Abstract
BACKGROUND
Successful peripheral blood stem cell transplantation (PBSCT) depends on the collection and infusion of adequate numbers of peripheral blood progenitor cells (PBPCs). Several predictors of PBPC yield are used currently, including white blood cell (WBC) count and CD34 analysis. This study evaluated the utility of the new automated hematopoietic progenitor cell count available on Sysmex XN hematology analyzers (XN-HPCs) in PBSCT.
STUDY DESIGN AND METHODS
The performance characteristics of XN-HPC, CD34+, and WBC analysis were compared using 107 matched peripheral blood and apheresis samples.
RESULTS
Good correlation was observed between XN-HPC and CD34+ cell counts in peripheral blood (r = 0.88; slope, 0.81) and apheresis collections (r = 0.91; slope, 0.89). Moreover, peripheral blood XN-HPC and CD34 analysis showed comparable ability to predict successful PBPC harvests (≥ 2 × 106 CD34+ cells/kg). At a cutoff of 20 × 106 progenitor cells/L, peripheral blood XN- HPC and CD34 analysis both showed negative predictive values (NPVs) of 100% and positive predictive values (PPVs) of 55.4 and 63%, respectively. Using an optimized cutoff of 38 × 106 progenitor cells/L, derived from receiver operating characteristic analysis, the PPV for XN-HPC and CD34 analysis increased to 71.4 and 78.9%, respectively, with relatively unchanged NPVs (XN-HPC 97.7%, CD34+ 98.0%). In contrast, the correlation between peripheral blood WBC and CD34 analysis was poor (r = 0.48; slope, 669.85), and the peripheral blood WBC count (cutoff, 10 × 109/L) was a poor predictor of PBPC harvest (NPV 60%, PPV 43.1%).
CONCLUSION
XN-HPC compares favorably with CD34 analysis and may be a surrogate for CD34 analysis to predict optimal timing of PBPC collections.
Peripheral blood stem cell transplantation (PBSCT) is used increasingly to treat patients who have undergone high-dose chemotherapy for hematologic or solid organ malignancies. Successful transplantation and engraftment of stem cells requires the infusion of an adequate number of progenitor cells.1-5 Stem cells are traditionally identified as CD34+ cells by flow cytometry. The minimum threshold value of CD34+ progenitor cells recommended to induce rapid and successful engraftment of hematopoietic recovery is at least 2 × 106 CD34+ cells/kg patient body weight.3-5
Hematopoietic progenitor cells (HPCs) are mobilized from the marrow into the peripheral blood using various regimens and are harvested subsequently by apheresis. Patient responses to stem cell mobilization regimens vary, however, and are influenced by a number of variables, including age, diagnosis, marrow involvement, and preceding chemotherapy.6-10 Thus, determining the optimal time for initiating peripheral blood stem cell collection is often challenging.
Historically, the peripheral blood white blood cell (WBC) count has been used as a marker of marrow response to stem cell mobilization, given the convenience of its availability as part of automated complete blood count analysis. However, a growing number of studies confirm that there is little correlation between the peripheral blood WBC count and the number of CD34+ stem cells in circulation.11 Thus, reliance on the WBC count to initiate apheresis may result in inadequate peripheral blood stem cell harvests and the need for an increased number of apheresis procedures. In contrast, peripheral blood CD34 analysis, performed before initiation of apheresis, correlates well with the number of CD34+ cells collected during apheresis.3-5,12-14 CD34 analysis, however, is a labor-intensive and time-consuming laboratory procedure, requiring highly specialized staff. This often creates delays and challenges in patient management.
Automated platforms have been developed to identify HPC on Sysmex SE and XE series analyzers.15-20 Analysis is rapid and inexpensive, and performed on the same instruments as are used for complete blood count and automated differential testing. HPCs are detected in the immature myeloid information channel of the analyzers, where all WBCs, except immature myeloid cells, are lysed by the action of surfactants-detergents on the lipid components of the cell membrane. The immature cells are analyzed using radiofrequency and direct current. The radiofrequency signal conveys information regarding cell complexity such as nuclear size and the presence of granules, whereas the direct current signal reflects the size or volume of the cell.
Using this technology, moderate correlations between HPC measurements and CD34+ cell counts have been observed.17-19 Although HPC appears to be a useful positive predictor of when to initiate apheresis to obtain desired CD34+ cell yields, HPC levels below predefined cutoffs have not reliably predicted poor CD34+ cell collections. The latter has limited the use of HPC as a surrogate for CD34 analysis in PBSCT. However, strategies for conserving laboratory resources have been proposed, which use HPCs to screen peripheral blood to perform CD34 analysis only on samples with HPC counts below a predetermined cutoff,16,21 thus preventing unsuccessful stem cell harvests while minimizing the risk of missing an adequate stem cell collection.
Recently, improved HPC detection (XN-HPC) was developed on a new-generation Sysmex analyzer (Sysmex XN). HPC detection was optimized based on improved sample hemolysis conditions and fluorescent staining. Moreover flow cytometry–based optical detection of XN-HPC was referenced to CD34+ cells.22 Preliminary data from 18 allogeneic and six autologous stem cell donors suggest a good correlation between the new XN-HPC analysis and CD34 analysis by flow cytometry.22 The goal of the present study was to evaluate XN-HPC testing in a larger clinical PBSCT setting. The results demonstrate a strong correlation between XN-HPC and CD34+ cell counts in preharvest peripheral blood and postharvest apheresis products and support the use of XN-HPC as a suitable surrogate for CD34 analysis to determine optimal timing for the collection of stem cells.
MATERIALS AND METHODS
A single-center, observational case study was performed from November 2013 through June 2014.
Samples
Peripheral blood and apheresis samples were obtained from the Clinical Laboratory Service at Memorial Sloan Kettering Cancer Center after all diagnostic testing had been completed. Samples were fully deidentified before enrollment into the study. A separate secure link to patient identity was maintained for medical record review. This study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center for the Protection of Human Subjects.
Peripheral blood samples (n = 107) were anticoagulated with K2EDTA. Samples from 99 patients undergoing autologous transplant and eight healthy allogeneic donors were included in the study. In addition, matched apheresis samples (n = 107), diluted 1:10 with RPMI 1640 containing l-glutamine and 25 mmol/L HEPES (Mediatech, Inc., Manassas VA), were obtained from the flow cytometry laboratory after CD34 analysis had been completed.
Patient population
Samples were from patients and healthy stem cell donors ranging in age from 16 months to 76 years (median age, 54 years). Approximately equal numbers of males and females (55% male, 45% female) were represented. The majority of patients carried a diagnosis of myeloma (approx. 43%) or lymphoma (approx. 42%). The remaining diagnoses included germ cell tumors, amyloidosis, β-thalassemia major, neuroblastoma, desmoid round cell tumor, and melanoma. Patients were mobilized with a variety of regimens including granulocyte–colony-stimulating factor (G-CSF), G-CSF with chemotherapy, or G-CSF with plerixafor (Mozobil, Sanofi US, Bridgewater, NJ). Apheresis products were collected using apheresis systems COBE Optia or COBE Spectra (Terumo BCT, Lakewood, CO).
Enumeration of nucleated cells in peripheral blood and apheresis products
WBC counts in peripheral blood and apheresis collections were determined using one of two hematology analyzers (Advia 2120, Siemens [Tarrytown, NY] for clinical testing; and Sysmex XN 1000, Sysmex Corporation of America [Lincolnshire, IL] for research purposes).
Enumeration of XN-HPCs and CD34+ cells
Peripheral blood and matched apheresis products were evaluated. HPC enumeration was performed using the Sysmex XN 1000. CD34 analysis was performed using ISHAGE guidelines.23
Statistical analysis
The correlation between XN-HPC and CD34 analysis was evaluated in preharvest peripheral blood samples and matched postharvest apheresis products. Linear regression models were fitted to correlate XN-HPCs with CD34+ cell counts using Passing and Bablok analysis.24 Correlations with WBC counts are presented for comparison.
In addition, the ability of preharvest peripheral blood XN-HPC and CD34+ cell counts to predict optimal timing to initiate apheresis to obtain an adequate CD34+ cell collection (≥2 × 106 CD34+ cells/kg5) was evaluated. Positive and negative predictive values (PPVs and NPVs, respectively)25,26 for XN-HPC and CD34 analysis were compared at different cutoffs. Receiver operating characteristic (ROC) statistics27,28 were used to determine the optimal cutoff for XN-HPC and CD34+ cell counts (≤38 × 106 HPC/L for both methods). In addition, XN-HPC and CD34 analyses were compared using a widely applied cutoff of fewer than 20 × 106 peripheral blood HPCs/L, suggested by Yu and colleagues,17 as well as several locally applied cutoff values. Comparisons were made with WBC analysis using a cutoff of 10 × 109 cells/L.29
RESULTS
Correlation between XN-HPC and CD34+ cell counts in peripheral blood
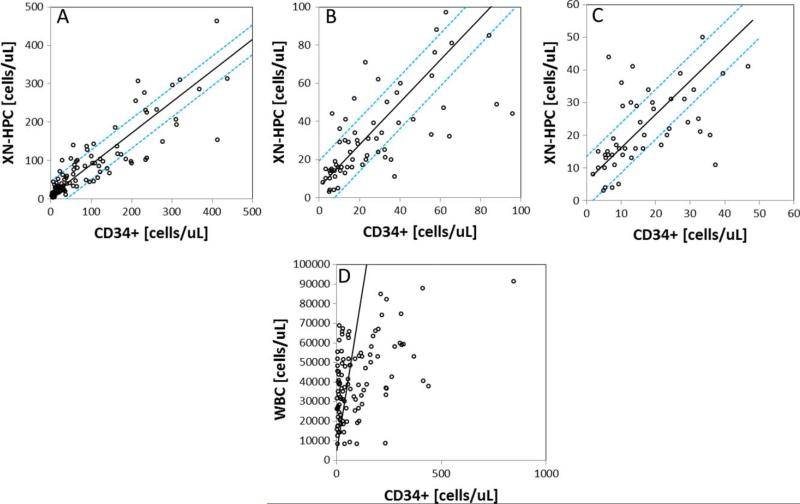
Comparison of CD34+ cell counts with XN-HPC and WBC counts, determined for 107 preapheresis peripheral blood samples, is shown in Fig. 1. The correlation between WBC and CD34+ cell counts was poor (Fig. 1D; r = 0.48; slope, 659.85), as reported previously.11 In contrast, XN-HPC and CD34+ cell counts correlated well (Fig. 1A; r = 0.88; slope, 0.815). Using an all plot regression analysis to identify outliers, 13 and 15% of samples were identified for which the XN-HPC count was greater than one standard deviation (SD) above or below the regression line, respectively, indicating discordantly high or low counts relative to the CD34+ cell count. Allowing for a 2 SD difference between XN-HPC and CD34+ cell counts, 4 and 6% of XN-HPCs fell above and below the expected regression line, respectively. Differences in XN-HPC and CD34+ cell counts occurred more frequently at high cell counts (>250 × 106 cells/L), where the clinical impact would likely be minimal.
Fig. 1.
Correlation between peripheral blood CD34 and XN-HPC (A-C) or WBC (D) analysis of 107 preharvest peripheral blood samples. Passing-Bablok24 linear regression analysis is shown. The dashed line represents ±1 SD from the regression line. (A) Correlation over the entire measured range; (B and C) correlations at CD34+ cell counts of fewer than 100 × 106/L and fewer than 50 × 106/L, respectively.
At CD34+ cell counts of less than 100 × 106/L (Fig. 1B) and 50 × 106/L (Fig. 1C), the correlation between XN-HPCs and CD34+ cells in peripheral blood was very good (Table 1). XN-HPC scattergrams for all outliers were reviewed. In all cases, the XN-HPC population was clearly identified, and no analytical errors were detected to explain the discrepant XN-HPC and CD34+ cell counts.
TABLE 1.
Correlation between XN-HPC and CD34+ cell counts in preharvest peripheral blood and apheresis products
| Comparison | Slope | Intercept | r value | Sample number |
|---|---|---|---|---|
| Peripheral blood | ||||
| XN-HPC and CD34 (all) | 0.81 | 7.84 | 0.88 | 107 |
| XN-HPC and CD34 (≤100 × 106 cells/L) | 1.11 | 5.51 | 0.70 | 64 |
| XN-HPC and CD34 (≤50 × 106 cells/L) | 1.03 | 5.88 | 0.55 | 48 |
| Apheresis product | ||||
| XN-HPC and CD34 (all) | 0.89 | 119.29 | 0.91 | 107 |
Correlation between XN-HPC and CD34+ cell counts in apheresis collections
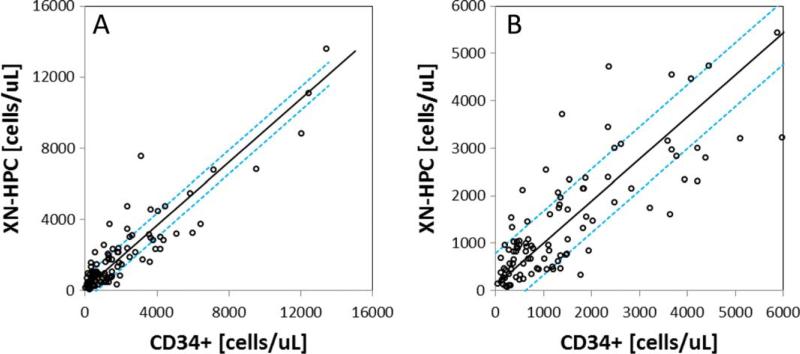
Correlation of XN-HPC and CD34+ cell counts in 107 apheresis collections from autologous and allogeneic stem cell donors showed excellent correlation (r = 0.91; slope, 0.89) over a wide range of CD34+ cell concentrations (Fig. 2). Using an all plot regression analysis to identify outliers, 13 and 16% of samples were identified for which the XN-HPC count differed from CD34+ cell counts by greater than 1 SD above or below the regression line. Allowing for a 2 SD difference between XN-HPC and CD34+ cell counts, 6 and 8% of XN-HPCs fell above and below the expected mean, respectively.
Fig. 2.
Correlation between CD34+ cell counts and XN-HPC analysis of 107 apheresis collections. Passing-Bablok24 linear regression analysis is shown. The dashed line represents ±1 SD from the regression line. (A) Correlation over the entire measured range; (B) correlation observed at low numbers of measured cells (≤6000 × 106 cells/L).
XN-HPC scattergrams were reviewed for all outliers. In many cases, an increased forward scatter intensity was noted, which resulted in a portion of the WBC population being included with the XN-HPC population. The reason for this phenomenon is not apparent. Because apheresis samples were received already diluted from the clinical laboratory, cellular changes affecting sample analysis cannot be ruled out.30
Correlation between peripheral blood HPC count and apheresis product CD34+ cell yield
The ability of preharvest peripheral blood XN-HPC counts to predict the ability to achieve a target CD34+ cell collection of more than 2 × 106 cells/kg is summarized in Table 2. Comparisons between performance characteristics of CD34+ and XN-HPC cell counts were made at several cutoffs. Data are shown for all apheresis samples (n = 107) and stratified by autologous (n = 99) and allogeneic donations (n = 8). Overall, the performance characteristics of XN-HPC and CD34+ cell counts were similar.
TABLE 2.
Comparison of performance characteristics of preharvest peripheral blood XN-HPC and CD34 analysis at selected cutoff points to predict successful apheresis meeting target CD34+ cell collections (≥2 × 106/kg)
| Donor type | Number | Cutoff (×106/L for XN-HPCs and CD34 cells | XN-HPC |
CD34 |
||
|---|---|---|---|---|---|---|
| PPV (%) | NPV (%) | PPV (%) | NPV (%) | |||
| All | 107 | 38* | 71.4 | 97.7 | 78.9 | 98.0 |
| Autologous | 99 | 68.4 | 100 | 76.5 | 100 | |
| Allogeneic | 8 | 100 | 50 | 100 | 50 | |
| All | 107 | 20† | 55.4 | 100 | 63.0 | 100 |
| Autologous | 99 | 52.0 | 100 | 59.1 | 100 | |
| Allogeneic | 8 | 87.5 | ‡ | 100 | 100 | |
| All | 107 | 15 | 50.0 | 100 | 57.5 | 100 |
| Autologous | 99 | 46.4 | 100 | 54.2 | 100 | |
| Allogeneic | 8 | 87.5 | ‡ | 87.5 | ‡ | |
| All | 107 | 10 | 45.1 | 100 | 51.7 | 100 |
| Autologous | 99 | 41.5 | 100 | 48.1 | 100 | |
| Allogeneic | 8 | 87.5 | ‡ | 87.5 | ‡ | |
| All | 107 | 5 | 44.2 | 100 | 44.2 | 100 |
| Autologous | 99 | 40.6 | 100 | 40.6 | 100 | |
| Allogeneic | 8 | 87.5 | ‡ | 87.5 | ‡ | |
Cutoff derived from ROC analysis.
Cutoff based on Armitage et al.14
Unable to calculated NPV due to absence of true-negative population.
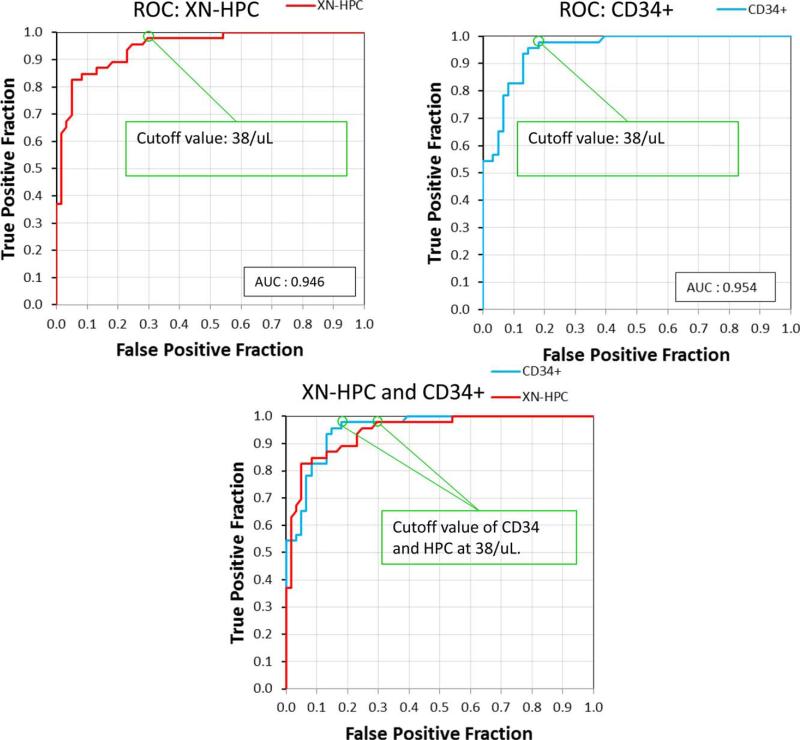
ROC analysis, comparing the ability of peripheral blood XN-HPC and CD34+ cell counts to predict an adequate stem cell harvest (≥2 × 106 CD34+ cells/kg) confirmed similar performance characteristics for both methods. The area under the curve for XN-HPC was 0.946, compared to 0.954 for CD34+ cell counts. ROC analysis of XN-HPC and CD34 analysis identified the same optimal cutoff (38 × 106 cells/L) for both methods (Fig. 3). At this cutoff (Table 2), the PPV of XN-HPC was 71.4% with a NPV of 97.7%. In comparison, PPV and NPV for CD34 analysis were 78.9 and 98%, respectively. In contrast, the peripheral blood WBC count, at a traditionally used cutoff of fewer than 10 × 109 WBCs/L,29 was a poor predictor of CD34+ cell yield (Table 3).
Fig. 3.
ROC curves,28 comparing the performance of peripheral blood XN-HPC (A) and CD34 analysis by flow cytometry (B) to predict successful stem cell collection (≥2 × 106 CD34+ cells/kg).5 The calculated optimal cutoff and area under the curve (AUC) for both methods are indicated. Merged ROC plots for XN-HPC and CD34+ cell counts (C) show similar test performance.
TABLE 3.
Performance characteristics of preharvest peripheral blood WBC analysis at selected cutoff points to predict successful apheresis meeting target CD34+ cell collections (≥2 × 106/kg)
| Donor type | Number | WBC Cutoff (×109/L) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| All | 107 | 10* | 43.1 | 60.0 |
| Autologous | 99 | 39.4 | 60.0 | |
| Allogeneic | 8 | 87.5 | † | |
| All | 107 | 5 | 43 | † |
| Autologous | 99 | |||
| Allogeneic | 8 |
WBC cutoff based on Elias et al.29
Unable to calculated NPV due to absence of true-negative population.
DISCUSSION
This study evaluated the performance characteristics of the new XN-HPC measurement available on Sysmex XN series analyzers in a large PBSCT setting. Previous comparisons of HPC analysis by Sysmex SE and XE series analyzers with CD34+ cell counting showed only moderate correlations,19,20 and the relationship between preharvest peripheral blood HPC counts and CD34+ yields in apheresis products was not strong.31 Although high peripheral blood HPC counts were useful for determining when to initiate stem cell collection, low HPC counts (<5 × 106/L) often underestimated peripheral blood CD34+ cells, and thus CD34 analysis was required.21 In marked contrast, results from this study demonstrate that the new automated XN-HPC analysis22 compares favorably with CD34 analysis by flow cytometry. Moreover, the ability of pre-harvest peripheral blood XN-HPC and CD34+ cell counts to predict postharvest CD34+ stem cell yield is comparable. Based on these findings, the new XN-HPC measurement represents a convenient surrogate for CD34+ cell counting for PBSCT applications.
Indeed, when the cell population corresponding to XN-HPCs was evaluated previously,22 the majority of HPCs overlapped with the CD34+ cell population identified by flow cytometry in both peripheral blood and peripheral blood stem cell collections. CD34+ cells were isolated using anti-CD34–conjugated magnetic beads and subjected subsequently for HPC analysis. The data demonstrated a single cell population around the HPC gate of the XN scattergram. Because of detergent effects on HPCs, HPCs cannot be isolated in an intact state for subsequent CD34 analysis.
Despite excellent correspondence between XN-HPC and CD34+ cell populations, these two cell populations are not entirely equivalent.32-35 Whereas cell surface CD34 expression has been used as a marker to detect and select hematopoietic stem cells, additional evidence supports the existence of early stem cell populations that are CD34−.33,34 During HPC analysis, CD34− hematopoietic progenitors appear in the same area of the Sysmex immature myeloid information channel as CD34+ cells, suggesting that the Sysmex HPC may include both CD34− and CD34+ progenitor cells.36 This is also consistent with the slight positive bias of XNHPC results compared to CD34 analysis in this study. Since the new automated XN-HPC analysis has been referenced to CD34+ cells, however, this bias is small and preharvest peripheral blood XN-HPC and CD34+cell counts appear to be equally predictive of adequate stem cell harvests.
The correlation between XN-HPC and CD34+ cell counts was particularly strong at low cell counts, supporting an excellent NPV. Review of false-positive preharvest XN-HPC cell counts (n = 18, using an XN-HPC cutoff of >38 × 106/L) with an unexpected low CD34+ cell recovery after harvest (<2 × 106/kg) failed to demonstrate any technical failure in XN-HPC enumeration. The peripheral blood XN-HPC count in one patient with a germ cell tumor was consistently false positive over four successive collections. Conceivably, the pretransplant patient characteristics including chemotherapy regimens6-10 may have affected stem cell characteristics and detection.
Indeed, the optimal timing and strategy for harvesting peripheral blood stem cells differs according to patients’ premobilization characteristics. Patients who have had exposure to alkylating agents and radiation therapy are known to have poor peripheral blood progenitor cell (PBPC) collections.7-10 In patients with diseases where treatment relies heavily on the use of alkylating agents and radiation therapy, applying a peripheral blood stem cell cutoff with the lowest false-negative rate, such as XNHPC or CD34+ cell counts of not more than 20 × 106/L would be appropriate, to avoid missing possibly adequate stem cell collections. At this cutoff, both XN-HPC and CD34 analysis demonstrated a NPV of 100%. In contrast, a different cutoff may be indicated for patients who are likely to demonstrate a good response to stem cell mobilization, based on treatment and diagnosis. In those cases, the use of a peripheral blood XN-HPC count with the highest PPV and the lowest false-positive rate, such as an XN- HPC cutoff of not more than 38 × 106/L would be indicated, to avoid harvests with poor CD34+ cell yields. Whereas this cutoff is higher than currently used by most centers, data from this study demonstrate similar performance characteristics for CD34+ cell and HPC cell counts at lower clinically utilized cutoffs.
Despite the known poor correlation between preharvest peripheral blood WBC counts and postharvest CD34+ cell yields,11,29 the WBC continues to be used to select mobilized patients for apheresis. This is due to the complexity of CD34 analysis by flow cytometry and the current inability to provide timely results. This study confirms the poor predictive value of peripheral blood WBC counts in the PBSCT setting and demonstrates that XN-HPC, performed on the same automated hematology analyzer (Sysmex XN-1000) as the hemogram and WBC differential, offers a significant advantage and may supplant the need for CD34 analysis.
The timing of apheresis is a critical issue for the efficient and cost-effective collection of sufficient peripheral blood stem cells for transplantation. Depending on the number of stem cells harvested, this may require one or more apheresis procedures. The ability to rapidly predict the likelihood of a successful stem cell harvest is a significant advantage both economically and in terms of patient management, patient satisfaction, and expenditure of resources by the apheresis facility and the processing laboratory. This study demonstrates similar performance characteristics for CD34 analysis and XN-HPC counting, supporting the conclusion that the new XN-HPC count would serve as an acceptable surrogate for CD34 analysis. Additional studies to validate current findings in a larger allogeneic donor population and to define XN-HPC pre-harvest cutoff values based on cancer diagnosis and/or pretransplant chemotherapy regimens, as well as to correlate XN-HPC counts in peripheral blood stem cell collections with engraftment information would allow defining optimal XN-HPC collections (number of XN-HPCs/kg) and to establish distinct preharvest peripheral blood XNHPC cell counts. These studies will further enhance the use of XN-HPC as an alternate to CD34 analysis.
ACKNOWLEDGMENTS
The authors thank Ms Nenita Francisco and Ms Kajal Kothadia for technical assistance.
This work was supported in part by a grant from Sysmex Corporation, Kobe, Japan.
ABBREVIATIONS
- NPV(s)
negative predictive value(s)
- PBSCT
peripheral blood stem cell transplantation
- PPV(s)
positive predictive value(s)
- ROC
receiver operating characteristic
- XN-HPC(s)
hematopoietic progenitor cells detected on the Sysmex XN hematology analyzer
Footnotes
CONFLICT OF INTEREST
EIP and PM have received research support from Sysmex Corporation, Kobe, Japan. CM and MSP have disclosed no conflicts of interest.
REFERENCES
- 1.Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–9. [PubMed] [Google Scholar]
- 2.Ho A, Gluck S, Germond C, et al. Optimal timing for collections of blood progenitor cells following induction chemo-therapy and granulocyte-macrophage colony-stimulating factor for autologous transplantation in advanced breast cancer. Leukemia. 1993;7:1738–46. [PubMed] [Google Scholar]
- 3.Bender JG, To LB, Williams S, et al. Defining a therapeutic dose of peripheral blood stem cells. J Hematother. 1992;1:329–34. doi: 10.1089/scd.1.1992.1.329. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman TM, Lee WJ, Bender JG, et al. Quantitative CD34 analysis may be used to guide peripheral blood stem cell harvests. Bone Marrow Transplant. 1995;15:439–44. [PubMed] [Google Scholar]
- 5.Mavroudis D, Read E, Cottler-Fox M, et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996;88:3223–9. [PubMed] [Google Scholar]
- 6.Bensinger WI, Appelbaum FR, Rowley S, et al. Factors that influence collection and engraftment of autologous peripheral blood stem cells. J Clin Oncol. 1995;13:2547–55. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 7.Jones HM, Jones SA, Watts MJ, et al. Development of a simplified single-apheresis approach for peripheral blood progenitor cell transplantation in previously treated patients with lymphoma. J Clin Oncol. 1994;12:1693–702. doi: 10.1200/JCO.1994.12.8.1693. [DOI] [PubMed] [Google Scholar]
- 8.Goldschmidt H, Hegenbart U, Wallmeier M, et al. Factors influencing collection of peripheral blood progenitor cells following high dose cyclophosphamide and granulocyte colony stimulating factor in patients with multiple myeloma. Br J Haematol. 1997;98:736–44. doi: 10.1046/j.1365-2141.1997.2783095.x. [DOI] [PubMed] [Google Scholar]
- 9.Haas R, Möhle R, Frühauf S, et al. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood. 1994;83:3787–94. [PubMed] [Google Scholar]
- 10.Dreger P, Klöss M, Petersen B, et al. Autologous progenitor cell transplantation: prior exposure to stem cell-toxic drugs determines yield and engraftment of peripheral blood progenitor cell but not of bone marrow grafts. Blood. 1995;86:3970–8. [PubMed] [Google Scholar]
- 11.Yu J, Leisenring W, Rowley SD, et al. The predictive value of white cell or CD34+ cell count in peripheral blood for timing of apheresis and maximizing yield. Transfusion. 1999;39:442–50. doi: 10.1046/j.1537-2995.1999.39050442.x. [DOI] [PubMed] [Google Scholar]
- 12.Elliott C, Samson DM, Armitage S, et al. When to harvest peripheral blood stem cells after mobilization therapy: prediction of CD34-positive cell yield by preceding day CD34-positive concentration in peripheral blood. J Clin Oncol. 1996;14:970–3. doi: 10.1200/JCO.1996.14.3.970. [DOI] [PubMed] [Google Scholar]
- 13.Schots R, Van Riet I, Damiaens S, et al. The absolute number of circulating CD34+ cells predicts the number of hematopoietic stem cells that can be collected by apheresis. Bone Marrow Transplant. 1996;17:509–15. [PubMed] [Google Scholar]
- 14.Armitage S, Hargreaves R, Samson D, et al. CD 34 counts to predict the adequate collection of peripheral blood progenitor cells. Bone Marrow Transplant. 1997;20:587–91. doi: 10.1038/sj.bmt.1700938. [DOI] [PubMed] [Google Scholar]
- 15.Takekawa K, Yamane T, Suzuki K, et al. Identification of hematopoietic stem cells by the SE-9000 automated hematology analyzer in peripheral blood stem cell harvest samples. Acta Hematol. 1997;98:54–5. doi: 10.1159/000203564. [DOI] [PubMed] [Google Scholar]
- 16.Pollard Y, Watts MJ, Grant D, et al. Use of the haemopoietic progenitor cell count of the Sysmex SE-9500 to refine apheresis timing of peripheral blood stem cells. Br J Haematol. 1999;106:538–44. doi: 10.1046/j.1365-2141.1999.01584.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Leisenring W, Fritschle W, et al. Enumeration of HPC in mobilized peripheral blood with the Sysmex SE9500 predicts final CD34+ cell yield in the apheresis collection. Bone Marrow Transplant. 2000;25:1157–64. doi: 10.1038/sj.bmt.1702406. [DOI] [PubMed] [Google Scholar]
- 18.Kraai R, Reymer AG, Brouwer-Mandema GG, et al. Hemopoietic stem and precursor cell analysis in umbilical cord blood using the Sysmex SE-9000 IMI channel. Cytometry. 2001;46:114–8. doi: 10.1002/cyto.1073. [DOI] [PubMed] [Google Scholar]
- 19.Vogel W, Kopp HG, Kanz L, et al. Correlations between hematopoietic progenitor cell counts as measured by Sysmex and CD34+ cell harvest yields following mobilization with different regimens. J Cancer Res Clin Oncol. 2002;128:380–4. doi: 10.1007/s00432-002-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang SH, Wang TF, Tsai HH, et al. Preharvest hematopoietic progenitor cell counts predict CD34+ cell yields in granulocyte-colony-stimulating factor mobilized peripheral blood stem cell harvest in healthy donors. Transfusion. 2010;50:1088–95. doi: 10.1111/j.1537-2995.2009.02546.x. [DOI] [PubMed] [Google Scholar]
- 21.Lefrère F, Zohar S, Beaudier S, et al. Evaluation of an algorithm based on peripheral blood hematopoietic progenitor cell and CD34+ cell concentrations to optimize peripheral blood progenitor cell collection by apheresis. Transfusion. 2007;47:1851–7. doi: 10.1111/j.1537-2995.2007.01407.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanosaki R, Kumazawa T, Yoshida A, et al. Novel and rapid enumeration method of peripheral blood stem cells using automated hematology analyzer. Int J Lab Hematol. 2014;36:521–30. doi: 10.1111/ijlh.12182. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland DR, Anderson L, Keeney M, et al. The ISHAGE guidelines for 34+ cell determination of flow cytometry. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [PMC][8817388] [DOI] [PubMed] [Google Scholar]
- 24.Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–20. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 25.Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ. 1994;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loong TW. Understanding sensitivity and specificity with the right side of the brain. BMJ. 2003;327:716–9. doi: 10.1136/bmj.327.7417.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyd JC. Mathematical tools for demonstrating the clinical usefulness of biochemical markers. Scand J Clin Lab Invest Suppl. 1997;227:46–63. [PubMed] [Google Scholar]
- 28.Eng J. ROC analysis: web-based calculator for ROC curves. Johns Hopkins University; Baltimore (MD): 2006. [Google Scholar]
- 29.Elias AD, Ayash L, Anderson KC, et al. Mobilization of peripheral blood progenitor cells by chemotherapy and granulocyte-macrophage colony-stimulating factor for hematologic support after high-dose intensification for breast cancer. Blood. 1992;79:3036–44. [PubMed] [Google Scholar]
- 30.Saigo K, Sugimoto T, Takeuchi S, et al. Estimation of stem cell fractions in peripheral blood stem cell harvest by using an SE-9000 hematology analyzer. Acta Haematol. 2000;103:157–61. doi: 10.1159/000041039. [DOI] [PubMed] [Google Scholar]
- 31.Yang SH, Wang TF, Tsai HH, et al. Preharvest hematopoietic progenitor cell counts predict CD 34+ cell yields in granulocyte-colony-stimulating factor-mobilized peripheral blood stem cell harvest in healthy donors. Transfusion. 2010;50:1088–95. doi: 10.1111/j.1537-2995.2009.02546.x. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet D. Normal and leukemic CD34 negative human hematopoietic cells. Rev Clin Exp Hematol. 2001;5:42–61. doi: 10.1046/j.1468-0734.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 33.Huss R. CD34− stem cells as the earliest precursors of hematopoietic progeny. Exp Hematol. 1998;26:1022–3. [PubMed] [Google Scholar]
- 34.Zanjani ED, Almeida-Porada G, Livingston AG, et al. Human bone marrow CD34− cells engraft in vivo and undergo multi-lineage expression that includes giving rise to CD34+ cells. Exp Hematol. 1998;26:353–60. [PubMed] [Google Scholar]
- 35.Huss R. Perspective on the morphology and biology of CD34-negative stem cells. J Hematother Stem Cell Res. 2000;9:783–93. doi: 10.1089/152581600750062228. [DOI] [PubMed] [Google Scholar]
- 36.Wang FS, Rowan RM, Creer M, et al. Detecting human CD34+ and CD34− hematopoietic stem and progenitor cells using a Sysmex automated hematology analyzer. Lab Hematol. 2004;10:200–5. [PubMed] [Google Scholar]