Abstract
Genistein, an estrogenic, soy-derived isoflavone, may play a protective role against hormone-related cancers. We have reported that a high concentration of genistein inhibits cell proliferation and induces apoptosis in human uterine smooth muscle cells, but not in leiomyoma (fibroid) cells. To better understand the differential cell death responses of normal and tumor cells to a high concentration of genistein, we treated uterine smooth muscle cells and uterine leiomyoma cells with 50 μg/ml of genistein for 72 h and 168 h, and assessed for mediators of apoptosis, cytotoxicity and autophagy. We found that leiomyoma cells had increased protection from apoptosis by expressing an increased ratio of Bcl-2: bak at 72 h and 168 h; however, in smooth muscle cells, the Bcl-2: bak ratio was decreased at 72 h, but significantly rebounded by 168 h. The apoptosis extrinsic factors, Fas ligand and Fas receptor, were highly expressed in uterine smooth muscle cells following genistein treatment at both time points as evidenced by confocal microscopy. This was not seen in the uterine leiomyoma cells; however, cytotoxicity as indicated by elevated lactate dehydrogenase levels was significantly enhanced at 168 h. Increased immunoexpression of an autophagy/autophagosome marker was also observed in the leiomyoma cells, although minimally present in smooth muscle cells at 72 h. Ultrastructurally, there was evidence of autophagic vacuoles in the leiomyoma cells; whereas, the normal smooth muscle cells showed nuclear fragmentation indicative of apoptosis. In summary, our data show differential cell death pathways induced by genistein in tumor and normal uterine smooth muscle cells, and suggest novel cell death pathways that can be targeted for preventive and intervention strategies for inhibiting fibroid tumor cell growth in vivo.
Keywords: Autophagy, Cell death, Concentration, Genistein, Uterine leiomyoma cells
Introduction
Uterine fibroids (leiomyomas or myomas) are benign tumors that arise from the smooth muscle cells of the uterus, and generally occur in women 30-50 years of age [1,2]. These tumors clinically affect approximately 25% of all women in the United States, with African American women having a higher risk of development of fibroids compared to Caucasian women [3]. Uterine fibroids are also prevalent worldwide affecting women in Europe [4], Africa [5], Asia [6] and South America [7]. Fibroids can be asymptomatic or symptomatic depending on the tumor size and location in the uterus, with common symptoms such as menorrhagia and pelvic pressure. Additionally, fibroids significantly impact fertility and pregnancy, and have been associated with obstetric complications, which cause a significant economic burden [8]. It is been estimated that uterine fibroids cost the United States $5.9-34.4 billion annually [8].
Although the etiology of fibroids is unknown, studies have shown that they are hormonally dependent similar to breast and ovarian cancer. Also, both estrogen and progesterone appear to promote the development and growth of fibroids [1]. Genistein, an isoflavone naturally found in soy products, is an estrogenic compound that has been studied extensively due to its possible protective role in tumor growth in cancer [9,10]. In the past decade or so, epidemiological evidence supports the association of the lower incidence of hormone-related cancers such as breast, prostate, and ovarian cancer in Asian countries with high consumption of soy products [9,11-15]. Furthermore, the emergence of genistein as a possible chemotherapeutic or anti-cancer agent is promising due its growth inhibitory or cell death effects in many cancer cell types [16-19] and its varied molecular mechanisms of cell death [20]. In vitro studies have shown that genistein, at concentrations >10 μM, inhibits cell proliferation [21], specific tyrosine-kinase proteins [22], and DNA topoisomerase II [23]; it induces G2/M cell cycle arrest and apoptosis in human breast cancer cells [24], and autophagy in ovarian cancer cells [25]. Also, some studies attribute the induction of apoptosis to caspase-3 activation and increased Bax protein expression as a result of high concentrations of genistein [24,26]. We have found that in uterine leiomyoma (UtLM) cells a high concentration (50 μg/ml; 185 μM) of genistein downregulates activin A, Smad 3 and other TGF-beta genes, all thought to be important in fibroid growth [27]. Additionally, another of our previous studies has shown that 50 μg/ml of genistein inhibits cell proliferation in both UtLM cells and uterine smooth muscle cells (UtSMC), and induces apoptosis in UtSMC, but not in UtLM cells [28]. These data suggest that genistein may activate a non-apoptotic pathway as part of its inhibitory and death effects in UtLM cells. The ability of genistein to induce differential cell death in UtLM and UtSMC, and understanding the mechanisms whereby genistein elicits its inhibitory/death effects in tumor versus normal cells is important in delineating novel pathways and identifying molecules that can be targeted for growth inhibition in clinical cases of fibroid tumors, and possibly other neoplasms.
Cell death can occur by different mechanisms [29]. One such mechanism is apoptosis which can be regulated by two pathways: (a) the intrinsic pathway, triggered by internal signals that affect mitochondrial outer membrane pro-apoptotic proteins such as bax, and bak or the anti-apoptotic Bcl-2; or (b) the extrinsic pathway, triggered by external signals that activate regulators such as the Fas ligand (Fas-L) and its receptor, Fas. Initiation of either of these pathways leads to the activation or cleavage of caspases which, in turn, activate other caspases resulting in apoptosis and phagocytosis of the cell [30,31]. Another cell death pathway is autophagy, a lysosomal degradation pathway, that plays an important role in cellular responses to environmental stressors, such as nutrient starvation that can ultimately lead to cellular adaptation and survival, or cell death [32,33]. Lastly, necrosis involves early plasma membrane rupture, inflammation, dilatation of the cytoplasmic organelles, and internalization of cells by a macropinocytotic mechanism [34,35]. Therefore, by identifying specific molecular mechanisms by which genistein, a phytoestrogen, elicits its inhibitory/death effects in UtLM cells, makes it possible to identify novel cell death pathways that can be targeted for preventive and intervention strategies for inhibiting tumor cell growth in vivo.
Materials and Methods
Culture of human uterine leiomyoma and myometrial cells
Human uterine leiomyoma (UtLM) cells (GM10964; Coriell Institute for Medical Research, Camden, NJ, USA) and uterine smooth muscle cells (UtSMC) (Clonetics Corporation, San Diego, CA, USA) were kept in a standard tissue culture incubator at 37°C, with 95% humidity and 5% carbon dioxide. The UtLM cells were routinely cultured in Minimum Essential Medium (MEM) Eagle (Sigma Chemical Company, St. Louis, MO, USA) as previously described [28]. The UtSMC were cultured in Smooth Muscle Cell Growth Media System (SmGM-2 BulletKit®) (Lonza, Basel, Switzerland) as previously reported [28]. The media were changed 24 h prior to genistein treatment to DMEM/F-12 (Sigma) phenol red free with charcoal/dextran treated fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT, USA) for both cell types. The cells were then treated with genistein (4’, 5, 7-Trihydroxyisoflavone; ≥ 98% purity by HPLC) (Sigma) every two days until day seven. Genistein was reconstituted using DMSO (≥ 99.7 % purity by HPLC) (Sigma) before it was diluted into the media.
Western blot analysis
Determination of bak and Bcl-2 protein expression in UtLM cells and UtSMC after treatment with 0 μg/ml (control; DMSO) and 50 μg/ml genistein (genistein-treated cells) for 72 h and 168 h was performed by Western blot analysis. Briefly, protein concentrations of cell lysates were determined by a BCA (bicinchoninic acid) protein assay kit (Pierce, Rockford, IL, USA). Cell lysates (40 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE) using a 4-12% NuPAGE Bis-Tris gel (Invitrogen, Carlsbad, CA, USA). The gels were run at 160 v for 1 h. Proteins were transferred from the gel to a 0.45 um polyvinylidene fluoride membrane (Millipore Billerica, MA, USA) for 1 h and 30 min at 88 v, and the membrane was blocked with 5% bovine serum albumin (BSA) (Sigma) for 2 h. Next, the membrane was probed with mouse monoclonal anti-Bcl-2 primary antibody (05-729, 1 μg/ml, Upstate, Lake Placid, NY, USA) in 5% BSA overnight. The membrane was subsequently stripped using western re-probe reagent (G-Biosciences, St. Louis, MO. USA) and re-probed separately with rabbit polyclonal anti-bak antibody (06-536, 0.5 μg/ml, Upstate) in 5% BSA overnight. At the end of each primary antibody incubation, the membrane was washed with Tris-Buffered Saline and Tween 20, and incubated with the appropriate secondary biotinylated horseradish peroxidase (HRP)-conjugated mouse IgG for Bcl-2 (NA931V, 1:5000, Amersham Bioscience, Arlington, IL, USA) or HRP-conjugated rabbit IgG for bak (NA934V, 1:5000, Amersham) for 1 h. The target proteins were visualized using an ECL detection kit (Amersham) and the densities of the bands were measured using a densitometer (Fluor 224 ChemTM8900, Alpha Innotech, San Leandro, CA, USA).
Immunofluorescence analysis
UtLM cells and UtSMC were grown in glass bottom microwell dishes (Mat-Tek Corporation Ashland, Massachusetts, USA) at a density of 5×104 cells/dish and incubated with genistein for 72 h and 168 h. The cells were then fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 30 min, permeabilized with 0.1% Triton X-100 (Sigma) for 10 min, and blocked with 5% BSA (Sigma) for 1 h. The cells were incubated overnight with a primary antibody, rabbit monoclonal anti-Fas Antibody (5709-1, 1:1000, Epitomics, Burlingame, CA, USA) or mouse monoclonal IgG1 anti-Fas-L (sc-73974, 1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA). This was followed by incubation with the secondary antibody Alexa Fluor 488 goat anti-rabbit (A11008, 1:4000, Molecular Probes, Eugene, Oregon, USA) or Alexa Fluor 594 donkey anti-mouse (A21203, 1:4000, Molecular Probes) for 1 h. Confocal images were taken on a Zeiss LSM510-UV meta (Carl Zeiss Inc, Oberkochen, Germany) using a Plan-Apochromat 40×/1.3 Oil DIC objective. The 488 nm laser line from a Krypton/Argon laser was used for excitation of the Alexa 488. A 505 nm-550 nm band pass emission filter was used to collect this image with a pinhole setting of 1.09 airy units. For the second channel, the 543 nm laser line from a Helium Neon laser was used for excitation of the Alexa 594. A 560 nm long pass emission filter was used to collect the images with a pinhole setting of 1 airy unit. All images were taken with a zoom of 1.0, a 2.51-μs pixel dwell time, a 0.44 μm pixel size, and with the line averaging set to 8. Quantitative measurements of green and red fluorescence intensity were done by using a MetaMorph Imaging System (v7.7.5.0; Molecular Devices, Center Valley, PA, USA). The average pixel intensity was corrected by subtracting the average pixel intensity of the background.
Lactase Dehydrogenase (LDH) Fluor metric Assay
UtLM cells and UtSMC were seeded at 5×103 cells/well and 4×103 cells/well, respectively, in 96-well culturing plates (Corning, Corning, NY, USA). The cells were incubated with genistein for 72 h and 168 h. After treatment, cytotoxicity of the cells was measured using CytoTox-ONE™ Homogeneous Membrane Integrity Assay (Promega, Madison, WI, USA). CytoTox-ONE is a fluorometric assay that measures the release of lactate dehydrogenase (LDH) in the media of cultured cells that have a damaged membrane. The cells were incubated with 100 μl of CytoTox-ONE reagent at room temperature for 5 min. Followed by the addition of 50 μl of Stop Solution to each well. Using a plate reader (Molecular Devices Corporation, Sunnyvale, CA, USA), the fluorescence was recorded at an excitation and emission wavelength of 560 nm and 590 nm, respectively.
Immunocytochemistry for autophagy
To determine if cells were undergoing autophagy, expression of MAP1LC3A, one of the proteins involved in the formation of autophagosomes, was determined [36,37]. The cells were grown on chamber slides (Lab-tek; Nalge Nunc International, Naperville, IL, USA) at a density of 10×104 cells/slide. At 24 h prior to genistein treatment the media were changed to DMEM/F-12 (Sigma) phenol red free with charcoal/dextran treated FBS (Hyclone) for both cell types. At 72 h, genistein-treated and control cells were used for immunostaining. The cells were washed with 1X automation buffer (Biomeda Corporation, Foster City, CA, USA), then fixed in 4% paraformaldehyde (Electron Microscopy Sciences), permeabilized with 0.2% triton X-100 (Sigma) and quenched with 0.3% hydrogen peroxide (Sigma), for 20 min each. Both cell types were blocked with 10% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in 5% BSA (Sigma) and an Avidin/Biotin blocking kit (Vector Laboratories, Burlingame, CA, USA) for 30 min. The cells were incubated overnight in the primary antibody, Authopagy APG8a (MAP1LC3A) rabbit polyclonal antibody (AP1801a, 1:200, Abgent, San Diego, CA, USA), in 5% BSA (Sigma), followed by a 30 min incubation with the secondary antibody, a biotinylated donkey anti-rabbit (711-065-152, 1:500, Jackson) that was subsequently labeled with the Vectastain Standard Elite ABC kit (Vector) for 30 min. Cells were incubated with 3,3’-diaminobenzidine (DAB) chromogen (DAKO, Carpinteria, CA, USA) for 6 min and counterstained for 30 sec using Mayer's hematoxylin (Poly Scientific, Bayshore, NY, USA). During the staining procedure, nonimmune rabbit serum (Jackson), at the same concentration as the primary antibody, served as the negative control. Lastly, the slides were scanned and images were captured at 40X using an [38] Aperio ScanScopeXT and the Aperio ImageScope software (v11.0.2.716; Aperio Technologies, Vista, CA, USA).
Electron microscopy
UtLM cells and UtSMC were treated with genistein for 72 h and 168 h in 75 cm2 cell culture flask. After treatment, the cells were washed once with PBS, dissociated from cell culture flask with 3 ml of 0.25% Trypsin-EDTA (1X) for 3 min, and then transferred with 4 ml of media into a 10 ml centrifuge tube. The cells were centrifuged at 1,000 rpm (or 120×g) for 5 min to form a pellet. The supernatant was removed from the pellet and the cells were resuspended by adding 1 ml of a Modified Karnovsky's solution (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer) and fixed for 5 min. The cells were then transferred to a 1.5 ml microcentrifuge tube and re-spun into a pellet. The cells remained in the fixative at 4°C until processing. A 4% agar was added to solidify the cell pellets, and pellets were cut into cubes ≤ 1 mm3. The pieces of agar-containing cell pellets were loaded into a Leica® Automatic Tissue Processor that began with 4 washes of 0.1M sodium phosphate buffer, followed by postfixation in 1% osmium tetroxide, dehydration through a series of graded alcohols, followed by 100% acetone, and finally embedded in 100% polybed 812® resin and placed in a 60°C-70°C oven for 24 h to 72 h for polymerization. Plastic embedded samples were thick sectioned (700 nm), stained with 1% Toluidine blue, and examined with a light microscope. UtLM and UtSMC were thin sectioned (≤ 90 nm), placed on 100 mesh copper grids, and then stained with uranyl acetate and lead citrate. The grids were then examined on a FEI Tecnai 120KV Transmission electron microscope (Hillsboro, Oregon).
Statistical analysis
The LDH data were not normally distributed, so Mann-Whitney tests were used to compare genistein treated cells to respective controls. For the Western blot data, normality could not be rejected for ratios of Bcl-2: bak, but sample sizes were small. Therefore, randomization t-tests were used to compare genistein treated cells to respective controls. The randomization t-test is a nonparametric test that evaluates the observed t-statistic relative to all possible allocations of the data to two groups [38]. P-values are one-sided and were considered significant at the 0.05 level.
Results
Analysis of protein expression of Bcl-2 and bak in genistein-treated UtLM cells and UtSMC
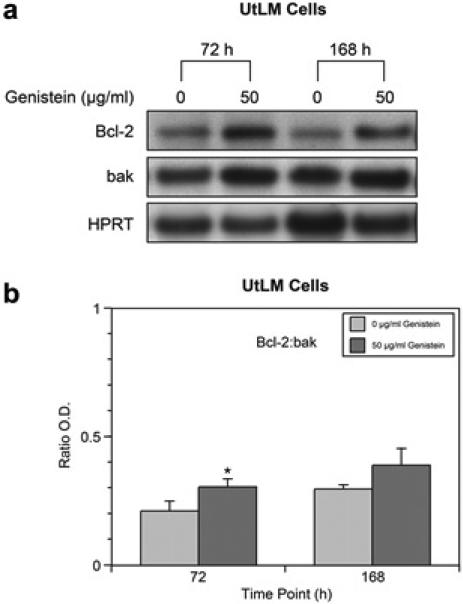
Western blot analysis of genistein-treated UtLM cells at 72 h and 168 h, showed an increase in Bcl-2 protein expression (Figure 1a). There were no significant changes in bak protein expression at both 72 h and 168 h, although levels were minimally increased at both time points. When Bcl-2: bak ratios were determined, we found that at 72 h the Bcl-2: bak ratio was significantly (p<0.05) increased in genistein-treated UtLM cells (Figure 1b). At 168 h, there was an increase in the Bcl-2: bak ratio; however, this increase was not statistically significant (Figure 1b). Hypoxanthine phosphoribosyl-transferase (HPRT) served as a loading control.
Figure 1.
Western blot analyses and ratio of Bcl-2 and bak in genistein-treated (50 μg/ml) UtLM cells and controls (0 μg/ml) at 72 h and/or 168 h. (a) Protein expression of bcl-2 and bak; (b) Bcl-2:bak ratios. The results are represented as mean ± SEM of three independent experiments. *P<0.05.
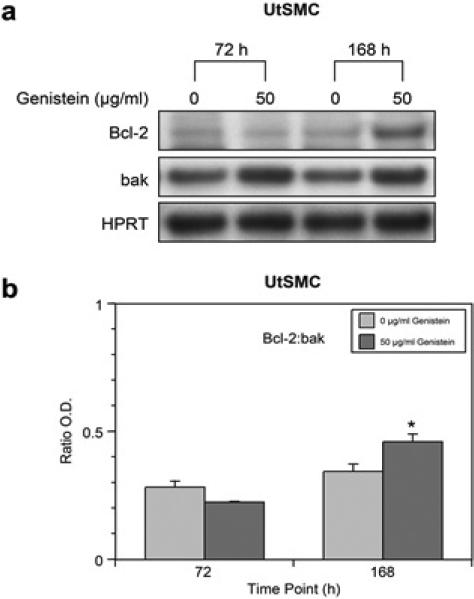
Western blot analysis of genistein-treated UtSMC showed no significant changes in Bcl-2 protein expression at 72 h; however, at 168 h the expression was increased (Figure 2a). Also, there was a minimal increase in bak protein expression at 72 h, but expression increased considerably by 168 h (Figure 2a). Evaluation of Bcl-2: bak ratios at 72 h showed a slight decrease; whereas, at 168 h the ratio was significantly (p<0.05) increased in genistein-treated UtSMC (Figure 2b). HPRT served as a loading control.
Figure 2.
Western blot analyses and ratio of Bcl-2 and bak in genistein-treated (50 μg/ml) UtLM cells and control (0 μ/ml) at 72 h and/or 168 h. (a) Protein expression of bcl-2 and bak (b) Bcl-2:bak ratios. The results are represented as mean ± SEM of three independent experiments. *P<0.05.
Expression and colocalization of the Fas receptor and its ligand, Fas-L in genistein-treated UtLM cells and UtSMC at 72 h and 168 h
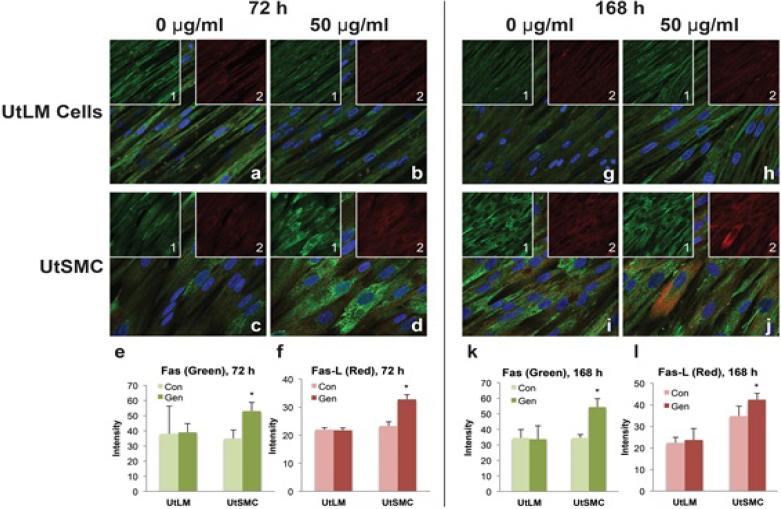
To assess involvement of the extrinsic apoptotic pathway in genistein induced cell death, colocalization and expression of the regulators Fas and Fas ligand (Fas-L) were determined. Control UtLM cells and UtSMC expressed both the Fas receptor and its ligand, Fas-L. The Fas receptor (green fluorescence) was expressed primarily in the cytoplasm with varying intensities in both control cell lines at 72 h and/or 168 h (Figures 3a, 3c, 3g and 3i). Fas-L (red fluorescence) was expressed in the plasma membrane, cytoplasm, and perinuclear regions of control UtSMC more so at 168 h versus 72 h, although, minimal expression was observed in the perinuclear regions of control UtLM cells at 72 h. Genistein-treated UtSMC (Figures 3d and 3j) showed upregulation and colocalization of Fas and Fas-L in the plasma membrane and cytoplasm at 72 h (Figure 3d), which was increased by 168 h (Figure 3j). In contrast, genistein-treated UtLM cells showed less expression and colocalization of Fas and FasL (Figures 3b and 3h) that did not differ significantly from the UtLM controls at 72 h and 168 h (Figures 3a and 3g). Quantitative measurements of fluorescence intensity at 72 h Fas (Figure 3e) and Fas-L (Figure 3f) were both expressed in genistein-treated and non-treated UtLM cells with no apparent difference in expression; however, there was a statistically significant increase in the fluorescence intensity of Fas (Figure 3e) and Fas-L (Figure 3f) in genistein-treated UtSMC compared to control UtSMC. Also, at 168 h the expression intensity patterns of Fas (Figure 3k) and Fas-L (Figure 3l) in non-treated and treated UtLM cells and UtSMC were similar to those observed in the respective cell types at 72 h (Figures 3e and 3f).
Figure 3.
Expression of the receptor, Fas and its ligand, Fas-L in genistein-treated (50 μg/ml) UtLM cells and UtSMC at 72 h (left panel) and 168 h (right panel). Control (0 μg/ml) cells (a, c, g, i) and genistein-treated cells (b, d, h, j). Fas=green fluorescence (inset 1); Fas-L=red fluorescence (inset 2); Fas and Fas-L=merged (a-j). Quantitative measurements of fluorescence intensity of Fas (e and k) and Fas-L (f and l). Nuclei shown as blue fluorescence by DAPI. The results are represented as mean±SEM of three independent experiments *P<0.05 versus control.
Release of lactase dehydrogenase (LDH) measurements in non-treated and genistein-treated UtLM cells and UtSMC at 72 h and 168 h
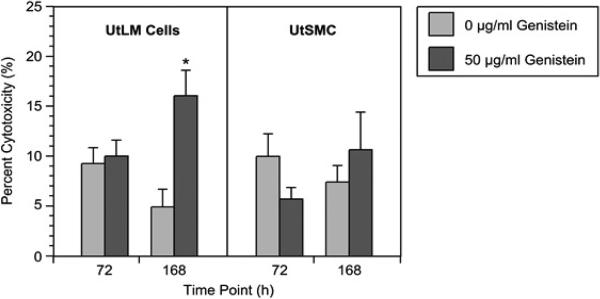
To test for cytotoxicity, media concentrations of LDH were determined in control and treated UtSMC and UtLM cells. At 72 h, genistein-treated UtLM cells showed a minimal, increase of LDH in media; however, by 168 h there was a significant (p<0.05) increase in LDH release (Figure 4). An initial decrease and then subsequent increase of LDH was observed in the media of genistein-treated UtSMC at 72 h and 168 h, respectively, although these changes were not significant (Figure 4). The increase of LDH observed in the media of genistein-treated UtLM cells was nearly doubled that of genistein-treated UtSMC at 168 h (Figure 4).
Figure 4.
Fluorometric measurements of the cytotoxicity in genistein-treated (50 μg/ml) and control (0 μg/ml) UtLM cells and UtSMC at 72 h and 168 h. Left panel UtLM cells and right panel UtSMC. The results are represented as mean±SEM of three or more independent experiments. *P<0.05.
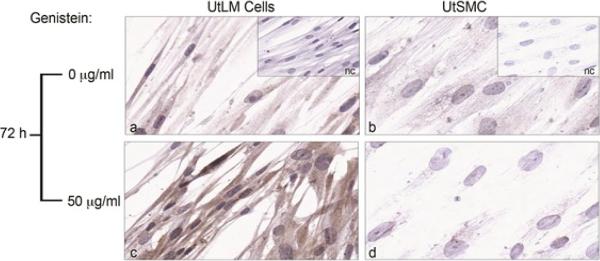
Expression of autophagy marker in genistein-treated UtLM cells and UtSMC at 72 h
The expression of the autophagy/autophagosome marker, MAP1LC3A, was minimally expressed in control UtLM cells (Figure 5a), but increased in the cytoplasm of genistein-treated UtLM cells (Figure 5c). However, control (Figure 5b) and genistein-treated UtSMC (Figure 5d) showed a minimal difference in the expression of MAP1LC3A, and expression was overall far less in both treated and non-treated UtSMC when compared to genistein–treated UtLM cells.
Figure 5.
Immunoexpression of an autophagy/autophagosome marker (MAP1LC3A) in genistein-treated (50 μg/ml) UtLM cells and UtSMC at 72 h. (a) Control UtLM cells (b) Control UtSMC. (c) Genistein-treated UtLM cells. (d) Genistein-treated UtSMC. Insets: Negative control (nc) for genistein-treated UtLM cells and UtSMC, respectively.
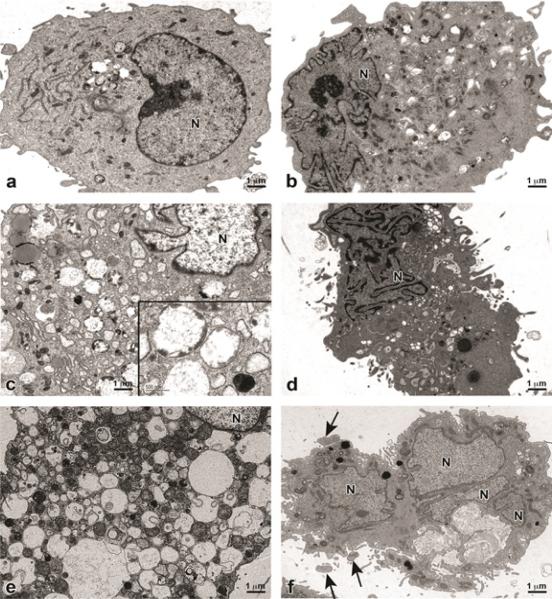
Transmission Electron Microscopy (TEM) of genistein-treated UtLM cells and UtSMC for 72 h and 168 h
Control UtLM Cells (Figure 6a) and UtSMC (Figure 6b) appear healthy with intact cytoplasmic membranes, organellar components, nuclei and nuclear membranes. At 72 h, genistein-treated UtLM cells began to accumulate various-sized membrane-bound cytoplasmic vacuoles, some of which contained cytoplasmic components and/or degenerated organelles and electron dense debris (Figure 6c). Genistein-treated UtSMC, at 72 h had convolution of the cell surface and nucleus, with peripheralized and focal areas of highly condensed chromatin in the nucleus (Figure 6d). At 168 h, surviving UtLM cells were severely vacuolated, with double (suggestive of autophagolysosomes) and single membraned vacuoles containing degenerated cellular organelles and other cytoplasmic debris (Figure 6e). The UtSMC at 168 h, showed nuclear fragmentation and membrane blebbing (Figure 6f).
Figure 6.
Transmission electron microscopy images of genistein-treated (50 μg/ml) UtLM cells and UtSMC at 72 h and 168 h. (a) Control UtLM cells (b) Control UtSMC. (c) Genistein-treated UtLM cells at 72 h. Inset: Higher magnification of double and single membraned vacuoles with cytoplasmic debris. (d) Genistein-treated UtSMC at 72 h. (e) Genistein-treated UtLM cells at 168 h. (f) Genistein-treated UtSMC at 168 h. Note: UtSMC have fragmented nucleus (N) and cellular blebbing (arrows). Image, scale bar=1 μm; Inset, scale bar=500 nm.
Discussion
Fibroids (uterine leiomyomas; myomas) are responsible for over 200,000 hysterectomies annually in reproductive-aged women in the United States [39], and are prevalent in women in Western Europe (including France, Germany, Italy, Spain, and the United Kingdom) [4], Africa [5], Asia [6] and South America [7]. Hysterectomy is the most common treatment modality for symptoms of bleeding and pelvic discomfort associated with these tumors. Other treatments for fibroids such as uterine artery embolization (UAE), magnetic resonance imaging-guided focused ultrasound surgery (MRgFUS) and GnRH are innovative, but their success in preclinical trials has been tenuous [40]. Consequently, the push for the development of drugs to treat uterine leiomyomas has increased over the past few years. Genistein is an estrogenic compound found in soy products that has been associated with beneficial health outcomes in humans, with hopeful prospects for breast cancer prevention and/or possible treatment [9,14]. In our previous studies, we found that 50 μg/ml genistein induced apoptosis and early and sustained caspase-3 activity in UtSMC [28]. However, in UtLM cells apoptosis was minimal and a late event, which suggested that apoptosis was not the major cause of cell death in these cells, and that a non-apoptotic pathway may be involved in the early inhibitory and/or death effects observed in UtLM cells following genistein treatment, although apoptosis was a major finding in UtSMC [28].
Apoptosis is regulated by many proteins including major proteins such as Bcl-2 [41], bax [41], bak [41], Fas [42] and Fas-L [43]. In this study we analyzed the effects of 50 μg/ml genistein on the expression of major pro-apoptotic (bak, Fas and Fas-L) and anti-apoptotic (Bcl-2) regulators in genistein-treated and non-treated UtLM cells and UtSMC. It has been reported that over expression of Bcl-2 functions as a suppressor of apoptosis and induces cell survival [44]; whereas, over expression of bax in relation to Bcl-2 determines the response of the cell to apoptotic stimuli [44,45]. Western blot analyses showed a decrease in bax protein expression after genistein treatment in both of our cells lines (data not shown) suggesting that bax may not play a major role in apoptosis for both cell lines in response to genistein; however, bak protein expression was increased in genistein-treated UtSMC compared to controls. In order to determine which of these proteins was predominant, a Bcl-2: bak protein ratio was measured. We found that the Bcl-2:bak ratio was increased in genistein-treated UtLM cells compared to controls at 72 h and 168 h, which suggests that Bcl-2 may play a role in the inhibition of apoptosis [28]. Our results for the UtLM cells are different from the inhibitory and/or death effects of 30 μM of genistein in MDA-MB-231 breast cancer cells in that genistein induced apoptosis by an upregulation of bax and a downregulation of Bcl-2 [45]. In contrast, Wang et al., demonstrated that treatment of MCF-7 cells with 17β-estradiol resulted in an increase of Bcl-2 mRNA levels and no changes in bax mRNA levels [46]. Our results are similar to Wang et al., in that treatment of UtLM cells with a weak estrogen, genistein, increased Bcl-2 expression and protected the cells from undergoing apoptosis. Conversely, the Bcl-2: bak ratios in genistein-treated UtSMC were decreased at 72 h, but increased at 168 h. These results suggest that the intrinsic pathway may be playing a dual role in these cells, in that at 72 h bak may be involved in inducing apoptosis, but at 168 h this effect is blocked by the increased expression of Bcl-2 and possibly a late attempt at induction of cell survival in genistein-treated UtSMC. Moore et al.,2007 reported that high concentrations of genistein inevitably induce apoptosis in UtSMC at 168 h as evidenced by cell cycle analysis/flow cytometry and increased caspase-3 activity [28]. These earlier findings and our current results, suggest that the apoptosis observed in genistein-treated UtSMC may be, in part, through the intrinsic pathway as an immediate and early response, but mainly by the extrinsic apoptotic pathway at later time points. Fas-L and its receptor Fas play an important role in inducing apoptosis via the extrinsic pathway [43]. Fas (APO-1, CD95) is a type I membrane protein and Fas ligand (Fas-L) is a type II membrane protein that belongs to the tumor necrosis factor (TNF) family [23]. In this study, treatmen of UtLM cells with genistein resulted in minimal change in Fas or Fas-L expression, whereas, genistein-treated UtSMC showed an increase in both of these proteins at both time points. Our results confirm that 50 μg/ml genistein induces apoptosis in UtSMC through both the intrinsic and extrinsic pathways.
Studies have also shown that genistein can induce cytotoxicity, inhibit glucose uptake and produce autophagy in ovarian cancer cells [25,47]. Cytotoxicity with loss of plasma membrane integrity in genistein-treated UtLM cells and UtSMC was measured by the release of lactase dehydrogenase (LDH) into the cell culture medium. LDH release was significantly increased in genistein-treated UtLM cells only, suggesting that cytotoxicity was a major feature of cell death in genistein-treated UtLM cells, and not in UtSMC. Our results are similar to Choi et al., in that 5 μM-100 μM genistein induced significant LDH release in the ovarian cancer cells, SK-OV-3 [47]. Additionally, we assessed autophagy which is a massive degradation pathway resulting in the uptake of proteins and organelles in the cells by autophagosomes, with localization of microtubules-associated light chain 3 (LC3) protein into autophagosomal membranes [36,37]. Increased autophagy is typically observed during nutrient deprivation and starvation, which plays an important role in cellular responses to environmental stressors that lead to cellular adaptation and survival, or cell death [32,33,48]. Also, autophagy can induce caspase activation and/or DNA fragmentation; however, this is usually a late event [36,49]. Using a marker for autophagy APG8a (MAP1LC3A), we demonstrated that 50 μg/ml genistein increased expression of the autophagy/autophagosome marker in the cytoplasm of the UtLM cells, but not to any great extent in the UtSMC. Gossner et al. demonstrated that genistein at 50 μM-100 μM induced apoptosis and cleavage of caspase-3 and caspase-9 in ovarian cancer cells, but it also induced a nonapoptotic cell death mechanism identified as autophagy. UtLM cells after treatment with genistein at both time points showed an increase and the presence of double membrane autophagic vacuoles or autophagosomes [50,51] in the cytoplasm. However, genistein treated UtSMC showed blebbing of the cell membranes; peripheral chromatin condensation in nuclei, nuclear fragmentation and cell shrinkage compared to the non-treated UtSMC, which are characteristic ultrastructural features of apoptosis [51,52].
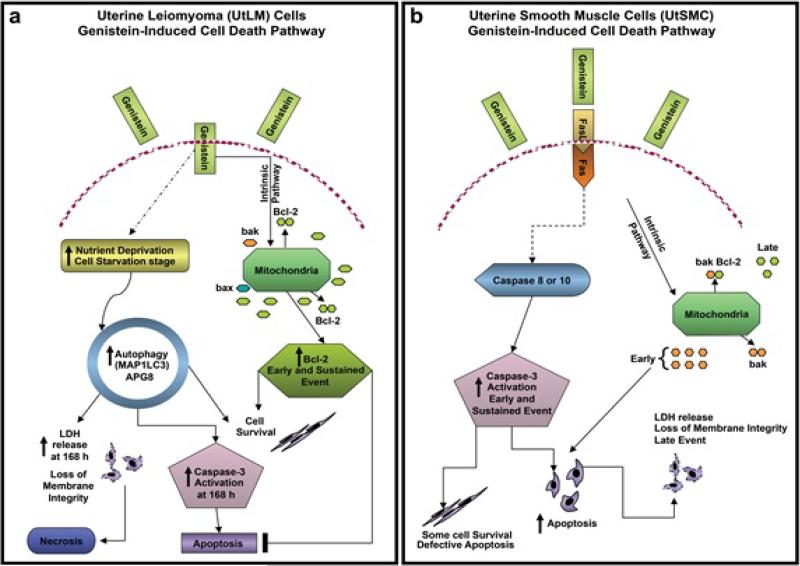
In conclusion, the inhibitory and/or cell death effects of genistein occurred in both UtLM cells and UtSMC, but the mechanisms of cell death appear to be different as shown in Figure 7. In UtSMC, genistein induced apoptosis by stimulating the extrinsic apoptotic pathway, via Fas-L and its receptor Fas and through activation of caspase-3, and possibly other caspases that resulted in the stimulation of the apoptotic extrinsic pathway. In contrast, in UtLM tumor cells, genistein's inhibitory and/or death effects were mostly through autophagy, initially. It could be speculated that in the UtLM cells genistein's effects on the intrinsic apoptotic pathway may have been blocked by inducing increased Bcl-2 expression, in an attempt towards cell survival leading to conservation of energy by self-digestion (autophagy) to provide an alternative energy source. On the other hand autophagy, apoptosis and necrosis are different forms of cell death, but as a late event autophagy can cause caspase activation and therefore also induce DNA fragmentation (apoptosis). Additionally, inhibition of specific proteins involved in autophagy or a depletion of external resources (nutrients or organelles) of the cells can cause a switch from autophagy to necrosis, leading to cytotoxicity and the release of LDH, similar to what was observed at 168 h in the UtLM cells in this study. These findings help to underscore the differential effects of exogenous estrogens on normal and tumor cells and help to delineate cell death regulatory proteins and pathways that may be strategically manipulated for the nonsurgical treatment of clinical cases of fibroids.
Figure 7.
Summary of gensitein-induced cell death. (a) In UtLM cells, genistein's inhibitory and/or death effects were mostly through autophagy. It could be speculated that genistein may have blocked the intrinsic apoptotic pathway in UtLM cells by inducing increased expression of Bcl-2 leading to starvation and self-digestion (autophagy) and cell survival. As a late event, autophagy can cause caspase activation and DNA fragmentation (apoptosis). Alternatively, inhibition of proteins involved in autophagy or a depletion of resources (nutrients or organelles) of the cell can cause a switch from autophagy to necrosis, leading to cytotoxicity and the release of lactate dehydrogenase (LDH). (b) In UtSMC, genistein induced apoptosis by primarily stimulating the extrinsic apoptotic pathway, via Fas-L and its receptor Fas with activation of caspases and induction of apoptosis. Also, initially the Bcl-2: bak ratios in genistein-treated UtSMC were decreased at 72 h (early event) due to elevated expression of the proapoptotic mediator, bak; however, the Bcl-2: bak ratio was increased at 168 h (late event) by incremental expression of Bcl-2. These findings suggest that the intrinsic pathway may be playing a dual role in UtSMC after genistein treatment; in that, bak may be involved in inducing apoptosis as an early event, whereas, this effect is abrogated by increased expression of Bcl-2 as a late event, which may be a last attempt at induction of cell survival in genistein-treated UtSMC.
Acknowledgements
The authors would like to thank Drs. Wendy Jefferson and Tonia Hermon for their critical review of this manuscript, and Dr. Connie Cummings for her expert technical assistance with capturing the electron microscopy images. This research was supported by the Intramural Research Program of the NIH, NIEHS and DNTP.
Footnotes
Citation:
Castro L, Gao X, Moore AB, Yu L, Di X, et al. (2016) A High Concentration of Genistein Induces Cell Death in Human Uterine Leiomyoma Cells by Autophagy. Expert Opin Environ Biol. S1.
References
- 1.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds A. Diagnosis and management of uterine fibroids. Radiol Technol. 2007;79:157–178. [PubMed] [Google Scholar]
- 3.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 4.Downes E, Sikirica V, Gilabert-Estelles J, Bolge SC, Dodd SL, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol. 2010;152:96–102. doi: 10.1016/j.ejogrb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Emembolu JO. Uterine fibromyomata: presentation and management in northern Nigeria. Int J Gynaecol Obstet. 1987;25:413–416. doi: 10.1016/0020-7292(87)90349-3. [DOI] [PubMed] [Google Scholar]
- 6.Sato F, Mori M, Nishi M, Kudo R, Miyake H. Familial aggregation of uterine myomas in Japanese women. J Epidemiol. 2002;12:249–253. doi: 10.2188/jea.12.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldblum PJ, Wheeless A, Trujillo V, Guzman S, Halpern V, et al. Pelvic surgery and hospitalization among Chilean women after nonsurgical sterilization with quinacrine pellets between 1977 and 1989. Contraception. 2012;86:106–109. doi: 10.1016/j.contraception.2011.11.072. [DOI] [PubMed] [Google Scholar]
- 8.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar FH, Li Y. The role of isoflavones in cancer chemoprevention. Front Biosci. 2004;9:2714–2724. doi: 10.2741/1430. [DOI] [PubMed] [Google Scholar]
- 10.Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adlercreutz H. Phytoestrogens: epidemiology and a possible role in cancer protection. Environ Health Perspect 103 Suppl. 1995;7:103–112. doi: 10.1289/ehp.95103s7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AH, Su D, Pasalich M, Tang L, Binns CW, et al. Soy and isoflavone intake associated with reduced risk of ovarian cancer in southern Chinese women. Nutr Res. 2014;34:302–307. doi: 10.1016/j.nutres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, et al. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12:665–668. [PubMed] [Google Scholar]
- 14.Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–3350. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]
- 15.Zhu YY, Zhou L, Jiao SC, Xu LZ. Relationship between soy food intake and breast cancer in China. Asian Pac J Cancer Prev. 2011;12:2837–2840. [PubMed] [Google Scholar]
- 16.Dastjerdi MN, Kavoosi F, Valiani A, Esfandiari E, Sanaei M, et al. Inhibitory Effect of Genistein on PLC/PRF5 Hepatocellular Carcinoma Cell Line. Int J Prev Med. 2015;6:54. doi: 10.4103/2008-7802.158914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian F, Bhuiyan M, Li YW, Wall N, Kraut M, et al. Genistein-induced G2-M arrest, p21WAF1 upregulation, and apoptosis in a non-small-cell lung cancer cell line. Nutr Cancer. 1998;31:184–191. doi: 10.1080/01635589809514701. [DOI] [PubMed] [Google Scholar]
- 18.Mizushina Y, Shiomi K, Kuriyama I, Takahashi Y, Yoshida H. Inhibitory effects of a major soy isoflavone, genistein, on human DNA topoisomerase II activity and cancer cell proliferation. Int J Oncol. 2013;43:1117–1124. doi: 10.3892/ijo.2013.2032. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Wang SS, Wong TC, Fung MC. Genistein promotes cell death of ethanol-stressed HeLa cells through the continuation of apoptosis or secondary necrosis. Cancer Cell Int. 2013;13:63. doi: 10.1186/1475-2867-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015;20:1531–1562. doi: 10.1007/s10495-015-1169-2. [DOI] [PubMed] [Google Scholar]
- 21.Chen WF, Huang MH, Tzang CH, Yang M, Wong MS. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim Biophys Acta. 2003;1638:187–196. doi: 10.1016/s0925-4439(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- 23.Markovits J, Linassier C, Fossé P, Couprie J, Pierre J, et al. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- 24.Leung LK, Wang TT. Bcl-2 is not reduced in the death of MCF-7 cells at low genistein concentration. J Nutr. 2000;130:2922–2926. doi: 10.1093/jn/130.12.2922. [DOI] [PubMed] [Google Scholar]
- 25.Gossner G, Choi M, Tan L, Fogoros S, Griffith KA, et al. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol. 2007;105:23–30. doi: 10.1016/j.ygyno.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa H, Yamamoto D, Kiyozuka Y, Tsuta K, Uemura Y, et al. Effects of genistein and synergistic action in combination with eicosapentaenoic acid on the growth of breast cancer cell lines. J Cancer Res Clin Oncol. 2000;126:448–454. [PubMed] [Google Scholar]
- 27.Di X, Andrews DM, Tucker CJ, Yu L, Moore AB, et al. A high concentration of genistein down-regulates activin A, Smad3 and other TGF-beta pathway genes in human uterine leiomyoma cells. Exp Mol Med. 2012;44:281–292. doi: 10.3858/emm.2012.44.4.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore AB, Castro L, Yu L, Zheng X, Di X, et al. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–2631. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taatjes DJ, Sobel BE, Budd RC. Morphological and cytochemical determination of cell death by apoptosis. Histochem Cell Biol. 2008;129:33–43. doi: 10.1007/s00418-007-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martel KM, Ko AC, Christman GM, Stribley JM. Apoptosis in human uterine leiomyomas. Semin Reprod Med. 2004;22:91–103. doi: 10.1055/s-2004-828615. [DOI] [PubMed] [Google Scholar]
- 31.Schafer ZT, Kornbluth S. The apoptosome: physiological, developmental, and pathological modes of regulation. Dev Cell. 2006;10:549–561. doi: 10.1016/j.devcel.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 36.Singletary K, Milner J. Diet, autophagy, and cancer: a review. Cancer Epidemiol. Biomarkers Prev. 2008;17:1596–610. doi: 10.1158/1055-9965.EPI-07-2917. [DOI] [PubMed] [Google Scholar]
- 37.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conover W. Practical Nonparametric Statistics. John Wiley & Sons Inc; New York, USA: 1971. [Google Scholar]
- 39.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 40.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 41.Zapata JM, Krajewska M, Krajewski S, Huang RP, Takayama S, et al. Expression of multiple apoptosis-regulatory genes in human breast cancer cell lines and primary tumors. Breast Cancer Res Treat. 1998;47:129–140. doi: 10.1023/a:1005940832123. [DOI] [PubMed] [Google Scholar]
- 42.Keane MM, Ettenberg SA, Lowrey GA, Russell EK, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res. 1996;56:4791–4798. [PubMed] [Google Scholar]
- 43.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 44.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 46.Wang TT, Phang JM. Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res. 1995;55:2487–2489. [PubMed] [Google Scholar]
- 47.Choi EJ, Kim T, Lee MS. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 2007;80:1403–1408. doi: 10.1016/j.lfs.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 48.Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy. 2007;3:28–31. doi: 10.4161/auto.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol. 2004;36:2405–2419. doi: 10.1016/j.biocel.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Eskelinen EL. Maturation of autophagic vacuoles in Mammalian cells. Autophagy. 2005;1:1–10. doi: 10.4161/auto.1.1.1270. [DOI] [PubMed] [Google Scholar]
- 51.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Krysko DV, Vanden Berghe T, D'Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]