Abstract
Manual therapy (MT) is a passive, skilled movement applied by clinicians that directly or indirectly targets a variety of anatomical structures or systems, which is utilized with the intent to create beneficial changes in some aspect of the patient pain experience. Collectively, the process of MT is grounded on clinical reasoning to enhance patient management for musculoskeletal pain by influencing factors from a multidimensional perspective that have potential to positively impact clinical outcomes. The influence of biomechanical, neurophysiological, psychological and nonspecific patient factors as treatment mediators and/or moderators provides additional information related to the process and potential mechanisms by which MT may be effective. As healthcare delivery advances toward personalized approaches there is a crucial need to advance our understanding of the underlying mechanisms associated with MT effectiveness.
KEYWORDS : biomechanical, clinical reasoning, effectiveness, expectation, neurophysiological, placebo, preference, psychological, treatment mediation, treatment moderation
Practice points.
Background
Manual therapies (MTs) are centuries old and practiced by many professions worldwide.
Techniques are generally classified as joint, muscle and connective tissue, or neurovascular-biased techniques based on the primary tissue focus of the technique.
MT is effective for managing musculoskeletal pain.
Mediating factors for effectiveness of MT
-
Biomechanical:
– MT causes measurable movement in targeted tissues;
– Some structural changes occur within the targeted tissues in response to MT;
– Limitations to a strictly biomechanical model explaining the effectiveness of MT result from low interpractitioner reliability of application of technique parameters (force and magnitude, among others).
-
Neurophysiological:
-
– Immediate changes in neurophysiological function observed after MT:
– Reduction in inflammatory markers;
– Decreased spinal excitability and pain sensitivity;
– Modification to cortical areas involved in pain processing;
– Excitation of the sympathetic nervous system.
-
Moderating factors for effectiveness of MT
Patient and provider expectation, therapeutic alliance, and context of the intervention heavily influence the clinical outcomes of MT.
Psychological factors (e.g., catastrophizing) interact with technique provision enhancing or reducing benefit.
Future directions
Additional work is needed to link immediate changes in neurophysiological measures with clinical outcomes.
The appropriate dosing of MT remains undetermined.
Genetic characteristics of patients may also be linked to response to MT.
This perspective is really about two things. First, it is about what ‘manual therapy’ (MT) is. Second, it is about how MT affects the patient's whole pain experience.
So what is MT? In general terms, MT is most often described (particularly by manual therapists) by the tissue targeted by the practitioner; which can be joint-biased, muscle and connective tissue-biased, and/or those techniques biased toward the neurovascular system. Joint-biased techniques target articular structures; muscle and connective tissue techniques apply manual stress to these tissues; and techniques focused on the neurovascular system place stress on neurovascular bundles. However, there is considerable overlap among practitioners in the targeted tissues that serve as the focus of the therapies provided and the techniques that are used. For example, chiropractors, physiotherapists and osteopaths all provide therapies that target each of these areas.
The MTs are a very old discipline that developed in parallel in many cultures across the world [1]. Muscle-biased techniques have been represented in Egyptian pictographs, foundational documents of traditional Chinese medicine, and Sanskrit writings from India. Early texts by Hippocrates describe the use of joint and muscle-biased techniques. Today there exist quite a staggering variety of schools of thought within MT practiced by many different professions including but not limited to osteopathy, chiropractic, physiotherapy and massage therapy.
Often discussions of MT, focus specifically on the ‘manual’ part of MT – the use of a practitioners’ hands with the intent to effect beneficial change in some part of a patient. However, MT is not just the application of a technique but an entire ‘process’ for patient management based on a reasoning model [2]. In its simplest form, MT encompasses a philosophy of caring for the patient that is similar to many other treatment strategies. As such MT involves not only aspects related to the interventions; for example, passive movement of a joint, but consistent with other complex interventions [3] also includes surrounding issues related to patient management (e.g., the diagnostic process, patient/practitioner interaction, movement re-education, advice and cognitive–behavioral factors, among others) which are often influential factors for clinical improvement in patients with musculoskeletal pain.
The pain experience
The International Association for the Study of Pain defines pain as "…unpleasant sensory and emotional experience that is associated with actual or potential tissue damage or described in such terms." That definition continues: "Pain is always subjective. Each individual learns the application of the word through experiences related to injury in early life" [4]. This suggests that as clinicians, we should not question patients perception or nature of pain, rather acknowledge that it is an individual unique experience; that is, the individual has the last word as to whether he or she is in pain or not, and what the nature and amount of his or her pain is.
Melzack and Casey (1968) proposed that the pain experience has three dimensions [5]. The sensory-discriminative dimension identifies the location on or within the body, the characteristics (mechanical, chemical and heat, among others) of the stimulus, and prompts reflex withdrawal to prevent or limit tissue damage. Next, the affective-motivational dimension is associated with those emotions related to pain. This dimension engages behaviors related to escape and recuperation. Last, the cognitive–evaluative dimension considers the consequences and meanings of a noxious stimulus. Together, these dimensions interact with one another and influence the experience of pain and pain-related behavior.
In the last 20 years, there has been an evolution of our knowledge about pain. We have evolved from a model wherein pain and nociception were considered synonymous to a new more complex but also more attractive view whereby pain is always a brain response in which nociception plays a variable role [6]. Notably, this pain experience involves the CNS. We modify the adage ‘no brain, no pain’ to be ‘no brain, no pain experience’ for without the cortex the experience cannot occur.
Clinical anecdotes and innumerable patient stories support the effectiveness of MT in treating a great variety of musculoskeletal conditions. MT is cost effective in comparison to other commonly provided interventions [7] and is rarely associated with serious complications [8]. In fact, MT has a similar risk profile for adverse events as exercise and a smaller risk profile than most medications [9]. MT is also a commonly sought treatment, and its use in USA has been fairly stable from 1999 to 2012; for example, in the most recent survey 8.4% of the general population used joint-based manipulations and 6.9% used massage within the last year. In addition, our work and that of others, suggests that patients with pain have both high expectations for benefit from MT.
Studies have been performed in several different musculoskeletal disorders; for example, low back pain [10], shoulder pain [11] and cervical pain [12]. These studies of MT have mainly focused on providing direct evidence supporting its clinical effects [13] with the primary outcome being reduction in both pain at rest and pain with activity. Thus, the most studied aspects of MT suggest a change in the sensory discriminate domain of the pain experience; that is, MT produces a reduction in pain intensity and unpleasantness in the pain experience and ultimately improved clinical outcomes. But how does this occur?
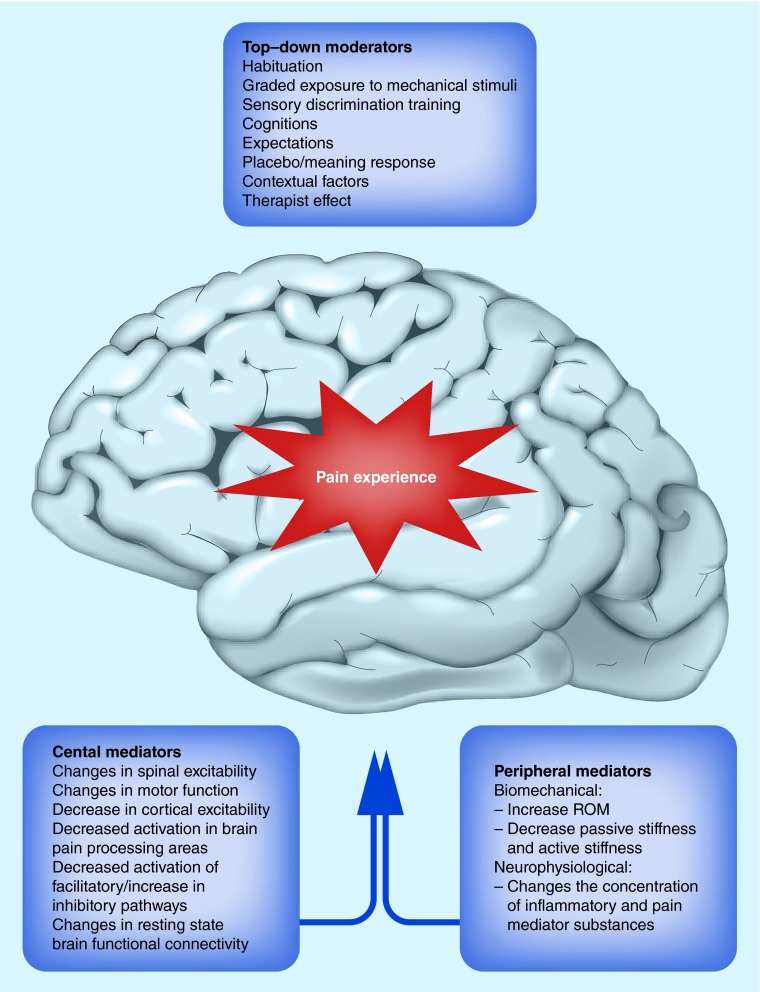
The mechanisms underpinning clinical outcomes associated with MT are not yet well established to date. Understanding the mechanisms of action is essential prior to identifying and selecting appropriate patients to receive MT; that is, those who will respond favorably. The identification of mechanisms of action would likely also provide greater acceptance of MT techniques and more appropriate use of MT by healthcare providers [14]. In this paper, we consider mediating and moderating factors that influence the outcomes from MT. These are summarized in Figure 1.
Figure 1. . Moderators and mediators of the pain experience resulting from manual therapy interventions.
Mediating factors are those aspects of an intervention that are a component of the mechanism through which the intervention impacts the outcome. As such treatment effect mediators are measured during treatment to determine if changes in the mediating variable in question impact a particular outcome. Once identified, mediating variables are capable of providing additional information related to the process and potential mechanisms by which an intervention may be effective (or ineffective) [15]. In addition, treatment aimed at influencing a mediating variable (assuming it can be modified through direct treatment) may be used to improve the effectiveness of other interventions (e.g., MT).
The mediating mechanisms of MT likely combine biomechanical and neurophysiological effects [16]. The mechanical stimulus provided by the MT and the series of neurophysiological effects initiated, in conjunction with the context or manner in which it is provided, are responsible for the clinical outcomes observed.
Mediating factors for effectiveness of MT
• Biomechanical mediators
Historically many MT approaches have been based on an identification of biomechanical dysfunction and interventions applied using biomechanical principles to correct the noted dysfunction. Accordingly, evaluation techniques are used to determine the tissue dysfunction responsible for the patient's pain according to these approaches. The subsequent selection of technique usually depends on the therapist's previous training or own preferences and overall conception of practice [17]. This need to choose a particular technique is often reinforced by MT educators, emphasizing that a mistake in choosing the ‘right’ technique (e.g., in terms of the degree of force, direction and segmental level, among others), can result in poor clinical outcomes and even be potentially harmful to the patient. The implication behind this kind of approach is that the success of the MT depends on the correction of biomechanical abnormalities detected during clinical examination in accordance with theoretical biomechanical constructs. The ‘conditions’ affected by MT also have been/are couched in such terms. Perhaps some of the best-known conceptual models are the ‘vertebral subluxation’ model [18], ‘stiffness’ on passive movement [19], the intervertebral disc pathology hypothesis [20] and ‘trigger points’ in muscle. Additionally, specific conceptual models explain the mechanism of action of MT in biomechanical terms. For instance, terms such as ‘rupture of joint adhesions’, ‘tissue lubrication’, ‘correction of subluxation’, ‘reduction (disc reduction)’ or ‘adjustment’ are used to explain the action of MT on joints, muscles, nerves or connective tissues.
However, as we report below, many of these conceptual biomechanical theories have not been supported empirically. It is very true that in humans MT is capable of causing movement of or stresses within the structures to which it is applied. These movements have been quantified for treatments targeting the joint [21] muscle or nerve [22]. In the studies of joint-biased techniques considerable motion and force are imparted on tissues [23]. During manipulation (a high velocity, small amplitude technique targeting a joint) of the spine, for example, these forces range from 200 to 800 N and approximately 6 mm of posterior to anterior translation of the vertebral segment occurs [24]. During techniques purported to primarily target the neurovascular structures there may be as much as 16 mm of excursion in the median nerve during some techniques [22]. Structural changes in tissues are also reported after select interventions. For example, report increased fluid uptake in the intervertebral disc is associated with clinical pain relief after joint-biased interventions to the lumbar spine [25] and, in a feline model, changes in spinal stiffness were dependent upon the specific location of a joint biased MT intervention provided to the spine [26].
Techniques that primarily target muscles and other soft tissues, such as massage, use mechanical pressure. This pressure is hypothesized to increase tissue extensibility with resulting increases in joint motion. Pressure to the tissues might also help to increase blood flow [27]. Few studies have examined changes in human connective tissues after muscle and connective tissue-biased techniques.
However, several limitations to using biomechanical effects as the sole explanation for mechanisms of effective pain relief have been reported. The reliability of some biomechanical assessments (e.g., palpation of anatomical references, evaluation of intersegmental spinal mobility) used during MT assessment and often the planning the subsequent intervention have been questioned [28]. Positional changes reported after joint-biased techniques do not last beyond the intervention [21,29]. Further, studies indicate less precision and accuracy than expected by the practitioner [30,31] with forces being dissipated over a large area [30] and movement effects measured at sites distant to the area of ‘focus’ for the intervention [30]. For example, spinal mobilization of the third lumbar vertebrae causes segmental effects at the first lumbar vertebrae [31] and effects of spinal manipulation may occur 14 cm away from the site of the application.
The forces used by practitioners also vary considerably with a systematic review of these studies indicating poor to moderate interpractitioner application of force (intraclass correlation [ICC]: -0.04–0.70) but good reliability (ICC: 0.75–0.99) for intrapractitioner application [32]. This is coupled with the findings that the use of MT to randomly chosen areas other than the area of dysfunction [33,34], render similar results as interventions targeting specific dysfunction. Furthermore, therapeutic effects can occur in remote locations relative to the site of treatment [35].
Therefore, while MT produces definite, measurable biomechanical effects, these do not completely explain pain relief observed after applying MT. Despite the limitations of a strictly biomechanical explanation, MT is effective, so additional mechanisms need to be considered.
Studies have established that the parameters of mechanical stimulus generated by MT appear to have some relationship with subsequent neurophysiological effects – that is, dose-dependent neurophysiological response. For example, the magnitude of the manual pressure applied affects the degree of analgesia during active movement [36], and changes the electromyographic response in the lumbar paraspinal muscles [21,36] during spinal manipulation; that is, increasing electromyographic response during manipulation with increasing force and impulse.
• Neurophysiological mediators
MT can affect the interaction between inflammatory mediators and peripheral nociceptors that occurs after tissue injury by modifying the concentration of mediator substances of inflammation and pain. Teodorczyk-Injeyan, Injeyan et al. 2006 [37], for example, identified a 20% reduction in cytokine concentration (e.g., TNF-α and IL-1β) that persisted 2 h after joint-biased interventions. Small but statistically significant increases in serotonin and β-endorphins occur 5 min after spinal manipulation [38] and a 168% increase in endogenous cannabinoids was noted immediately post manipulation [39]. These endogenous hormones are essential to endogenous pain relief mechanisms.
MT appears to also modify the state of spinal excitability as indicated by immediately decreased nociceptive flexion reflexes [40] and reduced temporal sensory summation [10,41], representing a combination of reduced facilitation and increased inhibition of nociceptive input in the CNS. Systematic reviews also indicate reductions in pressure pain thresholds in response to both joint and muscle/connective tissue biased MT [35,42]. The clinical ramifications of these short-term changes are not entirely clear, however, provide preliminary support for neurophysiological effects associated with MT. Changes in motor function have been also reported following the application of MT. Suppression of motor neuron pool activity [43,44], decreases in resting activity in muscle [45] and reduced motor responses are all reported effects [46].
Going above the spinal cord, animal and human imaging results lend some support toward a supraspinal effect. MT appears to have an immediate effect on cortical regions that integrate sensory inputs with higher cognitive and emotional regions. In the animal imaging studies, findings indicate decreased cortical activity in response to noxious stimuli following manual joint mobilization [47]. Recently, supraspinal effects were investigated in humans using spinal manipulation [48] – a joint-biased technique. Immediately after applying spinal manipulation a reduction in cerebral activity was observed in areas associated with the pain processing. In addition, there was a significant correlation between reduced activation in the insular cortex and decreased subjective pain ratings on the numeric pain rating scale. This study provides preliminary evidence of supraspinal mechanisms mediating hypoalgesia achieved with thoracic thrust manipulation [48].
Another study used functional magnetic resonance imaging to investigate the immediate changes in functional connectivity between brain regions that process and modulate the pain experience following different types of MT techniques (spinal manipulation, spinal mobilization and therapeutic touch) [49]. Each MT technique resulted in an immediate reduction in clinical pain reports. Changes in resting-state functional connectivity were found between several brain regions that were common to all three MT interventions. This finding also suggests specific mechanical parameters may not be as important and that a shared mechanism common to varying MT techniques exists that may be an underlying mechanism of pain relief.
The involvement of supraspinal systems in mediating the treatment effects of MT has been corroborated through the observation of concurrent hypoalgesia (reduction in pain in response to a standard stimulus) and excitation of the sympathetic nervous system in relation with the application of MT techniques [50]; for example, changes in heart rate, blood pressure, skin conductance or skin blood flow [51]. Decreases heart rate variability [52], salivary amylase [53] and salivary cortisol and insulin levels [52] are also noted after MT. These changes are similar to those observed in animals upon the artificial stimulation of higher centers responsible for descending pain modulation such as the PGA or RVM [54]. Additionally, hypoalgesia through the application of MT is obtained both locally and remotely from the site of application of the stimulus [35] and the duration of the hypoalgesia achieved with MT may last up to 24 h [54].
Persistent pain may also be a product of a ‘pain memory’. By way of example, consider a patient with chronic musculoskeletal pain. Even though the original pathology has likely healed, the patient is continuing to complain of pain and show indications of ongoing altered (protective) movements and perhaps even avoidance. Zusman [55] proposes that MT may assist in the acquisition of a new painless memory by exposure to new and less threatening stimuli, thereby removing aversive memories previously associated with that stimulus. Therefore, MT acts through the CNS to desensitize itself, both physically (e.g., exposure to nonthreatening mechanical stimuli), and cognitive–emotionally (e.g., through patient education), helping to remove acquired aversive memories of pain. These concepts have been recently extrapolated to exercise therapy for chronic musculoskeletal pain [56].
To the best of our knowledge, studies that evaluate psychological factors as treatment mediators for MT interventions are lacking which presents an opportunity for future research. Evaluating the influence of baseline variables are more appropriate for identifying prognostic factors (through single arm study designs) or treatment effect modifiers (through randomized clinical trials) and not for treatment mediators which require evaluation of ‘changes’ in the variable of interest during or as a consequence of treatment [57].
Collectively, this body of literature suggests the biomechanical stimulus provided by a MT intervention results in neurophysiological responses with relevance to the sensory discriminative, affective-motivational, and cognitive–evaluative dimensions of the pain experience.
Moderating for effectiveness of MT
Many of the physiological changes identified after MT may also be initiated by treatment modifiers. A treatment effect modifier is a factor that results in a greater treatment effect in one group compared with another and is best identified through randomized controlled trials. Identification of treatment effect moderators provides information about which patients and under which conditions a particular treatment is most effective [15]. The mechanisms of action underpinning these moderating factors are similar and overlap supraspinal regions mediating MT pain relief. Synergistic effects through these common pathways may underlie individual variations in the magnitude of clinical response.
The mechanical stimulus and resultant neurophysiological effects are modified by nonspecific factors such as expectation of the patient [58,59], equipoise of the practitioner [60,61], placebo effects [62], contextual factors such as the setting and therapeutic alliance between provider and patient [63]. All of these factors can be decisive in treatment outcomes. These effects are patient-dependent, therapist-dependent, mediated by the context of the intervention and obviously by the clinical condition and are an integral to all complex interventions such as MT to the extent they may be considered constituent parts of the treatment approach rather than a separate entity [3]. These effects are not unique to MT but discussion of them is pertinent to understanding the effects of MT on the pain experience.
Patient-related issues include patient expectations, especially if they have had previous positive experiences with the treatment received. The patient's expectations on a given kind of manual intervention may be more decisive in the therapeutic result than the actual manual intervention applied [59]. Therefore, it is essential to consider the patient's expectations and preferences when choosing the patient's MT treatment. The effectiveness of MT maybe enhanced when, based on the evidence of the effectiveness of that treatment, patient expectation is increased in view of the possibility of a positive response to treatment. Alternatively, outcomes may worsen based on the interaction of patient and therapist.
Findings from single arm studies provide conflicting results for relationships between pre-intervention psychological factors and short-term clinical outcomes following MT joint based techniques [64–66]. Findings from randomized clinical trials also provide conflicting results for this relationship. For example, Lopez-Lopez et al. [12] reported statistical interactions between pre-intervention trait anxiety and different MT techniques, such low and high levels of anxiety were associated with varying levels of clinical outcome based on the MT technique received. A secondary analysis of the UK BEAM dataset [67] did not however find any statistical interactions when evaluating for similar relationships with pre-intervention back pain beliefs and treatment response.
A previous review study indicated some evidence that spinal manipulation improved psychological outcomes compared with verbal interventions [68]. In that study, the authors provided a unique perspective on the influence that psychological factors may have on a patient's pain experience and the difficulty in evaluating treatment effectiveness associated with MT interventions. For example, the changes in psychological factors that may (or may not) occur in response to administering MT interventions ‘are not just incidental effects, but contribute to its characteristic treatment effect by reducing distressing symptoms such as pain and fear’ [68].
As we come to understand more regarding the factors that produce clinical benefit from specific MT interventions the likelihood of improved clinical measures increases. Identifying underlying mechanisms by which MT relieves pain (treatment mediators) will improve the clinical effectiveness of this approach by determining the clinical presentation of individuals likely to benefit from the established mechanisms and will increase both acceptability and utilization by patients and healthcare providers. In addition, if we can identify other mediators that are capable of being addressed through direct treatment (e.g., psychological factors), clinicians should consider supplementing MT interventions with other treatment approaches to increase the likelihood of achieving the most optimal MT clinical outcomes. The recognition of patient and therapist characteristics that modify treatment outcomes will also improve the application and implementation of MT approaches to the management of the pain experience by determining the psychological profile of individuals likely to benefit from these interventions and the best context in which to provide these interventions (Figure 1).
Future perspective
MT is an effective treatment contributing to the recovery of functional capabilities, but it should be included within a multimodal approach targeting the functional recovery of the patient. Current evidence is suggesting that a multimodal approach, including MT, exercise and education, seems to provide better outcomes than MT alone. A genuine multimodal approach should include not only physical management but a consideration of the psychological and psychosocial aspects of the patient's unique pain experience.
As we continue to uncover more about the management of pain conditions using MT, especially chronic pain, it becomes more noticeable that they appear to resemble a mosaic of phenotypes that may be further influenced by genetic factors related to peripheral and central neural plasticity (e.g., polymorphisms in BDNF), nociceptive processing (COMT variations) and/or environmental events and exposures. Moving forward, investigations will continue to uncover biomarkers that underlie the complex pathophysiology of pain conditions and the transition of acute to chronic pain states. As healthcare moves toward mechanism-based personalized treatments, it will become ever more important to understand the extent to which MT influences these underlying mechanisms. In addition, studies of MT must link the many immediate changes in neurophysiological function (e.g., changes in sympathetic nervous system function and the endogenous pain inhibitory systems, among others) more closely to the clinical complaints of our patients.
Footnotes
Financial & competing interests disclosure
This work was supported by funding from the National Institutes of Health National Center for Complementary and Integrative Health (R01AT006334 – MD Bishop, J Bialosky; F32 AT007729 – CW Gay) and National Center for Medical Rehabilitation Research (K12HD055929 – JM Beneciuk). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Pettman E. A history of manipulative therapy. J. Man. Manip. Ther. 2007;15(3):165–174. doi: 10.1179/106698107790819873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvernail J. Manual therapy: process or product? J. Man. Manip. Ther. 2012;20(2):109–110. doi: 10.1179/1066981712Z.00000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ. 2005;330(7501):1202–1205. doi: 10.1136/bmj.330.7501.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merskey H. Logic, truth and language in concepts of pain. Qual. Life Res. 1994;3(Suppl. 1):S69–S76. doi: 10.1007/BF00433379. [DOI] [PubMed] [Google Scholar]
- 5.Melzack R, Casey KL. Sensory, motivational and central control determinants of chronic pain: a new conceptual model. In: Kenshalo DR, editor. The Skin Senses. Thomas; IL, USA: 1968. pp. 423–443. [Google Scholar]
- 6.Institute of Medicine (US) Committee on Advancing Pain Research C, And Education. Relieving Pain in America. A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press; Washington, DC, USA: 2011. [PubMed] [Google Scholar]
- 7.Michaleff ZA, Lin CW, Maher CG, Van Tulder MW. Spinal manipulation epidemiology: Systematic review of cost effectiveness studies. J. Electromyogr. Kinesiol. 2012;22(5):655–662. doi: 10.1016/j.jelekin.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Rubinstein SM. Adverse events following chiropractic care for subjects with neck or low-back pain: do the benefits outweigh the risks? J. Manipulative Physiol. Ther. 2008;31(6):461–464. doi: 10.1016/j.jmpt.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Carnes D, Mars TS, Mullinger B, Froud R, Underwood M. Adverse events and manual therapy: a systematic review. Man. Ther. 2010;15(4):355–363. doi: 10.1016/j.math.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Bialosky JE, George SZ, Horn ME, et al. Spinal manipulative therapy-specific changes in pain sensitivity in individuals with low back pain ( NCT01168999) J. Pain. 2014;15(2):136–148. doi: 10.1016/j.jpain.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain TK, Sharma NK. The effectiveness of physiotherapeutic interventions in treatment of frozen shoulder/adhesive capsulitis: a systematic review. J. Back Musculoskelet. Rehabil. 2014;27(3):247–273. doi: 10.3233/BMR-130443. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Lopez A, Alonso Perez JL, Gonzalez Gutierez JL, et al. Mobilization versus manipulations versus sustain appophyseal natural glide techniques and interaction with psychological factors for patients with chronic neck pain: randomized control trial. Eur. J. Phys. Rehabil. Med. 2014;51(2):121–132. [PubMed] [Google Scholar]
- 13.Fisher BE, Davenport TE, Kulig K, Wu AD. Identification of potential neuromotor mechanisms of manual therapy in patients with musculoskeletal disablement: rationale and description of a clinical trial. BMC Neurol. 2009;9:20. doi: 10.1186/1471-2377-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahner-Roedler DL, Vincent A, Elkin PL, et al. Physicians’ attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid. Based Complement. Alternat. Med. 2006;3(4):495–501. doi: 10.1093/ecam/nel036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pincus T, Miles C, Froud R, et al. Methodological criteria for the assessment of moderators in systematic reviews of randomised controlled trials: a consensus study. BMC Med. Res. Methodol. 2011;11:14. doi: 10.1186/1471-2288-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man. Ther. 2009;14(5):531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson OP, Petty NJ, Moore AP. Clinical decision-making and therapeutic approaches in osteopathy – a qualitative grounded theory study. Man. Ther. 2014;19(1):44–51. doi: 10.1016/j.math.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Henderson CN. The basis for spinal manipulation: chiropractic perspective of indications and theory. J. Electromyogr. Kinesiol. 2012;22(5):632–642. doi: 10.1016/j.jelekin.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Maitland GD, Hengeveld E. Maitland's Vertebral Manipulation. Butterworth-Heinemann; Oxford, UK: 2001. [Google Scholar]
- 20.Mckenzie R, May S. The Lumbar Spine Mechanical Diagnosis and Therapy. Spinal Publications; New Zealand: 2003. [Google Scholar]
- 21.Colloca CJ, Keller TS, Gunzburg R. Neuromechanical characterization of in vivo lumbar spinal manipulation. Part II. Neurophysiological response. J. Manipulative Physiol. Ther. 2003;26(9):579–591. doi: 10.1016/j.jmpt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man. Ther. 2008;13(3):213–221. doi: 10.1016/j.math.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Herzog W, Kats M, Symons B. The effective forces transmitted by high-speed, low-amplitude thoracic manipulation. Spine (Phila. Pa 1976) 2001;26(19):2105–2110. doi: 10.1097/00007632-200110010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Herzog W. The biomechanics of spinal manipulation. J. Bodyw. Mov. Ther. 2010;14(3):280–286. doi: 10.1016/j.jbmt.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Beattie PF, Arnot CF, Donley JW, Noda H, Bailey L. The immediate reduction in low back pain intensity following lumbar joint mobilization and prone press-ups is associated with increased diffusion of water in the l5–s1 intervertebral disc. J. Orthop. Sports Phys. Ther. 2010;40(5):256–264. doi: 10.2519/jospt.2010.3284. [DOI] [PubMed] [Google Scholar]
- 26.Edgecombe TL, Kawchuk GN, Long CR, Pickar JG. The effect of application site of spinal manipulative therapy (SMT) on spinal stiffness. Spine J. 2013;15(6):1332–1338. doi: 10.1016/j.spinee.2013.07.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–256. doi: 10.2165/00007256-200535030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Pattyn E, Rajendran D. Anatomical landmark position – can we trust what we see? Results from an online reliability and validity study of osteopaths. Man. Ther. 2014;19(2):158–164. doi: 10.1016/j.math.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Tullberg T, Blomberg S, Branth B, Johnsson R. Manipulation does not alter the position of the sacroiliac joint. A roentgen stereophotogrammetric analysis. Spine (Phila. Pa 1976) 1998;23(10):1124–1128. doi: 10.1097/00007632-199805150-00010. [DOI] [PubMed] [Google Scholar]
- 30.Ross JK, Bereznick DE, Mcgill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine (Phila Pa 1976) 2004;29(13):1452–1457. doi: 10.1097/01.brs.0000129024.95630.57. [DOI] [PubMed] [Google Scholar]
- 31.Kulig K, Landel R, Powers CM. Assessment of lumbar spine kinematics using dynamic mri: a proposed mechanism of sagittal plane motion induced by manual posterior-to-anterior mobilization. J. Orthop. Sports Phys. Ther. 2004;34(2):57–64. doi: 10.2519/jospt.2004.34.2.57. [DOI] [PubMed] [Google Scholar]
- 32.Gorgos KS, Wasylyk NT, Van Lunen BL, Hoch MC. Inter-clinician and intra-clinician reliability of force application during joint mobilization: a systematic review. Man. Ther. 2014;19(2):90–96. doi: 10.1016/j.math.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Kanlayanaphotporn R, Chiradejnant A, Vachalathiti R. The immediate effects of mobilization technique on pain and range of motion in patients presenting with unilateral neck pain: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2009;90(2):187–192. doi: 10.1016/j.apmr.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Chiradejnant A, Latimer J, Maher CG. Forces applied during manual therapy to patients with low back pain. J. Manipulative Physiol. Ther. 2002;25(6):362–369. doi: 10.1067/mmt.2002.126131. [DOI] [PubMed] [Google Scholar]
- 35.Coronado RA, Gay CW, Bialosky JE, et al. Changes in pain sensitivity following spinal manipulation: A systematic review and meta-analysis. J. Electromyogr. Kinesiol. 2012;22(5):752–767. doi: 10.1016/j.jelekin.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This analysis when taken together with [42] indicates that both joint and muscle-biased manual therapy (MT) reduce pain sensitivity; and the parameters of the intervention determine the magnitude of the hypoalgesia.
- 36.Nougarou F, Dugas C, Deslauriers C, Page I, Descarreaux M. Physiological responses to spinal manipulation therapy: Investigation of the relationship between electromyographic responses and peak force. J. Manipulative Physiol. Ther. 2013;36(9):557–563. doi: 10.1016/j.jmpt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance p production in normal subjects. J. Manipulative Physiol. Ther. 2006;29(1):14–21. doi: 10.1016/j.jmpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Vernon HT, Dhami MS, Howley TP, Annett R. Spinal manipulation and beta-endorphin: A controlled study of the effect of a spinal manipulation on plasma beta-endorphin levels in normal males. J. Manipulative Physiol. Ther. 1986;9(2):115–123. [PubMed] [Google Scholar]
- 39.Mcpartland JM, Giuffrida A, King J, et al. Cannabimimetic effects of osteopathic manipulative treatment. J. Am. Osteopath. Assoc. 2005;105(6):283–291. [PubMed] [Google Scholar]
- 40.Courtney CA, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal response in individuals with knee osteoarthritis is modulated by joint compression and joint mobilization. J. Pain. 2010;11(2):179–185. doi: 10.1016/j.jpain.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Bishop MD, Beneciuk JM, George SZ. Immediate reduction in temporal sensory summation after thoracic spinal manipulation. Spine J. 2011;11(5):440–446. doi: 10.1016/j.spinee.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gay CW, Alappattu MJ, Coronado RA, Horn ME, Bishop MD. Effect of a single session of muscle-biased therapy on pain sensitivity: a systematic review and meta-analysis of randomized controlled trials. J. Pain Res. 2013;6:7–22. doi: 10.2147/JPR.S37272. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This analysis when taken together with [35] indicates that both joint and muscle-biased MT reduce pain sensitivity, and the parameters of the intervention determine the magnitude of the hypoalgesia.
- 43.Bulbulian R, Burke J, Dishman JD. Spinal reflex excitability changes after lumbar spine passive flexion mobilization. J. Manipulative Physiol. Ther. 2002;25(8):526–532. doi: 10.1067/mmt.2002.127073. [DOI] [PubMed] [Google Scholar]
- 44.Dishman JD, Burke J. Spinal reflex excitability changes after cervical and lumbar spinal manipulation: a comparative study. Spine J. 2003;3(3):204–212. doi: 10.1016/s1529-9430(02)00587-9. [DOI] [PubMed] [Google Scholar]
- 45.Devocht JW, Pickar JG, Wilder DG. Spinal manipulation alters electromyographic activity of paraspinal muscles: a descriptive study. J. Manipulative Physiol. Ther. 2005;28(7):465–471. doi: 10.1016/j.jmpt.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Coppieters MW, Stappaerts KH, Wouters LL, Janssens K. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicobrachial pain. J. Orthop. Sports Phys. Ther. 2003;33(7):369–378. doi: 10.2519/jospt.2003.33.7.369. [DOI] [PubMed] [Google Scholar]
- 47.Malisza KL, Gregorash L, Turner A, et al. Functional mri involving painful stimulation of the ankle and the effect of physiotherapy joint mobilization. Magn. Reson. Imaging. 2003;21(5):489–496. doi: 10.1016/s0730-725x(03)00074-2. [DOI] [PubMed] [Google Scholar]
- 48.Sparks C, Cleland JA, Elliott JM, Zagardo M, Liu WC. Using functional magnetic resonance imaging to determine if cerebral hemodynamic responses to pain change following thoracic spine thrust manipulation in healthy individuals. J. Orthop. Sports Phys. Ther. 2013;43(5):340–348. doi: 10.2519/jospt.2013.4631. [DOI] [PubMed] [Google Scholar]
- 49.Gay CW, Robinson ME, George SZ, Perlstein WM, Bishop MD. Immediate changes after manual therapy in resting-state functional connectivity as measured by functional magnetic resonance imaging in participants with induced low back pain. J. Manipulative Physiol. Ther. 2014;37(9):614–627. doi: 10.1016/j.jmpt.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this study, similar changes in cortical functional connectivity occur in response to three different MT techniques suggesting a common mechanism of action for these differing techniques.
- 50.Kingston L, Claydon L, Tumilty S. The effects of spinal mobilizations on the sympathetic nervous system: a systematic review. Man. Ther. 2014;19(4):281–287. doi: 10.1016/j.math.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Zegarra-Parodi R, Park PY, Heath DM, et al. Assessment of skin blood flow following spinal manual therapy: a systematic review. Man. Ther. 2015;20(2):228–249. doi: 10.1016/j.math.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Lindgren L, Rundgren S, Winso O, et al. Physiological responses to touch massage in healthy volunteers. Auton. Neurosci. 2010;158(1–2):105–110. doi: 10.1016/j.autneu.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Ogura T, Tashiro M, Masud M, et al. Cerebral metabolic changes in men after chiropractic spinal manipulation for neck pain. Altern. Ther. Health Med. 2011;17(6):12–17. [PubMed] [Google Scholar]
- 54.Hegedus EJ, Goode A, Butler RJ, Slaven E. The neurophysiological effects of a single session of spinal joint mobilization: does the effect last? J. Man. Manip. Ther. 2011;19(3):143–151. doi: 10.1179/2042618611Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zusman M. Mechanisms of musculoskeletal physiotherapy. Phys. Ther. Rev. 2004;9:39–49. [Google Scholar]
- 56.Nijs J, Lluch Girbes E, Lundberg M, Malfliet A, Sterling M. Exercise therapy for chronic musculoskeletal pain: innovation by altering pain memories. Man. Ther. 2015;20(1):216–220. doi: 10.1016/j.math.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Hill JC, Fritz JM. Psychosocial influences on low back pain, disability, and response to treatment. Phys. Ther. 2011;91(5):712–721. doi: 10.2522/ptj.20100280. [DOI] [PubMed] [Google Scholar]
- 58.Bishop MD, Bialosky JE, Cleland JA. Patient expectations of benefit from common interventions for low back pain and effects on outcome: secondary analysis of a clinical trial of manual therapy interventions. J. Man. Manip. Ther. 2011;19(1):20–25. doi: 10.1179/106698110X12804993426929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bishop MD, Mintken PE, Bialosky JE, Cleland JA. Patient expectations of benefit from interventions for neck pain and resulting influence on outcomes. J. Orthop. Sports Phys. Ther. 2013;43(7):457–465. doi: 10.2519/jospt.2013.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Supports that the expectations of the patient and those of the practitioner influence outcomes of MT.
- 60.Cook C, Learman K, Showalter C, Kabbaz V, O'Halloran B. Early use of thrust manipulation versus non-thrust manipulation: a randomized clinical trial. Man. Ther. 2013;18(3):191–198. doi: 10.1016/j.math.2012.08.005. [DOI] [PubMed] [Google Scholar]; • Supports that the expectations of the patient and those of the practitioner influence outcomes of MT.
- 61.Cook C, Sheets C. Clinical equipoise and personal equipoise: two necessary ingredients for reducing bias in manual therapy trials. J. Man. Manip. Ther. 2011;19(1):55–57. doi: 10.1179/106698111X12899036752014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bialosky JE, Bishop MD, George SZ, Robinson ME. Placebo response to manual therapy: Something out of nothing? J. Man. Manip. Ther. 2011;19(1):11–19. doi: 10.1179/2042618610Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuentes J, Armijo-Olivo S, Funabashi M, et al. Enhanced therapeutic alliance modulates pain intensity and muscle pain sensitivity in patients with chronic low back pain: an experimental controlled study. Phys. Ther. 2014;94(4):477–489. doi: 10.2522/ptj.20130118. [DOI] [PubMed] [Google Scholar]
- 64.Flynn T, Fritz J, Whitman J, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine (Phila. Pa 1976) 2002;27(24):2835–2843. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]
- 65.Cleland JA, Childs JD, Fritz JM, Whitman JM, Eberhart SL. Development of a clinical prediction rule for guiding treatment of a subgroup of patients with neck pain: use of thoracic spine manipulation, exercise, and patient education. Phys. Ther. 2007;87(1):9–23. doi: 10.2522/ptj.20060155. [DOI] [PubMed] [Google Scholar]
- 66.Mintken PE, Cleland JA, Carpenter KJ, et al. Some factors predict successful short-term outcomes in individuals with shoulder pain receiving cervicothoracic manipulation: a single-arm trial. Phys. Ther. 2010;90(1):26–42. doi: 10.2522/ptj.20090095. [DOI] [PubMed] [Google Scholar]
- 67.Underwood MR, Morton V, Farrin A, Team UBT. Do baseline characteristics predict response to treatment for low back pain? Secondary analysis of the UK Beam Dataset [ISRCTN32683578] Rheumatology (Oxford) 2007;46(8):1297–1302. doi: 10.1093/rheumatology/kem113. [DOI] [PubMed] [Google Scholar]
- 68.Williams NH, Hendry M, Lewis R, et al. Psychological response in spinal manipulation (PRISM): a systematic review of psychological outcomes in randomised controlled trials. Complement. Ther. Med. 2007;15(4):271–283. doi: 10.1016/j.ctim.2007.01.008. [DOI] [PubMed] [Google Scholar]