Abstract
The occurrence of venous thromboembolism (VTE) in acute lymphocytic leukemia patients receiving L-asparaginase therapy may cause significant morbidity, neurological sequela and possibly worse outcomes. The prophylactic use of antithrombin infusion (to keep antithrombin activity >60%) or low molecular weight heparin (LMWH) may reduce the risk of VTE. The decision to continue L-asparaginase therapy after the development of VTE should be based on anticipated benefits, severity of VTE and the ability to continue therapeutic anticoagulation. In patients receiving asparaginase rechallenge, the use of therapeutic LMWH, monitoring of anti-Xa level and antithrombin level are important. Novel oral anticoagulants are not dependent on antithrombin level, hence offer theoretical advantages over LMWH for the prevention and therapy of asparaginase-related VTE.
KEYWORDS : acute lymphocytic leukemia, asparaginase, thrombosis, venous thromboembolism
The use of L-asparaginase, along with multiagent systemic chemotherapy including anthracycline, vincristine and steroid, has made acute lymphocytic leukemia (ALL) a curable malignancy, particularly in children [1,2]. L-asparaginase catalyzes the conversion of asparagine (useful for protein synthesis) to aspartic acid and ammonia, thereby depleting the supply of asparagine to the leukemic cells that rely on an exogenous source due to their inability to synthesize it [3]. This leads to cell cycle arrest in the G1 phase and DNA strand breaks, thereby promoting apoptosis of leukemic cells [4]. L-asparaginase also deaminates glutamine to glutamic acid, which decreases the glutamine levels in the circulation [5]. Glutamine is believed to be a nitrogen donor for synthesis of RNA and DNA by the tumor cells, hence its depletion halts tumor growth and enhances cell apoptosis [6]. L-asparaginase is available in three forms, which include Escherichia coli asparaginase, Erwinia asparaginase and pegylated form of the native E. coli asparaginase (pegaspargase) [7]. Although a useful agent, the use of L-asparaginase has several potential toxicities including the risk of thrombosis [8–10]. In this review, we summarize the key studies on L-asparaginase-related venous thromboembolism (VTE) in patients with ALL with a focus on identification of high-risk patients, approaches to prevent VTE and management of patients who develop VTE.
Epidemiology
The incidence of VTE in ALL patients has been reported to vary from 1 to 36% depending upon the age group of the patients, study designs, treatment protocols, symptomatic thromboembolism versus detection with screening radiography [10–12]. Caruso et al. reported a meta-analysis of 17 prospective studies on thrombotic complications in childhood ALL and demonstrated a thrombosis rate of 5.2%; more than half of the patients had CNS thrombotic events [12]. A 2007 review suggested that the majority of symptomatic thrombotic events occur in the venous system, predominantly in the upper extremity, with CNS thrombosis being a unique association with L-asparaginase therapy [10]. The data on thrombosis in 548 adult and pediatric patients treated with asparaginase-based therapy on Dana-Farber Cancer Institute Consortium ALL protocols reported VTE in 8% of patients including 5% pediatric and 34% adult patients. The most common sites of thrombosis were venous circulation of upper extremity/central venous catheters (CVCs) (36%), followed by lower extremities (19%) and CNS (19%). Pulmonary embolism was also observed in 15% of patients. The sites of thrombosis were mostly similar for both adult and pediatric patients, with numerically increased predilection for pulmonary embolism in adults than children (22 vs 10%; p = 0.40) and cerebral sinus venous thrombosis in children than adults (24 vs 11%; p = 0.45). The median time to occurrence of VTE was 3.5 months [11]. In a retrospective analysis of Dutch–Belgian HOVON-37 multicenter study of 240 adult patients, symptomatic VTE occurred in 10% of patients during the first cycle of L-asparaginase-based induction therapy, with a median time to thrombosis of 23 days (range: 6–46 days). Upper extremity vein thrombosis (46%; all related to CVC) and CNS thrombosis (37%) were the most common sites of VTE. The development of VTE, particularly CNS thrombosis, in cycle one was associated with significantly worse complete remission rate [13]. Other studies discussed later have also demonstrated a possible association between worse outcomes and the occurrence of VTE with L-asparaginase-based therapy [14] but this finding is not consistent, particularly if the patients complete L-asparaginase therapy [11]. These studies indicate that VTE is common with L-asparaginase therapy, frequently involves upper extremity and CNS and may influence outcomes.
Mechanism of thrombosis
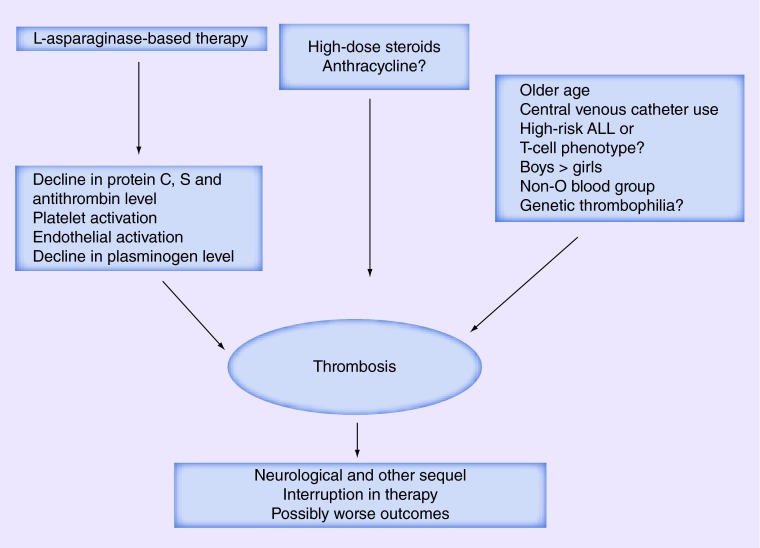
The mechanism of induction of thrombosis by L-asparaginase is likely complex. L-asparaginase is believed to disrupt the physiologic balance between the hemostatic and anticoagulation pathways, along with activation of platelets and endothelial cells (Figure 1) [9–11,14–15]. Although L-asparaginase appears to decrease the synthesis of both procoagulant and anticoagulant proteins by the liver, the decline in the levels of anticoagulant proteins C, S and antithrombin III contributes to an increased risk of thrombosis [9–11,14–15]. In a retrospective study, the median pretreatment antithrombin level of 120% was reduced to 59% in approximately half of the adult patients after the fourth infusion of L-asparaginase [14]. An earlier study suggested that L-asparaginase directly affects the antithrombin levels and other proteins of the coagulation system [15]. However, later studies have demonstrated that L-asparaginase prevents the secretion of proteins from the cells and leads to retention of protein aggregates intracellularly, thus indirectly decreasing the plasma levels of these proteins [16,17]. L-asparaginase may also increase platelet aggregation in response to adenosine diphosphate [18], but this finding is not consistent [19]. Another study showed enhanced endothelial activation by increasing the levels of soluble P-selectin, high molecular weight von Willebrand factor antigen and plasminogen activator inhibitor-1 [20]. The net results of the decline in anticoagulant proteins, platelet activation and endothelial activation is the development of thrombosis.
Figure 1. . Risk factors, pathogenesis and effect of asparaginase-associated thrombosis in acute lymphocytic leukemia.
ALL: Acute lymphocytic leukemia.
Identification of high-risk patients
The ability to identify patients at high-risk of VTE can allow close monitoring and the use of preventive measures. A few studies have assessed the risk factors of VTE in ALL patients treated with L-asparaginase. In the Dana-Farber consortium study, high-risk ALL group, older age at diagnosis and T-cell phenotype predicted a higher risk of VTE in a univariate analysis; however, increasing age was the only predictor of VTE in multivariate analysis. Patients aged >30 years had a VTE rate of 42% [11]. Another Canadian pediatric study also suggested that the risk of CNS thrombosis may be higher in high-risk ALL patients [21]. In an Italian pediatric study, boys had a higher risk of symptomatic VTE, as compared with girls (38 vs 10%; p = 0.001) [22]. In a Canadian study, non-O blood group (hazard ratio of 2.3; p = 0.006) was identified as an independent risk factor for VTE [23].
In the pediatric ALL patients, the meta-analysis by Caruso et al. demonstrated a higher incidence rate of thrombosis during induction versus after induction phase (4.8 vs 2.0%; p = 0.004), with daily asparaginase doses of ≤6000 versus ≥10,000 U/m2 (8.0 vs 2.9%; p < 0.001) and the use of asparaginase for ≥9 days versus <9 days (9.6 vs 3.1%; p < 0.001). This was the first study to show an association between lower daily doses of L-asparaginase and higher rates of thrombosis, which could be related to the intensification of regimens in patients who received lower doses of L-asparaginase. There may also be other differences between patients receiving different doses of L-asparaginase [12]. Although there is a relative paucity of high-quality data, randomized trials comparing the outcomes of ALL using different forms of L-asparaginase fail to demonstrate a difference in thrombotic risks between the three forms of asparaginase [12,24–26]. However, in one study, the use of E. coli asparaginase was associated with a greater likelihood of coagulation abnormalities (defined as ‘any clinical or biologic abnormality, e.g., hypofibrinogenemia requiring a modification of chemotherapy or supportive care’) [25]. In another small study (n = 20), the use of E. coli asparaginase was associated with a higher risk of decline in protein C level below 70%, compared with Erwinia asparaginase (50 vs 0%; p = 0.03) [27]. The use of L-asparaginase encapsulated within erythrocytes, compared with the native form of the enzyme, is thought to allow gradual serum asparagine depletion with lower doses. This is speculated to be associated with a lower risk of toxicities including thrombotic complications [28]; however, it remains to be established in larger studies.
The use of CVC may be a potential risk factor for site-specific thrombotic events with 25 out of 91 VTE events (27.5%) associated with CVC in the Caruso study; CVC-associated thrombosis accounted for more than half of non-CNS thrombosis. Although unclear in asparaginase-related VTE, the risk of line-related thrombosis in general appears to be higher with the use of peripherally inserted central catheter than CVC [29]. The use of anthracycline as well as prednisone (vs dexamethasone), particularly during postinduction therapy, were other potential risk factors [12]. In another pediatric study, all of the CNS thromboses occurred during the induction phase and were associated with the use of prednisone as steroid [21].
Prior studies have assessed the presence of any correlation between the level of coagulation proteins or inherited thrombophilia and the risk of L-asparaginase-related thrombosis. In a small study of pediatric patients treated with L-asparaginase-based therapy, levels of protein C, S and vitamin K-dependent factors (such as factors IX and X) of eight patients with thrombosis were compared with those of nine patients without any thrombosis. The frequency of protein C deficiency (37 vs 33%; p = 0.38) and protein S deficiency (83 vs 28%; p = 0.10) was numerically different but statistically similar between patients with versus without thrombosis. The lower levels of protein C and S were also associated with reduced levels of factors IX and X. Furthermore, the ratio of protein C and S to each other and to factors IX and X was similar in the two groups [30]. Although this study demonstrated no clear evidence of the reduced levels of these proteins in identifying at-risk patients, this may have been related to small sample size. An Israeli pediatric study (n = 41) utilizing enoxaparin prophylaxis (discussed later in detail) demonstrated a high incidence of genetic thrombophilia in 27 children (11% prothrombin G20210A and 18% factor V Leiden), who were Arabs and Jews in ethnic origin; however, no one had any VTE [31].
Another underpowered prospective trial of 85 pediatric patients demonstrated no significant association between thrombosis and the presence of factor V Leiden mutation, prothrombin gene 20210A and antiphospholipid antibodies [32]. An Italian study of 48 children demonstrated a numerically higher prevalence of factor V Leiden (20 vs 3–7%; p-value unavailable) and prothrombin mutation (10 vs 1–3%; p-value unavailable) in the ALL patients with VTE, as compared with the general population [22]. In the Dana-Farber study, the prevalence of activated protein C resistance (9% of 23 patients tested) and factor V Leiden mutation (4%) was felt to be similar to that expected in the general population [11]. In an adult study, none of the patients with VTE who underwent thrombophilia testing (54% of 24), were found to have factor V Leiden mutation or prothrombin gene 20210A [13]. The aforementioned meta-analysis assessed the impact of genetic thrombophilia in 557 children; the presence of genetic prothrombotic abnormalities was similar to that of general population. In pooled analysis, the presence of genetic thrombophilia increased the thrombotic risk by approximately eightfold [12]. Thus, genetic thrombophilia, although controversial, may be a potential risk factor for VTE in children treated with L-asparaginase. Since the benefit of thrombophilia testing in such context has not been ascertained, its routine evaluation cannot be recommended outside of a trial.
Prevention
The use of L-asparaginase therapy is associated with reduced levels of antithrombin and fibrinogen; hence studies have investigated the role of fresh frozen plasma (FFP) or cryoprecipitate supplementation to reduce the thrombohemorrhagic risk of L-asparaginase therapy (Table 1). A large pediatric study from Canada (n = 719) utilized antithrombin level of 40–60 U/ml or less and fibrinogen level of 1 g/l or less as general thresholds for transfusing FFP and cryoprecipitate respectively. Approximately 37 and 68% of children in the supplementation group received FFP and cryoprecipitate, respectively. The risk of CNS thrombosis was numerically lower but statistically similar (0 vs 1.5%; p-value unavailable) between patients who did versus did not receive FFP or cryoprecipitate supplementation [21]. Conversely, in the aforementioned Dutch–Belgian study, the incidence of symptomatic VTE in cycle one of L-asparaginase-based induction therapy was lower with FFP supplementation versus no prophylaxis (6 vs 19%; p = 0.02), even though FFP supplementation did not influence antithrombin levels. Prophylactic FFP was used in 82% of total patients at the dose of 10–15 ml/kg for each treatment day with L-asparaginase. The development of VTE, particularly CNS thrombosis, (but not the use of FFP) was associated with significantly worse complete remission rate (54 vs 84%; odds ratio of 0.22; 95% CI: 0.09–0.53) even after adjustment for ALL risk categories but not worse overall survival [13].
Table 1. . Prevention of asparaginase-related venous thrombosis.
| Study (year) | Study design | Number of patients, age group | Prophylaxis | Thrombosis incidence | Ref. |
|---|---|---|---|---|---|
| Abbott et al. (2009) |
Retrospective study |
719 children |
FFP/cryoprecipitate vs none |
CNS thrombosis 0 vs 1.5%† |
[21] |
| Lauw et al. (2013) |
Retrospective study |
240 adults |
FFP vs none |
6 vs 19%; p = 0.02 |
[13] |
| Mitchell et al. (2003) |
Randomized control trial |
85 children |
Antithrombin vs none |
28 vs 37%; p = 0.43 |
[32] |
| Hunault-Berger et al. (2008) |
Retrospective study |
214 adults |
Antithrombin vs none |
4.8 vs 12.2%; p = 0.04 |
[14] |
| Elhasid et al. (2001) |
Prospective study with historical comparison |
91 children |
Enoxaparin vs none |
2 vs 4%† |
[31] |
| Meister et al. (2008) | Prospective study | 112 children | Enoxaparin and antithrombin vs antithrombin | 0 vs 12.7%; p < 0.05 | [33] |
†p-values are not available.
FFP: Fresh frozen plasma.
Initial laboratory studies suggested that L-asparaginase treatment was associated with enhanced thrombin generation, increased levels of d-dimers and plasminogen activator inhibitor as well as marked reduction in protein C, protein S and antithrombin activity levels. The use of antithrombin supplementation resulted in normalization of enhanced thrombin generation as well as levels of antithrombin, d-dimers and plasminogen activator inhibitor. However, the levels of protein C and S did not change with antithrombin supplementation [34,35]. In an Italian study of 25 adult patients with ALL, antithrombin III therapy at the dose of 50 U/kg was given for 10 days along with 7-day course of L-asparaginase therapy. A significant increase in the levels of antithrombin was observed with no thrombosis and no increase in markers of hypercoagulability; the rate of VTE appeared to be lower than the historical control (0 vs 8%; p-value unavailable) [36]. These studies, although hypothesis generating, did not aim to determine the efficacy of antithrombin supplementation in preventing VTEs. A subsequent underpowered randomized control trial (RCT), showed a numerically lower but statistically similar rate of thrombosis in children receiving weekly antithrombin versus the control arm (28 vs 37%; p = 0.43). However, a quarter of patients had lower than targeted antithrombin level, thus indicating that twice weekly dosing may be preferred over weekly dosing. Antithrombin replacement did not increase the risk of bleeding [32]. A multicenter retrospective study of 214 adult ALL patients utilized FFP, fibrinogen and antithrombin concentrates to maintain fibrinogen > 1g/l and antithrombin activity of 60% during L-asparaginase-based induction therapy; L-asparaginase was held for 48 h if these targets were not achieved. The infusion of these products in about half of the patients resulted in an increase in the mean levels of antithrombin from 61 to 88% after antithrombin infusion, fibrinogen from 1.0 to 1.4 g/l after fibrinogen infusion and no increase in antithrombin or fibrinogen levels following FFP infusion. The study reported a lower rate of omission or delay of L-asparaginase (53 vs 72%; p = 0.005) as well as reduced thrombosis (4.8 vs 12.2%; p = 0.04) in the patients, who did versus did not receive antithrombin concentrates. The occurrence of thrombosis was associated with a trend toward lower disease-free survival (14 vs 58 months; p = 0.05) and median overall survival (19 vs 53 months; p = 0.06) [14].
A few studies have utilized anticoagulant prophylaxis to reduce the risk of VTE. In a pediatric study (n = 41), enoxaparin prophylaxis (0.45–1.33 mg/kg/day) was started at the first dose of L-asparaginase until 7 days after the last dose. No thrombotic (versus 4% VTE in the historical control of 50 patients; p-value unavailable) and bleeding episodes were noted; however, one patient (2%) developed brain infarct 1 week after the cessation of enoxaparin [31]. Another pediatric study compared the effect of enoxaparin prophylaxis (0.75–1.2 mg/kg/day) and antithrombin use to antithrombin supplementation alone. Antithrombin supplementation was used in approximately 60% children to maintain a plasma antithrombin level above 50%. Although combined prophylaxis group were more likely to have a CVC placed within 2 weeks (92 vs 62%; p = 0.002), symptomatic VTE was lower in combined prophylaxis group versus antithrombin prophylaxis group (0 vs 12.7%; p < 0.05). None of the patients in either group had major bleeding episodes [33]. Given the fact that the use of enoxaparin and antithrombin may be more effective than antithrombin alone in VTE prevention [33], we hypothesize that direct thrombin inhibitors, which do not require the presence of antithrombin [37] may be as effective as or perhaps more effective than enoxaparin in preventing the risk of VTE; this approach may possibly also obviate the need of antithrombin supplementation.
Whether the use of specific forms of L-asparaginase can reduce the risk of thrombosis remains unclear; however, E. coli asparaginase is superior to Erwinia asparaginase in terms of disease control and overall survival [24,25], hence may be preferentially used in the absence of allergies or neutralizing antibodies. In a small study of 18 patients, L-asparaginase encapsulated within erythrocytes appeared to have lower toxicities, compared with six patients treated with E. coli asparaginase. The former preparation demonstrated similar levels of asparagine depletion but a trend toward less allergic reactions and coagulation disorders [28]. However, this remains to be proven in larger studies.
Management of VTE & rechallenge with L-asparaginase
In cancer patients in general, options for anticoagulation may include vitamin K antagonists (VKAs), LMWH, fondaparinux and unfractionated heparin (UFH). Most experts recommend weight-adjusted parenteral use of LMWH over other agents (Table 2) [38]. Although not widely accepted, in patients with severe thrombocytopenia (n = 10), reduced dose of LMWH for prophylaxis or treatment of thrombosis may be safe and may not result in significant bleeding episodes [39]. A Cochrane meta-analysis of 16 RCTs for the treatment of VTE in cancer patients showed a reduction in mortality but not in the recurrence of VTE with LMWH versus UFH at the end of 3 months of follow-up [40]. The CLOT trial showed a lower incidence of recurrent VTE in LMWH group versus warfarin group (8 vs 16%; p = 0.002) [41]. Further, a meta-analysis of seven RCTs in cancer patients showed a reduction in VTE but not in mortality with long-term use of LMWH versus VKA [42]. LMWH also has advantages of fewer drug and food interactions, lack of need for frequent blood monitoring for anticoagulant effect and flexibility of use during invasive procedures due to its shorter half-life. Conversely, VKAs may be better agents in patients with cost considerations, preference of an oral agent and those with renal insufficiency. A recent post hoc analysis of the Matisse clinical trials, although not confirmatory, also suggested that fondaparinux may be largely similar in efficacy and safety to LMWH in cancer patients [43]. The novel oral anticoagulants such as rivaroxaban, dabigatran, apixaban and edoxaban are other attractive alternatives, which offer the added advantage of fixed oral dose and a lack of requirement for regular laboratory monitoring [44]. A subgroup analysis of the EINSTEIN DVT and PE trials demonstrated no difference in the risk of VTE recurrence (5 vs 7%; p = 0.24) or clinically relevant bleeding (14 vs 16%; p = 0.28) with rivaroxaban, compared with warfarin in cancer patients [45]. A meta-analysis of six RCT reported no difference in the risk of VTE recurrence (3.8 vs 5.9%; p = 0.99) with the use of novel oral anticoagulants, as compared with conventional anticoagulation in cancer patients [46].
Table 2. . Select studies comparing the management of thrombosis in cancer patients.
| Study (year) | Study design | Management | Recurrence risk/rate | Major bleeding risk | Mortality risk | Ref. |
|---|---|---|---|---|---|---|
| Akl et al. (2004) |
Meta-analysis |
LMWH vs UFH in the initial management |
RR: 0.78; 95% CI: 0.29–2.08; p = 0.62 |
RR: 0.79; 95% CI: 0.39–1.63; p = 0.53 |
RR: 0.71; 95% CI: 0.52–0.98; p = 0.04 |
[40] |
| Akl et al. (2014) |
Meta-analysis |
LMWH vs VKA for the long-term management |
HR: 0.47; 95% CI: 0.32–0.71; p < 0.001 |
RR: 1.07; 95% CI: 0.52–2.19; p = 0.85 |
HR: 0.96; 95% CI: 0.81–1.14; p = 0.31 |
[42] |
| van Doormal et al. (2009) |
Post hoc analysis of a randomized study |
Fondaparinux vs LMWH in DVT |
12.7 vs 5.4% at 3 months† |
7.1 vs 7.2%† |
HR: 1.25; 95% CI: 0.67–2.34; p = 0.49 |
[43] |
| Lee et al. (2003) |
Randomized control trial |
LMWH vs warfarin in VTE |
8 vs 16%; p = 0.002 |
6 vs 4%; p = 0.27 |
39 vs 41%; p = 0.53 |
[41] |
| Prins et al. (2014) |
Randomized control trial |
Rivaroxaban vs warfarin |
5 vs 7%; p = 0.24 |
2 vs 5%; p = 0.047 |
16 vs 18%; p = 0.70 |
[45] |
| Vedovati et al. (2015) | Meta-analysis | Novel oral anticoagulants vs conventional anticoagulation | 3.8 vs 5.9%; p = 0.10 | 3.2 vs 4.2%; p = 0.42 | Not reported | [46] |
†Absolute difference in VTE recurrence was 7.3% (95% CI: 0.1–14.5; p = 0. 0.046). Absolute difference in major bleeding risk recurrence was -0.1% (95% CI: -6.7–6.5; p = 0.99).
DVT: Deep vein thrombosis; HR: Hazard ratio; LMWH: Low molecular weight heparin; RR: Risk ratio; UFH: Unfractionated heparin; VKA: Vitamin K antagonists; VTE: Venous thromboembolism.
The anticoagulant effect of UFH or LMWH is dependent upon antithrombin levels, which may be reduced with the use of L-asparaginase. The use of L-asparaginase may, therefore, create antithrombin-dependent heparin resistance with possible indication of antithrombin level monitoring and substitution [47]. An in vitro study assessed the endogenous thrombin generation capacity of direct thrombin inhibitor and LMWH in plasma from children with L-asparaginase-induced antithrombin deficient state. The study demonstrated a consistent anticoagulant effect of direct thrombin inhibitors (melagatran) independent of antithrombin level in plasma. Conversely, the anticoagulant effect of LMWH was markedly dependent on antithrombin level [37]. In this context, novel oral anticoagulants such as rivaroxaban or dabigatran, which are direct inhibitors of thrombin or factor Xa, do not require monitoring of antithrombin level and may presumably have a lower risk of failure; however, further studies are warranted to confirm these speculations.
Prior reports have frequently utilized LMWH and VKA for management of VTE in ALL patients for 3–6 months or till the completion of L-asparaginase therapy or entire chemotherapy [11,13,48]. In select patients, particularly children, it may be possible to safely continue the use of L-asparaginase along with anticoagulation (Table 3). In UK ALL 2003 trial, 3.2% (n = 59) developed VTE, predominantly during exposure to pegylated asparaginase (90%) and during induction (70%). The majority (n = 47; 89%) were treated with LMWH, with antithrombin replacement in only one patient. All patients with VTE recovered fully without any major thrombohemorrhagic sequela; however, ALL therapy was delayed for a median of 8 days in 19% patients. Of 50 patients, who required further L-asparaginase therapy, 73% (n = 38) of patients (predominantly children) including ten patients with CNS thrombosis continued L-asparaginase therapy with concurrent LMWH prophylaxis (goal anti-Xa level of 0.1–0.3 IU/ml). This resulted in no further episodes of thrombosis or bleeding, suggesting that re-exposure to the agent may be safe in some children [48]. In the aforementioned Dana-Farber consortium study, 74% of 43 patients with VTE were initially treated with LMWH (goal anti-Xa level of 0.4–0.6 IU/ml in thrombocytopenic patients and 0.5–1 IU/ml in others), whereas 19% were treated with Coumadin (goal international normalized ratio of 1–2 in half of patients and 2–3 in others). Three quarters of monitored patients received antithrombin infusion to keep activity above 60%. Such anticoagulation approach resulted in epistaxis (9%), bruising (2%) and major bleeding (4.6%; only in two adults). Of patients who were reimaged after initiation of anticoagulation, 96% of pediatric patients and 58% of adult patients had resolution or stabilization of VTE. Ten percent of patients (n = 3/31) had extension of thrombosis, two of whom had restarted L-asparaginase prior to reimaging. L-asparaginase was held in all patients after VTE for a median of 9 weeks in children and 4 weeks in adults. L-asparaginase was restarted along with anticoagulation in 77% patients (n = 33) with thrombosis, with the subsequent development of VTE in 33% patients (n = 11) (17% pediatric vs 47% adult patients, p = 0.07). Only two patients had been reimaged prior to resuming L-asparaginase. The site of recurrence/extension tended to coincide with the original site of VTE. Of patients with VTE recurrence, L-asparaginase was continued in five patients, whereas others had already completed the therapy. VTE-related complications included postphlebitic syndrome (7%, only in adults) and neurological changes (20%, ranging from chronic headaches to seizures). None of the patients died of VTE. At 4 years, the event-free survival (85 vs 88%; p = 0.36) and overall survival (86 vs 95%; p = 0.12) did not differ between patients with or without VTE. The authors concluded that in patients who develop VTE, L-asparaginase may be restarted, along with anticoagulation and antithrombin infusion as needed, if the imaging demonstrates either stabilization or resolution of clot [11]. In the Canadian study, six of seven pediatric patients with CNS thrombosis continued L-asparaginase therapy after resolution of symptoms with or without partial or complete resolution of clot. All patients were alive in remission at a median follow-up of 11 years (Table 3) [21].
Table 3. . Safety of rechallenging with asparaginase after a thrombotic event.
| Study (year) | Study design | Number of patients†, age group | Thrombosis recurrence rate | Bleeding rate | Ref. |
|---|---|---|---|---|---|
| Qureshi et al. (2010) |
Post hoc analysis of a prospective study |
38 children and adults |
0 |
0 |
[48] |
| Grace et al. (2011) |
Post hoc analysis of a prospective study |
33 children and adults |
17% of children and 47% of adults |
‡ |
[11] |
| Abbott et al. (2009) |
Retrospective study |
6 children with CNS thrombosis |
Not reported§ |
Not reported§ |
[21] |
| Lauw et al. (2013) | Retrospective study | 4 adults | Not reported§ | Not reported§ | [13] |
Patients received anticoagulation during the re-exposure of asparaginase.
†Number of patients indicates the number of patients with asparaginase-related venous thromboembolism, who were re-exposed to asparaginase. Only a fraction of patients were re-exposed to asparaginase; the criteria for continuation versus discontinuation of asparaginase are not provided.
‡For 43 patients who developed asparaginase-related venous thromboembolism, 11% had minor bleeds and 4.6% had major bleeds.
§Patients appeared to have no major thrombohemorrhagic complications but details not provided.
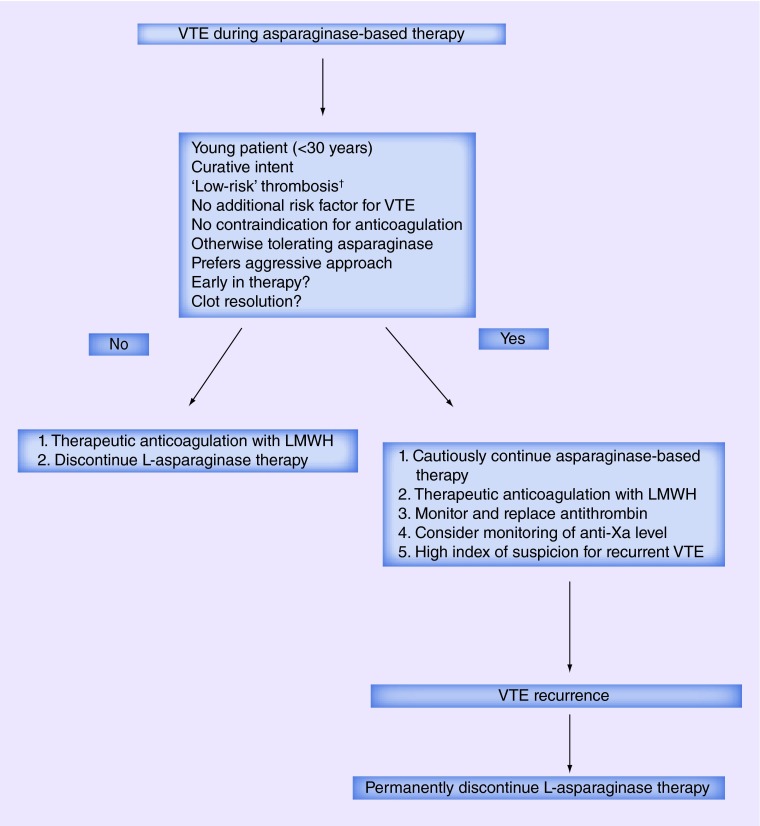
Clearly, the decision on resuming L-asparaginase should take into consideration the clinical severity of initial VTE event, risk of VTE recurrence (controlled cancer, presence of additional risk factors), age of the patients and perceived benefit of L-asparaginase (Figure 2). L-asparaginase, thus may be continued after an extremity deep vein thrombosis (DVT), VTE in the presence of additional transient risk factors and clot resolution, particularly in pediatric patients being treated with curative intent. Patients, who are not a candidate for anticoagulation (e.g., secondary to severe thrombocytopenia) or those with a history of life-threatening VTE or other irreversible risk factors for VTE may not be a good candidate for continuation of L-asparaginase. Further prospective studies are needed to identify the group of patients who are at high risk of complications from VTE recurrence on continuation of L-asparaginase.
Figure 2. . Algorithm for management of asparaginase-associated thrombosis.
†Low-risk VTE indicates asymptomatic or mildly symptomatic VTE, VTE involving extremities or catheter-associated VTE. A VTE that was not organ or life threatening and now has completely resolved perhaps could also be considered a low-risk VTE.
VTE: Venous thromboembolism.
Conclusion
VTE in ALL patients receiving L-asparaginase therapy is a common event, particularly in adults, during induction and among high-risk ALL groups [11–12,21]. Although VTE often involves upper extremity veins particularly when a CVC is used [12], CNS thrombosis and other major VTE may cause significant morbidity including long-term neurological consequences [11]. Additionally, the development of VTE is associated with a delay in therapy and possibly lower remission rate and survival [13,14]. A paucity of high quality data limits a definite conclusion regarding the role of several preventive measures and optimal management of VTE; however, the prophylactic use of antithrombin infusion (to keep antithrombin activity >60%) or LMWH may be effective in reducing the risk of VTE [14,31,33]. Such approach may particularly be cost–effective in patients at high risk of developing thrombosis [21] such as high-risk ALL patients receiving L-asparaginase and prednisone during induction therapy [21].
Patients who develop VTE should hold further L-asparaginase therapy for a few weeks and may be anticoagulated with LMWH based on data for cancer patients in general; however, periodic monitoring of antithrombin levels and replacement may be necessary in patients who continue to receive L-asparaginase therapy. Patients should be anticoagulated at least for the duration of L-asparaginase therapy or till the completion of chemotherapy. In select patients with a prior history of VTE, it may be safe to continue L-asparaginase therapy [11,48]; however, such decision should balance the risk of VTE recurrence and potential survival benefit with the continuation of therapy. L-asparaginase therapy may be continued after the resolution of VTE-related symptoms, with or without repeat imaging for clot resolution, if the goal of therapy is curative. VTE recurrence may involve the original site [11], hence, continuation of L-asparaginase therapy may be safer in patients with extremity DVT or those with no or minor symptoms; however, select patients with even a major VTE such as CNS thrombosis may be able to continue L-asparaginase therapy [11,48]. In patients, who continue L-asparaginase therapy, careful monitoring of anti-Xa level (to confirm adequacy of anticoagulation with LMWH) and antithrombin level (to maintain level >60%) is important.
Future perspective
The use of L-asparaginase is associated with decreased levels of antithrombin [14], which is considered as an important underlying mechanism for the development of VTE in these patients. This is further substantiated by the beneficial role of antithrombin replacement [14]. The full anticoagulant effect of LMWH requires the presence of antithrombin, whereas new oral anticoagulants are direct inhibitors of thrombin or Xa and are not dependent on antithrombin for their activity [37]. The aforementioned pediatric study demonstrated that the use of enoxaparin and antithrombin was more effective than antithrombin alone in preventive VTE during L-asparaginase therapy [33], which indicates that the new oral anticoagulants may be as effective as or perhaps more effective than LMWH and/or antithrombin replacement in the prevention of asparaginase-related VTE. Similarly, the new oral anticoagulants may have a unique role in patients with VTE, who are re-exposed to L-asparaginase therapy. In such circumstances, these agents may also obviate the need of monitoring of antithrombin levels or antithrombin supplementation. Although a lack of a reversible agent may be a concern, in cancer patients, the bleeding risk of these oral agents is at the least similar to LMWH and VKA [45,46]. Although remarkable advances in the last few decades has made ALL as one of the most curable malignancies in children, the risk of asparaginase-related VTE continues to be a problem. Further investigations of the preventive measures including the use of novel oral anticoagulants have a potential to reduce the incidence of VTE and its associated complications in ALL patients.
EXECUTIVE SUMMARY.
Background
The use of L-asparaginase, along with multiagent systemic chemotherapy including anthracycline, vincristine and steroid, has made acute lymphocytic leukemia (ALL) a curable malignancy, particularly in children.
Although a useful agent, the use of L-asparaginase has several potential toxicities including the risk of thrombosis.
Epidemiology
The incidence of venous thromboembolism (VTE) in ALL patients has been reported to vary from 1 to 36% depending upon the age group of the patients, study designs, treatment protocols, symptomatic thromboembolism versus detection with screening radiography.
Although VTE often involves upper extremity veins, particularly when a central venous catheter is used, CNS thrombosis and other major VTE may cause significant morbidity including long-term neurological consequences, an interruption in therapy and possibly lower remission rate and survival.
Mechanism of thrombosis
L-asparaginase causes a decline in the levels of anticoagulant proteins, which along with platelet and endothelial activation, may result in the development of thrombosis.
Identification of high-risk patients
In ALL patients receiving L-asparaginase therapy, VTE is more common in adults, during induction and with the use of central venous catheters and asparaginase therapy for ≥9 days. High-risk ALL groups and the use of prednisone instead of dexamethasone are other potential risk factors for VTE; however, the role of inherited thrombophilia is controversial, more so in adults.
Prevention
A paucity of high-quality data limits a definite conclusion regarding the role of several preventive measures and optimal management of VTE; however, the prophylactic use of antithrombin infusion (to keep antithrombin activity >60%) or low molecular weight heparin (LMWH) may be effective in reducing the risk of VTE.
Management of VTE & rechallenge with L-asparaginase
Patients who develop VTE should hold further L-asparaginase therapy for a few weeks and may be anticoagulated with LMWH based on data for cancer patients in general.
In patients with a prior history of VTE, the decision to continue L-asparaginase therapy should balance the risk of VTE recurrence and potential survival benefit with the continuation of therapy.
VTE recurrence may involve the original site, hence, continuation of L-asparaginase therapy may be safer in patients with extremity deep vein thrombosis or those with no or minor symptoms; however, select patients with even a major VTE such as CNS thrombosis may be able to continue L-asparaginase therapy.
In patients, who continue L-asparaginase therapy, careful monitoring of anti-Xa level (to confirm adequacy of anticoagulation with LMWH) and antithrombin level is important.
Future perspective
Unlike LMWH, novel oral anticoagulants, which are direct inhibitors of thrombin or Xa, are not dependent on antithrombin level for their activity. Hence, we speculate that these agents may be as effective as or perhaps more effective than LMWH and/or antithrombin level in the prevention and therapy of asparaginase-related therapy.
Footnotes
Financial & competing interests disclosure
This work was supported in part by 2015–2016 Physician–Scientist Training Program Grant to VR Bhatt from the College of Medicine, University of Nebraska Medical Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin. Hematol. 2013;50(3):185–196. doi: 10.1053/j.seminhematol.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douer D. Is asparaginase a critical component in the treatment of acute lymphoblastic leukemia? Best Pract. Res. Clin. Haematol. 2008;21(4):647–658. doi: 10.1016/j.beha.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Earl M. Incidence and management of asparaginase-associated adverse events in patients with acute lymphoblastic leukemia. Clin. Adv. Hematol. Oncol. 2009;7(9):600–606. [PubMed] [Google Scholar]
- 4.Ueno T, Ohtawa K, Mitsui K, et al. Cell cycle arrest and apoptosis of leukemia cells induced by L-asparaginase. Leukemia. 1997;11(11):1858–1861. doi: 10.1038/sj.leu.2400834. [DOI] [PubMed] [Google Scholar]
- 5.Grigoryan RS, Panosyan EH, Seibel NL, Gaynon PS, Avramis IA, Avramis VI. Changes of amino acid serum levels in pediatric patients with higher-risk acute lymphoblastic leukemia (CCG-1961) In Vivo. 2004;18(2):107–112. [PubMed] [Google Scholar]
- 6.Cory JG, Cory AH. Critical roles of glutamine as nitrogen donors in purine and pyrimidine nucleotide synthesis: asparaginase treatment in childhood acute lymphoblastic leukemia. In Vivo. 2006;20(5):587–589. [PubMed] [Google Scholar]
- 7.Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv. Exp. Med. Biol. 1999;457:621–629. [PubMed] [Google Scholar]
- 8.Van Ommen CH, Chan AK. Supportive care in pediatric cancer: the road to prevention of thrombosis. Semin. Thromb. Hemost. 2014;40(3):371–381. doi: 10.1055/s-0034-1370795. [DOI] [PubMed] [Google Scholar]
- 9.Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia. 2013;27(3):553–559. doi: 10.1038/leu.2012.290. [DOI] [PubMed] [Google Scholar]
- 10.Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br. J. Haematol. 2007;138(4):430–445. doi: 10.1111/j.1365-2141.2007.06677.x. [DOI] [PubMed] [Google Scholar]
- 11.Grace RF, Dahlberg SE, Neuberg D, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br. J. Haematol. 2011;152(4):452–459. doi: 10.1111/j.1365-2141.2010.08524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides important epidemiological data and management insights.
- 12.Caruso V, Iacoviello L, Di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–2222. doi: 10.1182/blood-2006-04-015511. [DOI] [PubMed] [Google Scholar]; • This meta-analysis provides useful information regarding the incidence and risk of thrombotic complications in childhood acute lymphoblastic leukemia.
- 13.Lauw MN, Van Der Holt B, Middeldorp S, Meijers JC, Cornelissen JJ, Biemond BJ. Venous thromboembolism in adults treated for acute lymphoblastic leukaemia: effect of fresh frozen plasma supplementation. Thromb. Haemost. 2013;109(4):633–642. doi: 10.1160/TH12-11-0845. [DOI] [PubMed] [Google Scholar]; • Demonstrates a possible role of fresh frozen plasma in preventing the development of venous thromboembolism.
- 14.Hunault-Berger M, Chevallier P, Delain M, et al. Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: the CAPELAL study. Haematologica. 2008;93(10):1488–1494. doi: 10.3324/haematol.12948. [DOI] [PubMed] [Google Scholar]; • Demonstrates a possible role of antithrombin supplementation in preventing the development of venous thromboembolism.
- 15.Nowak-Gottl U, Boos J, Wolff JE, et al. Asparaginase decreases clotting factors in vitro: a possible pitfall? Int. J. Clin. Lab. Res. 1995;25(3):146–148. doi: 10.1007/BF02592556. [DOI] [PubMed] [Google Scholar]
- 16.Bushman JE, Palmieri D, Whinna HC, Church FC. Insight into the mechanism of asparaginase-induced depletion of antithrombin III in treatment of childhood acute lymphoblastic leukemia. Leuk. Res. 2000;24(7):559–565. doi: 10.1016/s0145-2126(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Espinosa D, Minano A, Martinez C, et al. L-asparaginase-induced antithrombin type I deficiency: implications for conformational diseases. Am. J. Pathol. 2006;169(1):142–153. doi: 10.2353/ajpath.2006.051238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantarjian HM, O'brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J. Clin. Oncol. 2000;18(3):547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 19.Homans AC, Rybak ME, Baglini RL, Tiarks C, Steiner ME, Forman EN. Effect of L-asparaginase administration on coagulation and platelet function in children with leukemia. J. Clin. Oncol. 1987;5(5):811–817. doi: 10.1200/JCO.1987.5.5.811. [DOI] [PubMed] [Google Scholar]
- 20.Giordano P, Molinari AC, Del Vecchio GC, et al. Prospective study of hemostatic alterations in children with acute lymphoblastic leukemia. Am. J. Hematol. 2010;85(5):325–330. doi: 10.1002/ajh.21665. [DOI] [PubMed] [Google Scholar]
- 21.Abbott LS, Deevska M, Fernandez CV, et al. The impact of prophylactic fresh-frozen plasma and cryoprecipitate on the incidence of central nervous system thrombosis and hemorrhage in children with acute lymphoblastic leukemia receiving asparaginase. Blood. 2009;114(25):5146–5151. doi: 10.1182/blood-2009-07-231084. [DOI] [PubMed] [Google Scholar]
- 22.Santoro N, Colombini A, Silvestri D, et al. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: an AIEOP experience. J. Pediatr. Hematol. Oncol. 2013;35(5):348–355. doi: 10.1097/MPH.0b013e31828dc614. [DOI] [PubMed] [Google Scholar]
- 23.Mizrahi T, Leclerc JM, David M, Ducruet T, Robitaille N. ABO group as a thrombotic risk factor in children with acute lymphoblastic leukemia: a retrospective study of 523 patients. J. Pediatr. Hematol. Oncol. 2015;37(5):e328–e332. doi: 10.1097/MPH.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 24.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duval M, Suciu S, Ferster A, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children's Leukemia Group Phase 3 trial. Blood. 2002;99(8):2734–2739. doi: 10.1182/blood.v99.8.2734. [DOI] [PubMed] [Google Scholar]
- 26.Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002;99(6):1986–1994. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 27.Risseeuw-Appel IM, Dekker I, Hop WC, Hahlen K. Minimal effects of E. coli and Erwinia asparaginase on the coagulation system in childhood acute lymphoblastic leukemia: a randomized study. Med. Pediatr. Oncol. 1994;23(4):335–343. doi: 10.1002/mpo.2950230404. [DOI] [PubMed] [Google Scholar]
- 28.Domenech C, Thomas X, Chabaud S, et al. L-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005–01 randomized trial. Br. J. Haematol. 2011;153(1):58–65. doi: 10.1111/j.1365-2141.2011.08588.x. [DOI] [PubMed] [Google Scholar]
- 29.Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311–325. doi: 10.1016/S0140-6736(13)60592-9. [DOI] [PubMed] [Google Scholar]
- 30.Pui CH, Chesney CM, Bergum PW, Jackson CW, Rapaport SI. Lack of pathogenetic role of proteins C and S in thrombosis associated with asparaginase-prednisone-vincristine therapy for leukaemia. Br. J. Haematol. 1986;64(2):283–290. doi: 10.1111/j.1365-2141.1986.tb04121.x. [DOI] [PubMed] [Google Scholar]
- 31.Elhasid R, Lanir N, Sharon R, et al. Prophylactic therapy with enoxaparin during L-asparaginase treatment in children with acute lymphoblastic leukemia. Blood Coagul. Fibrinolysis. 2001;12(5):367–370. doi: 10.1097/00001721-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell L, Andrew M, Hanna K, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thromb. Haemost. 2003;90(2):235–244. doi: 10.1160/TH02-11-0283. [DOI] [PubMed] [Google Scholar]
- 33.Meister B, Kropshofer G, Klein-Franke A, Strasak AM, Hager J, Streif W. Comparison of low-molecular-weight heparin and antithrombin versus antithrombin alone for the prevention of symptomatic venous thromboembolism in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2008;50(2):298–303. doi: 10.1002/pbc.21222. [DOI] [PubMed] [Google Scholar]; •• Demonstrates that the combination of enoxaparin and antithrombin prophylaxis is more effective than antithrombin alone in preventing the development of venous thromboembolism.
- 34.Gugliotta L, D'Angelo A, Mattioli Belmonte M, et al. Hypercoagulability during L-asparaginase treatment: the effect of antithrombin III supplementation in vivo . Br. J. Haematol. 1990;74(4):465–470. doi: 10.1111/j.1365-2141.1990.tb06336.x. [DOI] [PubMed] [Google Scholar]
- 35.Nowak-Gottl U, Kuhn N, Wolff JE, et al. Inhibition of hypercoagulation by antithrombin substitution in E. coli L-asparaginase-treated children. Eur. J. Haematol. 1996;56(1–2):35–38. doi: 10.1111/j.1600-0609.1996.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 36.Mazzucconi MG, Gugliotta L, Leone G, et al. Antithrombin III infusion suppresses the hypercoagulable state in adult acute lymphoblastic leukaemia patients treated with a low dose of Escherichia coli L-asparaginase. A GIMEMA study. Blood Coagul. Fibrinolysis. 1994;5(1):23–28. doi: 10.1097/00001721-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kuhle S, Lau A, Bajzar L, et al. Comparison of the anticoagulant effect of a direct thrombin inhibitor and a low molecular weight heparin in an acquired antithrombin deficiency in children with acute lymphoblastic leukaemia treated with L-asparaginase: an in vitro study. Br. J. Haematol. 2006;134(5):526–531. doi: 10.1111/j.1365-2141.2006.06209.x. [DOI] [PubMed] [Google Scholar]
- 38.Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2013;11(1):56–70. doi: 10.1111/jth.12070. [DOI] [PubMed] [Google Scholar]
- 39.Herishanu Y, Misgav M, Kirgner I, Ben-Tal O, Eldor A, Naparstek E. Enoxaparin can be used safely in patients with severe thrombocytopenia due to intensive chemotherapy regimens. Leuk. Lymphoma. 2004;45(7):1407–1411. doi: 10.1080/10428190410001663671. [DOI] [PubMed] [Google Scholar]
- 40.Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst. Rev. 2014;6:CD006649. doi: 10.1002/14651858.CD006649.pub6. [DOI] [PubMed] [Google Scholar]
- 41.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 42.Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst. Rev. 2014;7:CD006650. doi: 10.1002/14651858.CD006650.pub4. [DOI] [PubMed] [Google Scholar]
- 43.Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb. Haemost. 2009;101(4):762–769. [PubMed] [Google Scholar]
- 44.Wharin C, Tagalakis V. Management of venous thromboembolism in cancer patients and the role of the new oral anticoagulants. Blood Rev. 2014;28(1):1–8. doi: 10.1016/j.blre.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Prins MH, Lensing AWA, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37–e46. doi: 10.1016/S2352-3026(14)70018-3. [DOI] [PubMed] [Google Scholar]
- 46.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 47.Olson JD, Arkin CF, Brandt JT, et al. College of american pathologists conference XXXI on laboratory monitoring of anticoagulant therapy: laboratory monitoring of unfractionated heparin therapy. Arch. Pathol. Lab. Med. 1998;122(9):782–798. [PubMed] [Google Scholar]
- 48.Qureshi A, Mitchell C, Richards S, Vora A, Goulden N. Asparaginase-related venous thrombosis in UKALL 2003- re-exposure to asparaginase is feasible and safe. Br. J. Haematol. 2010;149(3):410–413. doi: 10.1111/j.1365-2141.2010.08132.x. [DOI] [PubMed] [Google Scholar]; • Demonstrates the safety of using L-asparaginase after the development of venous thromboembolism in select patients.