Abstract
Animal models of hematopoietic and gastrointestinal acute radiation syndromes (ARS) have been characterized to develop medical countermeasures. Acute radiation-induced decrease of intestinal absorptive function has been correlated to a decrease in the number of intestinal crypt cells resulting from apoptosis and enterocyte mass reduction. Citrulline, a noncoded amino acid, is produced almost exclusively by the enterocytes of the small intestine. Citrullinemia has been identified as a simple, sensitive and suitable biomarker for radiation-induced injury associated with gastrointestinal ARS (GI-ARS). Here we discuss the effect of radiation on plasma citrulline levels in three different species, C57BL/6 mice, Göttingen minipigs and rhesus nonhuman primates (NHPs), measured by liquid chromatography tandem mass spectrometry (LC-MS/MS). The effects of experimental study conditions such as feeding and anesthesia were also examined on plasma citrulline levels in the NHPs. Both the mice and Göttingen minipigs were partial-body irradiated (PBI) with doses from 13–17 Gy and 8–16 Gy, respectively, whereas NHPs were total-body irradiated (TBI) with doses from 6.72–13 Gy. Blood samples were taken at different time points and plasma citrulline levels were measured in the three species at baseline and after irradiation. Basal plasma citrulline concentrations (mean 6 SEM) in mice and minipigs were 57.8 ± 2.8 μM and 63.1 ± 2.1 μM, respectively. NHPs showed a basal plasma citrulline concentration of 32.6 ± 0.7 μM, very similar to that of humans (~40 μM). Plasma citrulline progressively decreased after irradiation, reaching nadir values between day 3.5 and 7. The onset of citrulline recovery was observed earlier at lower radiation doses, while only partial citrulline recovery was noted at higher radiation doses in minipigs and NHPs, complete recovery was noted in mice at all doses. Plasma citrulline levels in NHPs anesthetized with ketamine and acepromazine significantly decreased by 35.5% (P = 0.0017), compared to unanesthetized NHPs. In the postprandial state, citrulline concentrations in NHPs were slightly but significantly decreased by 12.2% (P = 0.0287). These results suggest that plasma citrulline is affected by experimental conditions such as anesthesia and feeding.
INTRODUCTION
Development of effective medical countermeasures for the treatment of large populations in the event of deliberate and/or accidental radiological catastrophes requires well characterized animal models (1). Biomarkers are a cornerstone of safety and efficacy assessments when using animal models of acute radiation syndrome (ARS) and qualification of relevant end points is part of the foundations in biodefense research. At least four sub-syndromes that are associated with ARS are currently being actively investigated in the context of accidental ionizing radiation exposure. These include the delayed effects of acute radiation exposure (DEARE), both hematopoietic syndrome (H-ARS) and gastrointestinal syndrome (GI-ARS), which are specific targets for the development of novel medical countermeasures, and cerebrovascular/central nervous system (CV/CNS) syndrome, which occurs within a radiation exposure range considered incurable (2). Radiation-induced cell death, mostly due to DNA damage, results in functional alterations in various tissues. H-ARS is characterized by leukopenia and thrombocytopenia, increased susceptibility to infection, hemorrhage and anemia (2, 3). Radiation-induced damage to the gastrointestinal tract is characterized by intestinal crypt cell apoptosis and mitotic cell death leading to loss of mucosal barrier function, villous atrophy, alterations in the normal intestinal bacterial flora and enterocyte mass reduction (4). Owing to a high cell-division rate, the small intestine is highly sensitive to radiation exposure and biomarkers of small bowel radiation-induced injury are valuable both for diagnostic purposes in the clinic, as well as for drug development of radiation-induced injury medical countermeasures.
In humans, circulating citrulline levels can be used as a biomarker indicative of a functional intestine (5). Citrulline, a non-DNA coded amino acid, is a byproduct of many different cellular enzyme reactions including glutamine metabolism in small bowel enterocytes (6, 7), however, it is not a component of either proteins or nutritional products (8). Citrulline is almost exclusively synthetized by the small intestine and is important in the subsequent synthesis of arginine, a critical precursor of vascular nitric oxide (9). Citrulline synthesis is abundant within the small intestine enterocytes because of the presence of high levels of the enzymes needed for synthesis (e.g., arginase II and ornithine carbamoyl transferase) coupled with the low activity of citrulline catabolic enzymes (e.g., argininosuccinate synthase and argininosuccinate lyase) (10). Citrulline is then released from the enterocytes into the circulation and primarily taken up by the kidney, avoiding liver metabolism (11). Thus, plasma citrulline concentration is highly proportional to intestinal enterocyte mass (12–14). Because of this relationship, citrullinemia has been identified as a simple assay for radiation-induced small bowel epithelial cell loss after exposure (15, 16). In this context, it is important to explore what, if any, additional independent factors (other than radiation) may alter plasma citrulline levels.
Herein, we discuss the effect of radiation on plasma citrulline levels in different species [C57BL/6 mice, Göttingen minipigs and rhesus nonhuman primates (NHPs)] and the effect that experimental study conditions such as feeding and anesthesia can have on plasma citrulline levels, specifically in the rhesus model.
MATERIALS AND METHODS
Animal Models, Experimental Environment and Radiation Exposure
All experimental procedures were performed in accordance with Institutional Animal Care and Use Committee (IACUC) and the Canadian Council on Animal Care guidelines for use of experimental animals. All protocols included strict euthanasia criteria reviewed and approved by the IACUC. All animals were monitored continuously (i.e., technical staff and veterinarians were available 24 h a day) for development of any untoward clinical signs.
For all species, the animal room environment was maintained at a temperature of 21 ± 3°C with a relative humidity of 50 ± 20%, a 12:12 h light-dark schedule and 10–15 air changes per hour. Temperature and relative humidity were monitored continuously. For all animal irradiations a Cobalt-60 gamma source (Theratron®-1000; MDS Nordion™ Inc., Ottawa, Canada) was used. Prior to all irradiations, dose was calibrated using an acrylic phantom placed in the same experimental setup as that used for the animals. Body measurements were taken to deliver midline to tissue dose. The measurements were taken using an ion chamber with a solid water phantom buildup. Dosimetry was performed using a Farmer ionization chamber connected to an electrometer that was included in each radiation treatment session. Nanodots were also used but only as backup in case the electrometer was not functional.
Mice (C57BL/6) acquired from Jackson Laboratory (Bar Harbor, ME) were acclimated for a minimum of 13 days prior to irradiation. A standard certified commercial chow (Certified Irradiated Global Rodent Diet, cat. no. 2918C; Harlan Teklad, Madison, WI) was provided to the animals ad libitum. Certified treats from Bio-Serv (yogurt drops) was provided as part of the animal enrichment program at least once a week. Acidified municipal tap water (which had been exposed to ultraviolet light and purified by reverse osmosis) was provided to the animals ad libitum. Mice were irradiated with partial shielding (PBI) in subsets of up to 12 mice in a custom designed restrainer where their left pelvic limb (distal to the mid-femur; approximately 3%) was extended and maintained in position with an elastic band. The left pelvic limb was shielded with a cerrobend structure. The animals were not anesthetized during irradiation and were not acclimated to the irradiation devices. During PBI, the mice were immobilized and the restraining devices and irradiation source did not move. The dose was between 13 to 17 Gy at 0.60 Gy/min and the Cobalt-60 source was on top of the animals. Plasma citrulline levels were measured after irradiation or sham procedures. Whole blood was collected into EDTA-coated tubes at scheduled termination on day 1, 3.5, 7, 10 and 30, and centrifuged at 1,500 rpm for 10 min at 4°C. Plasma was harvested and stored at −70°C.
Göttingen minipigs acquired from Marshall BioResources (North Rose, NY) were acclimated for a minimum of 30 days prior to irradiation. A standard certified commercial chow (Harlan Teklad Certified Miniswine Diet, cat. no. 7037C) was provided to the animals twice daily in the morning and in the afternoon. Treats or fruits/vegetables were provided as part of the animal enrichment program. Municipal tap water (which had been exposed to ultraviolet light and purified by reverse osmosis) was provided to the animals ad libitum. The Göttingen minipigs were partial-body irradiated at doses ranging from 8 to 16 Gy at a dose rate of approximately 0.50 Gy/min. A cerrobend structure was used for partial-body shielding, including the head, thorax and pelvic limb (approximately 50% of bone marrow based on CT-scan estimates in control animals). The animals were anesthetized [ketamine 15 mg/kg, intramuscular (IM) injection] during irradiation and were not acclimated to the irradiation devices. To produce homogenous dose distribution, exposure was divided in two parts. Half of the dose was delivered by right lateral irradiation followed by the second half of the dose delivered by left lateral irradiation. Whole blood was collected into EDTA-coated tubes prior to irradiation and on day 1, 3, 5, 7, 9, 15, 19, 20, 25, 30, 35, 40 and 45.
Rhesus macaques were acclimated for a minimum of 6 weeks prior to irradiation. A standard certified commercial chow (Harlan Teklad certified hi-fiber primate diet no. 7195C) was provided twice daily in the morning and afternoon. Treats or fruits/vegetables were provided as part of the animal enrichment program. Municipal tap water (which had been exposed to ultraviolet light and purified by reverse osmosis) was provided to the animals ad libitum. NHPs were total-body irradiated (TBI) with doses ranging from 6.7 to 13 Gy at a dose rate of 0.60–0.80 Gy/min. NHPs were anesthetized (ketamine hydrochloride 100 mg/ml and acepromazine 10 mg/ml at a dose of 0.1 ml/kg) during exposure and the animals were acclimated to the irradiation devices. To produce homogenous dose distribution, exposure was divided in two parts. First, the animals received half of the dose by anteroposterior irradiation followed by the second half of the dose delivered by posteroanterior irradiation. Whole blood was collected into EDTA-coated tubes prior to irradiation and between days 1–10. In nonirradiated NHPs, blood samples were obtained after a period at least 12 h of fasting and within 2 h after feeding from two animal cohorts to assess the impact of food consumption on plasma citrulline levels. To assess the impact of anesthesia, blood samples were obtained for plasma citrulline from nonirradiated animals that were anesthetized (ketamine 9.09 mg/kg and acepromazine 0.9 mg/kg, IM) and compared to plasma citrulline concentrations obtained from the same animals without anesthesia. Blood samples collected within 3 h of the anesthetic regimen were considered to be from an anesthetized animal and were subsequently pooled. Blood samples collected from animals not previously anesthetized or beyond the post-anesthetic 3 h window were considered for the “no anesthesia” group. A summary of the animals used in this comparative assessment is shown in Table 1.
TABLE 1.
Summary of Animal Demographics for Citrulline Analysis
| Species | Total (n) | Age range preirradiation |
Gender (n) | Radiation dose range (Gy) |
Radiation dose rate (cGy/min) |
Shielding conditions |
|---|---|---|---|---|---|---|
| Mouse (C57BL/6) | 20 | 9 weeks | Male | 13–17 | 0.60 | Left leg distal to mid-femur |
| Minipig (Göttingen) | 81 | 5–8 months | Male (38) Female (43) |
8–16 | 0.50 | Head, thorax and pelvic limb |
| Nonhuman primate (rhesus macaques) | 209 | 2.5–6 years | Male (153) Female (56) |
6.72–13 | 0.60–0.80 | None |
Chemicals and Materials
L-Citrulline (98% purity) was purchased from Toronto Research Chemicals Inc. (Toronto, Canada) and D7-citrulline (l-Citrulline-2,3,3,4,4,5,5-d7, 99% purity) was purchased from C/D/N Isotopes Inc. (Pointe-Claire, Canada). HPLC grade acetonitrile, methanol, 2-propanol, hydrochloric acid (1N) and formic acid (88%) were purchased from Fisher Scientific (Pittsburgh, PA). Type 1 water was used throughout the qualification.
Citrulline Detection in the Mice
Citrulline in mouse plasma (K3-EDTA) was quantified by hydrophilic interaction liquid chromatography (HILIC)-MS/MS (Biodosimetry Diagnostic Core Laboratories, UAMS, Little Rock, AR) as previously described (17).
Method Qualification for Citrulline Detection in the Minipigs and Rhesus Nonhuman Primates
A protein precipitation extraction method using a high performance liquid chromatographic mass spectrophotometric (LC-MS/MS) detection method for the determination of endogenous citrulline levels in rhesus monkey plasma (K3EDTA) and Göttingen minipig plasma (K2EDTA) was qualified. Calibration standards and quality control (QC) samples were prepared by spiking known concentrations of standards and QC samples in reverse osmosis filtered and deionized water. The calibration curve consisted of seven standards ranging from 1.00 to 50.0 μg/ml (5.7–285.4 μM). The endogenous citrulline concentrations in pooled lots of nonirradiated rhesus monkey plasma (K3EDTA) and Göttingen minipig plasma (K2EDTA) donors were quantified using the standard curve and QCs in reverse osmosis filtered and deionized water. QCs were prepared in plasma (rhesus monkey and Göttingen minipig) by addition of three concentrations (17.1, 57.1 and 114.2 μM) to the predetermined endogenous levels, using the appropriate spiking solutions. The plasma QCs were quantified relative to standard curve and QCs in water. Any interference due to the presence of arginine was investigated to ensure assay detection of citrulline was selective and sensitive. It was determined that arginine did not interfere with citrulline quantitation, as spiked concentrations of arginine eluted at a different retention time than citrulline, using the LC-MS/MS system in both minipig and NHP plasma.
Minipig and NHP Sample Preparation
Internal standard (500 μl; d7-citrulline, 500.0 ng/ml) was added to 20 μl of sample, followed by mixing and centrifugation for 10 min at 4,000 rpm. Supernatant (20 μl) was added to injection vials containing 400 μl of 80/20 (v/v) acetonitrile/water and thoroughly mixed.
Minipig and NHP Liquid Chromatography Tandem Mass Spectrometry
LC-MS/MS analyses were performed on an Applied Biosystems® API4000 Mass Spectrometer (Carlsbad, CA) coupled with a Shimadzu Prominence UFLC system (Shimadzu Corp., Kyoto, Japan). Mobile phases A and B consisted of 0.1% formic acid in water and 0.1% formic acid in acetonitrile, respectively. LC gradient separation was performed on a Zorbax HILIC Plus column (2.1 × 50 mm, 3.5 μm) (Agilent Technologies Inc., Palo Alto, CA) operated at 40°C, with a flow rate of 0.500 ml/min. The retention time for both citrulline and the internal standard occurred at 2.35 min and the total run time was 5.0 min.
Detection was performed with the Multiple Reaction Monitoring (MRM) mode using the electro-spray ionization (ESI) technique in positive-ion mode with the following transitions: citrulline (m/z 177.1 → 160.2) and d7-citrulline (m/z 183.0 → 166.0). Mass spectrometry sensitivity was decreased using the 13C isotope for citrulline. The other state file parameters used were as follows: spray voltage +5,000 V; temperature 550°C; collision (CAD) gas pressure 6 psi; GS1 pressure 60 psi; GS2 pressure 60 psi; curtain gas 30 psi; collision energy (CE) 20 eV; entrance potential (EP) 10 V and collision cell exit potential (CXP) 12 V.
Statistical Analysis
Data are expressed as mean ± SEM. Comparisons between two groups were performed using the two-tailed unpaired Student’s t test with GraphPad Prism v5.00 for Windows (GraphPad Software, LaJolla, CA). Differences were considered significant at P < 0.05.
RESULTS
Citrullinemia in Different Species
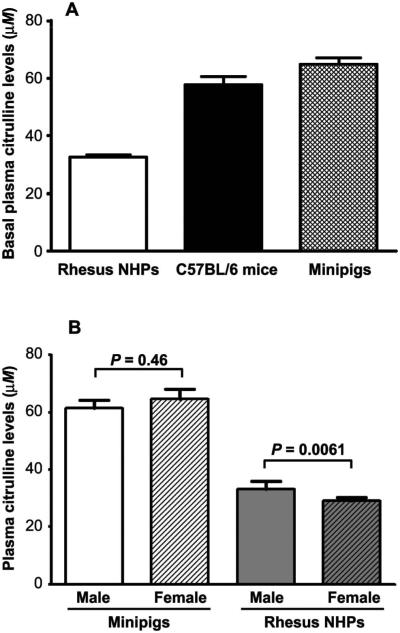
Plasma citrulline levels were measured in the three different species prior to irradiation and results were used as baseline values (Fig. 1A). The mean (±SEM) baseline plasma citrulline level in C57BL/6 mice (n = 20) was 57.8 ± 2.8 μM (range: 41.6–81.2 μM). In Göttingen minipigs (n = 81), the mean (± SEM) baseline plasma citrulline level was 63.1 ± 2.1 μM (range: 24.4–128.0 μM). In the NHPs (n = 209, males and females), the baseline plasma citrulline level was found to be lower than that in other species with a mean (± SEM) value of 32.6 ± 0.7 μM (range: 7.9–69.8 μM). Plasma citrulline levels in female NHPs were significantly lower than in males, 29.2 ± 1.2 μM compared to 33.7 ± 0.9 μM (P = 0.0061), an observation not seen in minipigs (Fig. 1B). In humans, no significant difference in plasma citrulline levels between genders has been reported according to the Geigy Scientific Tables, where values were 37.0 ± 9.0 μM and 35.0 ± 10.0 μM for males and females, respectively (18).
FIG. 1.
Plasma citrulline levels in different species. Panel A: Basal plasma citrulline levels in rhesus NHPs (n = 209), C57BL/6 mice (n = 20) and Göttingen minipigs (n = 81). Panel B: Plasma citrulline levels in male and female rhesus NHPs (n = 158 and 56, respectively), and male and female Göttingen minipigs (n = 33 and 38, respectively). Data are presented as mean ± SEM.
Plasma Citrulline Levels after Irradiation
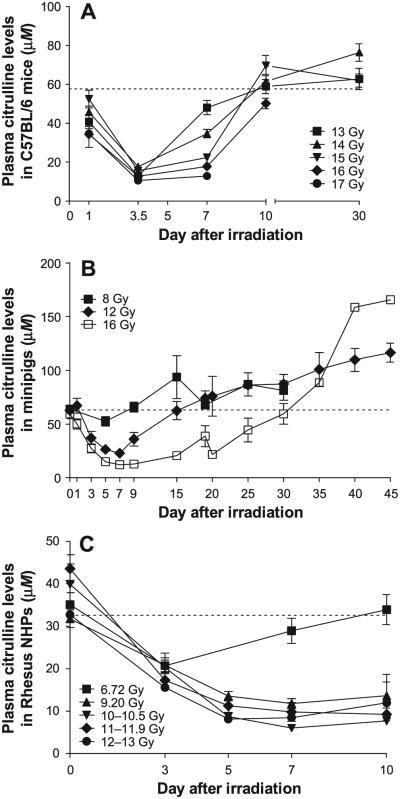
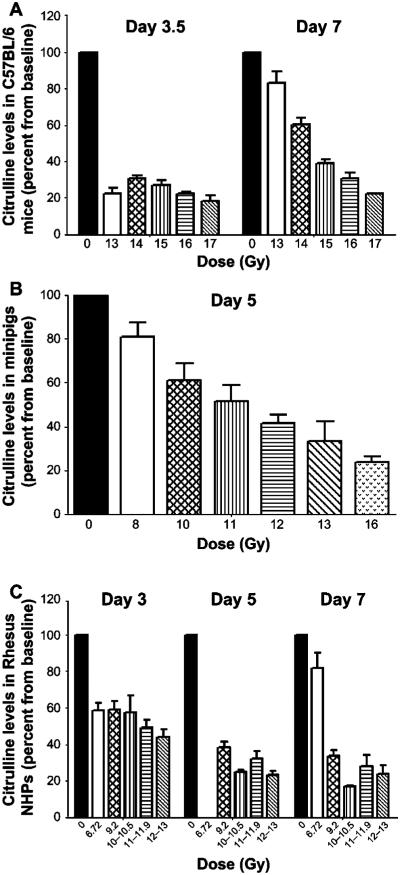
Plasma citrulline levels decreased in a dose- and time-dependent manner after irradiation with or without partial bone marrow shielding. Plasma citrulline levels decreased rapidly in the first days after irradiation to reach a minimum level at day 3.5 and 5 for the mice and the minipigs, respectively (Fig. 2A and B). However, the nadir in minipigs does appear to shift to a later time point at higher dose levels (day 7 at 12 and 16 Gy; Fig. 2B). While plasma citrulline recovery to the normal (or preirradiation) range was more rapid for the lowest radiation dose administered to both species, complete citrulline preirradiation level recovery was noted in the 16 Gy PBI minipigs only 30 days postirradiation, where 6 of the 7 animals survived to day 30. NHPs that were exposed to 6.72 Gy TBI reached a plasma citrulline nadir at day 3 postirradiation and returned to baseline within seven days postirradiation (Fig. 2C). NHPs exposed to radiation dose levels ranging from 9.2 to 13 Gy reached a plasma citrulline nadir level at day 7 with citrulline levels ranging from −62.8 to −84.8% compared to preirradiation values. Although a slight recovery was noted after day 7, plasma citrulline levels remained low at day 10 postirradiation with values ranging from 7.69 to 13.64 μM compared to baseline ranges between 31.77 to 43.55 μM in NHPs that received high-dose TBI. The plasma citrulline level decrease compared to baseline was radiation dose dependent in mice, minipigs and NHPs, as shown in Fig. 3, although the radiation dose dependence was time sensitive and in mice was more apparent during the recovery phase than at the nadir of the response.
FIG. 2.
Plasma citrulline levels after irradiation. Plasma citrulline levels decreased after irradiation with partial bone marrow shielding in C57BL/6 mice (panel A) and Göttingen minipigs (panel B). Plasma citrulline levels are also shown after total-body irradiation in rhesus NHPs (panel C). The dash line represents the average basal citrulline levels determined prior to study conduct and across all available animals. Values are presented as mean ± SEM. Individual sample sizes in the radiation dose groups ranged from 4 to 8 for mice, 1 to 20 for minipigs and 2 to 20 for NHPs, not accounting for any mortality during the study.
FIG. 3.
Plasma citrulline levels decrease in a dose-dependent manner. Plasma citrulline levels after irradiation in C57BL/6 mice at day 3.5 and 7 (panel A) and Göttingen minipigs at day 5 (panel B). Plasma citrulline levels also decreased as shown after total-body irradiation in rhesus NHPs at day 3, 5 and 7 (panel C). Values are presented as mean ± SEM.
Effect of Feeding on Plasma Citrulline Levels in Rhesus NHPs
In various studies, blood samples for plasma citrulline levels were collected in rhesus NHPs either in a fasted state or after feeding. Plasma citrulline levels were slightly but significantly lower in animals that were fed prior to blood collection compared to the second cohort of animals when fasted with concentrations of 30.6 ± 1.3 μM and 34.8 ± 1.2 μM, respectively (Fig. 4). The difference in plasma citrulline levels in postprandial animals compared to fasted animals without any radiation-induced effects was −12.2% (P = 0.0287).
FIG. 4.
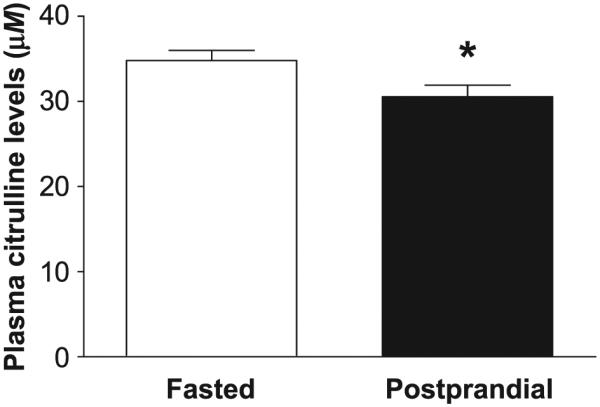
Effect of feeding on plasma citrulline levels in rhesus NHPs. Plasma citrulline levels were measured in rhesus NHPs either when fasted (n = 88) or postprandially (n = 47). Data are presented as mean ± SEM. *P < 0.05 when compared to the fasted animals.
Effect of Ketamine/Acepromazine Anesthesia on Plasma Citrulline Levels in Rhesus NHPs
Animals were considered anesthetized if blood was collected within 3 h of IM injection with ketamine (9.09 mg/kg) and acepromazine (0.9 mg/kg). The study results showed that anesthesia with ketamine/acepromazine significantly decreased plasma citrulline levels by −35.5% (P = 0.0017). After anesthesia, plasma citrulline levels were 21.7 ± 2.4 μM compared to 33.6 ± 2.5 μM in the same unanesthetized animals (Fig. 5).
FIG. 5.
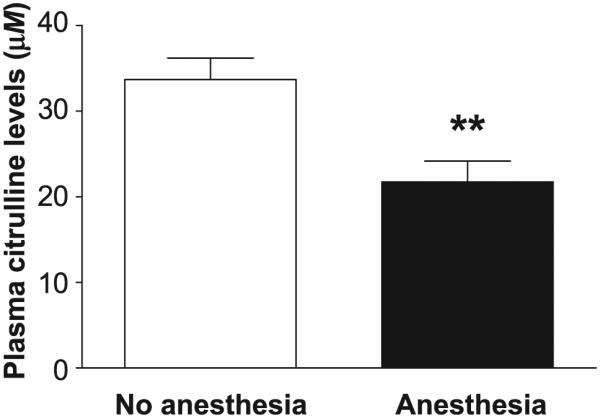
Effect of ketamine/acepromazine anesthesia on plasma citrulline levels in rhesus NHPs. Plasma citrulline levels were measured in rhesus NHPs with (n = 16) or without (n = 16) a ketamine/acepromazine anesthetic regimen. “Anesthesia” represents pooled data collected within 3 h of the anesthetic regimen. “No anesthesia” represents pooled data collected in excess of 3 h after the anesthetic regimen or from animals that were not previously anesthetized. Data are presented as mean ± SEM. **P < 0.01 when compared to unanesthetized animals.
DISCUSSION
Citrulline is a widely accepted biomarker in humans for quantifying enterocyte functional metabolic mass (19). In this study, the analysis of the plasma citrulline concentrations in mice, minipigs and NHPs highlights interesting species differences and assesses the impact of several experimental study conditions on this biomarker.
Healthy adult Caucasian subjects with normal intestinal mucosa and normal renal function have post-absorptive mean plasma citrulline levels of 40 μM (with a range between 20 and 60 μM) (6, 19, 20). Citrulline levels have typically varied with age as well as ethnicity of the subject group. For example, in elderly subjects (>70 years), citrulline levels were shown to increase, whereas in Asian compared to Caucasian subjects, they were reported to be lower (19, 21). Unfortunately, in this study there was insufficient data to address the effects of age on plasma citrulline levels after irradiation. Nevertheless, this represents an interesting consideration that should be assessed in future investigations. Plasma citrulline levels in mice, minipigs and NHPs are relevant since these species are commonly used as nonclinical models in the evaluation of citrulline as a GI biomarker. Recently, the minipig model has been increasingly considered in studies of radiation-induced GI-ARS (22, 23). The mean baseline citrulline level for NHPs in this study was 32.6 ± 0.7 μM, which is comparable to values reported in the literature (i.e., 10–80 μM) for this species. Basal citrulline levels in mice and minipigs were also similar to concentrations previously reported (17, 24–27). Plasma citrulline levels in NHPs were comparable to concentrations reported for healthy humans (6, 16, 19), whereas mice and minipigs presented mean concentrations that were generally higher than that observed in humans. Similarities between NHPs and humans may be attributed to physiological similarities between the two species.
Radiation-induced damage is generally proportional to the cell division rate. Cells with a high division rate such as the hematopoietic lineages (28) and the intestinal crypts (29) are more radiosensitive than cells in areas with low cell division rates, such as the central nervous system, skeletal muscle or the bones (30, 31). While crypt cell regeneration, cell apoptosis and mucosal surface measurements are the most commonly quantified parameters after irradiation (26), these morphologic/morphometric end points require tissue sampling and use of invasive methodologies. Conversely, as a plasma biomarker, citrulline allows for repeated measurements to be conducted (in time course studies) from the same individual animal in the larger species, thus reducing the number of animals, as is recommended by The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3R; London, UK). The radiation-induced effects on intestinal function have been correlated with impaired absorptive capacity due to epithelial cell loss (32–35) and citrulline can be used as a surrogate biomarker to quantify the intestinal epithelial cell mass (12, 16, 26). Plasma citrulline levels decreased rapidly after PBI in mice, reaching their lowest concentrations on day 3.5. The time course of plasma citrulline changes noted in mice was comparable to the kinetic profile characterized by others in this same species (26). In PBI minipigs, where shielding accounted for 50–55% of bone marrow, the nadir citrulline levels were noted to occur later with increasing radiation dose levels (i.e., day 5 at 8 Gy and day 7 at 12 and 16 Gy). The NHPs in this study presented a slower kinetic of post-exposure citrulline reduction compared to mice, reaching nadir values by day 7 at radiation dose levels higher than 9 Gy. Lower radiation dose levels were associated with an earlier nadir (day 5 at 6.72 Gy). As would be anticipated, allometric differences are widely reported among species for physiological parameters when determined at baseline (36); however, differences have also been observed during drug development studies (37) as well as during radiation biology studies (38) with smaller species presenting faster kinetic profiles typically proportional to body surface area.
In this study, citrulline recovery was more rapid with lower radiation doses in all species, although recovery was incomplete with TBI at 9.2–13 Gy in NHPs. Partial recovery of citrulline levels to preirradiation levels could be attributed, albeit to a moderate degree, to the short duration of monitoring (i.e., only 10 days for NHPs) in the current study. In the minipigs, citrulline levels appeared to rebound beyond basal levels, although given the low sample size (n = 2 and n = 1 beyond day 35 at 12 and 16 Gy, respectively) it is difficult to ascertain the cause of the excessive rebound. Plasma citrulline levels could potentially be used as a biomarker for biodosimetry that can be quantitatively related to the magnitude of the radiation dose received as shown by decreased citrulline levels with increasing radiation dose used in our study.
Citrullinemia is modulated by clinical factors such as renal and liver function, metabolic stress and inflammation (8). In ARS animal models, opportunistic bacterial infections, which are often associated with inflammation, could represent a potential confounding factor with regard to the interpretation of citrulline values. However, the timing of development of neutropenia (which typically coincides with the onset of opportunistic infections) occurs much later than the citrulline nadirs observed in our studies. The current study showed that experimental conditions such as feeding and anesthesia can modulate basal citrulline levels in NHPs. The plasma citrulline concentrations that were determined in these studies were slightly, but statistically significantly, decreased (−12.2%) in the postprandial state in NHPs, which is comparable to observations reported in humans (6). However, this change may not be clinically relevant against the backdrop of the extent of the radiation effect. As a consequence, a decreased appetite (which is a common occurrence after radiation exposure) needs to be monitored accurately for interpretation of citrulline levels in GI-ARS studies. In this study we also demonstrate that a standard anesthetic regimen in NHPs (i.e., ketamine and acepromazine) could also potentially modulate citrulline levels in these animals. To the best of our knowledge, this is the first time that an anesthetic regimen consisting of ketamine and acepromazine has been reported to decrease plasma citrulline concentrations. Anesthesia may be required in studies conducted with large NHPs and our results suggest that standardization of the interval between anesthesia and the blood collection time points for citrulline monitoring should be considered in the study design, as it could help reduce potential variability within and between study groups. The exact mechanisms by which anesthesia impacts citrulline levels or whether all anesthetics could have a similar effect remains unknown. However, in our studies the effect was noted as early as 8 min after induction of anesthesia by a ketamine/acepromazine regimen.
The data reported here suggest that dose-dependent changes in citrulline plasma concentrations occur after radiation exposure in different animal species used in nonclinical models of GI-ARS. However, when conducting such studies it should be noted that citrulline levels can also be modulated by other factors such as feeding regimens and anesthetic agents.
ACKNOWLEDGMENTS
This work was supported by the Biomedical Advanced Research and Development Authority (BARDA; contract nos. HHSO100201300023C, HHSO100201100027C, HHSO100201100045C and HHSO100201000051C), the National Institute of Allergy and Infectious Diseases (NIAID; contract no. HHSN272201300030C) and the U.S. Department of Veterans Affairs. The authors gratefully acknowledge Daniel Lambert for his contributions and support with statistical analyses.
Footnotes
None of the authors have any conflicts of interest, other than their employment in commercial pharmaceutical companies or contract research organizations.
REFERENCES
- 1.DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5:S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garau MM, Calduch AL, Lopez EC. Radiobiology of the acute radiation syndrome. Rep Prac Oncol Radiother. 2011;16:123–30. doi: 10.1016/j.rpor.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 4.Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–7. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119:1496–505. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 6.Rabier D, Kamoun P. Metabolism of citrulline in man. Amino Acids. 1995;9:299–316. doi: 10.1007/BF00807268. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Borbolla AG, Knabe DA. The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr. 1994;124:2437–44. doi: 10.1093/jn/124.12.437. [DOI] [PubMed] [Google Scholar]
- 8.Crenn P, Hanachi M, Neveux N, Cynober L. Circulating citrulline levels: a biomarker for intestinal functionality assessment. Ann Biol Clin (Paris) 2011;69:513–21. doi: 10.1684/abc.2011.0609. (French) [DOI] [PubMed] [Google Scholar]
- 9.Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–74. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 10.Moinard C, Cynober L. Citrulline: a new player in the control of nitrogen homeostasis. J Nutr. 2007;137:1621S–5S. doi: 10.1093/jn/137.6.1621S. [DOI] [PubMed] [Google Scholar]
- 11.Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, et al. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci U S A. 1993;90:7749–53. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology. 2003;124:1210–9. doi: 10.1016/s0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi Y, Yamada E, Yoshida T, Takahashi N. Effect of intestinal resection and arginine-free diet on rat physiology. Am J Physiol. 1995;269:G313–8. doi: 10.1152/ajpgi.1995.269.2.G313. [DOI] [PubMed] [Google Scholar]
- 14.Wakabayashi Y, Iwashima A, Yamada E, Yamada R. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. II. N-acetylglutamate synthase. Arch Biochem Biophys. 1991;291:9–14. doi: 10.1016/0003-9861(91)90098-4. [DOI] [PubMed] [Google Scholar]
- 15.Lutgens L, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: an emerging role for plasma citrulline. World J Gastroenterol. 2007;13:3033–42. doi: 10.3748/wjg.v13.i22.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutgens LC, Deutz N, Granzier-Peeters M, Beets-Tan R, De RD, Gueulette J, et al. Plasma citrulline concentration: a surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys. 2004;60:275–85. doi: 10.1016/j.ijrobp.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 17.Gupta PK, Brown J, Biju PG, Thaden J, Deutz NE, Kumar S, et al. Development of high-throughput HILIC-MS/MS methodology for plasma citrulline determination in multiple species. Anal Methods. 2011;3:1759–68. [Google Scholar]
- 18.Lentner C. Geigy scientific tables. 8th Ciba-Geigy; Basel, Switzerland: 1981. [Google Scholar]
- 19.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–39. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119:1496–505. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 21.Jianfeng G, Weiming Z, Ning L, Fangnan L, Li T, Nan L, et al. Serum citrulline is a simple quantitative marker for small intestinal enterocytes mass and absorption function in short bowel patients. J Surg Res. 2005;127:177–82. doi: 10.1016/j.jss.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim S, Jang WS, Lee SJ, Jin S, Kim J, Lee SS, et al. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res. 2014;181:387–95. doi: 10.1667/RR13207.1. [DOI] [PubMed] [Google Scholar]
- 24.Garg S, Wang W, Prabath BG, Boerma M, Wang J, Zhou D, et al. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiat Res. 2014;181:229–39. doi: 10.1667/RR13548.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones JW, Tudor G, Bennett A, Farese AM, Moroni M, Booth C, et al. Development and validation of a LC-MS/MS assay quantitation of plasma citrulline for application to animal models of the acute radiation syndrome across multiple species. Anal Bioanal Chem. 2014;406:4663–75. doi: 10.1007/s00216-014-7870-0. (Abstract) [DOI] [PubMed] [Google Scholar]
- 26.Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, et al. Citrulline: a physiologic marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol Biol Phys. 2003;57:1067–74. doi: 10.1016/s0360-3016(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 27.Moroni M, Elliott TB, Deutz NE, Olsen CH, Owens R, Christensen C, et al. Accelerated hematopoietic syndrome after radiation doses bridging hematopoietic (H-ARS) and gastrointestinal (GI-ARS) acute radiation syndrome: early hematological changes and systemic inflammatory response syndrome in minipig. Int J Radiat Biol. 2014;90:363–72. doi: 10.3109/09553002.2014.892226. [DOI] [PubMed] [Google Scholar]
- 28.Fliedner TM, Graessle D, Meineke V, Dorr H. Pathophysiological principles underlying the blood cell concentration responses used to assess the severity of effect after accidental whole-body radiation exposure: an essential basis for an evidence-based clinical triage. Exp Hematol. 2007;35:8–16. doi: 10.1016/j.exphem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 29.MacNaughton WK. Review article: new insights into the pathogenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther. 2000;14:523–8. doi: 10.1046/j.1365-2036.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 30.Rubin P, Casarett GW. Clinical radiation pathology as applied to curative radiotherapy. Cancer. 1968;22:767–78. doi: 10.1002/1097-0142(196810)22:4<767::aid-cncr2820220412>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Goans RE, Flynn DF. Acute radiation syndrome in humans. In: Lenhart MK, Mickelson AB, editors. Medical consequences of radiological and nuclear weapons. Office of The Surgeon General United States Army; Falls Church: 2012. pp. 17–38. http://1.usa.gov/1rUXEEI. [Google Scholar]
- 32.Overgaard J, Matsui M. Effect of radiation on glucose absorption in the mouse jejunum in vivo. Radiother Oncol. 1990;18:71–7. doi: 10.1016/0167-8140(90)90024-q. [DOI] [PubMed] [Google Scholar]
- 33.Juby LD, Dixon MF, Axon AT. Abnormal intestinal permeability and jejunal morphometry. J Clin Pathol. 1987;40:714–8. doi: 10.1136/jcp.40.7.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunter-Smith PJ. Gamma radiation affects active electrolyte transport by rabbit ileum. II. Correlation of alanine and theophylline response with morphology. Radiat Res. 1989;117:419–32. [PubMed] [Google Scholar]
- 35.Kirichenko AV, Mason KA, Straume M, Teates CD, Rich TA. Nuclear scintigraphic assessment of radiation-induced intestinal dysfunction. Radiat Res. 2000;153:164–72. doi: 10.1667/0033-7587(2000)153[0164:nsaori]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.West GB, Woodruff WH, Brown JH. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci U S A. 2002;99:S2473–8. doi: 10.1073/pnas.012579799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Office of New Drugs; Rockville, MD: 2005. http://1.usa.gov/24KS4D7. [Google Scholar]
- 38.Michaelson SM, Odland LT. Relationship between metabolic rate and recovery from radiation injury. Radiat Res. 1962;16:281–5. [PubMed] [Google Scholar]