Abstract
Alzheimer's disease (AD) is the most common cause of dementia with no cure at present. Cholesterol metabolism is closely associated with AD at several stages. ACAT1 converts free cholesterol to cholesteryl esters, and plays important roles in cellular cholesterol homeostasis. Recent studies show that in a mouse model, blocking ACAT1 provides multiple beneficial effects on AD. Here we review the current evidence that implicates ACAT1 as a therapeutic target for AD. We also discuss the potential usage of various ACAT inhibitors currently available to treat AD.
Cholesterol is an essential lipid molecule present in mammalian cell membranes. However, abnormally high levels of cholesterol, especially free (unesterified) cholesterol, are harmful to cells [1]. Thus, within a given cell, cellular cholesterol homeostasis is highly regulated by various control mechanisms as reviewed in [2]. The enzyme ACAT, also known as sterol O-acyltransferase, plays important roles in cellular cholesterol homeostasis. ACAT converts free cholesterol to cholesteryl esters to prevent overaccumulation of free cholesterol at cellular membranes. There are two different ACAT genes, ACAT1 and ACAT2, with different tissue expression patterns. In normal human tissues, the expression of ACAT1 predominates over that of ACAT2 except in small intestines [3]. In most cell types, cholesteryl esters exist as cytoplasmic lipid droplets. In small intestines, cholesteryl esters form part of the core lipid moieties present in the lipoprotein called chylomicrons for cholesterol transport in the blood.
Alzheimer's disease (AD) is a progressive neurodegenerative disease that causes difficulty in cognitive functions, including memory, speech and perception, etc. AD is the most common cause of dementia in developed countries. Currently available treatments for AD improve symptoms but they do not provide a cure. Cholesterol metabolism is closely associated with AD at different stages, as reviewed in [4,5]. Recent studies have shown that blocking ACAT activity, specifically ACAT1, in mouse models and in cell culture produces several beneficial effects on AD. These studies suggest that ACAT1 can be a novel therapeutic target to treat AD. In this review, we provide a brief overview on the enzyme properties of ACATs, and the experimental evidence supporting the notion that ACAT1 blockage is a promising approach for treating AD. We then describe various mechanisms that may account for the beneficial effects of ACAT1 blockage on AD. We also discuss the potential usage of currently available ACAT inhibitors (Figure 1A) to treat AD.
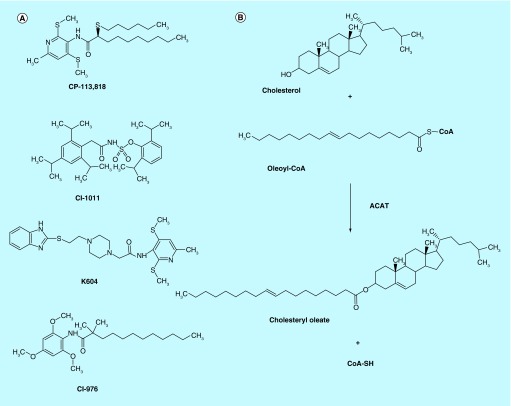
Figure 1. . The structures of various ACAT inhibitors and biosynthesis of cholesteryl ester by acyl-CoA:cholesterol acyltransferase.
(A) The structures of ACAT inhibitors, CP-113,181, CI-1011, K604 and CI-976. (B) ACAT transfers the fatty acyl group of long chain fatty acyl-CoA (oleoyl-CoA) to the 3β-hydroxy moiety of cholesterol to produce cholesteryl ester (cholesteryl oleate).
ACAT as drug targets
ACAT converts free cholesterol to cholesteryl esters by transferring the fatty acyl group of fatty acyl-CoA to the 3β-hydroxy moiety of cholesterol (Figure 1B). ACAT plays important roles in cellular cholesterol homeostasis. The first ACAT gene, ACAT1, was cloned by functional complementation of a Chinese hamster ovary (CHO) cell mutant lacking ACAT enzyme activity [6]. ACAT1 is located mainly at the endoplasmic reticulum (ER) [7], and is ubiquitously expressed in all human tissues examined [8]. ACAT1 is an allosteric enzyme; it can utilize a variety of sterols (including oxysterols, plant sterols, etc.) as a substrate as well as an activator, with cholesterol being the best substrate and the best activator [9]. The preferred fatty acyl-CoA as substrate for ACAT1 is oleoyl-CoA [10]. The second ACAT gene, ACAT2, was identified based on its sequence similarity to ACAT1 [11–13]. ACAT2 is mainly expressed in the intestines and hepatocytes, and it is also expressed in various other tissues at low level [3]. Its exact subcellular localization remains unknown. Similar to ACAT1, the activity of ACAT2 is controlled allosterically by cholesterol [9,14]. Unlike many enzymes in lipid metabolism, neither ACAT1 nor ACAT2 is transcriptionally regulated by sterols [14].
Traditionally, cholesterol metabolism has long been associated with the disease atherosclerosis. In the early stage of atherosclerosis, under dyslipidemia and chronic inflammation, monocytes adhere to the activated endothelium, and enter the intimal layer of the artery; the monocytes in the intima transform into resident macrophages and begin to acquire a large amount of cholesterol. At the macrophage cell interior, most of the cholesterol is esterifed by ACAT1; the cholesteryl esters accumulate as lipid droplets, causing the macrophages to be foamy in appearance. In human atherosclerotic plaques, ACAT1 is highly expressed in macrophage foam cells [15]. Foam cells may cause the atherosclerotic plaques to be more vulnerable to rupture. Thus, ACAT1 has long been studied as a drug target to treat atherosclerosis. In mouse studies, the Acat1 knockout (KO) mouse lines, which were independently created by Farese and co-workers, and by Ishibashi and co-workers, were employed to study the roles of ACAT1 in atherosclerosis; the results produced from these two laboratories were equivocal [16,17]. A more recent study showed that in mouse, global deletion of the Acat1 gene, including cells in the bone marrow, causes an increase in hematopoietic progenitor cell proliferation and lead to leukocytosis [18]. Leukocytosis may alter atherosclerosis progression. To study the roles of ACAT1 in atherosclerosis, tissue-specific Acat1 KO mice may provide better models. Studies in mouse suggest that ACAT2 is also a potential drug target for treating atherosclerosis [19,20]. In addition, recent evidence suggests that both ACAT1 and ACAT2 may be viable targets to treat various types of cancer, as reviewed in [21].
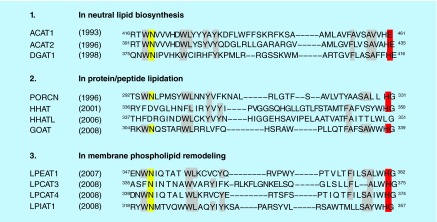
ACAT1, ACAT2 and acyl-CoA:diacylglycerol acyltransferase 1, which catalyzes the final step of triglyceride biosynthesis, are founding members of the membrane-bound O-acyltransferase (MBOAT) enzyme family [22]. There are 11 enzymes in the MBOAT family in humans. MBOATs contain multiple transmembrane domains, and share two similar catalytic sites, with an invariant histidine within a long stretch of hydrophobic domain, and a highly conserved asparagine within a long stretch of hydrophilic domain (Figure 2). MBOATs participate in diverse biological processes [23]. For instance, an MBOAT family enzyme, ghrelin O-acyltransferase catalyzes protein acylation of ghrelin [24], which stimulates appetite and increases food intake [25].
Figure 2. . Alignment of the two highly conserved catalytic sites of 11 membrane-bound O-acyltransferase enzymes in human.
The highly conserved histidine residues and asparagine residues are highlighted in red and in yellow, respectively. The residues highlighted in gray are conserved in more than 50% of the all membrane-bound O-acyltransferases. Membrane-bound O-acyltransferases can be classified into three groups based on biochemical reactions they catalyze.
Reproduced with permission from [23] © Springer (2011).
Amyloid plaques, neurofibrillary tangles & strategies to produce drugs to diminish amyloid β and pathological tau
The pathological hallmarks of AD in the brain consist of extracellular amyloid plaques, mainly composed of amyloid-β (Aβ) peptides and intracellular neurofibrillary tangles (NFTs), mainly consisting of hyperphosphorylated forms of tau. Aβ and tau can interact with each other synergistically to trigger neurotoxicity [26–29]. Thus, reducing brain levels of Aβ and/or misfolded/aggregated tau and/or hyperphosphorylated tau are attractive strategies to treat AD, as reviewed in [30].
Aβ is produced from the APP by sequential proteolytic cleavages; there are mainly two forms of Aβ peptide in the brain, Aβ1–40 and Aβ 1–42; Aβ1–42 is much more neurotoxic than Aβ1–40 [31]. Several mutations in the APP gene cause an increase in Aβ1–42, or the ratio of Aβ1–42 to Aβ1–40, and lead to familial AD, as reviewed in [32]. APP is a type-I membrane protein, and is highly expressed in neurons. The precise physiological roles of APP are not well understood. On the contrary, APP processing has been extensively characterized, as reviewed in [31,32]. After APP is synthesized at the ER, the nascent APP becomes glycosylated at the Golgi complex, and then is transported to the cell surface via the secretary pathway. At the plasma membrane, APP is internalized via a clathrin-dependent endocytotic pathway and is processed by β-secretase followed by γ-secretase, which is present in intracellular compartments to produce Aβ (Figure 3). Alternatively, APP can be proteolyzed by α-secretase(s) present at the cell surface, and then internalized and processed by γ-secretase, which generates nonamyloidogenic (i.e., non-neurotoxic) products. Because proteolytic cleavage by α-secretase(s) occurs within the Aβ sequence in APP, this step prevents Aβ generation (Figure 3).
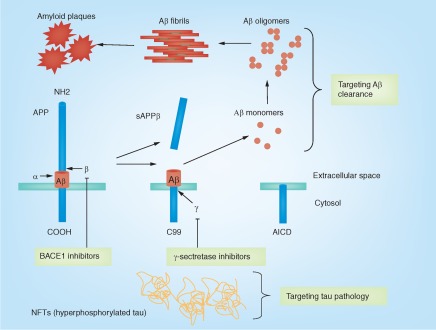
Figure 3. . Aβ production and clearance, neurofibrillary tangles and current drug development strategy to treat Alzheimer's disease.
The APP can undergo proteolytic processing by secretases. In the amyloidogenic pathway, APP is first cleaved by β-secretase (BACE1), releasing an ectodomain (sAPPβ) and retaining the last 99 amino acids of APP (C99) within the membrane. C99 is subsequently cleaved 38–43 amino acids from the amino terminus to produce Aβ, by the γ-secretase. In contrast, in the nonamyloidogenic pathway, cleavage by α-secretase occurs within the Aβ domain, thereby preventing the generation and release of the Aβ peptide. Current drug development includes targeting BACE1 and γ-secretase to reduce Aβ production. In addition, targeting Aβ clearance aims to increase Aβ turnover in the brain; targeting tau pathology aims to reduce tau toxicity in the brain.
α: α-cleavage site; AICD: APP intracellular domain; β: β-cleavage site; γ: γ-cleavage site.
The β-secretase, BACE1, is a membrane-bound aspartyl protease and the rate-limiting enzyme for Aβ generation. Thus, it has been considered as a good therapeutic target for AD (Figure 3). However, BACE1 KO mice have impaired cognitive functions [33], likely due to abnormal myelination in the CNS [34]. This result raises concerns on usage of a β-secretase inhibitor for AD treatment. The γ-secretase is a multiprotein complex composed of presenilin (PS), nicastrin, anterior pharynx-defective-1 and presenilin enhancer-2 [35]. There are two PS homologs in mammals, PS1 and PS2. Mutations in PS, particularly in PS1, cause familial AD. The γ-secretase has been considered as a drug target for AD (Figure 3). A variety of γ-secretase inhibitors has been developed to treat AD. However, these γ-secretase inhibitors also inhibit the processing of Notch and several other transmembrane proteins, which may cause serious mechanistic side effects [36].
Brain Aβ levels are determined by its production and its clearance. Studies in a mouse model of AD suggest that the brain's ability for Aβ clearance declines with age [37,38]. The clearance of Aβ in late-onset AD patients is less efficient than control subjects [39]. These lines of evidence implicate that Aβ clearance may be impaired in AD patients. Therefore, enhancing Aβ clearance to reduce brain Aβ levels may benefit AD (Figure 3). There has been intense research interest in developing molecules to enhance the clearance of Aβ. It is estimated that approximately 50% of Aβ is degraded within the brain while the rest is transported to the systemic tissues and degraded there [40]. Aβ immunotherapy is believed to increase the clearance of Aβ. Currently, Aβ immunotherapy using anti-Aβ monoclonal antibodies has been considered as one of the most promising therapeutic approaches for treating AD. In mouse models of AD, treatment with monoclonal antibodies against Aβ dramatically reduces AD pathology and improves cognitive deficits [41–44]. Thus, a variety of anti-Aβ monoclonal antibodies has been developed for the past decade, and some of them have moved to clinical trials [45,46].
NFTs are composed of hyperphophorylated tau. Tau is a soluble microtubule-associated protein, abundantly expressed in the axons of neurons. Tau contains numerous phosphorylation sites; phosphorylations of tau at certain sites modify the ability of tau to stabilize microtubules [47]. Uncontrolled phosphorylations alter the conformation of tau, which eventually leads to accumulation of pathological hyperphosphorylated tau in NFTs [48]. In addition to phosphorylations, tau protein can also be modified by site-specific acetylations [49,50]. In AD, most of the pathological tau is found in neuronal processes known as dystrophic neuritis [51]. In addition to AD, NFTs can be found in various other neurodegenerative diseases collectively called tauopathy, including frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) and Pick's disease. P301L is the most common tau mutation in FTDP-17. Mice expressing human P301L-tau exhibit development of NFTs and memory impairment, similar to those seen in human tauopathies [52,53], which suggests that dysfunctional tau alone without Aβ accumulation can cause neurodegeneration. In mouse models of AD, recent evidence shows that neurotoxicity of Aβ depends on tau, as suppressing tau levels attenuates Aβ-induced deficits [27,28]. These studies suggest that aiming to reduce pathological tau levels is also a potential therapeutic approach to treat AD (Figure 3).
AD, cholesterol metabolism & ACAT
A growing body of evidence suggests that cholesterol metabolism is closely associated with AD at several stages, as reviewed in [4,5]. Epidemiologic studies have identified hypercholesterolemia in mid-life as a risk factor for AD [54,55]. In a mouse model for AD, a high-fat/high-cholesterol diet accelerates AD pathology [56]. These studies implicate that high cholesterol levels in the plasma are correlated with initiation and progression of AD. Studies in vitro have indicated that cholesterol modulates Aβ production; increasing membrane cholesterol levels promotes activity of β-secretase and γ-secretase, which results in increased Aβ production [57–59]; in contrast, reducing membrane cholesterol strongly inhibits Aβ production in neuronal cells [60,61].
ApoE is the major cholesterol/lipid carrier in the CNS and has been most intensively studied as a key molecule that links cholesterol metabolism and AD. In humans, there are three ApoE isoforms (E2, E3 and E4), which differ from each other by only one or two amino acid at positions 112 and 158. The ApoE4 allele is the strongest genetic risk factor for late-onset AD. Although precisely how ApoE4 causes the increased risk for AD is not fully understood, numerous studies have suggested that ApoE has isoform-dependent effects on Aβ metabolism. For instance, AD model mice expressing human ApoE display an isoform-dependent difference (E4>E3>E2) in Aβ accumulation [62,63]. ApoE also affects Aβ clearance in a manner that likely depends on the isoforms and its level of lipidation [64–67]. The ATP-binding cassette (ABC) transporter ABCA1 plays a key role in the lipidation and the stability of ApoE [68,69]. Thus, increasing ABCA1 levels in the brain to promote ApoE lipidation is a potential therapeutic approach for treating AD [70–73].
It was estimated that in the brain, almost all of cholesterol is unesterified [74]. However, recent analyses have revealed that significant amount of cholesteryl esters exists in human and mouse brains. Importantly, in the brains of AD patients and in several mouse models for AD, the cholesteryl ester content is significantly elevated in the vulnerable regions affected by AD [75,76]. In different regions of the mouse brain, ACAT1 is the predominant enzyme for cellular cholesterol esterification [77]. Thus, ACAT1 may affect initiation and progression of AD. The first experimental evidence that linked ACAT activity and AD was provided at the cell culture level by Puglielli et al. [78]. This study showed that in a CHO cell model and in a neuronal cell model, cholesteryl ester levels were correlated with Aβ production; reducing cholesteryl ester content in these cell models by blocking ACAT activity diminished Aβ generation [78]. These results led to the hypothesis that inhibiting ACAT activity benefits AD. This hypothesis has been tested in cell-based and animal-based AD models by using different approaches.
ACAT inhibitors in a mouse model for AD
CP-113,818 (Figure 1A) is a small molecule, isotype-nonspecific ACAT inhibitor that inhibits both ACAT1 and ACAT2 [79]. In a mouse AD model expressing high levels of human APP751 containing the London (V717I) and Swedish (K670M/N671L) mutations (hAPP751 mice), systemic administration of CP-113,818 for 2 months starting at 4.5 months of age dramatically reduced the Aβ deposition in the brain, and rescued the cognitive deficits [80]. CI-1011 (also known as avasimibe) (Figure 1A) is an ACAT inhibitor structurally distinct from that of CP-113,818. It also inhibits both ACAT1 and ACAT2 [81]. CI-1011 was tested in clinical trials for atherosclerosis but failed to improve disease lesions [82]. When administered to a mouse model for AD, CI-1011 provided interesting result: 2 months treatment of CI-1011 diminished the amyoid plaques and Aβ levels in the brain of young hAPP751 mice [83]. Moreover, in old hAPP751 mice displaying robust amyloid deposits and sever cognitive impairment, CI-1011 treatment reduced the pre-existing amyloid burden and Aβ levels in the brains [83]. These animal studies suggest that ACAT inhibitors may improve amyloid pathology and ameliorate AD symptoms.
In the brain of hAPP751 mice treated with the ACAT inhibitors, there was a decrease in the ratio of mature APP to immature APP; the levels of α- and β-cleavage products of APP were also decreased [80,83]. On the other hand, CP-113,818 and CI-1011 did not affect BACE1 or γ-secretase activity in vitro [78,83]. These results suggest that both compounds downregulated the maturation of APP in the early secretory pathway, which led to a decrease in Aβ production [84]. More recently, it has been shown that a small portion of APP receives a post-translational lipid modification called palmitoylation, which plays an important role in APP maturation and subsequent Aβ generation; CP-113,818 and CI-1011 are shown to inhibit palmitoylation of APP [85]. These in vivo and in vitro data implicate that both CP-113,818 and CI-1011 reduce Aβ generation, possibly by inhibiting APP maturation, and ameliorate amyloid pathology in AD mice.
Genetic ablation of Acat1 in AD mice
Although the evidence described above suggests that ACAT inhibitors may prevent and/or slow down the progression of AD, several important questions were left unanswered. First of all, it was unclear whether the beneficial actions of CP-113,818 and CI-1011 required these compounds to block both ACAT1 and ACAT2. Secondly, in addition to inhibiting ACAT, these compounds may also inhibit other members of the MBOAT family; that is, the beneficial actions of these inhibitors may be due to their abilities to inhibit other enzymes. Thirdly, it was not clear whether the ACAT inhibitors systemically administrated to the AD mice had actually reached the brain interior. To answer these questions, Bryleva et al. [77] took a mouse genetic approach by deleting the Acat1 gene or the Acat2 gene in the triple transgenic AD (3XTg-AD) mouse line. The 3XTg-AD mouse model expresses mutant forms of human APP, PS1 and tau, and displays a phenocopy of human AD [86]. The results showed that Acat1 KO but not Acat2 KO reduced the Aβ1–42 levels as well as the amyloid plaque load, and improved the cognitive function in 12–month-old 3XTg-AD mice [77]. These data clearly indicate that inactivating Acat1 alone is sufficient to ameliorate the amyloid pathology in the 3XTg-AD mouse model. Importantly, Acat1 KO caused a decrease in the protein levels of full-length human APP (both the mature and immature forms); unlike CP-113,818 or CI-1011 described earlier, Acat1 KO did not alter the ratio of mature APP to immature APP [77]. In addition, Acat1 KO did not affect the mouse endogenous APP level [77]. This result indicates that the effects of Acat1 KO are distinct from the effects of CP-113,818 and CI-1011 [80,83]. The different effects between Acat1 KO and the ACAT inhibitors on APP may be due to different AD mouse models employed, and/or due to the abilities of CP-113,818 and CI-1011 to inhibit multiple enzymes in the MBOAT family. Other scenarios are also possible.
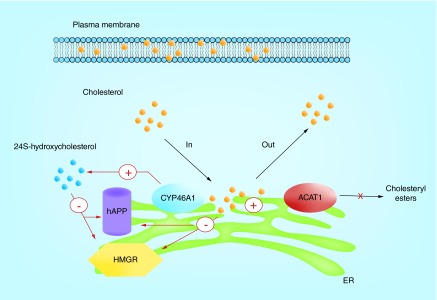
Bryleva et al. [77] reported that Acat1 KO caused an increase in the levels of 24S-hydorxycholesterol (24S-OH). 24S-OH is a key oxysterol in the brain synthesized from cholesterol by the ER resident enzyme Cyp46A1; under normal conditions, Cyp46A1 is almost exclusively expressed in neurons [87]. The Acat1 KO/3xTg-AD mice had decreased protein levels of HMG-CoA reductase, which is the rate-limiting enzyme in the cholesterol biosynthetic pathway, and decreased rate of cholesterol biosynthesis in the brain; in primary neurons isolated from the 3XTg-AD mice, Acat1 KO increased the 24S-OH biosynthesis; treating primary neurons with 24S-OH caused a decrease in the protein levels of full-length human APP and HMG-CoA reductase. Based on these findings, Bryleva et al. [77] hypothesized that increased levels of 24S-OH by Acat1 ablation leads to rapid downregulation of full length human APP protein content, which limits its capacity to produce Aβ in the brain of 3XTg-AD mice (Figure 4). The precise mechanism of 24S-OH-mediated reduction in full-length human APP in neurons is unknown at this point. Other groups have also reported that 24S-OH reduces Aβ production in vivo and in vitro [88,89]. Thus, increasing 24S-OH content in the brain may be a potential therapeutic strategy for AD treatment.
Figure 4. . A working model that links cholesterol trafficking with ACAT1, 24S-hydroxycholesterol biosynthesis and downregulations of human APP and HMG-CoA reductase in neurons.
This model predicts that ACAT1 blockage provides more cholesterol as substrate for the enzyme CYP46A1 to synthesize more 24S-hydroxycholesterol. The increase in cholesterol and in 24S-hydroxycholesterol at the ER serve as the signal(s) to downregulate the protein contents of hAPP and HMGR.
ACAT1: Acyl-CoA:cholesterol acyltransferase 1; ER: Endoplasmic reticulum; hAPP: Human APP.
Anti-Acat1 siRNA was shown to knockdown (KD) Acat1 and reduced Aβ generation in a cell culture model [90]. It is important to know whether blocking ACAT1 benefits AD in vivo, especially after the disease onset. Thus, Murphy et al. from this laboratory tested whether ACAT1 inhibition can still elicit beneficial effects in the 3XTg-AD mice at the postsymptomatic stage [91]. To inhibit ACAT1 specifically, we chose to inject Acat1 gene KD construct to the brains of 10-month-old 3XTg-AD mice, using a recombinant adeno-associated virus vector to express the anti-Acat1 siRNA. The 3XTg-AD mouse model displays memory dysfunction by 9 months of age and starts to develop significant Aβ1–42 accumulation approximately at 10 months of age [86]. The results by Murphy et al. [91] showed that Acat1 gene KD reduced the levels of full-length human APP protein and Aβ1–42 in the brains of the 3XTg-AD. These data are consistent with our earlier findings made in the Acat1 KO/3XTg-AD mice [77], supporting the conclusion that blocking ACAT1 is sufficient to benefit AD. The result of Murphy et al. [91] also demonstrated that after the AD onset, partial inactivation of ACAT1 (˜40%) in the brain was sufficient to cause a decrease in the levels of Aβ1–42 in the 3XTg-AD mice.
ACAT1 blockage on Aβ1–42 degradation in microglia
Aβ monomers can self-assemble to form small oligomers and then form the larger sized fibrils. Amyloid plaques are mainly composed of the fibrillar form of Aβ; however, the number and size of amyloid plaques correlate poorly with degree of neurodegeneration or severity of dementia in AD. Instead, recent studies suggest that the oligomeric forms of Aβ are the most toxic molecular species that cause synaptic loss [29,92–93]. Brain levels of Aβ are determined by its production and its clearance. As described above, blocking ACAT1 genetically decreases Aβ levels in the brains of AD mice, at least in part by reducing Aβ generation. However, it is possible that ACAT1 blockage also stimulates Aβ clearance, based on the following observations: Acat1 KO in the 3XTg-AD mice caused a more striking effect in reducing the Aβ1–42 content (by 80%) than in reducing the full-length human APP content (by 50–60%) [77]. In addition, Acat1 KD significantly diminished the oligomeric Aβ content in the brains of the 3XTg-AD mice [91]. These observations implicate that, in addition to its effect on reducing Aβ production, ACAT1 blockage may also promote the clearance of oligomeric Aβ. Microglia are the resident tissue macrophage-like cells in the CNS and play important roles in Aβ clearance in the brain. Cultured microglia are able to engulf and degrade Aβ in lysosomes [66,94]. In a mouse AD model, microglia are recruited to newly formed amyloid plaques within 1–2 days [95]. In addition, impaired microglial clearance of Aβ has been shown to be partially responsible for AD pathogenesis [96–98]. These studies emphasize the importance of microglia in Aβ turnover in the brain. Recently Shibuya et al. in this laboratory investigated whether ACAT1 blockage enhanced oligomeric Aβ1–42 clearance in microglia. We found that in cultured mouse microglia, Acat1 KO or an ACAT1 inhibitor K604 (Figure 1A) [81] upregulated lysosome biogenesis and enhanced degradation of oligomeric Aβ1–42 [99]. We also showed that Acat1 KO in microglia stimulated oligomeric Aβ1–42 clearance in the mouse brain in vivo [99]. These results suggest that in addition to reducing Aβ production, blocking ACAT1 may benefit AD by stimulating Aβ clearance in microglia.
ACAT1 blockage on autophagy
Macroautophagy (hereafter referred to as autophagy) is a cellular degradation process that delivers cytoplasmic contents to the lysosome to maintain cellular homeostasis. Autophagy begins with sequestration of the cytoplasm with a double-membrane structure called an autophagosome [100]. Autophagosomes eventually fuse with lysosomes to degrade sequestered cytoplasmic contents, including denatured and/or aggregation-prone proteins/peptides, such as Aβ [101]. Autophagy can be induced by inhibition of the mammalian target of rapamycin (mTOR), which is a serine/threonine protein kinase that regulates cell growth and proliferation [102]. Autophagy is closely associated with lysosome biogenesis [103]. The transcription factor EB (TFEB) coordinates lysosome biogenesis and autophagy. As a master regulator for lysosome biogenesis, it promotes transcription of target genes in the coordinated lysosomal expression and regulation network [104]. TFEB also upregulates the autophagic machinery [105,106]. Inhibition of mTOR signaling activates TFEB pathway to promote both autophagosome formation and lysosome biogenesis [106–108]. Shibuya et al. in this laboratory found that blocking ACAT1 in cultured microglia by gene KO or by the ACAT1-specific inhibitor K604 increased autophagy as well as TFEB-mediated lysosome biogenesis [99]. Acat1 KO microglia freshly isolated from adult mouse brains were also found to have increased mRNA levels of several genes in the coordinated lysosomal expression and regulation network [99]. Interestingly, ACAT1 blockage caused increases in autophagy and in TFEB-mediated lysosome biogenesis in an mTOR-independent manner; in fact, we found that blocking both ACAT1 and mTOR signaling in microglia showed additive effects in autophagy and in lysosome biogenesis [99]. Previous studies have shown that in mouse models of AD, inhibiting mTOR by rapamycin administration increases autophagy in the brain, reduces Aβ1–42 levels possibly by enhancing Aβ1–42 clearance and rescues cognitive deficits [109,110]. Thus, in AD brains, blocking both ACAT1 and mTOR signaling may provide additive effects on reducing Aβ levels.
ACAT1 blockage on autophagy-mediated human tau degradation in neurons
Enhancing autophagy in an mTOR-independent fashion can promote degradation of aggregate-prone proteins in various mammalian cells [111], [112]. Recently, Shibuya et al. in this laboratory showed that inhibiting ACAT1 by K604 stimulated autophagy in the mouse neuroblastoma N2a cells without altering mTOR signaling, and reduced human tau protein content ectopically expressed in these cells [113]. Acat1 KO also caused an increase in autophagy and decreased the P301L-tau content in primary cortical neurons isolated from 3XTg-AD mice [113]. These results suggest that ACAT1 blockage may provide additional benefit to AD by reducing the tau protein.
In the brains of 3XTg-AD mice, hyperphosphorylation of P301L-tau becomes apparent between 12 and 15 months of ages [86]. A previous study showed that in the 3XTg-AD mouse model, upregulating autophagy by rapamycin administration before the disease onset (2 months old), but not after the disease onset (15 months old), reduces hyperphosphorylated tau levels [114]. Similarly, Shibuya et al. [113] showed that Acat1 KO decreased the P301L-tau protein content in the brains of young 3XTg-AD mice (2–4 months old), but not in those of old mice (17–21 months old), even though Acat1 KO still promoted autophagy in the brains of the old mice. Thus, ACAT1 blockage could also benefit AD by attenuating tauopathy at early stage. It is currently unknown why enhancing autophagy by Acat1 KO is not effective in reducing P301L-tau levels in old 3XTg-AD mice. It is possible that in the old 3XTg-AD mouse brain, the hyperphosphorylated/aggregated P301L-tau may become much less susceptible to autophagy-mediated degradation in vivo.
Potential mechanisms
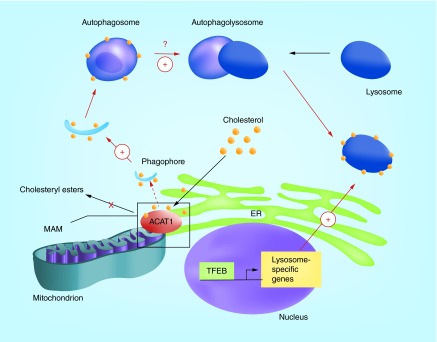
What is the mechanism responsible for the ACAT1 blockage-mediated decrease in full-length human APP protein and increase in autophagy? We believe that the answer may be the effect of ACAT1 blockage on local cholesterol content in the ER membranes. ER membranes contain ‘regulatory sterol pools’ that play important roles in cholesterol homeostasis [115]. Modulating ER cholesterol levels are shown to affect the processing of sterol regulatory element binding proteins [116], which are transcription factors that control cholesterol and fatty acid biosynthesis. ACAT1 resides in the ER, and converts free cholesterol to cholesteryl esters. Blocking ACAT1 is expected to lead to an increase in ER cholesterol pools. In macrophages and in CHO cells, blocking ACAT by using an ACAT inhibitor increases the ER regulatory sterol pool, and leads to downregulation of HMG-CoA reductase and sterol regulatory element binding protein processing [117,118]. In neurons, it is possible that Acat1 KO or KD increases a local cholesterol pool in the ER, which may directly downregulate full-length human APP levels (Figure 4). Recent evidence showed that a significant portion of ACAT1 is located at the mitochondria-associated ER membrane (MAM) [119]; MAM is rich in cholesterol and rich in sphingolipid (ceramide) contents [120]. MAM can serve as a platform for autophagosome formation during starvation [121]. Therefore, it is possible that blocking ACAT1 may modify the local cholesterol content in MAM, which signals an increase in autophagosome formation that leads to increase in lysosome volume (Figure 5). In addition, several studies in vitro have shown that cholesterol content in membranes facilitates membrane fusion [122,123]. Thus, in addition to promoting autophagosome formation at the MAM, ACAT1 blockage may also increase the cholesterol content in the autophagosome, which may promote fusion between autophagosome and lysosome (Figure 5).
Figure 5. . A working model to explain the ACAT1 blockage-dependent increase in autophagy and in lysosome biogenesis in microglia and in neurons.
ACAT1 blockage alters the local cholesterol content in MAM, which signals an increase in autophagosome formation. The increase in autophagosome formation leads to TFEB-mediated increase in lysosome biogenesis. ACAT1 blockage may also increase autophagosome-lysosome membrane fusion.
MAM: Mitochondria-associated ER membrane.
In addition to ACAT1, other resident ER enzymes can also use ER cholesterol as a substrate. In neurons, the ER enzyme Cyp46A1 produces 24S-OH from cholesterol. It is possible that lacking ACAT1 raises the substrate levels for Cyp46A1 and causes an increase in 24S-OH biosynthesis. A similar concept has been reported in macrophages: blocking ACAT enzyme activity by using an ACAT inhibitor increased the biosynthesis of a different oxysterol 27-hydroxycholesterol, presumably because of an increase in the substrate pool for the enzyme 27-hydroxylase Cyp27A1 [124]. In neurons, the increased 24S-OH content in the ER may reduce full-length human APP levels, possibly by upregulating its degradation in the ER (Figure 4).
Blocking ACAT1 activity also raises the overall free cholesterol levels in cells, which promotes excretion of excess cholesterol to various acceptors present in the cell exterior, such as high-density lipoprotein apoAI, and other proteins that bind to cholesterol [2]. A previous study showed that treating macrophages with isotype-nonspecific ACAT inhibitors causes ER stress and induces apoptosis, when the macrophages are cultured in the growth medium without any cholesterol acceptor [125]. Because ER stress can activate ER-associated degradation [126], it is possible that blocking ACAT1 may induce ER stress, which in turn leads to an increase in ER-associated degradation of APP in neurons. ER stress is also known to induce autophagy [127]. To test these possibilities, we examined the possible link between ER stress and ACAT1 blockage in our cell systems. The results showed that neither the ACAT1 specific-inhibitor K604 nor Acat1 gene KO-induced ER stress in mouse microglia and in mouse neuronal cells [99,113]. Thus, ER stress is unlikely to mediate the effects of ACAT1 blockage describe in neurons and in microglia. Earlier, Huttunen et al. [84] also showed that the isotype-nonspecific ACAT inhibitor CP-113,818 did not induce ER stress in CHO cells or in rat neuroblastoma cells.
Pitfalls of currently available ACAT inhibitors
Studies described in this review suggest the use of small molecule ACAT1-specific inhibitor to treat AD. However, there are concerns to use currently available ACAT inhibitors for clinical use. Recent evidence has shown that many ACAT inhibitors also inhibit other MBOAT family enzymes. This is because most of these inhibitors were discovered before the first ACAT gene (ACAT1) was identified in 1993 [6]. In 2000, the MBOAT family was discovered, with ACAT1 as the founding member [22]. Thus, the ACAT inhibitors developed prior to year 2000 may also inhibit other MBOAT members, which may cause undesirable side effects. For instance, the ‘ACAT inhibitor’ CI-976 (Figure 1A) was shown to inhibit multiple membrane trafficking steps, at least in part by inhibiting the enzyme activity of lysophospholipid acyltransferase 3 [128], which is an MBOAT member [23]. The ‘ACAT inhibitor’ CI-1011 orally fed to animals or to men decreased the plasma concentrations of total triglyceride [129], presumably because CI-1011 also inhibits the enzyme activity of diacylglycerol acyltransferase 1, a different MBOAT member [23]. Currently K604 is the only small molecule ACAT1-specific inhibitor available. In an in vitro study, K604 at 0.5 μM was shown to inhibit ACAT1 enzyme activity by 70% without significantly affecting the ACAT2 enzyme activity [81]. In cultured neurons and cultured microglial cells, we showed that when used at 0.5 μM, the effects of K604 faithfully mimic the effects of Acat1 KO [99,113]. However, these results do not eliminate the possibility that at higher concentration, K604 may also inhibit other member(s) of the MBOAT family.
A second concern for using the currently available ACAT inhibitors to treat AD is that many ACAT inhibitors are very hydrophobic compounds and possess ‘membrane-active’ property; the ‘membrane-active’ compounds are known to be sequestered within the lipid bilayer; they can reach high local concentration to affect the biophysical properties of membranes [130]. The short-term and long-term effects of these membrane active compounds in the CNS are unclear. To treat AD, it would be desirable to develop new small molecule ACAT1-specific inhibitors that are permeable to the blood–brain barrier without causing various off-target side effect(s) described above. In addition, we believe that the development of nonviral delivery of anti-Acat1 siRNA [91] for treating AD should also be seriously considered.
Conclusion & future perspective
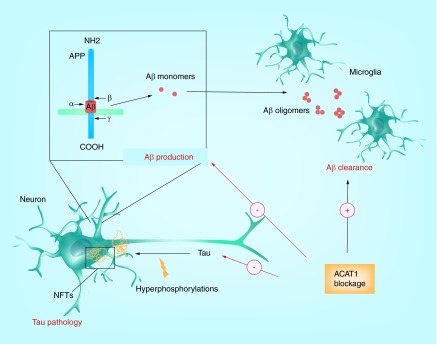
Over the past decade, a large amount of effort has been devoted to find agents for treating AD. At present, only a few of these agents have shown efficacy in clinical trials. As summarized in this review, in a mouse model for AD, blocking ACAT1 elicits multiple beneficial effects (Figure 6): first, it diminishes Aβ production by reducing full-length human APP protein. Second, ACAT1 blockage promotes autophagy-mediated lysosome biogenesis to enhance clearance of Aβ1–42 in microglia. Third, blocking ACAT1 upregulates autophagy in neurons and promote degradation of tau before it becomes hyperphosphorylated. Thus, we believe that ACAT1 can be a prominent therapeutic target for AD treatment. In the future, it will be important to test whether ACAT1 blockage also produces beneficial effects in the presence of ApoE4, because approximately 40% of all late-onset AD patients carry at least one copy of the ApoE4 allele [131]. A common event that occurs in several major neurodegenerative diseases in humans, including AD, Parkinson's disease, frontotemporal dementia, etc. is the presence of certain specific misfolded/aggregated proteins in a given region of the CNS; these misfolded/aggregated proteins are subjected to autophagy-mediated degradation [101]. Thus, in the future, it would be interesting to test if blocking ACAT1 can also benefit other neurodegenerative diseases.
Figure 6. . Beneficial effects of ACAT1 blockage on Alzheimer's disease.
In neurons, ACAT1 blockage reduces Aβ production and tau protein content; in microglia, it increases Aβ clearance.
Key terms.
Cholesteryl esters: Esterified forms of cholesterol where the carboxyl group of a long-chain fatty acid is linked to the hydroxyl group of cholesterol.
Lipid droplets: Lipid-rich intracellular organelles mainly consist of triacylglycerols and cholesteryl esters.
Neurodegenerative disease: A disease that results from structural and/or functional loss of neurons.
Oxysterols: Oxygenated derivatives of cholesterol.
Dyslipidemia: Abnormal elevation of cholesterol and/or triglycerides and/or their protein carriers in the plasma.
Familial AD: A very rare form of Alzheimer's disease, which happens to people who carry a genetic mutation leading to the disease.
Late-onset AD: The most common form of Alzheimer's disease that begins after age 65.
Executive summary.
ACAT converts cholesterol to cholesteryl esters.
There are ACAT1 and ACAT2 in human with different tissue expression patterns.
Blocking ACAT1 can be a promising therapeutic approach for Alzheimer's disease (AD).
ACAT as drug targets
ACAT1 and ACAT2 are members of the membrane-bound O-acyltransferase enzyme family.
ACAT1 and ACAT2 have been studied as drug targets to treat atherosclerosis.
Amyloid plaques, neurofibrillary tangles & strategies to produce drugs to diminish Aβ & pathological tau
Reducing Aβ levels and/or tau levels in the brain are currently the main therapeutic strategies for AD.
AD, cholesterol metabolism & ACAT
Cholesterol cholesterol metabolism is closely associated with AD.
The E4 of allele of apolipoprotein E, which is the major cholesterol/lipid carrier in the CNS, is the strongest genetic risk factor for late-onset AD.
ACAT inhibitors in a mouse model for AD
Treating a mouse AD model with isotype-nonspecific ACAT inhibitors, CP-113,818 and CI-1011 diminishes Aβ deposition in the brain.
Genetic ablation of ACAT1 in AD mice
Acat1 gene knockout (KO) and Acat1 knockdown in a mouse model for AD mouse model reduce Aβ levels in the brains.
ACAT1 blockage on Aβ1–42 degradation in microglia
Blocking ACAT1 by gene KO or by using an ACAT1-specific inhibitor K604 in microglia promotes lysosome biogenesis, and enhances oligomeric Aβ1–42 degradation in lysosomes.
ACAT1 blockage on autophagy
Blocking ACAT1 by gene KO or by using K604 stimulates autophagy and lysosome biogenesis in microglia.
ACAT1 blockage on autophagy-mediated human tau degradation in neurons
Inhibiting ACAT1 by K604 or by gene KO stimulates autophagy and promotes degradation of human tau in mouse neuronal cells.
Potential mechanisms
ACAT1 blockage alters local cholesterol content in the ER membranes, which may cause various beneficial effects on AD.
Pitfalls of currently available ACAT inhibitors
Many ACAT inhibitors currently available also block other membrae-bound O-acyltransferase family enzymes in addition to blocking ACAT1, which may cause undesirable side effects.
Future perspective
To develop new ACAT1 inhibitors without side effects.
To test whether blocking ACAT1 elicits beneficial effects to AD in the presence of ApoE4.
Acknowledgements
We thank members of the Chang laboratory at the Geisel School of Medicine at Dartmouth for stimulating discussions.
Footnotes
Financial & competing interests disclosure
T-Y Chang and C Chang are founders and owners of Theracat, LLC. This work was supported by NIH Grant AG37609 to T-Y Chang and C Chang. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J. Clin. Invest. 2002;110(7):905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang TY, Chang CCY, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 3.Chang TY, Li BL, Chang CCY, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 2009;297(1):E1–E9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann T, Kuchenbecker J, Grimm MOW. Alzheimer's disease: the lipid connection. J. Neurochem. 2007;103:159–170. doi: 10.1111/j.1471-4159.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- 5.Paolo GD, Kim TW. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat. Rev. Neurosci. 2011;12(5):284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An excellent review on the roles of lipids in Alzheimer's disease (AD).
- 6.Chang CCY, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 1993;268(28):20747–20755. [PubMed] [Google Scholar]
- 7.Chang CCY, Chen J, Thomas MA, et al. Regulation and immunolocalization of acyl-coenzyme A:cholesterol acyltransferase in mammalian cells as studied with specific antibodies. J. Biol. Chem. 1995;270(49):29532–29540. doi: 10.1074/jbc.270.49.29532. [DOI] [PubMed] [Google Scholar]
- 8.Lee O, Chang CCY, Lee W, Chang TY. Immunodepletion experiments suggest that acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1) protein plays a major catalytic role in adult human liver, adrenal gland, macrophages, and kidney, but not in intestines. J. Lipid Res. 1998;39(8):1722–1727. [PubMed] [Google Scholar]
- 9.Zhang Y, Yu C, Liu J, Spencer TA, Chang CCY, Chang TY. Cholesterol is superior to 7-Ketocholesterol or 7α-Hydroxycholesterol as an allosteric activator for acyl-coenzyme A: cholesterol acyltransferase 1. J. Biol. Chem. 2003;278(13):11642–11647. doi: 10.1074/jbc.M211559200. [DOI] [PubMed] [Google Scholar]
- 10.Chang CCY, Miyazaki A, Dong R, et al. Purification of Recombinant acyl-coenzyme A: cholesterol acyltransferase 1 (ACAT1) from H293 cells and binding studies between the enzyme and substrates using difference intrinsic fluorescence spectroscopy. Biochemistry (Mosc). 2010;49(46):9957–9963. doi: 10.1021/bi1013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RA, Joyce C, Davis M, et al. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 1998;273(41):26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 12.Cases S, Novak S, Zheng YW, et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 1998;273(41):26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 13.Oelkers P, Behari A, Cromley D, Billheimer JT, Sturley SL. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 1998;273(41):26765–26771. doi: 10.1074/jbc.273.41.26765. [DOI] [PubMed] [Google Scholar]
- 14.Rogers MA, Liu J, Kushnir MM, et al. Cellular pregnenolone esterification by acyl-CoA:cholesterol acyltransferase. J. Biol. Chem. 2012;287(21):17483–17492. doi: 10.1074/jbc.M111.331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki A, Sakashita N, Lee O, et al. Expression of ACAT-1 protein in human atherosclerotic lesions and cultured human monocytes-macrophages. Arterioscler. Thromb. Vasc. Biol. 1998;18(10):1568–1574. doi: 10.1161/01.atv.18.10.1568. [DOI] [PubMed] [Google Scholar]
- 16.Accad M, Smith SJ, Newland DL, et al. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J. Clin. Invest. 2000;105(6):711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagyu H, Kitamine T, Osuga J, et al. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J. Biol. Chem. 2000;275(28):21324–21330. doi: 10.1074/jbc.M002541200. [DOI] [PubMed] [Google Scholar]
- 18.Huang LH, Gui J, Artinger E, et al. Acat1 gene ablation in mice increases hematopoietic progenitor cell proliferation in bone marrow and causes leukocytosis. Arterioscler. Thromb. Vasc. Biol. 2013;33:2081–2087. doi: 10.1161/ATVBAHA.112.301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakashita N, Miyazaki A, Chang CCY, et al. Acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) is induced in monocyte-derived macrophages: in vivo and in vitro studies. Lab. Invest. 2003;83(11):1569–1581. doi: 10.1097/01.lab.0000095687.17383.39. [DOI] [PubMed] [Google Scholar]
- 20.Willner EL, Tow B, Buhman KK, et al. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA. 2003;100(3):1262–1267. doi: 10.1073/pnas.0336398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers MA, Liu J, Song BL, Li BL, Chang CCY, Chang TY. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): enzymes with multiple sterols as substrates and as activators. J. Steroid Biochem. Mol. Biol. 2015;151:102–107. doi: 10.1016/j.jsbmb.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25(3):111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 23.Chang CCY, Sun J, Chang TY. Membrane-bound O-acyltransferases (MBOATs) Front. Biol. 2011;6(3):177–182. [Google Scholar]
- 24.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 26.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc. Natl Acad. Sci. USA. 2002;99(9):6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid ß-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 28.Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce tau hyperphosphorylation and neuritic degeneration. Proc. Natl Acad. Sci. USA. 2011;108(14):5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Citron M. Alzheimer's disease: strategies for disease modification. Nat. Rev. Drug Discov. 2010;9(5):387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]; • A comprehensive review on current therapeutic strategies for AD.
- 31.Masters CL, Selkoe DJ. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 33.Laird FM, Cai H, Savonenko AV, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 2005;25(50):11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willem M, Garratt AN, Novak B, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314(5799):664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 35.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010;6(2):99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Strooper B, Chávez Gutiérrez L. Learning by failing: ideas and concepts to tackle γ-secretases in Alzheimer's disease and beyond. Annu. Rev. Pharmacol. Toxicol. 2015;55(1):419–437. doi: 10.1146/annurev-pharmtox-010814-124309. [DOI] [PubMed] [Google Scholar]
- 37.Cirrito JR, May PC, O'Dell MA, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J. Neurosci. 2003;23(26):8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong S, Quintero-Monzon O, Ostaszewski BL, et al. Dynamic analysis of amyloid β-protein in behaving mice reveals opposing changes in ISF versus parenchymal Aβ during age-related plaque formation. J. Neurosci. 2011;31(44):15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts KF, Elbert DL, Kasten TP, et al. Amyloid-β efflux from the central nervous system into the plasma. Ann. Neurol. 2014;76(6):837–844. doi: 10.1002/ana.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotilinek LA, Bacskai B, Westerman M, et al. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J. Neurosci. 2002;22(15):6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hock C, Konietzko U, Streffer JR, et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38(4):547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 43.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated Tau aggregates via the proteasome. Neuron. 2004;43(3):321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Wilcock DM, Rojiani A, Rosenthal A, et al. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J. Neuroinflammation. 2004;1(1):24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N. Engl. J. Med. 2014;370(4):311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 46.Salloway S, Sperling R, Fox NC, et al. Two Phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N. Engl. J. Med. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandelkow E-M, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012;2(7):a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer's disease. Neurobiol. Aging. 2000;21(5):719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 49.Min S-W, Cho SH, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen TJ, Guo JL, Hurtado DE, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 52.Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 53.Götz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J. Biol. Chem. 2001;276(1):529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- 54.Pappolla MA, Bryant-Thomas TK, Herbert D, et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61(2):199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein FC, Ashley AV, Endeshaw Y, Hanfelt J, Lah JJ, Levey AI. Effects of hypertension and hypercholesterolemia on cognitive functioning in patients with Alzheimer's disease. Alzheimer Dis. Assoc. Disord. 2008;22(4):336–342. doi: 10.1097/wad.0b013e318188e80d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Refolo LM, Pappolla MA, Malester B, et al. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7(4):321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 57.Wahrle S, Das P, Nyborg AC, et al. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 2002;9(1):11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- 58.Kalvodova L, Kahya N, Schwille P, et al. Lipids as modulators of proteolytic activity of bace involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro . J. Biol. Chem. 2005;280(44):36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- 59.Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of γ-secretase by its lipid microenvironment. J. Biol. Chem. 2008;283(33):22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simons M, Keller P, Strooper BD, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl Acad. Sci. USA. 1998;95(11):6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer's disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo . Proc. Natl Acad. Sci. USA. 2001;98(10):5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2000;97(6):2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bales KR, Liu F, Wu S, et al. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J. Neurosci. 2009;29(21):6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koistinaho M, Lin S, Wu X, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat. Med. 2004;10(7):719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 65.Deane R, Sagare A, Hamm K, et al. apoE isoform–specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 2008;118(12):4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang Q, Lee CYD, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Aβ. Neuron. 2008;58(5):681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellano JM, Kim J, Stewart FR, et al. Human apoE Isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011;3(89):89ra57–89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirsch-Reinshagen V, Zhou S, Burgess BL, et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 2004;279(39):41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 69.Wahrle SE, Jiang H, Parsadanian M, et al. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 2004;279(39):40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 70.Riddell DR, Zhou H, Comery TA, et al. The LXR agonist TO901317 selectively lowers hippocampal Aβ42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol. Cell. Neurosci. 2007;34(4):621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Wahrle SE, Jiang H, Parsadanian M, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J. Clin. Invest. 2008;18(2):671–682. doi: 10.1172/JCI33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donkin JJ, Stukas S, Hirsch-Reinshagen V, et al. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 2010;285(44):34144–34154. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cramer PE, Cirrito JR, Wesson DW, et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dietschy JM, Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004;45(8):1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Chan RB, Oliveira TG, Cortes EP, et al. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J. Biol. Chem. 2012;287(4):2678–2688. doi: 10.1074/jbc.M111.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important study showing elevated levels of cholesteryl ester in the brains of human AD patients and AD mouse models.
- 76.Tajima Y, Ishikawa M, Maekawa K, et al. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer's disease. Lipids Health Dis. 2013;12(1):68. doi: 10.1186/1476-511X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryleva EY, Rogers MA, Chang CCY, et al. ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc. Natl Acad. Sci. USA. 2010;107(7):3081–3086. doi: 10.1073/pnas.0913828107. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First paper demonstrating that blocking ACAT1 but not ACAT2 elicits the beneficial effects on AD.
- 78.Puglielli L, Konopka G, Pack-Chung E, et al. Acyl-coenzyme A:cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat. Cell Biol. 2001;3(10):905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 79.Chang CCY, Sakashita N, Ornvold K, et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 2000;275(36):28083–28092. doi: 10.1074/jbc.M003927200. [DOI] [PubMed] [Google Scholar]
- 80.Hutter-Paier B, Huttunen HJ, Puglielli L, et al. The ACAT Inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron. 2004;44(2):227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]; •• First animal study suggesting that ACAT can be a drug target for treating AD.
- 81.Ikenoya M, Yoshinaka Y, Kobayashi H, et al. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis. 2007;191(2):290–297. doi: 10.1016/j.atherosclerosis.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 82.Tardif J-C, Grégoire J, L'Allier PL, et al. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation. 2004;110(21):3372–3377. doi: 10.1161/01.CIR.0000147777.12010.EF. [DOI] [PubMed] [Google Scholar]
- 83.Huttunen HJ, Havas D, Peach C, et al. The acyl-coenzyme A:cholesterol acyltransferase inhibitor CI-1011 reverses diffuse brain amyloid pathology in aged amyloid precursor protein transgenic mice. J. Neuropathol. Exp. Neurol. 2010;69(8):777–788. doi: 10.1097/NEN.0b013e3181e77ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huttunen HJ, Peach C, Bhattacharyya R, et al. Inhibition of acyl-coenzyme A:cholesterol acyl transferase modulates amyloid precursor protein trafficking in the early secretory pathway. FASEB J. 2009;23(11):3819–3828. doi: 10.1096/fj.09-134999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhattacharyya R, Barren C, Kovacs DM. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. J. Neurosci. 2013;33(27):11169–11183. doi: 10.1523/JNEUROSCI.4704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 87.Russell DW, Halford RW, Ramirez DMO, Shah R, Kotti T. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 2009;78:1017–1040. doi: 10.1146/annurev.biochem.78.072407.103859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hudry E, Van Dam D, Kulik W, et al. Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer's disease. Mol. Ther. J. Am. Soc. Gene Ther. 2010;18(1):44–53. doi: 10.1038/mt.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Urano Y, Ochiai S, Noguchi N. Suppression of amyloid-β production by 24S-hydroxycholesterol via inhibition of intracellular amyloid precursor protein trafficking. FASEB J. 2013;27(10):4305–4315. doi: 10.1096/fj.13-231456. [DOI] [PubMed] [Google Scholar]
- 90.Huttunen HJ, Greco C, Kovacs DM. Knockdown of ACAT-1 reduces amyloidogenic processing of APP. FEBS Lett. 2007;581(8):1688–1692. doi: 10.1016/j.febslet.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murphy SR, Chang CC, Dogbevia G, et al. Acat1 knockdown gene therapy decreases amyloid-β in a mouse model of Alzheimer's disease. Mol. Ther. 2013;21(8):1497–1506. doi: 10.1038/mt.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shankar GM, Li S, Mehta TH, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lesné S, Koh MT, Kotilinek L, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 94.Yang C-N, Shiao Y-J, Shie F-S, et al. Mechanism mediating oligomeric Aβ clearance by naïve primary microglia. Neurobiol. Dis. 2011;42(3):221–230. doi: 10.1016/j.nbd.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 95.Meyer-Luehmann M, Spires-Jones TL, Prada C, et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer's disease. Nature. 2008;451(7179):720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hickman SE, Allison EK, Khoury JE. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer's disease mice. J. Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Griciuc A, Serrano-Pozo A, Parrado AR, et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78(4):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lucin KM, O'Brien CE, Bieri G, et al. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer's disease. Neuron. 2013;79(5):873–886. doi: 10.1016/j.neuron.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shibuya Y, Chang CCY, Huang LH, Bryleva EY, Chang TY. Inhibiting ACAT1/SOAT1 in microglia stimulates autophagy-mediated lysosomal proteolysis and increases Aβ1–42 clearance. J. Neurosci. 2014;34(43):14484–14501. doi: 10.1523/JNEUROSCI.2567-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First publication showing that blocking ACAT1 in microglia promotes autophagy and lysosome biogenesis.
- 100.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 101.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009;10(9):623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 104.Sardiello M, Palmieri M, di Ronza A, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 105.Settembre C, Malta CD, Polito VA, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332(6036):1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou J, Tan S-H, Nicolas V, et al. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013;23(4):508–523. doi: 10.1038/cr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roczniak-Ferguson A, Petit CS, Froehlich F, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 2012;5(228):ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Settembre C, Zoncu R, Medina DL, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31(5):1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mTOR, Aβ and tau: effects on cognitive impairments. J. Biol. Chem. 2010;285(17):13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem. 2007;282(8):5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 112.Krüger U, Wang Y, Kumar S, Mandelkow E-M. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol. Aging. 2012;33(10):2291–2305. doi: 10.1016/j.neurobiolaging.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 113.Shibuya Y, Niu Z, Bryleva EY, et al. Acyl-coenzyme A:cholesterol acyltransferase 1 blockage enhances autophagy in the neurons of triple transgenic Alzheimer's disease mouse and reduces human P301L-tau content at the presymptomatic stage. Neurobiol. Aging. 2015;36(7):2248–2259. doi: 10.1016/j.neurobiolaging.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS ONE. 2011;6(9):e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 116.Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell. 2002;10(2):237–245. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- 117.Scheek S, Brown MS, Goldstein JL. Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proc. Natl Acad. Sci. USA. 1997;94(21):11179–11183. doi: 10.1073/pnas.94.21.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tabas I, Weiland DA, Tall AR. Inhibition of acyl coenzyme A:cholesterol acyl transferase in J774 macrophages enhances down-regulation of the low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl-coenzyme A reductase and prevents low density lipoprotein-induced cholesterol accumulation. J. Biol. Chem. 1986;261(7):3147–3155. [PubMed] [Google Scholar]
- 119.Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31(21):4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important study demonstrating that a significant portion of ACAT1 is located in the mitochondria-associated estrogen receptor (ER) membrane.
- 120.Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol. Pharmacol. 2010;77(4):517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamasaki M, Furuta N, Matsuda A, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495(7441):389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]; • First paper suggesting that autophagosomes form at the mitochondria-associated ER membrane during starvation.
- 122.Churchward MA, Rogasevskaia T, Höfgen J, Bau J, Coorssen JR. Cholesterol facilitates the native mechanism of Ca2+-triggered membrane fusion. J. Cell Sci. 2005;118(20):4833–4848. doi: 10.1242/jcs.02601. [DOI] [PubMed] [Google Scholar]
- 123.Chang J, Kim SA, Lu X, Su Z, Kim SK, Shin YK. Fusion step-specific influence of cholesterol on SNARE-mediated membrane fusion. Biophys. J. 2009;96(5):1839–1846. doi: 10.1016/j.bpj.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown AJ, Watts GF, Burnett JR, Dean RT, Jessup W. Sterol 27-hydroxylase acts on 7-ketocholesterol in human atherosclerotic lesions and macrophages in culture. J. Biol. Chem. 2000;275(36):27627–27633. doi: 10.1074/jbc.M004060200. [DOI] [PubMed] [Google Scholar]
- 125.Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003;5(9):781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 126.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13(3):374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 127.Ogata M, Hino S, Saito A, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006;26(24):9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J. Cell Biol. 2009;186(2):211–218. doi: 10.1083/jcb.200904147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Llaverías G, Laguna JC, Alegret M. Pharmacology of the ACAT inhibitor avasimibe (CI-1011) Cardiovasc. Drug Rev. 2003;21(1):33–50. [PubMed] [Google Scholar]
- 130.Homan R, Hamelehle KL. Influence of membrane partitioning on inhibitors of membrane-bound enzymes. J. Pharm. Sci. 2001;90(11):1859–1867. doi: 10.1002/jps.1135. [DOI] [PubMed] [Google Scholar]
- 131.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]