Abstract
Platelets are circulating cellular sensors that express and release the damage-associated molecular pattern molecule (DAMP) high-mobility group box 1 (HMGB1) at sites of disrupted vascular and tissue integrity. We have recently identified platelet-derived HMGB1 as a critical mediator of thrombosis. The role of platelet-derived HMGB1 in mediating interactions with monocytes remains unknown. In transgenic mice with platelet-specific ablation of HMGB1 and neutralization studies, we show that HMGB1 derived from platelets promotes recruitment of monocytes and prevents monocytes from undergoing apoptosis. During experimental trauma and hemorrhagic shock, infiltrated monocytes in the lung and liver were significantly attenuated in mice lacking HMGB1 in platelets. Platelet-derived HMGB1 mediated monocyte migration via the receptor of advanced glycosylation end products (RAGE) and suppressed apoptosis via toll-like receptor 4 (TLR4)-dependent activation of MAPK/ERK (extracellular signal-regulated kinase) in monocytes. In conclusion, we identify platelet-derived HMGB1 as a critical regulator of monocyte recruitment and apoptosis, with potential implications in disease states associated with thrombosis and inflammation.
Keywords: platelets, high-mobility group box 1, monocytes, migration, apoptosis
Introduction
Beyond their role in mediating primary hemostasis and thrombosis, platelets have evolved as sentinel innate immune cells, which release a multitude of mediators upon activation and control tropism and apoptosis of other immune cells [1–4]. Platelets express high mobility group box 1 (HMGB1) [5–7], a highly conserved, non-histone, architectural DNA-binding protein that is typically abundant in the nucleus of mammalian cells [8]. HMGB1 may act as a damage-associated molecular pattern molecule (DAMP) [9] which initiates immune responses through recruitment/activation of monocytes/macrophages and dendritic cells [10–11] and downregulation of apoptosis of eosinophils, neutrophils, and other cells [12–13].
We have recently identified HMGB1 derived from platelets as a critical mediator of thrombosis and neutrophil extracellular traps (NET) formation [6]. In another study, we have shown that platelet-derived HMGB1 inhibits recruitment of regenerative mesenchymal stem cells to apoptotic tissue cells [7]. Thus, the release of HMGB1 from activated platelets favors thrombosis and inflammation and suppresses mechanisms that potentially promote tissue repair and regeneration, which we have recently validated for necrotic cell-derived HMGB1 [14].
Monocytes express HMGB1 receptors on the cell surface, including toll-like receptors (TLRs) and the receptor of advanced glycation end products (RAGE), a transmembrane multiligand receptor of the immunoglobulin superfamily [15–16]. However, the role of platelet-derived HMGB1 in regulating monocytes remained unknown. We now report that platelet-derived HMGB1 promotes recruitment of monocytes via RAGE and suppresses monocyte apoptosis via TLR4-dependent activation of MAPK/ERK (extracellular signal-regulated kinase).
Materials and Methods
Monocytes and platelets
Monocytes were isolated from leukocyte buffy coats or healthy volunteer donors as described previously [14]. Isolation of human and murine platelets was carried out as described previously [6]. For certain experiments, isolated platelets were activated in PBS (pH 7.4; Lonza) by treatment with 5 μg/ml collagen-related peptide (CRP; from Richard Farndale, University of Cambridge, Cambridge, UK) or 50 μM adenosine diphosphate (ADP; Chrono-Log, Havertown, PA) for 15 min and centrifuged at 800 × g for 5 min to obtain activated platelet supernatant.
Animals
Platelet-specific HMGB1 knockout mice were generated by crossing floxed HMGB1 (HMGB1fl/fl, termed HMGB1 Flox mice) with platelet factor (PF4)-Cre transgenic mice (Jackson Laboratories, Bar Harbor, ME) as we have described previously [6]. Both mouse strains were on C57BL/6 background. PF4-Cre HMGB1fl/fl (termed HMGB1 PF4 mice) offspring were confirmed using standard genomic PCR genotyping techniques. Male mice were used for experiments at an age of 8–12 weeks.
Cell migration assay
Monocyte migration was analyzed in a modified Boyden chamber (Neuro Probe Inc., Gaithersburg, MD) with a 5-μm pore polycarbonate membrane. 5×104 monocytes/well in RPMI-1640 medium (GIBCO/Invitrogen, Karlsruhe, Germany) supplemented with 0.5% bovine serum albumin (BSA; Roth, Karlsruhe, Germany) were loaded onto the upper chamber. Conditioned media (CM) derived from resting or activated (CRP/ADP) platelets (platelet supernatants supplemented with RPMI-1640/BSA 0.5%, ratio 1:1) were loaded onto the lower chamber. When indicated, neutralizing monoclonal anti-HMGB1 antibody (10 μg/ml, mouse IgG2b Kappa; Biolegend, San Diego, CA) or isotype control antibody (10 μg/ml, mouse IgG2b Kappa; Biolegend) were added to the targets. When indicated, HMGB1 receptors were blocked on monocytes with anti-human RAGE polyclonal antibody (20 μg/ml, goat IgG), anti-human TLR2 monoclonal antibody (2 μg/ml, mouse IgG2b) or anti-human TLR4 polyclonal antibody (10 μg/ml, goat IgG) (R&D Systems, Wiesbaden, Germany). In other experiments, CM of resting/activated platelets derived from HMGB1 Flox/HMGB1 PF4 mice were used as targets. Recombinant HMGB1 (rHMGB1, 40 ng/ml) served as positive control for monocyte migration [14]. Migrated cells were stained and counted after 4 h in selected microscopic view fields at 20× magnification.
HMGB1 ELISA
HMGB1 release from platelets was determined by measuring HMGB1 levels in CM derived from resting or CRP/ADP-activated platelets with ELISA (Shino Test Corporation, Kanagawa, Japan) following the manufacturer’s instructions.
Murine polytrauma/hemorrhagic shock model
HMGB1 PF4 and HMGB1 Flox control mice were subjected to experimental trauma and hemorrhagic shock, consisting of bone pseudofracture, tissue injury, and hemorrhagic shock, as described previously [6]. The animal research protocol complied with the regulation regarding the care and use of experimental animals published by the US NIH and was approved by the Institutional Animal Use and Care Committee of the University of Pittsburgh.
Immunofluorescence stainings of cryopreserved lung and liver tissue sections were performed using anti-F-actin monoclonal antibody (2 μg/ml, mouse IgM, Abcam, Cambridge, MA) and anti-F4/80 monoclonal antibody (5 μg/ml, rat IgG2a, eBioscience, San Diego, CA). Alexa Fluor 488-conjugated anti-mouse IgG (1:100; Invitrogen, San Diego, CA) and Cy3-conjugated anti-rat IgG (1:1000; Jackson Immunoresearch) were used as secondary antibodies. Nuclei were stained with Hoechst (1:10,000, Abcam). Imaging was performed using a Nikon A1 confocal microscope (Nikon, Tokyo, Japan). Quantification of mean fluorescence intensities (MFI) was performed using a ratio of F4/80 to total actin.
Induction and detection of apoptosis in monocytes
Monocyte apoptosis was induced with 300 nM staurosporine (Calbiochem, Bad Soden, Germany) or 25 μM ABT-737 (Selleckchem, Houston, TX) and evaluated by measuring TMRE (Molecular Probes, Eugene, OR) MFI or the frequencies of Annexin V (Immunotools, Friesoythe, Germany) positive cells with flow cytometry using a FACS Calibur flow cytometer and CellQuest software (BD Biosciences, Heidelberg, Germany). In certain experiments, monocytes were pretreated with the inhibitors wortmannin (WM; PI3K inhibitor, Sigma, St. Louis, MO), LY294002 (PI3K inhibitor, Sigma), SH-6 (AKT inhibitor, Santa Cruz, Heidelberg, Germany) or U0126 (MEK/ERK inhibitor, Sigma) at indicated concentrations for 1 h. For immunofluorescence staining, polyclonal anti-cleaved caspase 3 antibody (5 μg/ml; rabbit IgG; Abcam) and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:100; Invitrogen) were used. Nuclei were stained with TO-PRO-3 iodide (Molecular Probes). Confocal microscopic analysis was performed using a LSM510 META confocal laser scanning microscope and ZEN 2012 imaging software (Carl Zeiss MicroImaging, Jena, Germany).
Immunoblot for phosphorylation of ERK
Western blot analysis of MAPK/ERK phosphorylation was performed as described previously [6]. P44/42 MAPK (Erk1/2) monoclonal antibody (1:500; rabbit IgG; Cell Signaling, Danvers, MA) and Phospho-p44/42 MAPK (Erk1/2) monoclonal antibody (1:1000; rabbit IgG; Cell Signaling) served as primary antibodies. Anti-alpha-Tubulin monoclonal antibody (1:1000; mouse IgG1; Cell Signaling) was used as loading control. Corresponding secondary fluorochrome-labeled antibodies and the Odyssey infrared imaging system (LI-COR, Bad Homburg, Germany) were used.
Statistical analysis
All data are presented as mean±S.D. for n ≥ 3 unless stated otherwise. Statistical significance was determined with the Student’s t-test using Graph Pad Prism software (GraphPad, San Diego, CA, USA).
Results
Platelet-derived HMGB1 mediates monocyte recruitment via RAGE
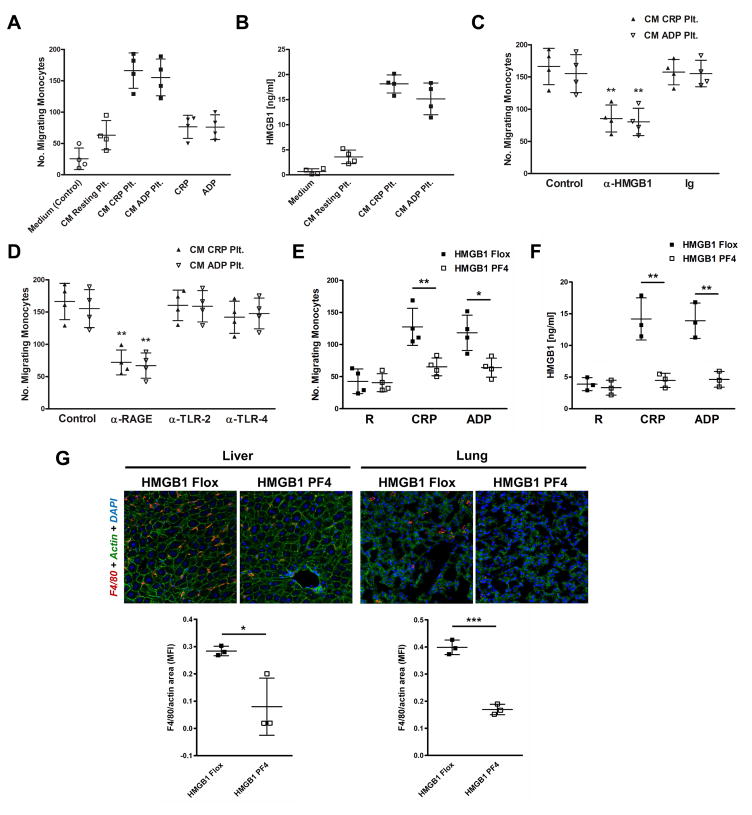
Migration of monocytes towards conditioned media (CM) derived from platelets was investigated in transwell migration experiments. CM of platelets activated by CRP or ADP induced a strong migratory response of monocytes whereas CM derived from non-activated (resting) platelets or the platelet agonists themselves only had moderate effects on monocyte recruitment (Fig. 1A). We detected significant amounts of HMGB1 in CM derived from CRP/ADP-activated platelets with ELISA (p<0.02 for either combination) (Fig. 1B), indicating HMGB1 release by activated platelets, which is in accordance with recently published data [6–7]. The HMGB1 receptors RAGE, TLR2, and TLR4 were expressed on all monocyte preparations, as evaluated by flow cytometry (data not shown). In the migration assay, the addition of a neutralizing anti-HMGB1 antibody to CM derived from CRP/ADP-activated platelets significantly inhibited monocyte recruitment (Fig. 1C). Moreover, blocking RAGE, but not TLR4 or TLR2 on monocytes with neutralizing antibodies significantly interfered with monocyte migration towards CM of CRP/ADP-activated platelets (Fig. 1D).
Figure 1. Platelet-derived HMGB1 mediates monocyte recruitment via RAGE.
(A) In transwell migration experiments, monocytes migrate towards CM derived from CRP-activated and ADP-activated platelets. CM derived from resting platelets or the platelet agonists themselves only have moderate effects on monocyte recruitment. (B) Substantial amounts of HMGB1 are detected in CM derived from CRP-activated and ADP-activated platelets using ELISA. (C) Migration of monocytes towards CM of CRP-activated or ADP-activated platelets is altered in the presence of a neutralizing anti-HMGB1 antibody. (D) Preincubation of monocytes with a blocking antibody against RAGE inhibits monocyte migration towards CM of CRP-activated and ADP-activated platelets. (E) Monocytes migrate towards CM derived from CRP-activated and ADP-activated platelets from HMGB1 Flox control mice, which is inhibited when CM of activated platelets derived from HMGB1 PF4 mice is used. (F) Substantial amounts of HMGB1 are detected in CM derived from CRP-activated and ADP-activated platelets from HMGB1 Flox control mice. HMGB1 levels are decreased in CM of activated HMGB1 PF4 platelets. (G) In a trauma/hemorrhagic shock model, F4/80-positive macrophage infiltrates are detected in the liver and lung of HMGB1 Flox control mice, which are attenuated in HMGB1 PF4 mice. Data are presented as mean ± SD for N≥3 and at least three separate experiments in all studies. * p<0.05, ** p<0.01, *** p<0.001 (Student’s t test).
To further substantiate a critical role of platelet-derived HMGB1 in monocyte recruitment, we used mice with platelet-specific ablation of HMGB1 (PF4-Cre HMGB1fl/fl mice, termed HMGB1 PF4 mice). CM of activated platelets from HMGB1 Flox control animals induced chemoattraction of monocytes, which was significantly reversed when HMGB1-deficient platelets were used (Fig. 1E). The addition of rHMGB1 served as a positive control and was followed by a strong migratory response of monocytes (data not shown). Substantial amounts of HMGB1 were only detected in CM derived from activated HMGB1 Flox control platelets, which were significantly decreased in CM derived from platelets lacking HMGB1 (Fig. 1F). During experimental trauma and hemorrhagic shock, consisting of soft tissue injury, bone pseudofracture, hemorrhage, and liver crush [6], F4/80-positive macrophage infiltrates were detected in the liver and lung of HMGB1 Flox control mice, which were significantly attenuated in HMGB1 PF4 mice (Fig. 1G), suggesting a critical role of platelet-derived HMGB1 in promoting the recruitment of monocytes/macrophages into injured tissues/organs in vivo.
Platelet-derived HMGB1 inhibits apoptosis of monocytes
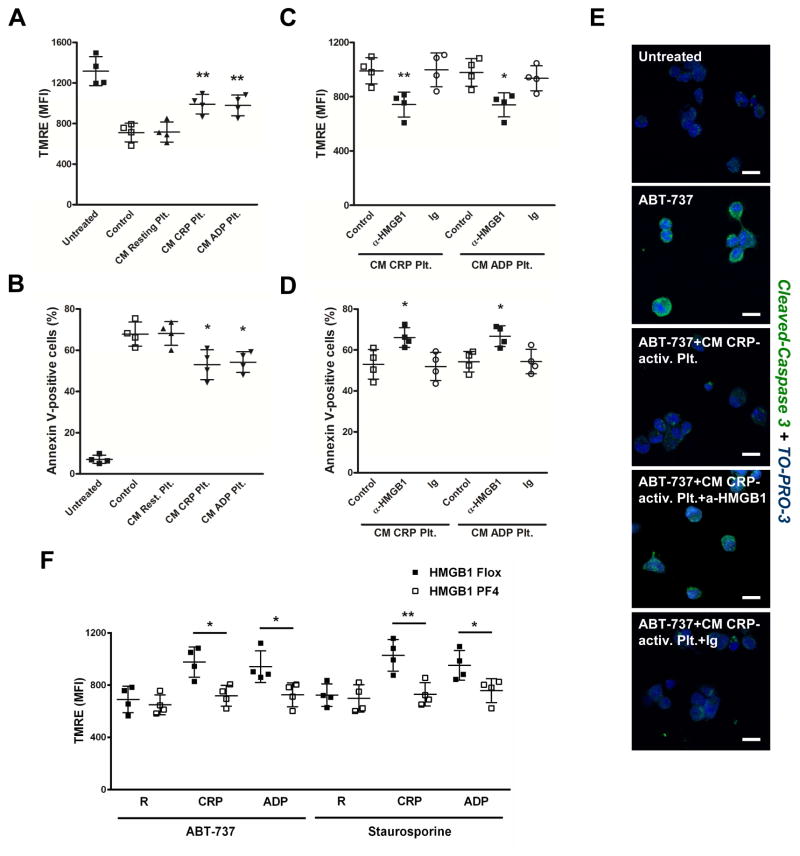
Next, we elucidated the effect of platelet-derived HMGB1 on monocyte apoptosis by flow cytometric analysis of mitochondrial transmembrane potential (tetramethylrhodamine ethyl ester fluorescence, TMRE) (Fig. 2A,C,F) and phosphatidylserine exposure (Annexin-V binding) (Fig. 2B,D). Preincubation of staurosporine-treated monocytes with CM derived from CRP/ADP-activated platelets, but not resting platelets significantly inhibited monocyte apoptosis (Fig. 2A,B). The addition of a neutralizing anti-HMGB1 antibody to CM derived from CRP/ADP-activated platelets significantly reversed the antiapoptotic effect (Fig. 2C,D). HMGB1-driven antiapoptotic effects were confirmed when ABT-737 was used to induce monocyte apoptosis (data not shown). In immunofluorescence stainings of intracellular cleaved caspase-3, which was only detected in apoptotic (ABT-737), but not vital monocytes, CM derived from CRP-activated platelets inhibited expression of the enzyme in apoptotic monocytes, which was reversed in the presence of a neutralizing HMGB1 specific antibody (Fig. 2E).
Figure 2. Platelet-derived HMGB1 suppresses monocyte apoptosis.
Preincubation of monocytes with CM derived from CRP-activated or ADP-activated, but not resting platelets reverses the effect of staurosporine on monocyte mitochondrial transmembrane potential (TMRE) (A) and Annexin V-binding (B). (C,D) The addition of a neutralizing anti-HMGB1 antibody to CM derived from CRP-activated or ADP-activated platelets reverses the antiapoptotic effects. (E) In immunofluorescence stainings and confocal laser scanning microscopy, intracellular cleaved caspase-3 is upregulated in ABT-737-treated apoptotic monocytes, which is suppressed by CM derived from CRP-activated platelets. In the presence of a neutralizing HMGB1 specific antibody, the effect exerted by the platelet media is markedly reversed. (F) CM derived from activated, but not resting platelets from HMGB1 Flox control mice increases TMRE fluorescence in ABT-737-treated and staurosporine-treated apoptotic monocytes, which is reversed when CM derived from HMGB1-deficient activated platelets is used. Data are presented as mean ± SD for N≥4 and at least three separate experiments in all studies. * p<0.05, ** p<0.01 (Student’s t test).
To validate a critical role of platelet-specific HMGB1 in regulating monocyte apoptosis, monocytes were incubated with CM of resting or CRP/ADP-activated platelets from HMGB1 Flox or HMGB1 PF4 mice prior to induction of apoptosis with either ABT-737 or staurosporine (Fig. 2F). CM derived from activated, but not resting platelets from HMGB1 Flox control animals markedly suppressed apoptosis of monocytes, which was significantly reversed when CM derived from HMGB1-deficient activated platelets was used.
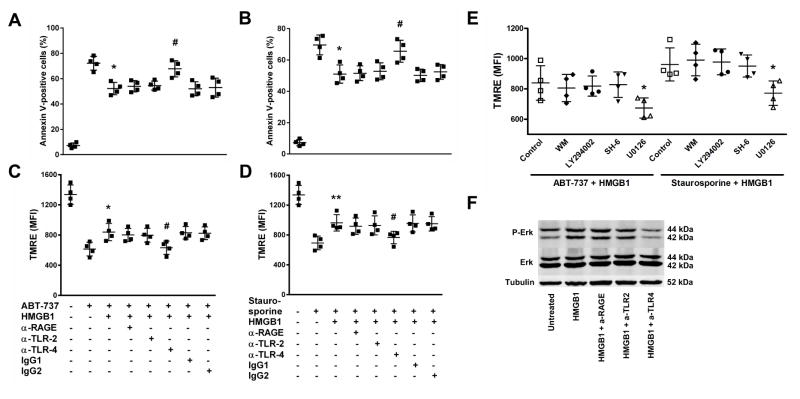
HMGB1-induced downregulation of monocyte apoptosis is mediated via TLR4-dependent activation of MAPK/ERK
To further study the underlying mechanism, we performed Annexin V (Fig. 3A,B) and TMRE stainings (Fig. 3C–E) on ABT-737 treated (Fig. 3A,C,E) or staurosporine treated (Fig. 3B,D,E) apoptotic monocytes in the presence or absence of rHMGB1. HMGB1 significantly inhibited monocyte apoptosis, which was significantly reversed when monocytes were pretreated with a blocking TLR4 antibody (Fig. 3A–D). Only the addition of U0126, a specific MEK/ERK inhibitor, but not wortmannin (WM; PI3K inhibitor), LY294002 (PI3K inhibitor) or SH-6 (AKT inhibitor) significantly reversed the effect of rHMGB1 on ABT-737/staurosporine treated monocytes (Fig. 3E). Western Blot analysis performed on monocyte lysates revealed that treatments with rHMGB1 induced phosphorylation of ERK in monocytes, which did not occur when monocytes were pretreated with a blocking TLR4 antibody (Fig. 3F). Thus, TLR4-mediated MAPK/ERK phosphorylation and activation in monocytes is critical for HMGB1-induced downregulation of apoptosis.
Figure 3. HMGB1-induced downregulation of monocyte apoptosis is mediated via TLR4-dependent activation of MAPK/ERK.
HMGB1 inhibits monocyte apoptosis induced by ABT-737 (A,C) or staurosporine (B,D), which is reversed when monocytes are pretreated with a blocking TLR4 antibody, as evaluated by Annexin V (A,B) and TMRE (C,D) stainings. (E) U0126, a specific MEK/ERK inhibitor, inhibits the effect of rHMGB1 on TMRE fluorescence in monocytes. (F) rHMGB1 (100 ng/ml) induces phosphorylation of ERK in monocytes, which does not occur when monocytes are pretreated with a blocking TLR4 antibody. Data are presented as mean ± SD for N≥4 and at least three separate experiments in all studies. * p<0.05, # p<0.05, ** p<0.01 (Student’s t test).
Discussion
Recruitment of monocytes from the blood to sites of inflammation or vascular and tissue lesions is regulated by multiple chemotactic factors and adhesion molecules [17]. Extracellular HMGB1 is known to induce tropism and activation of monocytes/macrophages [10] and other immune cells. In our study, we show that HMGB1 released by activated platelets mediates migration of monocytes via RAGE. Macrophage infiltrates in the lung and liver of mice subjected to experimental trauma and hemorrhagic shock were mediated by platelet-derived HMGB1. However, neither neutralizing HMGB1 bioactivity in conditioned platelet media in transwell migration experiments nor the ablation of HMGB1 in platelets themselves inhibited monocyte recruitment completely, indicating potential contribution of other factors to the platelet-mediated migratory response of monocytes. Indeed, various chemokines and growth factors, including CCL5 (RANTES) [18] and hepatocyte growth factor [19], are stored and, upon activation, secreted by platelets, which may contribute to monocyte migration in various disease models [20–21].
Monocytes undergo apoptosis when they are no longer needed, a process that may be prevented or inhibited by exogenous stimuli during inflammation [22]. Various cytokines and chemokines including IL1-beta, TNF-alpha, and CXCL4 have been identified to promote monocyte survival [22–23]. Platelets and their secretory products regulate leukocyte apoptosis/survival, as well [24]. Platelet-mediated downregulation of monocyte apoptosis may occur either directly by a specific phagocytosis-dependent process [3] or indirectly via secretion of soluble mediators by platelets [23]. Moreover, monocyte TLR4 ligation by LPS [25] and phosphorylation of ERK induced by oxidized LDL [26] or glucocorticoids [27] are known to suppress monocyte apoptosis. Here, HMGB1 release from activated platelets was critical for platelet-mediated downregulation of monocyte apoptosis, which occurred through TLR4-dependent activation of MAPK/ERK pathway in monocytes. In conclusion, we have identified platelet-derived HMGB1 as a novel regulator of monocyte migration and apoptosis. Further studies are needed to investigate HMGB1 derived from platelets as a potential therapeutic target in disease states associated with thrombosis and inflammation.
Supplementary Material
Highlights.
Platelet-derived high-mobility group box 1 induces migration of monocytes via RAGE
Platelet-derived high-mobility group box 1 suppresses monocyte apoptosis via TLR4
Decreased apoptosis is caused by TLR4-dependent activation of MAPK/ERK in monocytes
Acknowledgments
This work was supported by the DFG KFO 274 (VO 2126/1-1 to SV, BO 3786/2-2 to OB, GA 381/10-2 to MG); the NIH (P50GM053789 to TRB); an AAST Scholarship (to MDN); and a research grant of the German Cardiac Society (DGK to SV).
Abbreviations
- ADP
adenosine diphosphate
- CM
conditioned media
- CRP
collagen-related peptide
- DAMP
damage-associated molecular pattern molecule
- HMGB1
high-mobility group box 1
- MAPK/ERK
extracellular signal-regulated kinase
- NET
neutrophil extracellular trap
- RAGE
receptor of advanced glycosylation end products
- TLR
toll-like receptor
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andonegui G, Trevani AS, Lopez DH, Raiden S, Giordano M, Geffner JR. Inhibition of human neutrophil apoptosis by platelets. Journal of immunology. 1997;158:3372–3377. [PubMed] [Google Scholar]
- 2.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang D, Dohle F, Terstesse M, Bangen P, August C, Pauels HG, Heidenreich S. Down-regulation of monocyte apoptosis by phagocytosis of platelets: involvement of a caspase-9, caspase-3, and heat shock protein 70-dependent pathway. Journal of immunology. 2002;168:6152–6158. doi: 10.4049/jimmunol.168.12.6152. [DOI] [PubMed] [Google Scholar]
- 4.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouhiainen A, Imai S, Rauvala H, Parkkinen J. Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thrombosis and haemostasis. 2000;84:1087–1094. [PubMed] [Google Scholar]
- 6.Vogel S, Bodenstein R, Chen Q, Feil S, Feil R, Rheinlaender J, Schaffer TE, Bohn E, Frick JS, Borst O, Munzer P, Walker B, Markel J, Csanyi G, Pagano PJ, Loughran P, Jessup ME, Watkins SC, Bullock GC, Sperry JL, Zuckerbraun BS, Billiar TR, Lotze MT, Gawaz M, Neal MD. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest. 2015;125:4638–4654. doi: 10.1172/JCI81660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel S, Chatterjee M, Metzger K, Borst O, Geisler T, Seizer P, Muller I, Mack A, Schumann S, Buhring HJ, Lang F, Sorg RV, Langer H, Gawaz M. Activated Platelets Interfere with Recruitment of Mesenchymal Stem Cells to Apoptotic Cardiac Cells via High Mobility Group Box 1/Toll-like Receptor 4-mediated Down-regulation of Hepatocyte Growth Factor Receptor MET. The Journal of biological chemistry. 2014;289:11068–11082. doi: 10.1074/jbc.M113.530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochimica et biophysica acta. 2010;1799:101–113. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciucci A, Gabriele I, Percario ZA, Affabris E, Colizzi V, Mancino G. HMGB1 and cord blood: its role as immuno-adjuvant factor in innate immunity. PLoS One. 2011;6:e23766. doi: 10.1371/journal.pone.0023766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. Journal of leukocyte biology. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 12.Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. Journal of immunology. 2009;183:5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Messer JS, Wang Y, Lin F, Cham CM, Chang J, Billiar TR, Lotze MT, Boone DL, Chang EB. Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation. J Clin Invest. 2015;125:1098–1110. doi: 10.1172/JCI76344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel S, Borger V, Peters C, Forster M, Liebfried P, Metzger K, Meisel R, Daubener W, Trapp T, Fischer JC, Gawaz M, Sorg RV. Necrotic cell-derived high mobility group box 1 attracts antigen-presenting cells but inhibits hepatocyte growth factor-mediated tropism of mesenchymal stem cells for apoptotic cell death. Cell Death Differ. 2015;22:1219–1230. doi: 10.1038/cdd.2014.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kierdorf K, Fritz G. RAGE regulation and signaling in inflammation and beyond. Journal of leukocyte biology. 2013;94:55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 16.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. Journal of immunology. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 17.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beilmann M, Vande Woude GF, Dienes HP, Schirmacher P. Hepatocyte growth factor-stimulated invasiveness of monocytes. Blood. 2000;95:3964–3969. [PubMed] [Google Scholar]
- 21.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 22.Mangan DF, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. Journal of immunology. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 23.Scheuerer B, Ernst M, Durrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–1166. [PubMed] [Google Scholar]
- 24.Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122:2550–2554. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- 25.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Powell WS, Monneret G. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. Journal of immunology. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 26.Namgaladze D, Kollas A, Brune B. Oxidized LDL attenuates apoptosis in monocytic cells by activating ERK signaling. J Lipid Res. 2008;49:58–65. doi: 10.1194/jlr.M700100-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Barczyk K, Ehrchen J, Tenbrock K, Ahlmann M, Kneidl J, Viemann D, Roth J. Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood. 2010;116:446–455. doi: 10.1182/blood-2009-10-247106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.