Abstract
Background
The survival of patients with metastatic colorectal cancer (mCRC) could be improved with exposure to three active drugs, irinotecan, fluorouracil/leucovorin, and oxaliplatin, irrespective of their sequence. However, only 50%–80% of patients can be exposed to all the three drugs in a sequential strategy with two-drug combinations. We carried out this systematic assessment to compare the survival benefit and safety of FOLFOXIRI (irinotecan, fluorouracil/leucovorin, and oxaliplatin) ± bevacizumab (with or without bevacizumab) versus FOLFIRI (irinotecan and fluorouracil/leucovorin) ± bevacizumab (with or without bevacizumab) as first-line treatment for unresectable mCRC.
Methods
PubMed and EMBASE were searched for original articles written in English and published before December 2015. A total of 1,035 patients from three randomized controlled trials were included.
Results
Our results demonstrated that overall survival (hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.73–0.97), progression-free survival (HR, 0.69; 95% CI, 0.59–0.81), and overall response rate (odds ratio, 1.96; 95% CI, 1.28–2.98) were significantly improved in the FOLFOXIRI ± bevacizumab arm compared to the FOLFIRI ± bevacizumab arm. Significantly higher incidences of neutropenia, anemia, diarrhea, stomatitis, and neuropathy were observed in the FOLFOXIRI ± bevacizumab arm.
Conclusion
Current evidence shows that the combination of FOLFOXIRI ± bevacizumab significantly improves the overall survival, progression-free survival, and overall response rate of patients with mCRC, with an increased but manageable toxicity, compared with the combinations of FOLFIRI ± bevacizumab. The combination of FOLFOXIRI ± bevacizumab should be considered as a treatment option for these patients under the premise of reasonable selection of target population.
Keywords: meta-analysis, FOLFOXIRI, FOLFIRI, colorectal cancer, chemotherapy
Introduction
Colorectal cancer (CRC) continues to be the third most commonly diagnosed cancer, after lung and breast cancer, and the fourth most common cause of cancer death worldwide, albeit with a declining incidence and mortality rate in countries with programmatic screening.1 In Western countries, CRC is the leading cause of cancer morbidity and mortality, while in developed countries CRC incidence is higher, and accounts for an even higher proportion of cancer deaths.2 Early diagnosis of CRC is very important for improving treatment outcomes; however, most patients already have localized disease at diagnosis, and hence cannot receive curative (R0) surgical resection. Considering that inhibitors of vascular endothelial growth factor and epidermal growth factor receptor have limited activity in monotherapy, chemotherapy remains the primary therapeutic option for these patients with unresectable metastatic CRC (mCRC). Combination therapy is now the predominant approach in cancer chemotherapy. The two most commonly used cytotoxic combinations for unresectable mCRC are infused 5-fluorouracil (FU) (plus leucovorin [LV]), with irinotecan (CPT-11) (FOLFIRI) and infused FU (plus LV) with oxaliplatin (LOHP) (FOLFOX), with or without (±) bevacizumab. The comparisons of FOLFOX with FOLFIRI in randomized studies have reported equivalent activity and efficacy.3 The most widely used triplet combination includes CPT-11, FU/LV, and LOHP ± bevacizumab. A study suggested that this combination is associated with promising survival, when unresectable mCRC patients are exposed to all the three most active agents, irrespective of their sequence.3 In addition, a pooled analysis of seven Phase III trials demonstrated that survival has relationship with the proportion of patients who receive all the three active drugs but not with the proportion of patients who receive any second-line therapy.4 This analysis also showed that only 50%–80% of patients can be exposed to all the three drugs in a sequential strategy with two-drug combinations. Whether FOLFOXIRI ± bevacizumab combination is better or not than FOLFIRI ± bevacizumab combination as first-line treatment for the unresectable mCRC patients is not completely known. So, we performed this first meta-analysis to compare the survival benefit and safety between the combinations of FOLFOXIRI ± bevacizumab and FOLFIRI ± bevacizumab.
Materials and methods
Study search strategy
We performed a systematic assessment according to Preferred Reporting Items for Systematic Reviews and Meta-analysis criteria.5 We searched PubMed, EMBASE, and the annual meeting abstracts of the American Society of Clinical Oncology (from 1985 to December 2015) for original articles. We searched all published (full papers or abstracts in English language) and unpublished trials that compared FOLFOXIRI ± bevacizumab (experimental group) with FOLFIRI ± bevacizumab (control group) for the treatment of colorectal cancer (before December 2015). To minimize the risk of selection or information bias, only prospective studies were included in our assessment. We searched articles with various combinations of different terms: “fluorouracil, oxaliplatin, irinotecan”, “fluorouracil, irinotecan”, “unresectable”, “advanced”, “metastatic”, “first-line treatment”, “randomized controlled trial”, “colorectal cancer”, “colon cancer”, “rectal cancer”, and others. The reference lists of relevant studies were also reviewed to avoid missing potential studies.
Selection of trials
To be eligible for our analysis, the trials had to meet the following criteria: 1) subjects were patients with unresectable mCRC, with histological or cytological confirmation; 2) prospective Phase II and III randomized controlled trials (RCTs); 3) control arm patients received FOLFIRI or FOLFIRI + bevacizumab (collectively referred to as the FOLFIRI group), and experimental arm patients received FOLFOXIRI or FOLFOXIRI + bevacizumab (collectively referred to as the FOLFOXIRI group) as first-line treatment; and 4) control arm and experimental arm were compared without confounding by additional agents or interventions (ie, in the combination chemotherapy, the control and experimental arms had to differ only with respect to addition or lack of LOHP component).
Two independent reviewers (WX and MK) assessed all the identified abstracts according to the predefined inclusion criteria. If only one reviewer considered an abstract eligible, the full text of the article was retrieved, and both reviewers reviewed it in detail. All publications were included, but only the most recent and the most informative data were used.
Quality assessment
The quantitative five-point Jadad score was used to assess the quality of included trials based on the report of the methods and results of the studies.6
Data extraction
To avoid bias in the data extraction process, two reviewers (WX and MK) independently extracted the data from the trials and compared the results. The following information was extracted: 1) publication details such as first author, year of publication, country, phase of study, and form of publication (full/abstract); 2) information of treatment such as chemotherapy regimens, treatment line, median overall survival (OS), median progression-free survival (PFS), overall response rate (ORR), toxicity, and R0 secondary resection rate; and 3) characteristics of patients such as number of patients, age, sex, prior chemotherapy history, and Eastern Cooperative Oncology Group performance status (PS). Before performing the analyses, the extracted data of each published study were carefully double-checked by another reviewer (YG). Any discrepancies were resolved by group discussion or by contacting the authors of the original study. If multiple publications of the same trial were retrieved, or if there were data inconsistencies between publications of the same trial, all publications were included, but only the most recent and the most informative data were used.
Statistical analysis
The primary outcome measure was OS, which was defined as time from random assignment to death. Secondary outcome measures were PFS – defined as the time between date of random assignment and date of progression, or date of death for patients without progression, or last date of follow-up for censored patients – and ORR – defined as the sum of partial and complete response rates. A hazard ratio (HR) was calculated to assess the survival advantage of the experimental group as compared with the control group. Odds ratios (ORs) were calculated to assess objective response rate and toxic events. For safety profile, data on grade 3–4 adverse events were extracted and analyzed. Between-study heterogeneity was estimated by χ2-based Q test.7 Heterogeneity was considered statistically significant when P heterogeneity was ≤0.1 or I2 was >50%, or both. We applied a random-effects model; a fixed-effects model was used when there is lack of significant heterogeneity. The presence of publication and selection bias was evaluated through funnel plots using the Begg’s and Egger’s tests.8,9 All statistical analyses were performed using the STATA version 10.0 software (StataCorp LP, College Station, TX, USA).
All reported P-values were from two-sided versions of the respective tests. A P-value <0.05 was considered statistically significant. All confidence intervals (CIs) had two-sided probability coverage of 95%.
Results
Characteristics of included trials
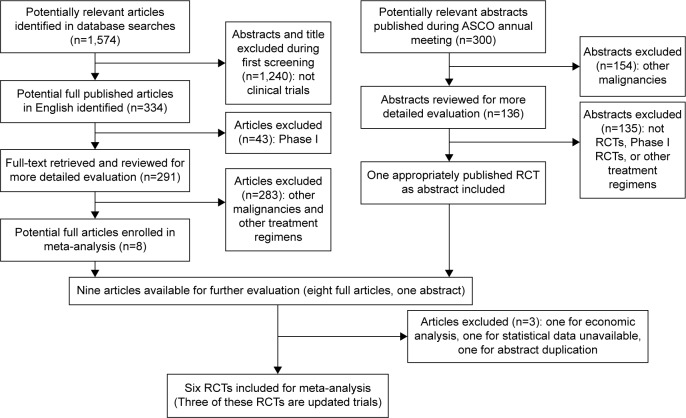
Six full-text articles10–15 were included in this meta-analysis; three of these articles were updated trials.10,13,15 The flow chart of inclusion and exclusion of studies is shown in Figure 1. There were 1,035 patients included in this assessment, of which 511 were randomly assigned to receive chemotherapy with FOLFOXIRI ± bevacizumab and 524 were randomly assigned to receive chemotherapy with FOLFIRI ± bevacizumab. The characteristics of the three included trials are summarized in Table 1.
Figure 1.
Flow chart of inclusion and exclusion of trials.
Abbreviations: ASCO, American Society of Clinical Oncology; RCT, randomized controlled trial.
Table 1.
Characteristics of the patients enrolled in the meta-analysis
| Characteristics | Study
|
|||||
|---|---|---|---|---|---|---|
| Loupakis et al12 and Cremolini et al10
|
Souglakos et al14 and Vamvakas et al15
|
Falcone et al11 and Masi et al13
|
||||
| FOLFOXIRI + bevacizumab (n=252) | FOLFIRI + bevacizumab (n=256) | FOLFOXIRI (n=137) | FOLFIRI (n=146) | FOLFOXIRI (n=122) | FOLFIRI (n=122) | |
| Median (range) | 60.5 (29–75) | 60 (29–75) | 66 (25–82) | 66 (39–84) | 62 (27–75) | 64 (21–75) |
| ≥65 years, n | – | – | 75 (55%) | 82 (56%) | – | – |
| Sex, n | ||||||
| Male | 150 (59.5%) | 156 (60.9%) | 76 (55%) | 85 (58%) | 75 (61%) | 69 (57%) |
| Female | 102 (40.5%) | 100 (39.1%) | 61 (45%) | 61 (42%) | 47 (39%) | 53 (43%) |
| ECOG performance status, n | ||||||
| 0 | 227 (90.1%) | 229 (89.5%) | 49 (36%) | 55 (38%) | 74 (60%) | 74 (61%) |
| 1–2 | 25 (9.9%) | 27 (10.5%) | 88 (64%) | 91 (62%) | 48 (40%) | 48 (39%) |
| Previous adjuvant therapy, n | 32 (12.7%) | 32 (12.5%) | 92 (63%) | 89 (65%) | 29 (24%) | 29 (24%) |
| Time to metastases, n | ||||||
| Synchronous | 197 (78.2%) | 207 (80.9%) | 66 (48%) | 63 (43%) | – | – |
| Metachronous | 55 (21.8%) | 49 (19.1%) | 70 (52%) | 83 (57%) | – | – |
| Köhne prognostic score, n | ||||||
| High risk | 18 (7.1%) | 29 (11.3%) | 37 (27%) | 35 (24%) | – | – |
| Intermediate risk | 111 (44.0%) | 113 (44.2%) | 56 (41%) | 57 (39%) | – | – |
| Low risk | 108 (42.9%) | 105 (41.0%) | 44 (32%) | 54 (37%) | – | – |
| Localization, n | ||||||
| Colon | – | – | 100 (73%) | 110 (75%) | 81 (66%) | 95 (78%) |
| Rectum | – | – | 37 (27%) | 36 (25%) | 41 (34%) | 27 (22%) |
| Number of metastatic sites | ||||||
| ≤1 | 59 (23.4%) | 46 (18.0%) | 55 (40%) | 59 (40%) | 65 (53%) | 67 (55%) |
| >1 | 193 (76.6%) | 210 (82.0%) | 82 (60%) | 87 (60%) | 57 (47%) | 55 (45%) |
Efficacy
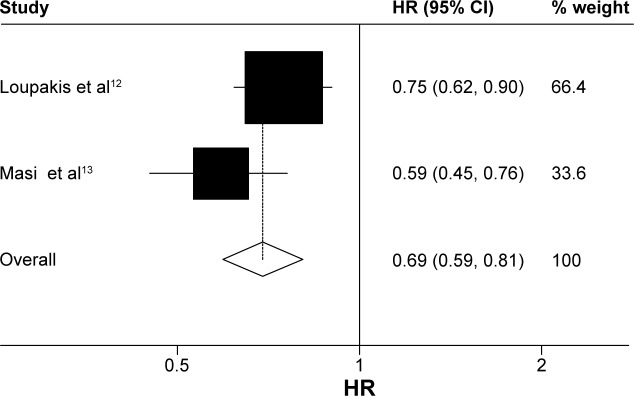
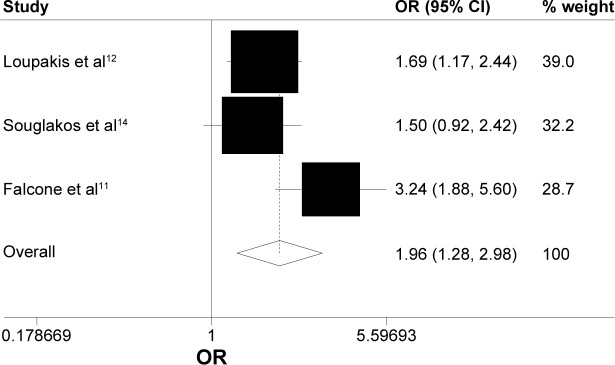
Data on OS were available for three trials (1,035 patients; Table 2). There was a statistically significant association with 16% reduction in the hazard of death in the FOLFOXIRI ± bevacizumab arm, as compared with FOLFIRI ± bevacizumab arm (HR, 0.84; 95% CI, 0.73–0.97; P=0.016; Figure 2). Data on PFS were available for two trials (752 patients; Table 2). The experimental group was also associated clinically with a 31% reduction in the hazard of death as compared with the control group; there was a significant improvement in the PFS (HR, 0.69; 95% CI, 0.59–0.81; P<0.0001; Figure 3). ORR was stated in three trials, which included 1,035 patients (Table 2). The experimental group was characterized by a significant 96% increase in the OR of response in comparison with the control group (OR, 1.96; 95% CI, 1.28–2.98; P=0.002; Figure 4). There was no statistically significant heterogeneity in the HR of both OS and PFS from the trials, and fixed-effects model was applied. Nevertheless, there was statistically significant heterogeneity in the OR of ORR, so a random-effects model was applied. In addition, there was no statistically significant improvement in radical resection of metastases in the experimental group compared to the control group in two trials; only Falcone et al’s trial reported a statistically significant improvement (P=0.033).11 We did not perform heterogeneity analysis due to of the lack of details. A summary of grade 3–4 adverse effects is reported in Table 3. With regard to the incidence of thrombocytopenia, febrile neutropenia, nausea/vomiting, and asthenia, no significant difference in any adverse event was observed between the experimental group and the control group. In addition, the incidence of each mentioned significant adverse effect was much lower than 6.3% in the control group. In contrast, the experimental group was characterized by a significantly higher incidence of neutropenia, anemia, diarrhea, stomatitis, and neuropathy (OR, 2.45, 4.92, 2.25, 1.96, and 18.64, respectively). Nevertheless, the incidence of each mentioned significant adverse effect was much lower than 35%. In addition, no significant difference was observed with regard to treatment-related death. Heterogeneity existed for some adverse effects among trials, possibly due to the different combinations and doses used.
Table 2.
Characteristics of enrolled trials, including treatment regimens and survival data
| Author | Country | Phase | Line | Age (years) | Number | Treatment | Male (%) | Median OS (months) | Median PFS (months) | ORR (%) | Postchemotherapy R0 surgical resections (%) | ECOG PS | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loupakis et al12 | Italy | III | 1st | ≥18 and ≤75 | 508 | FOLFOXIRI + bevacizumab | 59.5 | 31 | 12.1 | 65 | 15 | 0–2 | 4 |
| FOLFIRI + bevacizumab | 60.9 | 25.8 | 9.7 | 53 | 12 | – | |||||||
| Cremolini et al10 | Italy | III | 1st | ≥18 and ≤75 | 508 | FOLFOXIRI + bevacizumab | 59.5 | 29.8 | – | – | – | 0–2 | 4 |
| FOLFIRI + bevacizumab | 60.9 | 25.8 | – | – | – | ||||||||
| Souglakos et al14 | Greece | III | 1st | ≥18 | 283 | FOLFOXIRI | 55 | 21.5 | – | 43 | 10 | 0–2 | 4 |
| FOLFIRI | 58 | 19.5 | – | 33.6 | 4 | ||||||||
| Vamvakas et al15 | Greece | III | 1st | ≥18 | 283 | FOLFOXIRI | 55 | 21.9 | – | 43 | – | 0–2 | 4 |
| FOLFIRI | 58 | 19.9 | – | 33.6 | – | ||||||||
| Falcone et al11 | Italy | III | 1st | ≥18 and ≤75 | 244 | FOLFOXIRI | 61 | 22.6 | 9.8 | 60 | 15 | 0–2 | 4 |
| FOLFIRI | 57 | 16.7 | 6.9 | 34 | 6 | ||||||||
| Masi et al13 | Italy | III | 1st | ≥18 and ≤75 | 244 | FOLFOXIRI | 61 | 23.4 | 9.8 | – | – | 0–2 | 4 |
| FOLFIRI | 57 | 16.7 | 6.8 | – | – |
Abbreviations: OS, overall survival; PFS, progression-free survival; ORR, overall response rate; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Figure 2.
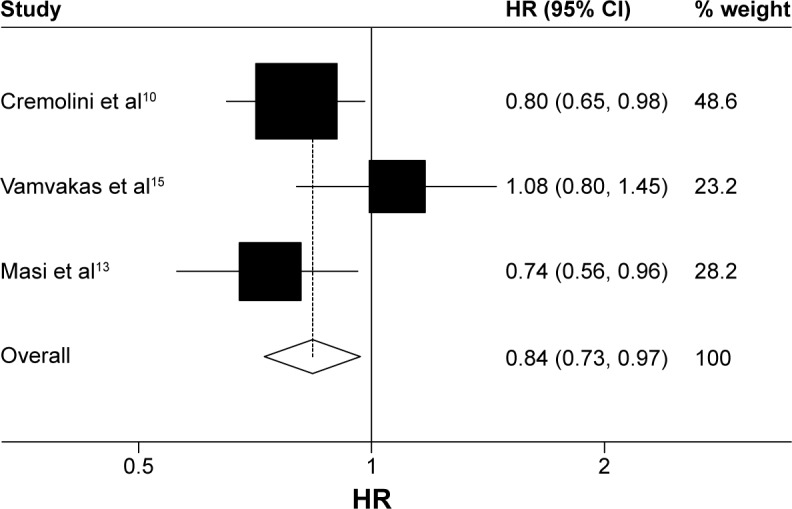
Fixed-effects model of HR (95% CI) of overall survival associated with FOLFOXIRI ± bevacizumab group compared with FOLFIRI ± bevacizumab group.
Notes: Heterogeneity chi-squared =3.81 (df =2); P=0.149. I-squared (variation in ES attributable to heterogeneity) =47.5%. Test of ES =1: z=2.40; P=0.016.
Abbreviations: HR, hazard ratio; CI, confidence interval; df, degrees of freedom; ES, effect size.
Figure 3.
Fixed-effects model of HR (95% CI) of progression-free survival associated with FOLFOXIRI ± bevacizumab group compared with FOLFIRI ± bevacizumab group.
Notes: Heterogeneity chi-squared =2.14 (df =1); P=0.144. I-squared (variation in ES attributable to heterogeneity) =53.3%. Test of ES =1: z=4.75; P=0.000.
Abbreviations: HR, hazard ratio; CI, confidence interval; df, degrees of freedom; ES, effect size.
Figure 4.
Random-effects model of hazard ratio (95% CI) of overall response rate associated with FOLFOXIRI ± bevacizumab group compared with FOLFIRI ± bevacizumab group.
Notes: Heterogeneity chi-squared =5.00 (df=2); P=0.082. I-squared (variation in OR attributable to heterogeneity) =60.0%. Estimate of between-study variance Tau-squared =0.0829. Test of OR =1: z=3.12; P=0.002.
Abbreviations: CI, confidence interval; OR, odds ratio; df, degrees of freedom.
Table 3.
Most common grade 3 or 4 AEs
| Study AEs (%) | Loupakis et al12
|
Souglakos et al14
|
Falcone et al11
|
Total
|
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| FOLFOXIRI + bevacizumab (n=250) | FOLFIRI + bevacizumab (n=254) | FOLFOXIRI (n=130) | FOLFIRI (n=146) | FOLFOXIRI (n=122) | FOLFIRI (n=122) | FOLFOXIRI ± bevacizumab (n=502) | FOLFIRI ± bevacizumab (n=522) | ||
| Hematologic | |||||||||
| Neutrophils | 50 | 20.5 | 35 | 28.1 | 50 | 28 | 46.2 | 24.3 | 0.004 |
| Platelets | – | – | 2 | 4 | 2 | 1 | 0.1 | 1.3 | 0.665 |
| Anemia | – | – | 4 | 1 | 3 | 1 | 1.8 | 0.4 | 0.043 |
| Nonhematologic | |||||||||
| Nausea/vomiting | 7.2 | 6.3 | 4.6 | 4.8 | 12.3 | 2.5 | 7.8 | 5 | 0.286 |
| Diarrhea | 18.8 | 10.6 | 27.7 | 10.9 | 20 | 12 | 21.5 | 10.9 | <0.0001 |
| Stomatitis | 8.8 | 4.3 | – | – | 5 | 3 | 5.6 | 2.9 | 0.04 |
| Febrile neutropenia | 8.8 | 6.3 | 7 | 4 | 5 | 3 | 7.4 | 5 | 0.115 |
| Asthenia | 12 | 9.1 | – | – | 6 | 3 | 7.4 | 5.2 | 0.173 |
| Neuropathy | 5.2 | 0 | 5.8 | 0 | 2 | 0 | 4.8 | 0 | 0.001 |
Abbreviation: AEs, adverse events.
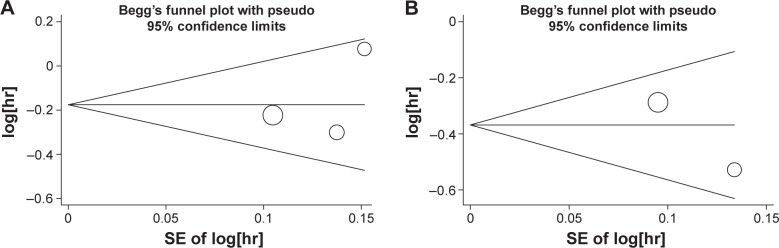
Publication bias
We constructed Begg’s funnel plot and performed Egger’s test to assess the publication bias in the literature. The shapes of the funnel plots indicated the absence of publication bias (Figure 5). Furthermore, Egger’s test was used to statistically confirm the symmetry of the funnel plots. The results also did not suggest any evidence of publication bias.
Figure 5.
Begg’s funnel plots of publication bias test.
Notes: Plots for (A) overall survival and (B) progression-free survival.
Abbreviation: SE, standard error.
Discussion
Many studies reported the feasibility of the combination of FOLFOXIRI ± bevacizumab.16–18 In addition, some studies suggested that FOLFOXIRI ± bevacizumab was more efficient compared to FOLFIRI ± bevacizumab as first-line treatment for unresectable mCRC. However, some studies showed contrasting results. Our overall analysis indicated that experimental group showed significant improvement in OS and PFS in comparison with the control group. Our data on ORR reinforced further the survival result due to of a higher response rate in the experimental group. In addition, there was an improvement in radical resection of metastases in the experimental group, but statistically significant difference was undefined due to the limited published data. Considering safety profile, there was no significant difference between the two groups with respect to all grade 3–4 adverse events except neutropenia, anemia, diarrhea, stomatitis, and neuropathy. However, the incidence rate of anemia, stomatitis, and neuropathy was low in both arms. Conversely, the incidence rate of other adverse events was high in the experimental group, but they were predictable, manageable, and acceptable, compared to the control group. With regard to treatment-related death, no significant difference was observed between the two groups. The experimental group was associated with a longer OS and PFS, a higher response rate, and almost equivalent safety compared with the control group. All trials provided data on OS, and we extracted updated results from the published articles via PubMed. The results of all trials10,13,15 showed that OS was prolonged in patients with unresectable mCRC in the experimental group, regardless of there being no significant difference in OS in one trial (Souglakos et al’s trial14). The median OS of patients assigned to FOLFOXIRI ± bevacizumab arm was significantly longer than that of patients assigned to FOLFIRI ± bevacizumab arm in all trials except Souglakos et al’s trial. Meanwhile, the forest plot of OS showed favorable results for the experimental group compared with the control group in all included trials except Souglakos et al’s trial. Souglakos et al’s trial reported that updated median OS was 21.9 months in the experimental group compared with 19.9 months in the control group (HR, 1.08; 95% CI, 0.8–1.45; P=0.41).15 In spite of this, statistically significant difference in OS in favor of the experimental group was still observed in our meta-analysis. In view of long survival benefit, FOLFOXIRI ± bevacizumab could be an optimal choice of treatment for patients with unresectable mCRC under the premise of reasonable selection of target population, including those who have never ever received adjuvant chemotherapy and who had a PS of 0–1. All trials11,12,14 reported that the response rate in the experimental group was significantly higher than that in the control group except Souglakos et al’s trial15 (43% versus 33.6% in each group; P=0.11). The ORRs were 58.1% in the experimental group and 43.1% in the control group in this analysis, which demonstrated a 96% increase in the OR of response in the experimental group than that in the control group, and the difference was significant (P=0.002). Data on PFS were available in two trials,12,13 and significant difference was observed in PFS in our meta-analysis (Figure 4). The PFS benefit of the combination of FOLFOXIRI ± bevacizumab should be further investigated in more trials.
In addition, the following issues may confound the assessment of survival and response rate, and they are worthy of further discussion. First, the different median age and the proportion of patients, the patients older than 65 years, and those with an Eastern Cooperative Oncology Group PS of 0–1 were higher in Souglakos et al’s trial in comparison with that reported in the other trials. In addition, the study enrolled patients >75 years old, which was relatively unusual compared with the previous randomized studies.3,19 However, Souglakos et al’s trial and some other trials18,20 revealed that age alone is not a negative predictor of the efficacy and the tolerance of currently used chemotherapy regimens, but the PS status and response to treatment are also independent prognostic factors of survival. Second, inconsistency in systemic therapy before and after the study among the three trials may affect the end points. Loupakis et al’s study12 suggested that patients who have received adjuvant chemotherapy are not ideal candidates for an intensified upfront chemotherapy. From Souglakos et al’s trial, we found that adjuvant chemotherapy is not a significant factor of the patients’ outcome, while Falcone et al’s study11 did not refer to adjuvant chemotherapy. The details could not be searched from the articles, and we got no updated information from the authors.
The findings of our study showed that almost equivalent tolerance was observed among the two treatment groups except significant increases in grade 3–4 neutropenia, anemia, diarrhea, stomatitis, and neuropathy in the experimental group. However, all toxicities including those mentioned were tolerable, predictable, and manageable. Treatment-related death, which was an important toxic indicator, was reported in all trials. Two trials reported that more than one person died in each group.12,14 Loupakis et al’s trial reported six and four persons died in each group, respectively. Souglakos et al’s trial reported two and two persons died in each group, respectively. In total, due to of the limited published data, significant difference in treatment-related death was undefined in our meta-analysis.
The pharmacokinetic data demonstrated that the dose of drugs used for the two groups was appropriate. In the chemotherapy regimens of Souglakos et al’s trial, the FU bolus was maintained, so the use of a dose of LOHP and CPT-11 significantly lower than in the other trials.
This meta-analysis presents the most systematic comparison of the efficiency and the safety between the experimental group and the control group on the basis of all relevant RCTs. All chosen articles were of high quality, in order to enhance the reliability of our analyses and reduce the inherent bias. However, limitations of these studies also need our attention. First, to our knowledge, the quality of the individual studies has an effect on the results of any meta-analysis. Although all trials were RCTs and we extracted information from the updated trials, no updated or confirmed results could be obtained from the authors. Therefore, our results should be interpreted with care. Second, our meta-analysis was based on abstracted data and not on individual patient data. Meta-analyses based on individual patient data tend to give a more robust estimation of the association compared with published data analyses. Third, the difference in treatment schedules among the trials (data not shown) might have resulted in increase in the clinical heterogeneity of the meta-analysis. Finally, lack of blinding, which is inevitable in any included studies, might have contributed to an overestimate of the treatment effects. Because the two treatment methods studied were very different (triple drug ± bevacizumab versus double drug ± bevacizumab), the treatment allocation could not be concealed from the investigators or patients.
In our meta-analysis, there remain some problems which should be illustrated and solved through further prospective trials. Firstly, to our knowledge, chemotherapy treatment combination molecular-targeted agents are more promising, which have been widely applied in the clinical treatment. However, two trials in our meta-analysis were conducted before the inclusion of monoclonal antibodies to standard of care, and thus, they were not optimized to present practice. Secondly, to our knowledge, the liver is the most common site of metastasis in patients with colorectal cancer. Treatment strategies that allow hepatic resection as part of an interdisciplinary consensus offer higher 5-year survival rates than palliative treatment alone.21 The trial by Loupakis et al did not focus on converting patients with liver metastases into candidates for surgical resection. The other trials performed higher rate of R0 metastatic surgery assessing the role of intensified therapy toward that goal. However, the proportion of patients with metastasis limited to the liver was very low. Thus, we are unable to make any conclusion regarding the benefit of treatment with FOLFOXIRI ± bevacizumab or FOLFIRI ± bevacizumab in these patients. Recently, the OLIVIA trial22 compared FOLFOXIRI + bevacizumab with FOLFOX-6 (FU, LV, and LOHP) + bevacizumab in mCRC patients with liver-limited metastasis. The trial found that FOLFOXIRI plus bevacizumab improved PFS and the secondary resection of metastases, but it did not mention the statistically significant difference. These problems need to be solved by further prospective trials.
Our meta-analysis indicates that triple-drug treatment is a good strategy to optimize the feasibility and efficacy of subsequent treatments. It also gives full answer to the question of whether the upfront use of FOLFOXIRI improves survival compared with the sequential use of LOHP and CPT-11.
In summary, FOLFOXIRI ± bevacizumab chemotherapy was not only superior to FOLFIRI ± bevacizumab chemotherapy in terms of OS and PFS but also led to increased responses. All of these results confirmed that FOLFOXIRI ± bevacizumab showed almost equivalent tolerance and much more effectiveness compared to FOLFIRI ± bevacizumab. Thus, this combination should be considered as a first-line treatment option for the patients with unresectable colorectal cancer under the premise of reasonable selection of target population and combination of chemotherapy drugs. The superiority of the FOLFOXIRI ± bevacizumab therapy compared to the other double-drug combinations needs to be further evaluated and confirmed through larger studies with longer observation period.
Acknowledgments
This study was supported by a grant from The Project of Plans for the Development of Science and Technology of Nanjing, China (Grant No 201208020).
Footnotes
Disclosure
The authors declare no conflict of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Lombardi L, Morelli F, Cinieri S, et al. Adjuvant colon cancer chemotherapy: where we are and where we’ll go. Cancer Treat Rev. 2010;36(Suppl 3):S34–S41. doi: 10.1016/S0305-7372(10)70018-9. [DOI] [PubMed] [Google Scholar]
- 3.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22(7):1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 7.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 8.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 9.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 11.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 12.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 13.Masi G, Vasile E, Loupakis F, et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103(1):21–30. doi: 10.1093/jnci/djq456. [DOI] [PubMed] [Google Scholar]
- 14.Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94(6):798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vamvakas L, Athanasiadis A, Karampeazis A, et al. Clinical outcome of elderly patients with metastatic colorectal cancer treated with FOLFOXIRI versus FOLFIRI: subgroup analysis of a randomized phase III trial from the Hellenic Oncology Research Group (HORG) Crit Rev Oncol Hematol. 2010;76(1):61–70. doi: 10.1016/j.critrevonc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Falcone A, Masi G, Allegrini G, et al. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional fluorouracil, and leucovorin: a pilot study in patients with metastatic colorectal cancer. J Clin Oncol. 2002;20(19):4006–4014. doi: 10.1200/JCO.2002.12.075. [DOI] [PubMed] [Google Scholar]
- 17.Masi G, Allegrini G, Cupini S, et al. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of a phase II study with a simplified biweekly schedule. Ann Oncol. 2004;15(12):1766–1772. doi: 10.1093/annonc/mdh470. [DOI] [PubMed] [Google Scholar]
- 18.Souglakos J, Mavroudis D, Kakolyris S, et al. Triplet combination with irinotecan plus oxaliplatin plus continuous-infusion fluorouracil and leucovorin as first-line treatment in metastatic colorectal cancer: a multicenter phase II trial. J Clin Oncol. 2002;20(11):2651–2657. doi: 10.1200/JCO.2002.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 20.Sastre J, Marcuello E, Masutti B, et al. Irinotecan in combination with fluorouracil in a 48-hour continuous infusion as first-line chemotherapy for elderly patients with metastatic colorectal cancer: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. J Clin Oncol. 2005;23(15):3545–3551. doi: 10.1200/JCO.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Ferrarotto R, Pathak P, Maru D, et al. Durable complete responses in metastatic colorectal cancer treated with chemotherapy alone. Clin Colorectal Cancer. 2011;10(3):178–182. doi: 10.1016/j.clcc.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Gruenberger T, Bridgewater J, Chau I, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702–708. doi: 10.1093/annonc/mdu580. [DOI] [PubMed] [Google Scholar]