TST and the FST when they were administered via intraperitoneally (ip.) at 5 and 10 mg/kg doses. The results were comparable to imipramine although at a higher dose (15 mg/kg). Both compounds did not stimulate locomotor activity at 5 mg/kg dose. However, PRC-050 produced significant locomotor activity at 10 mg/kg dose in rat. On the other hand, PRC-025 produced a significant increase in locomotor activity in mice at 10 mg/kg dose [114]. PRC200-SS, an optically pure enantiomer of PRC-050, inhibited uptake of serotonin, NE and dopamine with Ki values of 2.1, 1.5 and 61 nM, respectively. Similar to imipramine, PRC200-SS also reduced immobility in both FST in rats and tail suspension test in mice in a dose-dependent manner. Furthermore, peripheral administration of PRC200-SS (5 and 10 mg/kg ip.) significantly enhanced extracellular concentrations of NE and serotonin in the medial prefrontal cortex and dopamine and serotonin levels in the core of nucleus accumbens [115]. Its clinical development was terminated because of dose-propotional kidney toxicity observed in preclinical studies in cynomolgus monkeys [142]. Tropane scaffold-based compound WF-23 is an analog of cocaine and exhibited nearly 1000-fold potency for all three transporters [116]. It has very high bioavailability and exhibited slow metabolism [143]. With a dose of 1 mg/kg, it was able to produce significant increase in locomotor activity [144].
The Sunovion Pharmaceuticals has a series of TRIs based on novel cyclohexane alkyl amine scaffold. The N-monomethyl compound 1 is a dopamine and NE-preferring TRI which has a half-life of 0.53 h and inhibited hERG at 23 μM (IC50) [117]. Corresponding N-dimethyl compound 2 is a NE and serotonin-preferring TRI which has a half-life of 0.32 h and inhibited hERG at 40 μM (IC50). Compound 3 in which the 3,4-dichlorophenyl group was replaced by a 2-napthyl group, is a serotonin and NE-preferring TRI which has half-life of 0.28 h and inhibited hERG at 4.3 μM (IC50). The three compounds (1, 2 and 3) were tested at 5, 10 and 30 mg/kg in animal study, respectively. They have good BBB crossing capability and have shown good concentration in brain levels. All three compounds were able to reduce total immobility time by 30–33% in the TST at 30 mg/kg and also reduced locomotor activity to a great extent [117].
SKF83959, (3-methyl-6-chloro-7,8-hydroxy-1-(3-methylphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine), has been discovered as a competitive inhibitor of SERT but a noncompetitive inhibitor of DAT and NET [118]. SKF83959 was also active in animal models as it reduced immobility in the FST and the TST [118].
Han et al. have developed azetidine-based TRIs. 3-(Naphthalen-2-yl(propoxy)methyl)azetidine (4) and 3-(naphthalen-2-yl(phenoxy)methyl)azetidine (5) are serotonin- and norepinepherine-preferring TRIs. Both compounds have good half-life (4.8 and 3.3 h, respectively, p.o.) and the BBB crossing ability. In FST, compound 5 was able to reduce total immobility in a dose-dependent manner [119]. However, due to the presence of naphthyl groups, both compounds have inhibited hERG at low concentrations which may lead to serious side effects.
The Sunovion Pharmaceutical has been developing octahydro-1-H-isoindole-based TRIs which are structurally similar to DOV analogs. Compound 6 exhibited ‘balanced’ potency at all three transporters and was able to reduce total immobility time dose-dependently in the TST at 10 and 30 mg/kg doses. It did not increase locomotor activity compared with the control groups. The compound has good human microsomal stability (262 h) and inhibited hERG at 5.28 μM (IC50). In addition, the compound exhibited good BBB crossing ability and brain to plasma level (25:1) [120].
5-APB or 5-(2-aminopropyl)benzofuran was originally synthesized at the Eli Lilly. Most recently, it was reported by Iversen et al. that 5-APB has a ‘balanced’ TRI activity [121]. However, the compound may lead to severe cardiotoxicity because of its potent 5-HT2B agonist property. Due to its structural similarity with MDA, the compound is sold as a controlled substance in the market [145].
The Accenta Pharmaceuticals has utilized a hybrid approach to combine key pharmacophoric moieties of Nocaine (DAT/NET selective) and Modafinil (DAT preferred) to develop the TRI JZ-IV-10, which exhibited improved potency for all three transporters. The compound has ‘balanced’ affinity for all three transporters [122].
Lu 19–005 or Indatraline was developed by the Lundbeck company [146]. It is a serotonin and NE-preferring TRI [147]. It has a similar mode of action like cocaine, but slower onset of action and potentially be useful to treat various drug addiction issues [148].
JNJ-7925476 is an isoquinoline scaffold-based TRI that is being developed by the Johnson and Johnson company [124]. This racemic compound is a serotonin and dopamine-preferring TRI and corresponding (+)-diastereomer JNJ-39836966 is the most potent. JNJ-7925476 exhibited higher potency toward human transporter than rat. In microdialysis study, it was able to increase the extracellular level of all three neurotransmitters at 1 mg/kg dose; however, for DAT occupancy, a higher dose (10 mg/kg) was required to reach the same level as SERT and NET. The compound has good T max of 1 h and good BBB penetration ability. It has a half-life of 1.8 h in plasma and was able to increase extracellular level of all the neurotransmitters considerably [124].
Lee et al. have recently reported that tetrazole-containing compound 7 exhibited a ‘balanced’ TRI profile [125]. The compound has relatively higher half-life in human liver microsome compared with rat. It showed very moderate or minimal activity against other aminogenic receptor and CYP isoforms. It was able to increase 5-HTP-induced head-switches dose dependently. In FST and TST experiments, it was able to reduce the total immobility time significantly. In addition, the compound has a 5.48 h of elimination half-life and approximately 43% bioavailability when it was administered via PO [125].
Lucas et al. at the Roche company have developed pyrrolidine scaffold-based TRI. The 3,3-substituted pyrrolidine (8) has ‘balanced’ potency at all three neurotransmitter, however, it exhibited short half-life in human liver microsome [126].
Based on the scaffold of WF-23, the Eli Lilly company has developed TRIs. The 2-napthyl-based bicyclic analog (9) is a serotonin and NE-preferring TRI and was able to exhibit good oral activity [127]. However, it has very low oral bioavailability due to CYP-450-mediated hydroxylation of the naphthyl moiety. Several other analogs of this series were made by replacing the naphthyl moiety with benzothiophene; however, further development was halted due to unspecified reasons.
Researchers at the Sepracor company modified the tetrahydronapthalene-based structure of trans-setraline and developed a positional isomeric compounds, which exhibited TRI-like profile. However, majority of the TRIs in this series have shown strong inhibition of CYP and hERG. Overall, they chose compound 10 for in vivo studies based on its potency and liability. In TST, compound 10 was able to reduce overall immobility time in a dose-dependent manner and showed no effect on locomotor activity. The compound also showed good BBB crossing ability and at a dose of 30 mg/kg, a plasma ratio of 40 was observed [128].
Bettati et al. have developed oxa-azspiro derivatives as novel TRIs. Among these derivatives, compound 11 has exhibited high affinity for all three transporters. In functional potency assay, 11 showed comparable values similar to indatraline. In CYPEX bactosome P450 inhibition assay, 11 exhibited an excellent inhibition profile (IC50 >10 μM). The compound has long in vivo half-life (2.2 h) and exhibited a good brain to plasma ratio of 9.6. However, the compound undergoes extensive metabolism and as a result only overall 9% bioavailability was observed [129].
TRIs in clinical trials
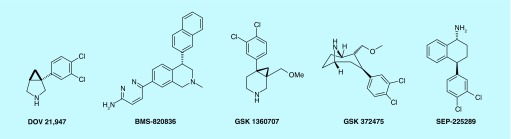
Based on the promising results from the animal model of depression, a number of TRIs drug candidates have been advanced to clinical development [109,115,149]. These include DOV 216,303, DOV 21,947, BMS 820836, GSK 1360707, GSK 372,475 and SEP 225289 (Figure 5).
Figure 5. . Triple reuptake inhibitors in clinical trials.
DOV 216,313 was safe and well tolerated in both single and multiple dose studies with healthy individuals. Safety and tolerability of DOV 216,303 was explored in a randomized, double-blind Phase II study on 67 subjects with moderate to severe MDD. Subjects received either 100 mg DOV 216,303 (50 mg b.i.d.) or 40 mg citalopram (20 mg, b.i.d.) for 2 weeks. Time-dependent reductions in HAM-D scores were observed in both the DOV 216,303 and citalopram groups compared with baseline scores (p < 0.0001). There was no significant difference in the side effect profiles of DOV 216,303 and citalopram. It should be noted that in this study a placebo control was not used [109,150].
DOV 21,947 (amtifadine) is the (+)-isomer of DOV 216,303 and has triple reuptake inhibitory profile with preference for SERT. It was able to reduce immobility both in the FST and in the TST [93]. It did not increase the locomotor activity significantly at doses 10 and 20 mg/kg compared with the positive control MK-801 [93]. In a 6-week randomized, double-blind, placebo-controlled study, amitifadine (EB-1010/DOV 21,947) was given in doses of 50 and 100 mg/day to a group of 63 patients with MDD [151]. The results demonstrated significant antidepressant activity and tolerability relative to placebo. Recently, amitifadine has undergone Phase IIb/IIIa clinical trial in the treatment-resistant 342 MDD patient group. The clinical trial (NCT01318434) involving paroxetine- and placebo-controlled study of 50 and 100 mg/day of amitifadine. The study did not show a statistically significant difference from placebo in the primary end point of a change in MADRS. However, the lack of side effects and signs of efficacy suggest that higher doses may show benefit in the target MDD patient group.
SEP-225289 was developed by the Sunovion Pharmaceuticals Inc. It has a very long half-life of 14 h and showed promising pharmacokinetic properties and tolerability in Phase I study. Although in in vitro study the compound exhibited good potency for all three transporters [152]; in vivo drug occupancy studies using PET revealed that it has low potency for serotonin and thus may not impart strong antidepressant effect [130]. SEP-225289 in a Phase II clinical trial (NCT00584974) on a double-blind, placebo- and active-controlled study on a 523-patient group with MDD did not result in reduction of symptoms of depression following 8 weeks of treatment. In contrast, positive control venlafaxine did achieve a significant reduction in MDD symptoms as compared with placebo. Furthermore, the serum concentration of SEP-225289 was observed to be lower than that expected at both doses (0.5 and 2 mg) as compared with exposure profiles observed in its Phase I study. Development of this drug has been discontinued.
In two placebo- and active-controlled, double-blind 10-week studies, the safety, efficacy and tolerability of GSK-372,475 were evaluated. Study 1 had patients with MDD while study 2 required patients with decreased pleasure and interest. In both studies, GSK-372,475 did not differ significantly from placebo. Furthermore, GSK-372,475 exhibited side effects like dry mouth, headache, insomnia and was not well tolerated [135].
In a Phase 1 PET study (NCT01153802) in healthy subjects pharmacokinetics and safety of single dose of GSK-1360707 was evaluated [153]. The study demonstrated that GSK-1360707 was well tolerated up to a dose of 150 mg. However, its further development was halted due to strategic reasons of the company.
BMS-820836 is a triple reuptake inhibitor developed by the Bristol–Myers Squibb. It exhibited high binding affinity for SERT, DAT and NET with IC50 values of 1.08, 5.67 and 7.99 nM, respectively, in human embryonic kidney 293 cells. It has a half-life of 34–57 h and T max of 5.0–7.2 h [154]. BMS-820836 had undergone two Phase IIb clinical trials. BMS-820836 indicated high level of safety and tolerability in human [154,155]. Development of BMS-820836 has been terminated as it turned out that the compound acted as a SNRI due to low occupancy of DAT, underscoring importance of in vivo receptor occupancy study [154].
It is promising that a number of TRIs drug candidates have entered Phase II clinical trials with a strong evidence of supporting the hypothesis that a TRI could be efficacious in patients with MDD. However, there have been set backs in Phase IIb clinical trials some of which are attributed to a number of assumptions not tested early on in the clinical studies. The doses selected for the SEP-225289 and DOV 21,947 studies were calculated on allometric scaling between rodents and humans and the target occupancy in humans was not determined. Furthermore, it was assumed that healthy volunteers and patients with treatment-resistant depression would have the similar level of target occupancy [156]. The success of a clinical trial also depends upon patient compliance. The Phase Ib study of DOV-21,947 faced an issue of patient noncompliance which had the effect in assessing the safety and efficacy of drug [157]. It is perceived that better understanding of the optimal inhibition of the three MAT, integration of translational research including a biomarker for functional neuroimaging of reward circuits, well-characterized dosing regimen and target population may provide a breakthrough in getting a TRI in clinic for the effective management of MDD.
Proposed transporter occupancy for a TRI
From various receptor occupancy, PET imaging studies with clinically approved antidepressants in humans, it has been established that an occupancy of 70–80% is required for the SERT and 50–70% is required for the NET for production of an antidepressant effect [153,158–160]. Also it has been established that to produce a dopamine effect without producing any addiction liability, an occupancy of 30–40% is required for the DAT [161]. This occupancy was exhibited by a DAT-blocking antidepressant, bupropion, in a PET imaging study in humans [161]. Therefore, it is hypothesized that an ideal TRI with above in vivo transporter occupancy at DAT, SERT and NET should produce optimal efficacy.
Potential of TRIs beyond antidepressant
Although the involvement of dopaminergic component in depression led to the development of TRIs having varied scaffold diversity, several recent clinical and preclinical studies revealed that they may have good potential to treat a broad range of diseases as described below.
Obesity: during the preclinical and clinical studies of tesofensine, weight loss was an adverse side effect [162]. This side effect had prompted the researchers at NeuroSearch to find out the efficacy of the compound in weight loss by using various doses and studying its metabolic profile. The compound proved to be efficacious in weight loss. Sibutramine, was approved by the US FDA to treat obesity but was later withdrawn from market owing to its cardiovascular side effects [163]. Antiobesity effect of another TRI, DOV 21,947 has also been demonstrated in both preclinical and clinical studies [164,165].
Antinociceptive effect: several preclinical studies indicated that the TRIs have strong potentials to act as antinociceptive agents. For example, bicifadine showed promising effect in animal model of pain and inflammation [103].
Treatment of addiction: DOV 102,677 has a ‘balanced’ TRI profile and in preclinical study, it was able to reduce alcohol consumption habits in rats without affecting others habits. Since dopamine has been linked to reward-related behaviors, it has been hypothesized that the TRIs can provide improved treatment regimen for addiction due to their dopamine reuptake inhibition property [166].
Treatment of ADHD: several dopamine and NE-preferring TRIs in preclinical trials for the potential use in ADHD [167].
Conclusion
The previous survey reveals that development of TRIs is an active field of research. Many compounds showed promising results in clinical as well as preclinical studies. Although the optimal potency ratio for all three transporters is yet to be established, it has become apparent that optimal in vivo occupancy of transporters has a greater significance to produce efficacy in the clinic. Thus, further systematic future clinical studies will be able to shed more light into it. In addition, these future clinical studies will also reveal whether the TRIs have any broader advantages over the SSRIs or SNRIs in terms of efficacy, early onset and side effects. We hope that this review will stimulate further interest in the development of TRIs.
Future perspective
Despite having about 30 drugs available for the treatment of MDD, there still exist a significant pressing unmet need for development of novel drugs with improved efficacy. The coming decade will present opportunities for major pharmaceutical players and researches to tap into this unmet need for developing efficacious agents demonstrating rapid antidepressant response with higher efficacy. Coming years may see a personalized treatment approach for patients with depression in order to increase remission rates. Development of suitable TRIs can provide solution to such challenges. Further research will lead to a better understanding of optimal receptor occupancy for the SERT, DAT and NET resulting in clinical success of future TRI as drug candidates. In the next decade or so, it is estimated that number of late-stage products will enter the MDD market including TRIs.
Key terms.
Anhedonia: A core symptom of major depressive disorder and other neuropsychiatric illness. It is characterized by an inability to feel pleasure from normally enjoyable acts.
Attention-deficit/hyperactivity disorder (ADHD): A common neurobehavioral disorder found mostly in children. It is characterized by difficulty in paying attention, hyperactivity and impulsive behavior.
Forced-swim test (FST): A widely used behavioral animal model for evaluation of efficacy of antidepressant drugs. The test involves forcing a rodent to swim in a tank filled with water and their mobility behavior for escape is then scored. The reduction of immobility is a measure of antidepressant activity of a drug given that it does not increase the locomotor activity.
Hamilton Rating Scale for Depression (HAM-D or HDRS): HAM-D is a widely used scale for measuring severity of depression in patients. It involves answering 17 or 21 questions related to mood disorder. The higher the HAM-D score the more severe is the depression.
Brain microdialysis: A widely used minimally invasive technique used for monitoring and quantifying neurotransmitters and hormones in the brain and periphery.
Executive summary.
Monoamine deficiency has been proposed as one of the validated hypotheses for development of major depressive disorder.
Structure of leucine transporter, from the bacterium Aquifex aeolicus is currently used as a surrogate model for human monoamine transporters.
Selective serotonin reuptake inhibitors and serotonin/norepinephrine reuptake inhibitors are the current first-line treatment options for major depressive disorder.
Preclinical and clinical studies have demonstrated that dopamine plays an important role in the pathophysiology and treatment of depression.
Triple reuptake inhibitors inhibiting uptake of SERT, NET and DAT in a calibrated way are a novel class of drug and has been hypothesized to produce greater efficacy than existing antidepressants.
D-473 is a novel triple reuptake inhibitor (TRI) with potent in vivo efficacy which is currently under development.
Several inhibitors have been investigated in preclinical stage. Few candidates including DOV 216,303, DOV 21,947, BMS 820836, GSK 1360707, GSK 372,475 and SEP 225289 have gone through clinical trials.
Proposed occupancy required for a TRI for producing superior antidepressant effect is 70–80% for SERT, 50–70% for NET and 30–40% for DAT.
Footnotes
Financial & competing interests disclosure
This work is supported by the National Institute of Mental Health/The NIH Grant MH084888 (A Dutta). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Belmaker RH, Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]; •• Underscores monoamine deficiency as one key hypothesis of major depressive disorder.
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (4th Edition) American Psychiatric Association; Washington, DC, USA: 2000. [Google Scholar]
- 3.Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc. Assoc. Am. Physicians. 1999;111(1):22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- 4.Millan MJ. Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs. Neurotherapeutics. 2009;6(1):53–77. doi: 10.1016/j.nurt.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 6.Blendy JA. The role of CREB in depression and antidepressant treatment. Biol. Psychiatry. 2006;59(12):1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Shimon H, Agam G, Belmaker RH, Hyde TM, Kleinman JE. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am. J. Psychiatry. 1997;154(8):1148–1150. doi: 10.1176/ajp.154.8.1148. [DOI] [PubMed] [Google Scholar]
- 8.Coupland NJ, Ogilvie CJ, Hegadoren KM, Seres P, Hanstock CC, Allen PS. Decreased prefrontal myo-inositol in major depressive disorder. Biol. Psychiatry. 2005;57(12):1526–1534. doi: 10.1016/j.biopsych.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Coppen A. The biochemistry of affective disorders. Br. J. Psychiatry. 1967;113(504):1237–1264. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- 10.Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci. 2003;72(15):1665–1682. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- 11.Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33(26):2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 12.Handley SL. 5-Hydroxytryptamine pathways in anxiety and its treatment. Pharmacol. Ther. 1995;66(1):103–148. doi: 10.1016/0163-7258(95)00004-z. [DOI] [PubMed] [Google Scholar]
- 13.Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004;7(2):193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- 14.Racagni G, Brunello N. Physiology to functionality: the brain and neurotransmitter activity. Int. Clin. Psychopharmacol. 1999;14(Suppl. 1):S3–S7. doi: 10.1097/00004850-199905001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasler G, Van Der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2007;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc. Natl Acad. Sci. USA. 2006;103(19):7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beasley CL, Honer WG, Bergmann K, Falkai P, Lutjohann D, Bayer TA. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449–455. doi: 10.1111/j.1399-5618.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 19.Bymaster FP, Felder CC. Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Mol. Psychiatry. 2002;7(Suppl. 1):S57–S63. doi: 10.1038/sj.mp.4001019. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch. Gen. Psychiatry. 2006;63(11):1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan GM, Mann JJ, Oquendo MA, Lo ES, Cooper TB, Gorman JM. Low cerebrospinal fluid transthyretin levels in depression: correlations with suicidal ideation and low serotonin function. Biol. Psychiatry. 2006;60(5):500–506. doi: 10.1016/j.biopsych.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol. Psychiatry. 2012;72(7):537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 24.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins introduction. Pflugers Arch. 2004;447(5):465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 25.Guastella J, Nelson N, Nelson H, et al. Cloning and expression of a rat brain GABA transporter. Science. 1990;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254(5031):579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- 27.Kilty JE, Lorang D, Amara SG. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991;254(5031):578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- 28.Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- 29.Bismuth Y, Kavanaugh MP, Kanner BI. Tyrosine 140 of the gamma-aminobutyric acid transporter GAT-1 plays a critical role in neurotransmitter recognition. J. Biol. Chem. 1997;272(26):16096–16102. doi: 10.1074/jbc.272.26.16096. [DOI] [PubMed] [Google Scholar]
- 30.Kitayama S, Shimada S, Xu H, Markham L, Donovan DM, Uhl GR. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc. Natl Acad. Sci. USA. 1992;89(16):7782–7785. doi: 10.1073/pnas.89.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JG, Rudnick G. Permeation and gating residues in serotonin transporter. Proc. Natl Acad. Sci. USA. 2000;97(3):1044–1049. doi: 10.1073/pnas.97.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature. 2005;437(7056):215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]; • Reports the first x-ray cyral structure of the membrane protein the leucine transporter (LeuT).
- 33.Zhou Z, Zhen J, Karpowich NK, et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science. 2007;317(5843):1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448(7156):952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- 35.Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322(5908):1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat. Struct. Mol. Biol. 2009;16(6):652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481(7382):469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristensen AS, Andersen J, Jorgensen TN, et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 2011;63(3):585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 39.Ban TA. The role of serendipity in drug discovery. Dialogues Clin. Neurosci. 2006;8(3):335–344. doi: 10.31887/DCNS.2006.8.3/tban. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789–797. doi: 10.1007/s40263-013-0097-3. [DOI] [PubMed] [Google Scholar]
- 41.Deftereos SN, Dodou E, Andronis C, Persidis A. From depression to neurodegeneration and heart failure: re-examining the potential of MAO inhibitors. Expert Rev. Clin. Pharmacol. 2012;5(4):413–425. doi: 10.1586/ecp.12.29. [DOI] [PubMed] [Google Scholar]
- 42.Rose JB. Tricyclic antidepressant toxicity. Clin. Toxicol. 1977;11(4):391–402. doi: 10.3109/15563657708988202. [DOI] [PubMed] [Google Scholar]
- 43.Spinks D, Spinks G. Serotonin reuptake inhibition: an update on current research strategies. Curr. Med. Chem. 2002;9(8):799–810. doi: 10.2174/0929867024606795. [DOI] [PubMed] [Google Scholar]
- 44.Wong DT, Bymaster FP. Development of antidepressant drugs. Fluoxetine (prozac) and other selective serotonin uptake inhibitors. Adv. Exp. Med. Biol. 1995;363:77–95. [PubMed] [Google Scholar]
- 45.Hiemke C, Hartter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol. Ther. 2000;85(1):11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 46.Owens MJ, Nemeroff CB. The serotonin transporter and depression. Depress. Anxiety. 1998;8(Suppl. 1):5–12. [PubMed] [Google Scholar]
- 47.Di Giovanni G, Esposito E, Di Matteo V. Role of serotonin in central dopamine dysfunction. CNS Neurosci. Ther. 2010;16(3):179–194. doi: 10.1111/j.1755-5949.2010.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wenthur CJ, Bennett MR, Lindsley CW. Classics in chemical neuroscience: fluoxetine (prozac) ACS Chem. Neurosci. 2014;5(1):14–23. [Google Scholar]
- 49.Danish University Antidepressant Group. Citalopram: clinical effect profile in comparison with clomipramine. A controlled multicenter study. Psychopharmacology (Berl.) 1986;90(1):131–138. doi: 10.1007/BF00172884. [DOI] [PubMed] [Google Scholar]
- 50.Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br. J. Psychiatry. 2001;178:234–241. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- 51.Nemeroff CB, Entsuah R, Benattia I, Demitrack M, Sloan DM, Thase ME. Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biol. Psychiatry. 2008;63(4):424–434. doi: 10.1016/j.biopsych.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 52.Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr. Drugs. 2009;11(3):203–226. doi: 10.2165/00148581-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 53.Ratner S, Laor N, Bronstein Y, Weizman A, Toren P. Six-week open-label reboxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(5):428–433. doi: 10.1097/01.chi.0000155327.30017.8c. [DOI] [PubMed] [Google Scholar]
- 54.Breder CD. 2010 US 20100069390 A1. [Google Scholar]
- 55.Barbey JT, Roose SP. SSRI safety in overdose. J. Clin. Psychiatry. 1998;59(Suppl. 15):42–48. [PubMed] [Google Scholar]
- 56.Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders – III. Tolerability, safety and pharmacoeconomics. J. Psychopharmacol. 1998;12(3 Suppl. B):S55–S87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- 57.Gumnick JF, Nemeroff CB. Problems with currently available antidepressants. J. Clin. Psychiatry. 2000;61(Suppl. 10):5–15. [PubMed] [Google Scholar]
- 58.Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress. Anxiety. 1997;6(1):10–18. doi: 10.1002/(sici)1520-6394(1997)6:1<10::aid-da2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 59.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 60.Andersen J, Kristensen AS, Bang-Andersen B, Stromgaard K. Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters. Chem. Commun. (Camb.) 2009;(25):3677–3692. doi: 10.1039/b903035m. [DOI] [PubMed] [Google Scholar]
- 61.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997;340(2–3):249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 62.Auclair AL, Martel JC, Assie MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338–347. doi: 10.1016/j.neuropharm.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 63.Owen RT. Vilazodone: a new treatment option for major depressive disorder. Drugs Today (Barc.) 2011;47(7):531–537. doi: 10.1358/dot.2011.47.7.1622076. [DOI] [PubMed] [Google Scholar]
- 64.Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int. J. Clin. Pract. 2014;68(1):60–82. doi: 10.1111/ijcp.12350. [DOI] [PubMed] [Google Scholar]
- 65.D’Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur. J. Pharmacol. 2000;405(1–3):365–373. doi: 10.1016/s0014-2999(00)00566-5. [DOI] [PubMed] [Google Scholar]; •• Provides a review of the role of dopamine in the treatment of depression.
- 66.Papakostas GI. Dopaminergic-based pharmacotherapies for depression. Eur. Neuropsychopharmacol. 2006;16(6):391–402. doi: 10.1016/j.euroneuro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 68.Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J. Psychopharmacol. 2007;21(5):461–471. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- 69.Mcdonald WM, Richard IH, Delong MR. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol. Psychiatry. 2003;54(3):363–375. doi: 10.1016/s0006-3223(03)00530-4. [DOI] [PubMed] [Google Scholar]
- 70.Brown AS, Gershon S. Dopamine and depression. J. Neural. Transm. Gen. Sect. 1993;91(2–3):75–109. doi: 10.1007/BF01245227. [DOI] [PubMed] [Google Scholar]
- 71.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 72.Willner P. Dopamine and depression: a review of recent evidence. I. Empirical studies. Brain Res. 1983;287(3):211–224. doi: 10.1016/0165-0173(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 73.Mischoulon D, Nierenberg AA, Kizilbash L, Rosenbaum JF, Fava M. Strategies for managing depression refractory to selective serotonin reuptake inhibitor treatment: a survey of clinicians. Can. J. Psychiatry. 2000;45(5):476–481. doi: 10.1177/070674370004500509. [DOI] [PubMed] [Google Scholar]
- 74.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J. Clin. Psychiatry. 1995;56(9):395–401. [PubMed] [Google Scholar]
- 75.Reneric JP, Lucki I. Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology (Berl.) 1998;136(2):190–197. doi: 10.1007/s002130050555. [DOI] [PubMed] [Google Scholar]
- 76.Prica C, Hascoet M, Bourin M. Is co-administration of bupropion with SSRIs and SNRIs in forced swimming test in mice, predictive of efficacy in resistant depression? Behav. Brain Res. 2008;194(1):92–99. doi: 10.1016/j.bbr.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 77.Sporn J, Ghaemi SN, Sambur MR, et al. Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann. Clin. Psychiatry. 2000;12(3):137–140. doi: 10.1023/a:1009060800999. [DOI] [PubMed] [Google Scholar]
- 78.Brocco M, Dekeyne A, Papp M, Millan MJ. Antidepressant-like properties of the anti-parkinson agent, piribedil, in rodents: mediation by dopamine D2 receptors. Behav. Pharmacol. 2006;17(7):559–572. doi: 10.1097/01.fbp.0000236267.41806.5b. [DOI] [PubMed] [Google Scholar]
- 79.Kinney JL. Nomifensine maleate: a new second-generation antidepressant. Clin. Pharm. 1985;4(6):625–636. [PubMed] [Google Scholar]
- 80.Marks DM, Pae CU, Patkar AA. Triple reuptake inhibitors: the next generation of antidepressants. Curr. Neuropharmacol. 2008;6(4):338–343. doi: 10.2174/157015908787386078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guiard BP, El Mansari M, Blier P. Prospect of a dopamine contribution in the next generation of antidepressant drugs: the triple reuptake inhibitors. Curr. Drug Targets. 2009;10(11):1069–1084. doi: 10.2174/138945009789735156. [DOI] [PubMed] [Google Scholar]; •• Focuses on rationale for developing triple uptake inhibitors as the next-generation antidepressants.
- 82.Fava M, Rankin M. Sexual functioning and SSRIs. J. Clin. Psychiatry. 2002;63(Suppl. 5):13–16. discussion 23–15. [PubMed] [Google Scholar]
- 83.Gitlin MJ, Suri R, Altshuler L, Zuckerbrow-Miller J, Fairbanks L. Bupropion-sustained release as a treatment for SSRI-induced sexual side effects. J. Sex Marital. Ther. 2002;28(2):131–138. doi: 10.1080/00926230252851870. [DOI] [PubMed] [Google Scholar]
- 84.Prins J, Olivier B, Korte SM. Triple reuptake inhibitors for treating subtypes of major depressive disorder: the monoamine hypothesis revisited. Expert Opin. Investig. Drugs. 2011;20(8):1107–1130. doi: 10.1517/13543784.2011.594039. [DOI] [PubMed] [Google Scholar]
- 85.Dutta AK, Meltzer PC, Madras BK. Positional importance of the nitrogen atom in novel piperidine analogues of GBR 12909: affinity and selectivity for the dopamine transporter. Med. Chem. Res. 1993;3(4):209–222. [Google Scholar]
- 86.Dutta AK, Xu C, Reith ME. Structure-activity relationship studies of novel 4-[2-[bis(4-fluorophenyl)methoxy]ethyl]-1-(3-phenylpropyl)piperidine analogs: synthesis and biological evaluation at the dopamine and serotonin transporter sites. J. Med. Chem. 1996;39(3):749–756. doi: 10.1021/jm9506581. [DOI] [PubMed] [Google Scholar]
- 87.Dutta AK, Coffey LL, Reith ME. Highly selective, novel analogs of 4-[2-(diphenylmethoxy)ethyl]- 1-benzylpiperidine for the dopamine transporter: effect of different aromatic substitutions on their affinity and selectivity. J. Med. Chem. 1997;40(1):35–43. doi: 10.1021/jm960638e. [DOI] [PubMed] [Google Scholar]
- 88.Dutta AK, Xu C, Reith ME. Tolerance in the replacement of the benzhydrylic O atom in 4-[2-(diphenylmethoxy)ethyl]-1-benzylpiperidine derivatives by an N atom: development of new-generation potent and selective N-analogue molecules for the dopamine transporter. J. Med. Chem. 1998;41(17):3293–3297. doi: 10.1021/jm980066t. [DOI] [PubMed] [Google Scholar]
- 89.Dutta AK, Davis MC, Reith ME. Rational design and synthesis of novel 2,5-disubstituted cis- and trans-piperidine derivatives exhibiting differential activity for the dopamine transporter. Bioorg. Med. Chem. Lett. 2001;11(17):2337–2340. doi: 10.1016/s0960-894x(01)00443-7. [DOI] [PubMed] [Google Scholar]
- 90.Ghorai SK, Cook C, Davis M, et al. High affinity hydroxypiperidine analogues of 4-(2-benzhydryloxyethyl)-1-(4-fluorobenzyl)piperidine for the dopamine transporter: stereospecific interactions in vitro and in vivo . J. Med. Chem. 2003;46(7):1220–1228. doi: 10.1021/jm020275k. [DOI] [PubMed] [Google Scholar]
- 91.Kolhatkar RB, Ghorai SK, George C, Reith ME, Dutta AK. Interaction of cis-(6-benzhydrylpiperidin-3-yl)benzylamine analogues with monoamine transporters: structure-activity relationship study of structurally constrained 3,6-disubstituted piperidine analogues of (2,2-diphenylethyl)-[1-(4-fluorobenzyl)piperidin-4-ylmethyl]amine. J. Med. Chem. 2003;46(11):2205–2215. doi: 10.1021/jm020561w. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, Reith ME, Dutta AK. Design, synthesis, and activity of novel cis- and trans-3,6-disubstituted pyran biomimetics of 3,6-disubstituted piperidine as potential ligands for the dopamine transporter. Bioorg. Med. Chem. Lett. 2003;13(9):1591–1595. doi: 10.1016/s0960-894x(03)00169-0. [DOI] [PubMed] [Google Scholar]
- 93.Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur. J. Pharmacol. 2003;461(2–3):99–104. doi: 10.1016/s0014-2999(03)01310-4. [DOI] [PubMed] [Google Scholar]
- 94.Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. “Broad spectrum” antidepressants: is more better for the treatment of depression? Life Sci. 2003;73(25):3175–3179. doi: 10.1016/j.lfs.2003.06.007. [DOI] [PubMed] [Google Scholar]; •• Suggests that broad spectrum antidepressants will produce faster onset of action and would be more efficacious than selective serotonin reuptake inhibitors or serotonin/norepinephrine reuptake inhibitors.
- 95.Zhang S, Zhen J, Reith ME, Dutta AK. Structural requirements for 2,4- and 3,6-disubstituted pyran biomimetics of cis-(6-benzhydryl-piperidin-3-yl)-benzylamine compounds to interact with monoamine transporters. Bioorg. Med. Chem. 2004;12(23):6301–6315. doi: 10.1016/j.bmc.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 96.Zhang S, Zhen J, Reith ME, Dutta AK. Discovery of novel trisubstituted asymmetric derivatives of (2s,4r,5r)-2-benzhydryl-5-benzylaminotetrahydropyran-4-ol, exhibiting high affinity for serotonin and norepinephrine transporters in a stereospecific manner. J. Med. Chem. 2005;48(15):4962–4971. doi: 10.1021/jm049021k. [DOI] [PubMed] [Google Scholar]; • Reports the discovery of tri-substituted tetrahydropyran derivatives as a novel class of triple uptake inhibitors.
- 97.Zhang S, Fernandez F, Hazeldine S, et al. Further structural exploration of trisubstituted asymmetric pyran derivatives (2S,4R,5R)-2-benzhydryl-5-benzylamino-tetrahydropyran-4-ol and their corresponding disubstituted (3S,6S) pyran derivatives: a proposed pharmacophore model for high-affinity interaction with the dopamine, serotonin, and norepinephrine transporters. J. Med. Chem. 2006;49(14):4239–4247. doi: 10.1021/jm0601699. [DOI] [PubMed] [Google Scholar]
- 98.Santra S, Gogoi S, Gopishetty B, et al. Structural exploration of (3S,6S)-6-benzhydryl-n-benzyltetrahydro-2h-pyran-3-amine analogues: identification of potent triple monoamine reuptake inhibitors as potential antidepressants. Chem. Med. Chem. 2012;7(12):2093–2100. doi: 10.1002/cmdc.201200352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gopishetty B, Hazeldine S, Santra S, et al. Further structure-activity relationship studies on 4-((((3s,6s)-6-benzhydryltetrahydro-2h-pyran-3-yl)amino)methyl)phenol: identification of compounds with triple uptake inhibitory activity as potential antidepressant agents. J. Med. Chem. 2011;54(8):2924–2932. doi: 10.1021/jm200020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dutta AK, Santra S, Sharma H, et al. Pharmacological and behavioral characterization of D-473, an orally active triple reuptake inhibitor targeting dopamine, serotonin and norepinephrine transporters. PLoS ONE. 2014;9(11):e113420. doi: 10.1371/journal.pone.0113420. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports biological and behavioral evaluation of an orally active triple uptake inhibitor, D-473.
- 101.Dutta AK, Gopishetty B, Gogoi S, Ali S, Zhen J, Reith M. The novel trisubstituted pyran derivative D-142 has triple monoamine reuptake inhibitory activity and exerts potent antidepressant-like activity in rodents. Eur. J. Pharmacol. 2011;671(1–3):39–44. doi: 10.1016/j.ejphar.2011.09.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma H, Santra S, Debnath J, Antonio T, Reith M, Dutta A. Flexible and biomimetic analogs of triple uptake inhibitor 4-((((3S,6S)-6-benzhydryltetrahydro-2h-pyran-3-yl)amino)methyl)phenol: synthesis, biological characterization, and development of a pharmacophore model. Bioorg. Med. Chem. 2014;22(1):311–324. doi: 10.1016/j.bmc.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Basile AS, Janowsky A, Golembiowska K, et al. Characterization of the antinociceptive actions of bicifadine in models of acute, persistent, and chronic pain. J. Pharmacol. Exp. Ther. 2007;321(3):1208–1225. doi: 10.1124/jpet.106.116483. [DOI] [PubMed] [Google Scholar]
- 104.Krieter PA, Gohdes M, Musick TJ, Duncanson FP, Bridson WE. Pharmacokinetics, disposition, and metabolism of bicifadine in humans. Drug Metab. Dispos. 2008;36(2):252–259. doi: 10.1124/dmd.107.017871. [DOI] [PubMed] [Google Scholar]
- 105.Andersen PH. The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur. J. Pharmacol. 1989;166(3):493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 106.Maryanoff BE, Mccomsey DF, Gardocki JF, et al. Pyrroloisoquinoline antidepressants. 2. In-depth exploration of structure-activity relationships. J. Med. Chem. 1987;30(8):1433–1454. doi: 10.1021/jm00391a028. [DOI] [PubMed] [Google Scholar]
- 107.Moldt P, Watjen F. 1998 US5736556 A. [Google Scholar]
- 108.Huot P, Fox SH, Brotchie JM. Monoamine reuptake inhibitors in Parkinson’s disease. Parkinson’s Disease. 2015;2015:1–71. doi: 10.1155/2015/609428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Skolnick P, Krieter P, Tizzano J, et al. Preclinical and clinical pharmacology of DOV 216,303, a “triple” reuptake inhibitor. CNS Drug Rev. 2006;12(2):123–134. doi: 10.1111/j.1527-3458.2006.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popik P, Krawczyk M, Golembiowska K, et al. Pharmacological profile of the “triple” monoamine neurotransmitter uptake inhibitor, DOV 102,677. Cell Mol. Neurobiol. 2006;26(4–6):857–873. doi: 10.1007/s10571-006-9012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bymaster FP, Golembiowska K, Kowalska M, Choi YK, Tarazi FI. Pharmacological characterization of the norepinephrine and dopamine reuptake inhibitor EB-1020: implications for treatment of attention-deficit hyperactivity disorder. Synapse. 2012;66(6):522–532. doi: 10.1002/syn.21538. [DOI] [PubMed] [Google Scholar]
- 112.Miller S, Stensbol TB. 2010 US20100144788 A1. [Google Scholar]
- 113.Bang-Andersen B, Ruhland T, Jorgensen M, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 2011;54(9):3206–3221. doi: 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]
- 114.Shaw AM, Boules M, Zhang Y, et al. Antidepressant-like effects of novel triple reuptake inhibitors, PRC025 and PRC050. Eur. J. Pharmacol. 2007;555(1):30–36. doi: 10.1016/j.ejphar.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 115.Liang Y, Shaw AM, Boules M, et al. Antidepressant-like pharmacological profile of a novel triple reuptake inhibitor, (1s,2s)-3-(methylamino)-2-(naphthalen-2-yl)-1-phenylpropan-1-ol (PRC200-SS) J. Pharmacol. Exp. Ther. 2008;327(2):573–583. doi: 10.1124/jpet.108.143610. [DOI] [PubMed] [Google Scholar]
- 116.Bennett BA, Wichems CH, Hollingsworth CK, et al. Novel 2-substituted cocaine analogs: uptake and ligand binding studies at dopamine, serotonin and norepinephrine transport sites in the rat brain. J. Pharmacol. Exp. Ther. 1995;272(3):1176–1186. [PubMed] [Google Scholar]
- 117.Shao L, Hewitt MC, Wang F, et al. Discovery of n-methyl-1-(1-phenylcyclohexyl)ethanamine, a novel triple serotonin, norepinephrine and dopamine reuptake inhibitor. Bioorg. Med. Chem. Lett. 2011;21(5):1434–1437. doi: 10.1016/j.bmcl.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 118.Fang X, Guo L, Jia J, et al. SKF83959 is a novel triple reuptake inhibitor that elicits anti-depressant activity. Acta. Pharmacol. Sin. 2013;34(9):1149–1155. doi: 10.1038/aps.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han Y, Han M, Shin D, Song C, Hahn HG. Exploration of novel 3-substituted azetidine derivatives as triple reuptake inhibitors. J. Med. Chem. 2012;55(18):8188–8192. doi: 10.1021/jm3008294. [DOI] [PubMed] [Google Scholar]
- 120.Shao L, Hewitt MC, Malcolm SC, et al. Synthesis and pharmacological characterization of bicyclic triple reuptake inhibitor 3-aryl octahydrocyclopenta[c]pyrrole analogues. J. Med. Chem. 2011;54(15):5283–5295. doi: 10.1021/jm101312a. [DOI] [PubMed] [Google Scholar]
- 121.Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 2013;700(1–3):147–151. doi: 10.1016/j.ejphar.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou J, He R, Johnson KM, Ye Y, Kozikowski AP. Piperidine-based nocaine/modafinil hybrid ligands as highly potent monoamine transporter inhibitors: efficient drug discovery by rational lead hybridization. J. Med. Chem. 2004;47(24):5821–5824. doi: 10.1021/jm040117o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gu XH, Yu H, Jacobson AE, et al. Design, synthesis, and monoamine transporter binding site affinities of methoxy derivatives of indatraline. J. Med. Chem. 2000;43(25):4868–4876. doi: 10.1021/jm000329v. [DOI] [PubMed] [Google Scholar]
- 124.Aluisio L, Lord B, Barbier AJ, et al. In-vitro and in-vivo characterization of JNJ-7925476, a novel triple monoamine uptake inhibitor. Eur. J. Pharmacol. 2008;587(1–3):141–146. doi: 10.1016/j.ejphar.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 125.Lee KH, Park CE, Min KH, et al. Synthesis and pharmacological evaluation of 3-aryl-3-azolylpropan-1-amines as selective triple serotonin/norepinephrine/dopamine reuptake inhibitors. Bioorg. Med. Chem. Lett. 2010;20(18):5567–5571. doi: 10.1016/j.bmcl.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 126.Lucas MC, Weikert RJ, Carter DS, et al. Design, synthesis, and biological evaluation of new monoamine reuptake inhibitors with potential therapeutic utility in depression and pain. Bioorg. Med. Chem. Lett. 2010;20(18):5559–5566. doi: 10.1016/j.bmcl.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 127.Axford L, Boot JR, Hotten TM, et al. Bicyclo[2.2.1]heptanes as novel triple re-uptake inhibitors for the treatment of depression. Bioorg. Med. Chem. Lett. 2003;13(19):3277–3280. doi: 10.1016/s0960-894x(03)00660-7. [DOI] [PubMed] [Google Scholar]
- 128.Shao L, Wang F, Malcolm SC, et al. Synthesis and pharmacological evaluation of 4-(3,4-dichlorophenyl)-n-methyl-1,2,3,4-tetrahydronaphthalenyl amines as triple reuptake inhibitors. Bioorg. Med. Chem. 2011;19(1):663–676. doi: 10.1016/j.bmc.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 129.Bettati M, Cavanni P, Di Fabio R, et al. Oxa-azaspiro derivatives: a novel class of triple re-uptake inhibitors. Chem. Med. Chem. 2010;5(3):361–366. doi: 10.1002/cmdc.200900482. [DOI] [PubMed] [Google Scholar]
- 130.Delorenzo C, Lichenstein S, Schaefer K, et al. SEP-225289 serotonin and dopamine transporter occupancy: a PET study. J. Nucl. Med. 2011;52(7):1150–1155. doi: 10.2967/jnumed.110.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Culig J, Ehsanullah RS, Hallett C, Iliopoulou A, Matheson I, Turner P. A clinical pharmacological comparison of diclofensine (Ro 8–4650) with nomifensine and amitriptyline in normal human volunteers. Br. J. Clin. Pharmacol. 1983;15(5):537–543. doi: 10.1111/j.1365-2125.1983.tb02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pearce RK, Smith LA, Jackson MJ, Banerji T, Scheel-Kruger J, Jenner P. The monoamine reuptake blocker brasofensine reverses akinesia without dyskinesia in MPTP-treated and levodopa-primed common marmosets. Mov. Disord. 2002;17(5):877–886. doi: 10.1002/mds.10238. [DOI] [PubMed] [Google Scholar]
- 133.Zhu M, Whigan DB, Chang SY, Dockens RC. Disposition and metabolism of [14c]brasofensine in rats, monkeys, and humans. Drug Metab. Dispos. 2008;36(1):24–35. doi: 10.1124/dmd.107.016139. [DOI] [PubMed] [Google Scholar]
- 134.Wilens TE, Klint T, Adler L, et al. A randomized controlled trial of a novel mixed monoamine reuptake inhibitor in adults with ADHD. Behav. Brain Funct. 2008;4:24. doi: 10.1186/1744-9081-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Learned S, Graff O, Roychowdhury S, et al. Efficacy, safety, and tolerability of a triple reuptake inhibitor GSK372475 in the treatment of patients with major depressive disorder: two randomized, placebo- and active-controlled clinical trials. J. Psychopharmacol. 2012;26(5):653–662. doi: 10.1177/0269881111424931. [DOI] [PubMed] [Google Scholar]
- 136.Thatte U. NS-2330 (Neurosearch) Curr. Opin. Investig. Drugs. 2001;2(11):1592–1594. [PubMed] [Google Scholar]
- 137.Sorensen G, Husum H, Brennum LT, et al. Addiction-related effects of DOV 216,303 and cocaine: a comparative study in the mouse. Basic Clin. Pharmacol. Toxicol. 2014;114(6):451–459. doi: 10.1111/bcpt.12182. [DOI] [PubMed] [Google Scholar]
- 138.Lu AA24530 shows positive results in major depressive disorder Phase II study. http://investor.lundbeck.com/releasedetail.cfm?ReleaseID=608619
- 139.Takeda and Lundbeck announce FDA approval of brintellix™ (vortioxetine) for treatment of adults with major depressive disorder. http://investor.lundbeck.com/releasedetail.cfm?ReleaseID=794050
- 140.Gibb A, Deeks ED. Vortioxetine: first global approval. Drugs. 2014;74(1):135–145. doi: 10.1007/s40265-013-0161-9. [DOI] [PubMed] [Google Scholar]
- 141.Hanna MM, Eid NM, George RF, Safwat HM. Synthesis of some tropane derivatives of anticipated activity on the reuptake of norepinephrine and/or serotonin. Bioorg. Med. Chem. 2007;15(24):7765–7772. doi: 10.1016/j.bmc.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 142.Guha M, Heier A, Price S, et al. Assessment of biomarkers of drug-induced kidney injury in cynomolgus monkeys treated with a triple reuptake inhibitor. Toxicol. Sci. 2011;120(2):269–283. doi: 10.1093/toxsci/kfr013. [DOI] [PubMed] [Google Scholar]
- 143.Bennett BA, Hollingsworth CK, Martin RS, et al. Prolonged dopamine and serotonin transporter inhibition after exposure to tropanes. Neuropharmacology. 1998;37(1):123–130. doi: 10.1016/s0028-3908(97)00194-9. [DOI] [PubMed] [Google Scholar]
- 144.Daunais JB, Hart SL, Smith HR, et al. Long-acting blockade of biogenic amine transporters in rat brain by administration of the potent novel tropane 2beta-propanoyl-3beta-(2-naphthyl)-tropane. J. Pharmacol. Exp. Ther. 1998;285(3):1246–1254. [PubMed] [Google Scholar]
- 145.Dawson P, Opacka-Juffry J, Moffatt JD, et al. The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:57–63. doi: 10.1016/j.pnpbp.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 146.Bogeso KP, Christensen AV, Hyttel J, Liljefors T. 3-Phenyl-1-indanamines. Potential antidepressant activity and potent inhibition of dopamine, norepinephrine, and serotonin uptake. J. Med. Chem. 1985;28(12):1817–1828. doi: 10.1021/jm00150a012. [DOI] [PubMed] [Google Scholar]
- 147.Lengyel K, Pieschl R, Strong T, et al. Ex vivo assessment of binding site occupancy of monoamine reuptake inhibitors: methodology and biological significance. Neuropharmacology. 2008;55(1):63–70. doi: 10.1016/j.neuropharm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 148.Schenk S. Effects of GBR 12909, WIN 35,428 and indatraline on cocaine self-administration and cocaine seeking in rats. Psychopharmacology (Berl.) 2002;160(3):263–270. doi: 10.1007/s00213-001-0972-3. [DOI] [PubMed] [Google Scholar]
- 149.Micheli F, Cavanni P, Andreotti D, et al. 6-(3,4-Dichlorophenyl)-1-[(methyloxy)methyl]-3-azabicyclo[4.1.0]heptane: a new potent and selective triple reuptake inhibitor. J. Med. Chem. 2010;53(13):4989–5001. doi: 10.1021/jm100481d. [DOI] [PubMed] [Google Scholar]
- 150.Beer B, Stark J, Krieter P, et al. DOV 216,303, a “triple” reuptake inhibitor: safety, tolerability, and pharmacokinetic profile. J. Clin. Pharmacol. 2004;44(12):1360–1367. doi: 10.1177/0091270004269560. [DOI] [PubMed] [Google Scholar]
- 151.Tran P, Skolnick P, Czobor P, et al. Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. J. Psychiatr. Res. 2012;46(1):64–71. doi: 10.1016/j.jpsychires.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 152.Schreiber R, Lew R, Hardy L, Cremers T, Fang QK, Campbell U. Meeting of Society for Neuroscience. Chicago, IL, USA: October 2009. Pharmacological characterization of the triple monoamine transporter inhibitor SEP-225289; pp. 17–21. Presented at. [Google Scholar]
- 153.Comley RA, Salinas CA, Slifstein M, et al. Monoamine transporter occupancy of a novel triple reuptake inhibitor in baboons and humans using positron emission tomography. J. Pharmacol. Exp. Ther. 2013;346(2):311–317. doi: 10.1124/jpet.112.202895. [DOI] [PubMed] [Google Scholar]
- 154.Risinger R, Bhagwagar Z, Luo F, et al. Evaluation of safety and tolerability, pharmacokinetics, and pharmacodynamics of BMS-820836 in healthy subjects: a placebo-controlled, ascending single-dose study. Psychopharmacology (Berl.) 2014;231(11):2299–2310. doi: 10.1007/s00213-013-3391-3. [DOI] [PubMed] [Google Scholar]
- 155.Tran P, Skolnick P, Czobor P, et al. Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. J. Psychiatr. Res. 2012;46(1):64–71. doi: 10.1016/j.jpsychires.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 156.Lane RM. Antidepressant drug development: focus on triple monoamine reuptake inhibition. J. Psychopharmacol. 2015;29(5):526–544. doi: 10.1177/0269881114553252. [DOI] [PubMed] [Google Scholar]; • Reviews antidepressant drugs under development.
- 157.Czobor P, Skolnick P. The secrets of a successful clinical trial: compliance, compliance, and compliance. Mol. Interv. 2011;11(2):107–110. doi: 10.1124/mi.11.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bourdet DL, Tsuruda PR, Obedencio GP, Smith JA. Prediction of human serotonin and norepinephrine transporter occupancy of duloxetine by pharmacokinetic/pharmacodynamic modeling in the rat. J. Pharmacol. Exp. Ther. 2012;341(1):137–145. doi: 10.1124/jpet.111.188417. [DOI] [PubMed] [Google Scholar]
- 159.Ding YS, Naganawa M, Gallezot JD, et al. Clinical doses of atomoxetine significantly occupy both norepinephrine and serotonin transports: implications on treatment of depression and ADHD. Neuroimage. 2014;86:164–171. doi: 10.1016/j.neuroimage.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 160.Smith JA, Patil D, Daniels O, et al. Preclinical to clinical translation of CNS transporter occupancy of TD-9855, a novel norepinephrine and serotonin reuptake inhibitor. Int. J. Neuropsychopharmacol. 2015;18(2) doi: 10.1093/ijnp/pyu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Learned-Coughlin SM, Bergstrom M, Savitcheva I, Ascher J, Schmith VD, Langstrom B. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol. Psychiatry. 2003;54(8):800–805. doi: 10.1016/s0006-3223(02)01834-6. [DOI] [PubMed] [Google Scholar]
- 162.Appel L, Bergstrom M, Buus Lassen J, Langstrom B. Tesofensine, a novel triple monoamine re-uptake inhibitor with anti-obesity effects: dopamine transporter occupancy as measured by PET. Eur. Neuropsychopharmacol. 2014;24(2):251–261. doi: 10.1016/j.euroneuro.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 163.George M, Rajaram M, Shanmugam E. New and emerging drug molecules against obesity. J. Cardiovasc. Pharmacol. Ther. 2014;19(1):65–76. doi: 10.1177/1074248413501017. [DOI] [PubMed] [Google Scholar]
- 164.Tizzano JP, Stribling DS, Perez-Tilve D, et al. The triple uptake inhibitor (1r,5s)-(+)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0] hexane hydrochloride (DOV 21947) reduces body weight and plasma triglycerides in rodent models of diet-induced obesity. J. Pharmacol. Exp. Ther. 2008;324(3):1111–1126. doi: 10.1124/jpet.107.133132. [DOI] [PubMed] [Google Scholar]
- 165.Dov 21,947 demonstrates significant body weight and BMI reductions in drug- compliant subjects in Phase Ib clinical study. www.sec.gov/Archives/edgar/data/1066833/000114420407051174/v088668_ex99–1.htm
- 166.O’tousa DS, Warnock KT, Matson LM, et al. Triple monoamine uptake inhibitors demonstrate a pharmacologic association between excessive drinking and impulsivity in high-alcohol-preferring (HAP) mice. Addict. Biol. 2013;20(2):236–247. doi: 10.1111/adb.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology. 2012;37(9):2057–2066. doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]