Abstract
Graphene is a multifunctional carbon nanomaterial and could be utilized to develop platform technologies for cancer therapies. Its surface can be covalently and noncovalently functionalized with anticancer drugs and functional groups that target cancer cells and tissue to improve treatment efficacies. Furthermore, its physicochemical properties can be harnessed to facilitate stimulus responsive therapeutics and drug delivery. This review article summarizes the recent literature specifically focused on development of graphene technologies to treat cancer. We will focus on advances at the interface of graphene based drug/gene delivery, photothermal/photodynamic therapy and combinations of these techniques. We also discuss the current understanding in cytocompatibility and biocompatibility issues related to graphene formulations and their implications pertinent to clinical cancer management.
Keywords: : biocompatibility, drug/gene loading, graphene, photodynamic therapy, photothermal therapy
One in four deaths in the USA is attributed to cancer; approximately 14 million new cancer cases are diagnosed each year worldwide, and the mean survival rate of all cancers between 2003 and 2009 was 68% [1,2]. The current methods of treatment for cancers include surgery, radiation therapy and chemotherapy. The challenges of these cancer treatments and therapies, with regards to their efficacies and side effects, are well documented [3]. Many times these shortcomings could elicit adverse effects on other organs or tissues (e.g., chemotherapeutic agents could be nephrotoxic) [4,5].
A wide variety of nanoparticles are being explored for cancer therapeutics [6–8]. Materials such as carbon, ceramic, polymers, lipids and metals have been used to prepare nanoparticle-based therapeutic systems, and these systems can be synthesized in a variety of configurations (e.g., spherical, tubular or branched structures) [8]. The physicochemical properties of various nanoparticles have been exploited for cancer therapy. For example, the electromagnetic absorption properties of gold and carbon nanoparticles have been exploited for targeted ablation of cancer cells or tissues by photothermal therapy [9]. Alternately, the nanoparticles have also been explored as ‘passive’ or ‘active’ drug delivery agents [10]. The passive diffusion or convection of nanoparticle through leaky tumor vasculature into the tumor is referred to as passive delivery. Nanoparticles, due to their sizes, gain access to the tumor interstitium and can retain in the tumor for extended times; this phenomenon is known as the enhanced permeability and retention (EPR) effect [11]. EPR has been proposed as the dominant phenomena responsible for the higher delivery efficacy of the nanoparticles by passive tumor uptake mechanisms. The active nanoparticle uptake mechanism is based on targeted delivery (via attachment of peptides or antibodies targeting specific tumor antigens).
Graphene is a 2D planar carbon nanostructure comprising of a one-atom thick, densely packed network of sp2-hybridized carbon atoms arranged in a hexagonal crystal lattice [12–14]. This nanomaterial has attracted a great deal of attention due to some of its unique nanoscopic properties [14], and shows potential for various material and biomedical science applications [12,15]. Its scientific significance and potential transformative impact have been recognized by the 2010 Nobel Prize in Physics [16]. Recent reports also predict that graphene may overtake carbon nanotubes in commercial applications [17]. Graphene nanoparticles, based on the manufacturing method, exhibit different structural features, physicochemical properties and biological responses [18]. Some examples of graphene nanoparticles include graphene nano-onions (spherically shaped concentric layers of graphene with both sp2 and sp3 hybridizations), graphene nanoribbons (ribbon-shaped graphene stacks synthesized by unzipping multiwalled carbon nanotubes) and graphene nanoplatelets (irregularly or disc-shaped multilayered graphene nanoparticles synthesized from graphite; also called graphene oxide [GO]) [18]. The physical and chemical properties of graphene make them particularly promising for a variety of imaging, therapeutic, and drug-delivery applications [15,19–21], and thus, it can be considered to be a multifunctional nanoparticle. There are now multiple reviews that document advances in the functionalization, physicochemical properties and material science applications of graphene [12]. Some recent reviews also focus on biomedical applications of graphene [21–27]. This review compliments these recent reviews, and focuses on graphene-based platforms for cancer therapeutics. We specifically reviewed published peer-reviewed literature on graphene-based technologies for chemo-, gene-, photodynamic or photothermal therapy. Furthermore, we discuss recent efforts to combine these therapeutic approaches and integrate them with the imaging capabilities of graphene. We also cover published articles on effects of graphene formulations in vitro on cancer cells and in vivo on animal models of cancer. We conclude by providing a future perspective and discussing the potential challenges graphene-based cancer technologies would need to address to translate into clinic.
Graphene platforms for anticancer drug delivery
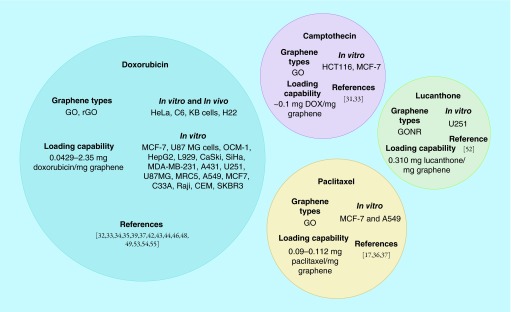
Analysis of our search results showed that the majority of the studies utilizing graphene for drug delivery is focused on cancer chemotherapeutics [24,28]. The hydrophobic chemical structure of graphene allows covalent and noncovalent tethering of various amphiphilic functionalities to improve aqueous dispersibility and facilitate cancer cell targeting. Graphene's high surface area-to-mass ratio and hydrophobic pi bond network allows incorporation of covalent and noncovalent functionalization strategies to increase loading of hydrophobic or aromatic anticancer drugs [29]. Below, we discuss some of salient points and Figure 1 summarizes the various drugs loaded on graphene for cancer therapeutic strategies.
Figure 1. . In vitro and in vivo cancer models, drug loading concentrations and graphene allotropes used in studies of doxorubicin, camptothecin, lucanthone and paclitaxel loaded onto graphene for cancer therapy.
Nontargeted cancer drug delivery
One of the earliest reports of graphene based cancer drug delivery was published by Liu et al. [30]. GO, covalently functionalized with polyethylene glycol (PEG) for improved water dispersibility, was loaded with camptothecin derivative SN38 (hydrophobic anticancer drug). The results showed 2–3 orders of magnitude increases in chemotherapeutic drug efficacy in colon cancer HCT-116 cell model when compared with irinotecan (water soluble prodrug of SN38) [30]. However, the loading SN38 on GO in an organic solvent (dimethyl sulfoxide) only yielded 0.1 g drug/g GO-PEG.
Yan et al. noncovalently loaded another hydrophobic cancer drug, doxorubicin (DOX), onto unmodified GO with loading efficiencies as high as 2.35 mg-DOX/mg-GO [31]. DOX is partially water soluble, thus, the drug loading protocol can be carried out in aqueous media without additional organic solvents such as dimethyl sulfoxide. The ability to load drugs in aqueous media is important to prevent residual organic solvent related toxicity. Other studies have shown that multiple drugs can be loaded on GO. Zhang et al. utilized GO loaded with DOX and camptothecin (CPT) to exploit both DNA intercalation (facilitated by DOX) and topoisomerase inhibition (induced by CPT) to elicit cytotoxicity in MCF-7 breast cancer cells at concentrations as low as 20 ng/ml CPT [32]. The results suggest drug combinations could improve therapeutic efficacy especially in cases of drug resistant tumors.
Other studies utilize graphene as a scaffold to append polymers and design composite nanoparticles with improved water dispersibility, ability to bind drugs or for controlled drug release due to changes in physiological conditions (pH or ionic strength). Zhou et al. reported synthesis of GO loaded with DOX and citraconic anhydride-functionalized poly(allylamine), a pH responsive charge-reversal polyelectrolyte, to bind and release DOX intracellularly at lower endosomal pH. They observed a 53% greater efficiency in 24 h with their formulation when the construct enters tumor cell endosomes (pH 5.0–6.5) [33]. Wei et al. covalently conjugated β-cyclodextrin to polyethylene imine (PEI) functionalized-GO as a carrier for DOX for pH modulated drug delivery [34]. Cyclodextrins are rings of glucose molecules which form a hydrophobic inner cavity that can interact with and increase loading capacity of other hydrophobic molecules and drugs such as DOX. Interestingly, the study found that over a 20 h timespan, at earlier timepoints DOX release was pH sensitive and at later timepoints salt sensitive. The effective drug release over the 20 h timespan was over 50% [34].
Jokar et al. reported albumin-conjugated GO onto which was loaded paclitaxel, an insoluble microtubulin stabilizer. The albumin-GO-paclitaxel complex also showed pH-dependent release of paclitaxel with 18% greater increase at pH = 5.4 compared with pH = 6.8 [16]. Xu et al. reported a study using covalently conjugated PEG-GO structures for delivering paclitaxel to A549 cells and MCF-7 cells in vitro [35]. They found that PEG-GO loaded with paclitaxel had approximately 50% cytotoxicity at paclitaxel concentrations of 20 nM while free paclitaxel at the same concentration had 82.5% viability with A549 cells [35]. Angelopoulou et al. reported noncovalent conjugation of polylactic acid-co-poly ethylene glycol to graphene oxide to also deliver paclitaxel to A549 cells in vitro [36]. The constructs could deliver 50% toxicity to A549 cells with only 25 ppm paclitaxel within 48 h [36]. Moore et al. reported in vitro delivery of paclitaxel loaded on graphene coated with polylactic acid to U-138 glioblastoma cells with efficacious doses as low as 24.6 nM of released drug [37]. They also reported that this graphene drug complex accumulates, after systemic injection, in U-138 glioblastoma intracranial xenografts induced in mice [37].
Targeted cancer drug delivery
Folic acid functionalization on GO (folic acid-GO) is one of the more common strategies employed for cancer cell targeting [38–41]. Many cancer cell types overexpress folic acid binding proteins on the cell surface, including ovarian, uterine, colon, meningeal, osteo- and lymphatic carcinomas [42]. Lin et al. showed that DOX, loaded onto folic acid functionalized GO, enhanced cytotoxicity toward OCM-1 human choroidal melanoma cells (<20% viability). At the same time the DOX-GO construct elicited significantly lesser cytotoxicity (˜40% viability) on ARPE-19 normal human retinal pigment epithelial cells after 24 h of incubation [41]. Furthermore, they showed the ability to load almost 100% of DOX to a concentration of 0.2 mg DOX/mg GO [41]. Similar cell-specific cytotoxic response was not noted for DOX loaded on GO without folic acid [41]. Zhao and Liu also synthesized folic acid-GO to deliver DOX [39]. Interestingly, even though they added folic acid to graphene, their results showed equivalent DOX loading capabilities as compared with only GO loaded with DOX (0.29–0.37 mg DOX/mg GO). The formulation selectively targeted and decreased cell viability of liver carcinoma HepG2 cells overexpressing folate receptors while not affecting healthy LSEC cells [39].
Targeting of other cancer cell surface receptors has also been reported. Hyaluronic acid (HA) has been shown to target breast, colon, basal cell, hepatic and renal cancer cells overexpressing transmembrane glycoprotein CD44 [43]. Miao et al. report in vitro and in vivo small animal results using cholesteryl HA conjugated to GO and noncovalently loaded with DOX formulations. In vitro studies were performed on KB epidermal carcinoma cells. DOX loaded onto GO conjugated with cholesteryl HA showed a 40.3% increase in cell death compared DOX loaded onto GO without cholesteryl HA conjugation. For in vivo studies, KB epidermal carcinoma cells were used to induce ectopic tumors in athymic mice. After subcutaneous injection, significant (14.1%) size reduction in tumor volume was observed after 24 days for cholesteryl HA conjugated GO loaded with DOX compared with GO without cholesteryl HA conjugation loaded with DOX [44]. Wu et al. reported small animal studies for adipic acid dihydrazide (ADH)-HA-GO loaded with DOX formulations, where adipic acid was used to introduce amino groups for tethering HA to GO [45]. Mice, with subcutaneous HeLa tumors, were intravenously injected with ADH-HA-GO loaded with DOX. By day 16 they showed approximately 12 and 17% greater tumor inhibition rates in mice treated with free DOX and GO loaded with DOX, respectively [45]. Song et al. also reported a method to make ADH-HA-GO loaded with DOX, and observed a pH responsive release of DOX at pH 5.3, endosomal cancer cell acidity [46]. This pH responsive release led to a 16–17% better tumor inhibition efficacy than only GO loaded DOX groups in H22 hepatic carcinoma cell tumors in mice [46].
Another widely targeted ligand for anticancer targeted delivery is transferrin (Tf), an iron-transporting serum glycoprotein that binds to transferrin receptors overexpressed on cancers of the bladder, breast, lung, lymphatic leukemia and glioma cells [47]. Liu et al. conjugated Tf to PEGylated GO (PEG-GO) nanoparticles and investigated their efficacy in mice with glioma tumors [48]. Mice intravenously administered with 3 mg DOX/kg animal weight of Tf-PEG-GO loaded with DOX showed 86% greater accumulation of DOX at the tumor site compared with PEG-GO loaded with DOX without Tf. Fourteen days after tumor growth, the relative tumor volumes were approximately 80.6% greater in mice injected with saline solution and 43.6% greater in PEG-GO loaded with DOX when compared with mice injected with the mice treated with Tf-PEG-GO loaded with DOX. Additionally, median survival time of the mice treated with Tf-PEG-GO loaded with DOX was 25 days, which is greater than mice treated with PEG-GO loaded with DOX and saline controls surviving only 4 and 8 days, respectively [49].
Wang et al. studied chlorotoxin based targeting of cancer cells, a toxin derived from Leiurus quinquestriatus which preferentially binds matrix metalloprotease-2 providing specificity to neuroectodermal tumors [50]. Wang et al. exclusively tested on glioma cells, demonstrating that their chlorotoxin coated GO loaded with DOX system, at 2.5 and 5.0 μg/ml DOX concentration, was more effective than only GO loaded with DOX [50].
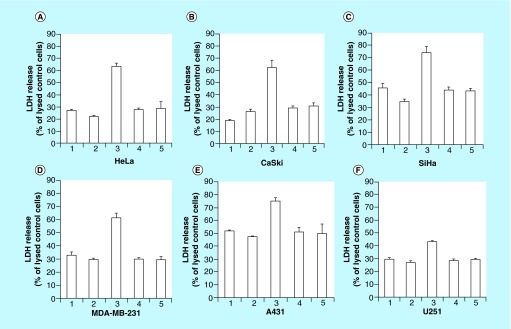
Until recently, majority of the graphene-based anticancer drug delivery agents work reported in literature are based on graphite-derived or chemically grown GO. In the past two years, reports have started emerging on the anticancer drug delivery capabilities of carbon nanotube-derived graphene oxide nanoribbons. Mullick-Chowdhury et al. observed that unlike GO nanoparticles, graphene oxide nanoribbons (GONR), noncovalently functionalized with PEG-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine) (GONR-PEG-DSPE) elicited cancer cell-specific cytotoxicity and drug delivery (Figure 2) [51,52]. They showed that cells overexpressing EGF receptors (EGFR) or with human papillomavirus genome (HPV; whether with regular or overexpressed EGFR) showed increased uptake of these nanoparticles and thus, enhanced drug delivery efficacy [53]. The cell-specific nanoparticle uptake was due to activation of EGFR by the GONR-PEG-DSPE. EGFR activation stimulated a macropinocytosis-like response giving rise to large amounts of GONR-PEG-DSPE uptake into the cells overexpressing EGFR. Cells with human papillomavirus genome, whether with regular or overexpressed EGFR, also showed greater cell uptake of the GONR-PEG-DSPE. This was attributed to E5 protein, a protein which is associated with EGFR activation, modulating and recycling activated EGFR back to the cell surface [54]. The results suggested that GONR-PEG-DSPE could be developed as targeted intracellular delivery agents without ligand functionalization for cancers that overexpress EGFR or mediated by HPV [54,55]. This type of delivery vehicle could potentially be beneficial for drug resistant cancer cells particularly tyrosine kinase inhibitors which target EGFR [56,57].
Figure 2. . Lactose dehydrogenase release (as a measure of cell toxicity) of HeLa, CaSki, SiHa, MDA-MB-231, A431 and U251 cancer cells when treated with GONR-PEG-DSPE at 50 μg/ml loaded with DOX.
Groups are identified as (A) untreated controls, (B) GONR-PEG-DSPE, (C) GONR-PEG-DSPE loaded with DOX, (D) Free DOX in PEG-DSPE (same concentration of DOX as group 3) and (E) Free DOX in PEG-DSPE (two-times the concentration of DOX as group 3).
Adapted with permission from [54] © Elsevier (2014).
Indeed in another study, Mullick-Chowdhury et al. loaded GONR-PEG-DSPE with lucanthone, an apurinic endonuclease-1 (APE-1) inhibitor which inhibits DNA repair and improves cytotoxic outcomes of ionizing radiation or other chemotherapeutics [58]. Lucanthone-GONR induced significantly greater cytotoxicity in glioblastoma multiforme cells (which overexpresses EGFR), while showing little to no toxicity to glial precursor cells (which do not over express EGFR) in an in vitro coculture model [52].
Other strategies for targeted delivery include the conjugation of antibodies to GO. Sun et al. reported one of the earliest studies of targeted GO drug delivery using anti-CD20 antibody to target Raji lymphoblasts in vitro. They report that tethering anti-CD20 to GO-PEG loaded with DOX selectively targets Raji CD20+ cancer cells at concentrations of DOX as low as 2 μg/ml [59]. Antibody attachment allows for highly selective binding and homing of drug delivery vehicles to target cells. However, antibody techniques face challenges in drug release upon antigen-binding, and intra-tumor delivery for solid tumors masses [60].
Other than antibodies, magnetic iron oxide nanoparticles conjugated with GO have also been explored for targeted drug delivery. Rather than passively targeting cancer cells, this method employs external magnetic fields to guide drug loaded nanoparticles to the site of a tumor. Yang et al. reported iron oxide conjugated to GO, by ionic interactions, with the ability to load DOX at concentrations of 1.08 mg DOX/mg iron oxide-GO [61]. In a follow-up study, the group reported the particles show superparamagnetic properties, with saturation magnetization of 8.57 emu/g, and after conjugating folic acid the particles selectively target SK3 breast cancer cells in vitro with graphene concentration of 10 μg/ml was demonstrated [62].
Gene delivery
Compared to drug delivery studies, relatively fewer studies have explored the efficacy of graphene nanoparticles for gene delivery applications. Most of these studies have involved GO. GO efficiently load aromatic drugs (e.g., chemotherapeutic drugs like doxorubicin, paclitaxel and lucanthone) via π-π interactions [63]. However, the same interactions are hindered between aromatic groups present in genetic material and GO. Both, nucleic acids (especially double stranded DNA) [64] and GO [65] carry a net negative charge causing electrostatic repulsion. To circumvent this issue, researchers have mainly used GO as a scaffold for functionalization of known positively charged transfecting agents such as PEI. PEI has been associated with high cytotoxicity [66,67], and hemolysis [68]. Thus, GO has mainly been employed to mitigate these adverse effects of PEI. Feng et al., Chen et al. and Kim et al. show that PEI functionalized GO graphene (GO-PEI) can be employed to bind plasmid DNA and this complex can transfect into cells [69–71]. Feng et al. noncovalently functionalized GO with branched PEI of two different molecular weights (1.2 kDa and 10 kDa). Plasmid DNA (enhanced green fluorescent protein, EGFP) delivery efficiency, measured qualitatively, was similar (˜0.1 mg plasmid/1 mg nanoparticle) for both PEI molecular weights in HeLa cells. However the GO-10 kDa PEI produced lower toxicity than the 10kDa PEI control at concentrations of approximately 100 μg/ml [69]. To further improve the transfection efficiency and open avenues for delivering other nucleic acids such as small interfering RNAs (siRNAs), Feng et al. employed a photo-thermal stimulation strategy [72]. Their study indicated that mild photothermal heating of GO-PEI increases membrane permeability of HeLa cells for delivering siRNA with increased transfection efficiencies of up to approximately 20% without eliciting a cytotoxic response [72].
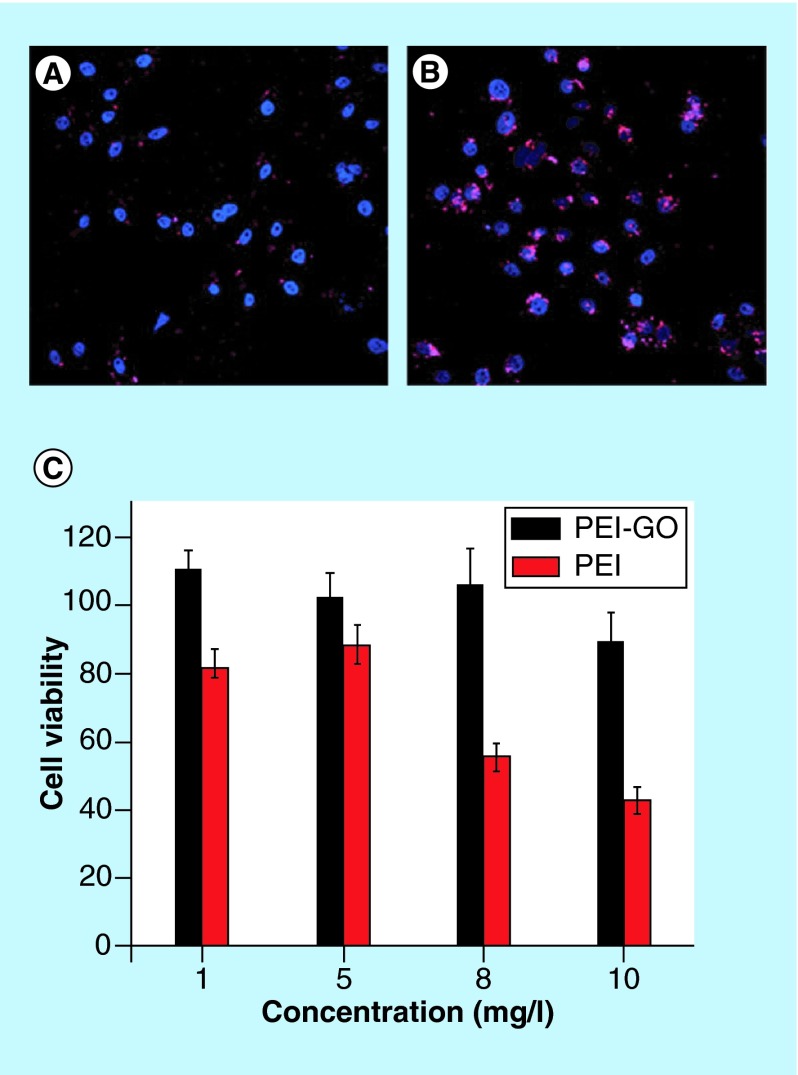
Chen et al. utilized higher molecular weight linear PEI (25 kDa) and showed GO-PEI complexes were approximately 20–50% less toxic than linear PEI alone in HeLa cells. The work also reported the complex could be delivered into the nucleus of the HeLa cells for transfection (loaded up to 1 mg plasmid/1 mg nanoparticle) (Figure 3) [70]. Ren et al. showed GO-branched PEI complexes could be used for nuclear localized gene delivery (at concentrations up to 1 mg DNA/1 mg nanoparticle) in HeLa cells with less inherent toxicity than PEI alone [73]. Furthermore, this study showed qualitatively, transfection with 25 kDa PEI was significantly greater than 10 kDa PEI and GO-10 kDa PEI. Transfection with GO-10 kDa PEI was significantly greater than only 10 kDa PEI by approximately 3–7% [73]. Kim et al. showed that GO functionalized with branched PEI can be used as a siRNA vector, with equivalent transfection capability of 25 kDa branched-PEI alone. Furthermore, they observed less cytotoxicity with the GO branched PEI construct than the control branched-PEI to HeLa and PC-3 cells [71].
Figure 3. .
PEI-GO loaded with plasmid DNA labeled with Cy3 in red in HeLa cells (blue nuclear DAPI staining) at (A) 4 h and (B) 24 h timepoints after transfection. (C) PEI-GO show less cytotoxicity to HeLa cells compared with branched-PEI controls.
Adapted with permission from [70] © The Royal Society of Chemistry (2011).
Zhang et al. demonstrated that sequential delivery of siRNA and chemotherapeutic drugs is possible using the GO-PEI complex [74]. Similarly, GO functionalized with PEI and poly(sodium 4-styrenesufonates) have been employed by Zhi et al. to codeliver doxorubicin and antimicroRNA-21 to overcome multidrug resistance in breast cancer cells [75]. Zhang et al. used dual functionalized graphene structures with PEG and PEI for transfection of plasmid DNA into Drosophila S2 cells [76]. PEG was used to increase the stability of graphene in physiological solutions [77]. The transfection efficiency for the Drosophila embryos was approximately 90% using this complex in comparison to approximately 30% produced in PEI alone. Yin et al. showed that PEG and PEI conjugated to GO can be used to transfect Stat3-specific siRNA into mouse for treatment of malignant melanoma resulting in significant regression of tumor growth and tumor weight as early as 18 days after administration [78].
GO have been functionalized with other polycations for gene delivery applications. Polyamidoamine (PAMAM) functionalized GO nanoparticles were used by Yang et al. to successfully deliver siRNA into HeLa cells [79]. Because of the highly positive surface charge on the PAMAM coated GO, the researchers were able to load Let-7g, a micro-RNA (miRNA) downregulated in many cancers which acts as a tumor suppressor, with up to 75% efficiency with an initial concentration of 0.2 nM of miRNA [79]. Gadolinium functionalized GO was recently used for delivery of Let-7g miRNA into glioblastoma cells for suppressing Ras oncogenes in the human genome [80]. The PAMAM and gadolinium, Gd3+, functionalization of the GO imparted a net positive charge that was exploited to load the negatively charged genetic material.
Hyperthermia & photodynamic therapy
The electromagnetic absorption of graphene has been harnessed for photodynamic therapy and photothermal therapy. Photodynamic therapy (PDT) employs photosensitizers that absorb incident light and generate free radicals that react with the local tissue in its periphery to further generate reactive oxygen species [81]. Photosensitizers have been covalently or noncovalently functionalized onto graphene nanoparticles. PDT studies have routinely employed macrocyclic porphyrin-based photosensitizers, including hemachrome and protoporphyrin, and have now expanded to chlorins, phthalocyanines and other macrocyclic porphin-like compounds [82]. Graphene nanoparticles have been explored to mitigate existing limitations of macrocyclic photosensitizers such as poor water solubility and tissue specificity [83]. GO has been noncovalently loaded with macrocyclic organic photosensitizers for PDT via weak π-π stacking and hydrophobic interactions. Yang et al. tethered Chlorin e6 (Ce6) to folic acid-GO for targeted delivery into MGC803 (human gastric cancer) cells. Their results showed less than 20% dark toxicity, or toxicity without light irradiation. After photo irradiation (λex = 632 nm) for 10 min, 90% cytotoxicity was observed with a 1:2 mass fraction of Ce6 to folic acid-GO [40]. Zhou et al. reported loading of hypocrellin-A, a hydrophobic nonporphyrin photosensitizer, on GO by noncovalent interactions at concentrations as high as 1 mg/ml. HeLa cells were treated with this complex showed little to no dark toxicity. After 1 min of photo irradiation, approximately 10% increase in cytotoxicity of HeLa cells was noted at drug concentrations of 1.65 μg/ml when compared with hypocrellin-A controls at the same concentration [84].
Metallic functionalities have also been investigated as a method to induce PDT in cancer cells. Zinc oxides have been shown to induce cancer cell death by ultraviolet-induced singlet oxygen (a radical) formation [85]. However, ultraviolet radiation has limited tissue penetration and induces DNA damage in a nonspecific manner [86]. Hu et al. demonstrated that folic acid-GO-zinc oxide conjugates eliminate over 80% of HeLa cells at concentrations of 75 μg/ml, predominately by activating apoptotic pathways under 15 min of visible light (48.6 J cm-2) irradiation [87].
Photothermal therapy (PTT) agents absorb electromagnetic energy and facilitate local significant increases in local temperatures (a.k.a hyperthermia) to ablate cells in their proximity [81]. Multiple studies have shown that graphene absorbs visible and near infrared radiation to elicit hyperthermic effects on cells and tissues [88,89]. Abdelsayed et al. report that approximately 16% of the excitation energy provided by a 532 nm laser was converted by the GO to heat deionized water, a process primarily attributed to the deoxygenation of GO [89]. Markovic et al. have compared the in vitro efficacy of GO and carbon nanotubes for PTT of cancer cells. They report, at various exposure times from 0.5 to 5 min, the concentration of nanoparticle required for IC50 values would be an order of magnitude less for GO (4.1 μM) compared with carbon nanotubes (49.3 μM). Also untreated controls showed no toxicity [90]. The greater efficiency was primarily attributed to better nanomaterial dispersions.
Surface chemistry may also contribute to the efficacy of graphene in PTT. In one study reduced GO sheets investigated for PTT resulted in sixfold increases in near infrared absorption compared with GO. This change in chemical characteristic leads to a ninefold increase in PTT induced toxicity against U87MG cells when functionalized with RGD peptide for cell uptake [91].
The first in vivo study exploring graphene-based PTT therapy was performed by Yang et al. [29]. In vivo fluorescence imaging of intravenously delivered PEG-graphene-Cy7 (a molecular fluorophore) showed high tumor uptake, by the EPR effect, while having low retention in the reticuloendothelial systems of tumor bearing mice intravenously injected with a 20 mg/kg dose [29].
Graphene and its derivatives have also been explored for radiofrequency-based ablation of cancer cells. Recently, Sasidharan et al. reported using carboxylic acid functionalized graphene for in vitro ablation of drug-resistant cancer cells using a radiofrequency electromagnetic radiation source [92]. Carboxylated-graphene was functionalized with transferrin to target cancer cells and stimulated with a 13.56 MHz radiofrequency power source. The transferrin-graphene complex was compared against single walled carbon nanotubes and 5 nm gold (Au) nanoparticles for their radiofrequency responsiveness and ablation capabilities. The graphene material outperformed both single walled carbon nanotubes and Au nanoparticles with approximately 300% greater thermal responsiveness. The graphene particles also showed increased toxicity in drug-resistant K562R cells at concentrations as low as 2.5 μg/ml while showing no toxicity to healthy BMMC cells [92]. This work opens avenues for PTT treatment of cancers at greater tissue depths.
Combination therapies & theranostics
PDT and PTT have also been combined for more effective cancer therapeutics. The photothermal effect can be utilized not only to heat and destroy cancer cells but also for increased cell permeability. Mild, low-power, photothermal heating to about 43°C has shown improved clathrin-dependent endocytosis in carbon nanotubes and graphitic structures [93]. Miao et al. recently synthesized PEG-GO-Ce6 and coloaded with DOX; evaluating its safety, tumor accumulation and efficacy in vivo [94]. At the day 28 timepoint, an approximately 31% volume reduction was noticed in both control groups (PEG-GO-Ce6 (PDT group) and PEG-GO loaded with DOX groups (chemotherapy group)). When combining PDT and chemotherapy, the PEG-GO-Ce6 loaded with DOX treated mice showed an even greater (73%) reduction in tumor volume [94].
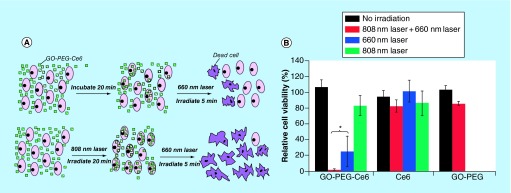
Tian et al. demonstrate improved efficacy of GO-PEG-Ce6 against KB cells when first pretreated with an 808 nm laser (photothermal permeabilized with a portion of dead cells) to allow GO-PEG-Ce6 uptake, followed by PDT (Figure 4) [95]. Unlike conventional hydrophobic photosensitizers, Sahu et al. utilized US FDA approved hydrophilic methylene blue as the photosensitizing agent with GO. Their study showed complete ablation of HeLa cell xenograft tumors in athymic mice by combined PDT and PTT effects without remission of cancer over a 15 day span. During the same time period, mice treated only with PDT or PTT exclusively, showed significant tumor regrowth, up to 30% in relative volume, compared with the PDT+PTT groups [96]. Interestingly, complete tumor regression, with both PTT and PDT combined, was accomplished at a lower dose and irradiation time (10 mg/kg, 3 min) than previous work with GO and PTT treatment only (20 mg/kg, 5 min) by Yang et al. [29].
Figure 4. .
(A) Top, KB cells treated with PDT after GO-PEG-Ce6 treatment. Bottom, KB cells pretreated for photothermal permeabilization followed by PDT. (B) Viability of KB cells treated with GO-PEG-Ce6, free Ce6, and GO-PEG without irradiation, with 660 nm laser for PDT, with 808 nm laser for PTT, and both 660 nm laser + 808 nm laser treatment.
Adapted with permission from [95] © American Chemical Society (2011).
Combinations of drugs and photothermal therapy employing graphene for synergistic effects have also been investigated. Known as chemo-photothermal therapy (CPTT), several systems have already been reported with gold [97] and silica [98] nanoparticles that deliver both PTT agents and drugs to tumors. The main cancer drug used in these CPTT strategies is DOX [93,99–103]. Zhang et al. explored GO-PEG loaded with DOX for CPTT based ablation of tumors in vivo [69]. The GO-PEG particles loaded with DOX used for CPTT resulted in complete tumor ablation, without recurrence for 30 days after treatment. Conversely, the groups treated with just DOX or PTT had tumor sizes between 60 and 90% of the control untreated groups [100]. Folic acid-GO loaded with DOX was assessed for CPTT by Qin et al. [104]. The results indicated that CPTT increased HeLa cell inhibition 20% more than folic acid-GO loaded with DOX and free DOX groups at 20 μg/ml concentrations [104].
Gene and drug delivery have also been combined for cancer therapy. Combining GO functionalized with chitosan for plasmid DNA delivery and loading cancer drug camptothecin has been demonstrated in one study [105]. By 24 h after treatment, Bao et al. have shown successful gene and drug delivery to eradicate HepG2 cells in concentrations between 10 and 100 μM with no toxicity in GO and chitosan controls [105].
The suitability of graphene-based agents for combined therapeutic and diagnostic (a.k.a. theranostics) has also been investigated. Graphene used for PDT, PTT, chemotherapy, gene delivery or any combination thereof can be further functionalized with bioimaging agents to also diagnose or track progress of treatment. Ma et al. synthesized GO-iron oxide nanocomposites for magnetically targeted PTT and T2 weighted MRI imaging of 4T1 murine breast tumors in BALB/c mice [101]. They report more than 50% cell death at 1 W/cm2 laser power for 5 min of treatment with the GO-iron oxide nanocomposites and no cell death in untreated controls. Furthermore, they also investigated magnetically-targeted DOX chemotherapy by introducing a magnetic field to attract the nanoparticle complexes to desired regions. They observed toxicity to cells at GO-iron oxide loaded with DOX concentrations of approximately 0.1 mg/l and no toxicity of the carrier at over 10 mg/l [101]. Along with PTT, with Sheng et al. utilized the inherent photoacoustic contrast from reduced GO for in vivo photoacoustic monitoring of subcutaneous injected MCF-7 breast cancer tumors in BALB/c mice [106]. The group studied the effect of PTT by calcein-AM (live cell) and propidium iodide (dead cell) staining. They observed increasing cell death from 0.01 mg/ml to 0.1 mg/ml reduced GO after 5 min of treatment at 1 W/cm2 treatment [106].
The effects of graphene formulations on cancer cells & animal models
A number of reviews summarizes the in vitro cytotoxicity and in vivo toxicity and biodistribution of graphene nanomaterials [23,28,107–110]. In this section, we provide a summary of the recent reports on graphene toxicology on cancer cells and animal models.
Graphene nanoparticles, depending on their chemical composition, synthesis method, external covalent or noncovalent functionalization, shape or size show diverse effects on cells and tissues [111]. GO-based formulations have been reported to elicit similar effects on different cancer cells. Zhou et al. reported effects of cancer cell migration on three different breast cancer cell lines (MDA-MB 231, MDA-MB-436 and SK-BR-3) when treated with 40 or 80 μg/ml of PEG-GO. They observed all breast cancer cells had attenuated cell migration and invasion capability due to obstructed mitochondrial phosphorylation and no similar effects on healthy mammary cells [112]. Further elaborating on previous work, Zhou et al. also reported a comprehensive evaluation of the migration and invasion capabilities of graphene treated MDA-MB-231 (human breast cancer cell line), PC3 (human prostate cancer cell line) and B16F10 cells (mouse melanoma cell line). With 20 μg/ml concentrations of GO, the metastatic capability of these cells reduced by disturbing electron transport chain activity in the mitochondria [113]. Jaworski et al. have reported the effect of graphene platelets on glioblastoma multiforme cells [114]. Human glioblastoma U87 and U118 cell lines were incubated with graphene platelets (diameter 450 nm to 1.5 μm) at concentrations between 5 and 100 μg/ml for 24 h. Results show that graphene is dose dependent toxic to glioma cells with approximately 50% toxicity at 100 μg/ml and activation of apoptosis (without the induction of necrosis) was observed in U118 cells [114].
Yuan et al. have investigated the cytotoxicity and distribution of three kinds of graphene quantum dot (GQD) functionalizations (NH2, COOH and CO-N(CH3)2) on human neural glioma C6 and A549 lung carcinoma cells [115]. Results show the absence of mortality and apoptosis or necrosis at all treatment concentrations (10–200 μg/ml) after 24 h for all three GQD groups. Furthermore, Raman spectroscopic analysis showed the intracellular accumulation of all three GQDs, nuclear translocation was absent.
Recently, Chng et al. reported a comparative study on the cytotoxicity of GONRs and GONPs [116]. In vitro cytotoxicity using human lung carcinoma (A549 cells) shows that GONRs exhibit a significantly higher cytotoxic response than GONPs over all concentrations (3–400 μg/ml). The increased cytotoxicity of GONRs was attributed to the presence of a greater amount of carbonyl groups (28.22% on GONRs vs 11.06% on GONPs) and the high aspect ratio (width × length of GONRs ˜310 × 5000 nm and GONPs ˜100 × 100 nm) of GONRs [116].
Mbeh et al. have reported the cytotoxicity of albumin functionalized GONRs against A549 cells revealing a dose-dependent cytotoxicity wherein albumin functionalized GONRs at concentrations <50 μg/ml did not exhibit significant cytotoxicity, whereas incubation of A549 cells with higher concentrations (100 μg/ml) resulted in loss of cell proliferation and induction of apoptosis [117].
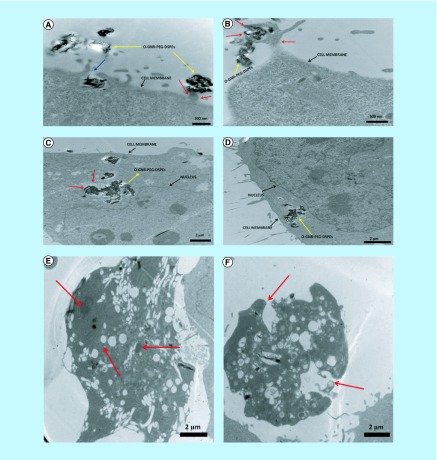
GONR-PEG-DSPE formulations have been reported to elicit cancer cell specific response. Mullick-Chowdhury et al. have reported the cytotoxicity of GONR-PEG-DSPE at various treatment doses (10–400 μg/ml) evaluated using three different cancer cell lines: HeLa, SKBR3 and MCF-7 [51]. Significant cell death (5–25% dead cells) was observed for HeLa cells at treatment concentrations of 10 μg/ml whereas approximately 100% cell viability was observed for other cell lines at similar treatment concentrations. The high cell death observed for HeLa cells was attributed to an increase in the intracellular uptake of GONRs, compared with other cells (Figure 5). The results suggested that GONR-PEG-DSPE exhibit heterogenous toxicity, which is dependent on the cell type under consideration.
Figure 5. . Representative TEM images of HeLa cells incubated with 20 μg/ml of PEG-DSPE dispersed GONRs for 3 h.
(A) Accumulation of GONRs toward cell periphery (blue arrows), (B) formation of cell elongation and protrusion for the internalization of GONR aggregates (red arrows), (C & D) GONR aggregated present inside large cytoplasmic vacuoles and (E) HeLa cells after exposure to 20 μg/ml of PEG-DSPE dispersed GONRs for 24 h. Red arrows in images (E & F) point toward swollen vacuoles and ruptured plasma membrane, respectively.
Adapted with permission from [53] © Elsevier (2013).
Processing and dispersing techniques used for graphene structures can also affect the size of the particles. Mullick Chowdhury et al. reported significant shortening of GONR as a function of probe sonication for 1, 5 and 10 min leading to average lengths of 323 nm, 201 nm and 100 nm, respectively [118]. The shortening of the GONR resulted in increased cellular metabolism in both A549 cells and MCF-7 cells and increased lactose dehydrogenase (marker for toxicity) release in MCF-7 cells treated GONR after 10 min of probe sonication [118].
Most in vivo safety pharmacological studies of graphene-based cancer therapeutics focus on biodistribution and blood circulation. Yang et al. investigated the effects of size and surface chemistry of GO-PEG in a breast cancer tumor model in Balb/c mice. They found blood half-life of the GO-PEG was about 0.29 h while reduced GO-PEG had a half-life of about 0.51 h. Blood half-life is an important factor for therapeutic effectiveness of PTT and PDT because it can increase the likelihood of the graphene constructs to accumulate at the tumor by the EPR effect [119]. Nurunnabi et al. investigated GQD toxicology in KB breast cancer cell model in SKH1 mice. They report serum biochemistry for mice injected with two doses, 5 and 10 mg/kg body weight, and found normal blood serum chemistry through 22 days of observation [120]. These studies show that the primary sites of biodistribution and accumulation for graphene cancer therapeutics are in the tumor, kidney, liver and spleen [119,120].
Conclusion
Graphene-based formulations show potential as multifunctional platforms for cancer therapeutics. In vitro investigations and in vivo proof of principle small animal studies provide evidence that graphene-based formulations could serve as versatile delivery agents. The results suggest that morphology of graphene as well as the functional groups employed to improve its water dispersibility may play an important role toward the development of an optimum formulations for efficacious anticancer therapies. The carboxylic, epoxy and hydroxyl group on the basal plane of the oxidized graphene nanoparticles such as GO or GONRs and hydrophobicity and pi bond network of graphene can be exploited for covalent or noncovalent functionalization of hydrophobic anticancer drugs, amphiphilic macromolecules, genetic material and any combination thereof. The electromagnetic absorption of graphene can be harnessed for photodynamic- and photothermal-based cancer therapy. Graphene-based formulations also allow simultaneous multipronged cancer treatment strategies that combine the above therapies to significantly improve cancer treatment outcomes. Graphene nanoparticles, depending on their chemical composition, synthesis method, external covalent or noncovalent functionalization, shape or size show diverse dose and time dependent cytotoxic effects on cancer cells and tumor tissues.
Future perspective
The vast array of drugs, genes and other therapeutic agents (e.g., PDT molecules) that can be loaded onto graphene nanoparticles for therapeutic delivery, along with its intrinsic electromagnetic properties, opens opportunities to develop treatment strategies to combat drug-resistant cancers. The hydrophobic structure of graphene is known to create bends, folds and wrinkles in aqueous solutions [121], which may limit the binding and even release bound therapeutic payload. Therefore employing design strategies to develop novel graphene assemblies that can encapsulate a therapeutic payload could be beneficial [122,123]. It has also been reported that drug release profiles of carbon nanotube are significantly different [124] than that of graphene [37]. However, studies comparing the drug release profiles of various types of graphene are unavailable and warrant further investigations. Availability of this information may allow identification of the appropriate type of graphene nanoparticle for burst or sustained release of cancer drugs. Furthermore, the ability to append onto or intercalate between graphene sheets imaging agents (paramagnetic ions for MRI, radiopaque ions for x-ray computed tomography or fluorescent labels for optical imaging) [125,126], or harness its intrinsic properties for photoacoustic [127] or Raman imaging [128] could facilitate image-guided tracking and monitoring of the biodistribution and clearance of the therapeutic cargo. Additionally, the use of graphene formulations as imaging agents will facilitate integration of the advantages of these various modalities. This integration should allow the same cancer tissue to be imaged at multiple scales, resolutions and depths. Additionally, this multimodal imaging could provide valuable insights during preclinical animal or eventually clinical imaging with applications including co-validation of target accumulation, and visualization of tumors before and/or during treatment.
Despite the aforementioned benefits of graphene for biomedical applications, compared with other carbon nanostructures (fullerenes, carbon nanotubes), relatively little work has been done to assess its in vivo efficacy for cancer therapeutics. All these studies are proof-of-principle studies mainly focused on GO-based formulations. Thus, potential of GONR and other graphene nanoparticles warrants further examination. For example, studies of protein adsorption and protein corona formation on graphene-drug and graphene-gene complexes are required to prevent inhibition of payload release [129]. Additionally, in vitro cytotoxicity of graphene-based formulation has been shown to be dependent on several factors such as functionalization state, shape and size distribution of graphene (lateral flake sizes and number of layers), dose and the cell type used for in vitro assays [18,51,108,114,130–133]. Along with these physiochemical properties, the route of administration and dose also play an important role in determining the in vivo toxicology, pharmacodynamics, biodistribution and metabolism of graphene nanoparticles [134]. Currently, no information is available regarding the small and large animal acute/subacute toxicity, maximum tolerable doses, metabolism or assessment of other important issues such as respiratory and cardiovascular pharmacology safety of these graphene-based formulations for cancer therapy. Consequently, the therapeutic indices or potential therapeutic dosages of these formulations remain unknown. Thus, significant amount of work still needs to be completed to translate the exciting promise of graphene-based cancer technologies into clinic.
Executive summary.
Background
Graphene-based formulations could serve as multifunctional platforms for cancer therapy.
Graphene platforms for anticancer drug delivery
Graphene nanoparticles, depending on their chemical composition, synthesis method, external covalent or noncovalent functionalization, shape or size show diverse effects on cells and tissues. Hydrophobic cancer drug delivery is facilitated by hydrophobic interactions between the drug and graphene oxide molecules, leading to water dispersible graphene loaded with cancer drugs with otherwise limited water solubility.
Gene delivery
Negative charges on both genomic material and graphene cause electrostatic repulsion. Positively charged polymers, grafted on graphene, allow for good binding of genomic material without the toxic effects of the free polymer in solution.
Hyperthermia & photodynamic therapy
Graphene's physiochemical properties can be harnessed to facilitate photothermal and photodynamic therapy to treat superficial or deep tissue cancers.
Hydrophobic, aromatic photosensitizers loaded on graphene can be utilized for photodynamic therapy. Graphene innately is an excellent photothermal agent for photothermal therapy. These processes can be simultaneously or systematically administered for improved, combinatorial effects.
The effects of graphene formulations on cancer cells & animal models
Graphene toxicity is well studied from many cell types to organ systems, and many times toxicity can be alleviated by surface functionalization of graphene.
Future perspectives
New strategies of graphene-based cancer therapeutics involve development of combinatorial techniques for fighting drug resistant cancers, for personalized medicine and for theranostic approaches to longitudinally track drug and tumor progression.
Footnotes
Financial & competing interests disclosure
This work was sponsored by a National Institutes of Health grants (1DP2OD007394 and AR61821). The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA-Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA-Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Potash J, Anderson KC. AACR Cancer Progress Report 2014: Transforming Lives Through Research. Clin. Cancer Res. 2014;20(19):4977. doi: 10.1158/1078-0432.CCR-14-2104. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA-Cancer J. Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 5.Sahni V, Choudhury D, Ahmed Z. Chemotherapy-associated renal dysfunction. Nat. Rev. Nephrol. 2009;5(8):450–462. doi: 10.1038/nrneph.2009.97. [DOI] [PubMed] [Google Scholar]
- 6.Alexis F, Rhee J-W, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol. Oncol. Semin. O I. 2008;26(1):74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol. Therapeut. 2010;128(2):324–335. doi: 10.1016/j.pharmthera.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. Semin. O I. 2008;26(1):57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Davis ME, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 10.Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J. Control. Release. 2010;141(3):265–276. doi: 10.1016/j.jconrel.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Nichols JW, Bae YH. Odyssey of a cancer nanoparticle: from injection site to site of action. Nano Today. 2012;7(6):606–618. doi: 10.1016/j.nantod.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen MJ, Tung VC, Kaner RB. Honeycomb carbon: a review of graphene. Chem. Rev. 2010;110:132–145. doi: 10.1021/cr900070d. [DOI] [PubMed] [Google Scholar]
- 13.Geim AK, Novoselov KS. The rise of graphene. Nat. Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 14.Geim AK. Graphene: status and prospects. Science. 2009;324:1530–1534. doi: 10.1126/science.1158877. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Li Z, Wang J, Lisend J, Lin Y. Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011;29:205–212. doi: 10.1016/j.tibtech.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jokar S, Pourjavadi A, Adeli M. Albumin-graphene oxide conjugates; carriers for anticancer drugs. RSC Adv. 2014;4(62):33001–33006. [Google Scholar]; • Reports pH responsive drug delivery using graphene particles.
- 17.Segal M. Selling graphene by the ton. Nat. Nanotechnol. 2009;4:612–614. doi: 10.1038/nnano.2009.279. [DOI] [PubMed] [Google Scholar]
- 18.Talukdar Y, Rashkow JT, Lalwani G, Kanakia S, Sitharaman B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials. 2014;35(18):4863–4877. doi: 10.1016/j.biomaterials.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Liu Z, Welsher K, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1(3):203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paratala BS, Jacobson BD, Kanakia S, Francis LD, Sitharaman B. Physicochemical characterization, and relaxometry studies of micro-graphite oxide, graphene nanoplatelets, and nanoribbons. PLoS ONE. 2012;7(6):e38185. doi: 10.1371/journal.pone.0038185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonelli FM, Goulart VA, Gomes KN, et al. Graphene-based nanomaterials: biological and medical applications and toxicity. Nanomedicine. 2015;10(15):2423–2450. doi: 10.2217/nnm.15.65. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Asiri AM, Tang Z, Du D, Lin Y. Graphene based materials for biomedical applications. Mater. Today. 2013;16(10):365–373. [Google Scholar]
- 23.Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev. 2013;42(2):530–547. doi: 10.1039/c2cs35342c. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, Zhang L, Liu M, Zhang Z. Biomedical applications of graphene. Theranostics. 2012;2(3):283. doi: 10.7150/thno.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Nayak TR, Hong H, Cai W. Graphene: a versatile nanoplatform for biomedical applications. Nanoscale. 2012;4(13):3833–3842. doi: 10.1039/c2nr31040f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XM, Zhang WH. Application of graphene derivatives in cancer therapy: a review. Carbon. 2014;67:795. [Google Scholar]
- 27.Chung C, Kim YK, Shin D, Ryoo SR, Hong BH, Min DH. Biomedical applications of graphene and graphene oxide. Accounts Chem. Res. 2013;46(10):2211–2224. doi: 10.1021/ar300159f. [DOI] [PubMed] [Google Scholar]
- 28.Bitounis D, Ali‐Boucetta H, Hong BH, Min DH, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013;25(16):2258–2268. doi: 10.1002/adma.201203700. [DOI] [PubMed] [Google Scholar]
- 29.Yang K, Zhang S, Zhang G, Sun X, Lee S-T, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008;130(33):10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Earliest report of graphene-based delivery agent of anticancer drugs.
- 31.Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J. Phys. Chem. C. 2008;112(45):17554–17558. [Google Scholar]
- 32.Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010;6(4):537–544. doi: 10.1002/smll.200901680. [DOI] [PubMed] [Google Scholar]
- 33.Zhou T, Zhou X, Xing D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials. 2014;35(13):4185–4194. doi: 10.1016/j.biomaterials.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Wei G, Dong R, Wang D, et al. Functional materials from the covalent modification of reduced graphene oxide and [small beta]-cyclodextrin as a drug delivery carrier. New J. Chem. 2014;38(1):140–145. [Google Scholar]
- 35.Xu Z, Zhu S, Wang M, Li Y, Shi P, Huang X. Delivery of paclitaxel using PEGylated graphene oxide as a nanocarrier. App. Mater. Interfaces. 2015;7(2):1355–1363. doi: 10.1021/am507798d. [DOI] [PubMed] [Google Scholar]
- 36.Angelopoulou A, Voulgari E, Diamanti EK, Gournis D, Avgoustakis K. Graphene oxide stabilized by PLA–PEG copolymers for the controlled delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2015;93:18–26. doi: 10.1016/j.ejpb.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Moore TL, Podilakrishna R, Rao A, Alexis F. Systemic administration of polymer‐coated nano‐graphene to deliver drugs to glioblastoma. Part. Part. Syst. Char. 2014;31(8):886–894. [Google Scholar]
- 38.Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, Yang BW. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. Photochem. Photobiol. 2013;120:156–162. doi: 10.1016/j.jphotobiol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Liu P. Biocompatible graphene oxide as a folate receptor-targeting drug delivery system for the controlled release of anti-cancer drugs. RSC Adv. 2014;4(46):24232–24239. [Google Scholar]
- 40.Huang P, Xu C, Lin J, et al. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2011;1:240–250. doi: 10.7150/thno/v01p0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Q, Huang X, Tang J, Han Y, Chen H. Environmentally friendly, one-pot synthesis of folic acid-decorated graphene oxide-based drug delivery system. J. Nanopart. Res. 2013;15(12):1–7. [Google Scholar]
- 42.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73(9):2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Yadav AK, Mishra P, Agrawal GP. An insight on hyaluronic acid in drug targeting and drug delivery. J. Drug Target. 2008;16(2):91–107. doi: 10.1080/10611860802095494 . [DOI] [PubMed] [Google Scholar]
- 44.Miao W, Shim G, Kang CM, et al. Cholesteryl hyaluronic acid-coated, reduced graphene oxide nanosheets for anti-cancer drug delivery. Biomaterials. 2013;34(37):9638–9647. doi: 10.1016/j.biomaterials.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 45.Wu H, Shi H, Wang Y, et al. Hyaluronic acid conjugated graphene oxide for targeted drug delivery. Carbon. 2014;69(0):379–389. [Google Scholar]
- 46.Song E, Han W, Li C, et al. Hyaluronic acid-decorated graphene oxide nanohybrids as nanocarriers for targeted and pH-responsive anticancer drug delivery. App. Mater. Interfaces. 2014;6(15):11882–11890. doi: 10.1021/am502423r. [DOI] [PubMed] [Google Scholar]
- 47.Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006;121(2):144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Liu G, Shen H, Mao J, et al. Transferrin modified graphene oxide for glioma-targeted drug delivery: in vitro and in vivo evaluations. App. Mater. Interfaces. 2013;5(15):6909–6914. doi: 10.1021/am402128s. [DOI] [PubMed] [Google Scholar]
- 49.Liu G, Shen H, Mao J, et al. Transferrin modified graphene oxide for glioma-targeted drug delivery: in vitro and in vivo evaluations. App. Mater. Interfaces. 2013;5(15):6909–6914. doi: 10.1021/am402128s. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Gu W, Xiao N, Ye L, Xu Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int. J. Nanomed. 2014;9:1433–1442. doi: 10.2147/IJN.S58783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mullick Chowdhury S, Lalwani G, Zhang K, Yang JY, Neville K, Sitharaman B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials. 2013;34(1):283–293. doi: 10.1016/j.biomaterials.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullick Chowdhury S, Surhland C, Sanchez Z, et al. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomed. Nanotechnol. 2015;11(1):109–118. doi: 10.1016/j.nano.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chowdhury SM, Lalwani G, Zhang K, Yang JY, Neville K, Sitharaman B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials. 2013;34(1):283–293. doi: 10.1016/j.biomaterials.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mullick Chowdhury S, Manepalli P, Sitharaman B. Graphene nanoribbons elicit cell specific uptake and delivery via activation of epidermal growth factor receptor enhanced by human papillomavirus E5 protein. Acta Biomater. 2014;10(10):4494–4504. doi: 10.1016/j.actbio.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses the uptake mechanism and drug delivery efficacy of graphene nanoribbons in various cancer cell types.
- 55.Siddiqui S, Fang M, Ni B, Lu D, Martin B, Maudsley S. Central role of the EGF receptor in neurometabolic aging. Int. J. Endocrinol. 2012;2012:739428. doi: 10.1155/2012/739428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogino A, Kitao H, Hirano S, et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non–small cell lung cancer cell line. Cancer Res. 2007;67(16):7807–7814. doi: 10.1158/0008-5472.CAN-07-0681. [DOI] [PubMed] [Google Scholar]
- 57.Lynch TJ, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor–associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12(5):610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 58.Luo M, Kelley MR. Inhibition of the human apurinic/apyrimidinic endonuclease (APE1) repair activity and sensitization of breast cancer cells to DNA alkylating agents with lucanthone. Anticancer Res. 2004;24(4):2127–2134. [PubMed] [Google Scholar]
- 59.Sun X, Liu Z, Welsher K, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1(3):203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005;23(9):1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Zhang X, Ma Y, Huang Y, Wang Y, Chen Y. Superparamagnetic graphene oxide-Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009;19(18):2710–2714. [Google Scholar]
- 62.Yang X, Wang Y, Huang X, et al. Multi-functionalized graphene oxide based anticancer drug-carrier with dual-targeting function and pH-sensitivity. J. Mater. Chem. 2011;21(10):3448–3454. [Google Scholar]
- 63.Sun X, Liu Z, Welsher K, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1(3):203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA. 1987;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Notley SM, Crawford RJ, Ivanova EP. Bacterial interaction with graphene particles and surfaces. Nanotechnol. Nanomater. 2013;5:99–118. [Google Scholar]
- 66.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release. 2006;114(1):100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Pérez-Martínez FC, Guerra J, Posadas I, Ceña V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm. Res. 2011;28(8):1843–1858. doi: 10.1007/s11095-010-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C, Zhong D, Zhang Y, et al. The effect of the gene carrier material polyethyleneimine on the structure and function of human red blood cells in vitro . J. Mater. Chem. B. 2013;1(14):1885–1893. doi: 10.1039/c3tb00024a. [DOI] [PubMed] [Google Scholar]
- 69.Feng L, Zhang S, Liu Z. Graphene based gene transfection. Nanoscale. 2011;3(3):1252–1257. doi: 10.1039/c0nr00680g. [DOI] [PubMed] [Google Scholar]; •• In vivo studies that investigate the potential of graphene-based formulations for combined drug delivery and photothermal therapy (chemo-photothermal therapy).
- 70.Chen B, Liu M, Zhang L, Huang J, Yao J, Zhang Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011;21(21):7736–7741. [Google Scholar]
- 71.Kim H, Namgung R, Singha K, Oh I-K, Kim WJ. Graphene oxide–polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjugate Chem. 2011;22(12):2558–2567. doi: 10.1021/bc200397j. [DOI] [PubMed] [Google Scholar]
- 72.Feng L, Yang X, Shi X, et al. Polyethylene glycol and polyethylenimine dual‐functionalized nano‐graphene oxide for photothermally enhanced gene delivery. Small. 2013;9(11):1989–1997. doi: 10.1002/smll.201202538. [DOI] [PubMed] [Google Scholar]
- 73.Ren T, Li L, Cai X, Dong H, Liu S, Li Y. Engineered polyethylenimine/graphene oxide nanocomposite for nuclear localized gene delivery. Polym. Chem. 2012;3(9):2561–2569. [Google Scholar]
- 74.Zhang L, Lu Z, Zhao Q, Huang J, Shen H, Zhang Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI‐grafted graphene oxide. Small. 2011;7(4):460–464. doi: 10.1002/smll.201001522. [DOI] [PubMed] [Google Scholar]
- 75.Zhi F, Dong H, Jia X, et al. Functionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitro . PLoS ONE. 2013;8(3):e60034. doi: 10.1371/journal.pone.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J, Feng L, Tan X, et al. Dual‐polymer‐functionalized nanoscale graphene oxide as a highly effective gene transfection agent for insect cells with cell‐type‐dependent cellular uptake mechanisms. Part. Part. Syst. Char. 2013;30(9):794–803. [Google Scholar]
- 77.Zhou X, Laroche F, Lamers GE, et al. Ultra-small graphene oxide functionalized with polyethylenimine (PEI) for very efficient gene delivery in cell and zebrafish embryos. Nano Res. 2012;5(10):703–709. [Google Scholar]
- 78.Yin D, Li Y, Lin H, et al. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo . Nanotechnologia. 2013;24(10):105102. doi: 10.1088/0957-4484/24/10/105102. [DOI] [PubMed] [Google Scholar]
- 79.Yang HW, Huang CY, Lin CW, et al. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials. 2014;35(24):6534–6542. doi: 10.1016/j.biomaterials.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 80.Lee ST, Chu K, Oh HJ, et al. Let-7 microRNA inhibits the proliferation of human glioblastoma cells. J. Neuro-Oncol. 2011;102(1):19–24. doi: 10.1007/s11060-010-0286-6. [DOI] [PubMed] [Google Scholar]
- 81.Gollavelli G, Ling YC. Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials. 2014;35(15):4499–4507. doi: 10.1016/j.biomaterials.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 82.Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995;24(1):19–33. [Google Scholar]
- 83.Mensudar R. Photodynamic therapy-A review. World J. Med. Sci. 2014;10(2):139–142. [Google Scholar]
- 84.Zhou L, Wang W, Tang J, Zhou JH, Jiang HJ, Shen J. Graphene oxide noncovalent photosensitizer and its anticancer activity In vitro . Chem. Eur. J. 2011;17(43):12084–12091. doi: 10.1002/chem.201003078. [DOI] [PubMed] [Google Scholar]; • Introduces the potential of nonporphyrin-based photosensitizers onto graphene substrates for photo dynamic therapy.
- 85.Wang S, Gao R, Zhou F, Selke M. Nanomaterials and singlet oxygen photosensitizers: potential applications in photodynamic therapy. J. Mater. Chem. 2004;14(4):487–493. [Google Scholar]
- 86.Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. Photochem. Photobiol. 2001;63(1):88–102. doi: 10.1016/s1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 87.Hu Z, Li J, Li C, et al. Folic acid-conjugated graphene-ZnO nanohybrid for targeting photodynamic therapy under visible light irradiation. J. Mater. Chem. 2013;1(38):5003–5013. doi: 10.1039/c3tb20849d. [DOI] [PubMed] [Google Scholar]
- 88.Gilje S, Dubin S, Badakhshan A, Farrar J, Danczyk S, Kaner RB. Photothermal deoxygenation of graphene oxide for patterning and distributed ignition applications. Adv. Mater. 2010;22(3):419–423. doi: 10.1002/adma.200901902. [DOI] [PubMed] [Google Scholar]
- 89.Abdelsayed V, Moussa S, Hassan HM, Aluri HS, Collinson MM, El-Shall MS. Photothermal deoxygenation of graphite oxide with laser excitation in solution and graphene-aided increase in water temperature. J. Phys. Chem. Lett. 2010;1(19):2804–2809. [Google Scholar]
- 90.Markovic ZM, Harhaji-Trajkovic LM, Todorovic-Markovic BM, et al. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials. 2011;32(4):1121–1129. doi: 10.1016/j.biomaterials.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 91.Robinson JT, Tabakman SM, Liang Y, et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011;133(17):6825–6831. doi: 10.1021/ja2010175. [DOI] [PubMed] [Google Scholar]
- 92.Sasidharan A, Sivaram AJ, Retnakumari AP, et al. Radiofrequency ablation of drug-resistant cancer cells using molecularly targeted carboxyl-functionalized biodegradable graphene. Adv. Health Mater. 2015;4(5):679–684. doi: 10.1002/adhm.201400670. [DOI] [PubMed] [Google Scholar]
- 93.Sherlock SP, Tabakman SM, Xie L, Dai H. Photothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystals. ACS Nano. 2011;5(2):1505–1512. doi: 10.1021/nn103415x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miao W, Shim G, Lee S, Lee S, Choe YS, Oh YK. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials. 2013;34(13):3402–3410. doi: 10.1016/j.biomaterials.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 95.Tian B, Wang C, Zhang S, Feng L, Liu Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano. 2011;5(9):7000–7009. doi: 10.1021/nn201560b. [DOI] [PubMed] [Google Scholar]
- 96.Sahu A, Choi WI, Lee JH, Tae G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials. 2013;34(26):6239–6248. doi: 10.1016/j.biomaterials.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 97.Park H, Yang J, Lee J, Haam S, Choi IH, Yoo KH. Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano. 2009;3(10):2919–2926. doi: 10.1021/nn900215k. [DOI] [PubMed] [Google Scholar]
- 98.Liu H, Chen D, Li L, et al. Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew. Chem. Int. Ed. Engl. 2011;123(4):921–925. doi: 10.1002/anie.201002820. [DOI] [PubMed] [Google Scholar]
- 99.Kim H, Lee D, Kim J, Kim TI, Kim WJ. Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano. 2013;7(8):6735–6746. doi: 10.1021/nn403096s. [DOI] [PubMed] [Google Scholar]
- 100.Zhang W, Guo Z, Huang D, Liu Z, Guo X, Zhong H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32(33):8555–8561. doi: 10.1016/j.biomaterials.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 101.Ma X, Tao H, Yang K, et al. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012;5(3):199–212. [Google Scholar]
- 102.Feng L, Li K, Shi X, Gao M, Liu J, Liu Z. Smart pH-responsive nanocarriers based on nano-graphene oxide for combined chemo- and photothermal therapy overcoming drug resistance. Adv. Health Mater. 2014;3(8):1261–1271. doi: 10.1002/adhm.201300549. [DOI] [PubMed] [Google Scholar]
- 103.Bian X, Song ZL, Qian Y, et al. Fabrication of graphene-isolated-Au-nanocrystal nanostructures for multimodal cell imaging and photothermal-enhanced chemotherapy. Sci. Rep. 2014;4:6093. doi: 10.1038/srep06093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, Yang BW. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. Photochem. Photobiol. 2013;120(0):156–162. doi: 10.1016/j.jphotobiol.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 105.Bao H, Pan Y, Ping Y, et al. Chitosan‐functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small. 2011;7(11):1569–1578. doi: 10.1002/smll.201100191. [DOI] [PubMed] [Google Scholar]
- 106.Sheng Z, Song L, Zheng J, et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials. 2013;34(21):5236–5243. doi: 10.1016/j.biomaterials.2013.03.090. [DOI] [PubMed] [Google Scholar]
- 107.Bianco A. Graphene: safe or toxic? The two faces of the medal. Angew. Chem. Int. Edit. 2013;52(19):4986–4997. doi: 10.1002/anie.201209099. [DOI] [PubMed] [Google Scholar]
- 108.Seabra AB, Paula AJ, de Lima R, Alves OL, Duraán N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol.. 2014;27(2):159–168. doi: 10.1021/tx400385x. [DOI] [PubMed] [Google Scholar]
- 109.Jastrzębska AM, Kurtycz P, Olszyna AR. Recent advances in graphene family materials toxicity investigations. J. Nanopart. Res. 2012;14(12):1–21. doi: 10.1007/s11051-012-1320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma Y, Shen H, Tu X, Zhang Z. Assessing in vivo toxicity of graphene materials: current methods and future outlook. Nanomedicine. 2014;9(10):1565–1580. doi: 10.2217/nnm.14.68. [DOI] [PubMed] [Google Scholar]
- 111.Feng L, Liu Z. Graphene in biomedicine: opportunities and challenges. Nanomedicine. 2011;6(2):317–324. doi: 10.2217/nnm.10.158. [DOI] [PubMed] [Google Scholar]
- 112.Zhou T, Zhang B, Wei P, et al. Energy metabolism analysis reveals the mechanism of inhibition of breast cancer cell metastasis by PEG-modified graphene oxide nanosheets. Biomaterials. 2014;35(37):9833–9843. doi: 10.1016/j.biomaterials.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 113.Zhou H, Zhang B, Zheng J, et al. The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials. 2014;35(5):1597–1607. doi: 10.1016/j.biomaterials.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Jaworski S, Sawosz E, Grodzik M, et al. In vitro evaluation of the effects of graphene platelets on glioblastoma multiforme cells. Int. J. Nanomed. 2013;8:413. doi: 10.2147/IJN.S39456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yuan X, Liu Z, Guo Z, Ji Y, Jin M, Wang X. Cellular distribution and cytotoxicity of graphene quantum dots with different functional groups. Nanoscale Res. Lett. 2014;9(1):1–9. doi: 10.1186/1556-276X-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chng ELK, Chua CK, Pumera M. Graphene oxide nanoribbons exhibit significantly greater toxicity than graphene oxide nanoplatelets. Nanoscale. 2014;6(18):10792–10797. doi: 10.1039/c4nr03608e. [DOI] [PubMed] [Google Scholar]; • Compares the in vitro toxicities of two different allotropes of graphene; graphene nanoplatelets and graphene nanoribbons.
- 117.Mbeh DA, Akhavan O, Javanbakht T, Mahmoudi M, Yahia LH. Cytotoxicity of protein corona-graphene oxide nanoribbons on human epithelial cells. Appl. Surf. Sci. 2014;320:596–601. [Google Scholar]
- 118.Mullick Chowdhury S, Dasgupta S, McElroy AE, Sitharaman B. Structural disruption increases toxicity of graphene nanoribbons. J. App. Toxicol. 2014;34(11):1235–1246. doi: 10.1002/jat.3066. [DOI] [PubMed] [Google Scholar]
- 119.Yang K, Wan J, Zhang S, Tian B, Zhang Y, Liu Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials. 2012;33(7):2206–2214. doi: 10.1016/j.biomaterials.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 120.Nurunnabi M, Khatun Z, Huh KM, et al. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano. 2013;7(8):6858–6867. doi: 10.1021/nn402043c. [DOI] [PubMed] [Google Scholar]
- 121.Meyer JC, Geim AK, Katsnelson MI, Novoselov KS, Booth TJ, Roth S. The structure of suspended graphene sheets. Nature. 2007;446(7131):60–63. doi: 10.1038/nature05545. [DOI] [PubMed] [Google Scholar]
- 122.Chen Y, Guo F, Jachak A, et al. Aerosol synthesis of cargo-filled graphene nanosacks. Nano Lett. 2012;12(4):1996–2002. doi: 10.1021/nl2045952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park SH, Kim HK, Yoon SB, et al. Spray-assisted deep-frying process for the In situ spherical assembly of graphene for energy-storage devices. Chem. Mater. 2015;27(2):457–465. [Google Scholar]
- 124.Moore TL, Pitzer JE, Podila R, et al. Multifunctional polymer-coated carbon nanotubes for safe drug delivery. Part. Part. Sys. Char. 2013;30(4):365–373. doi: 10.1002/ppsc.201200145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paratala B, Hill L, Mujica-Parodi L, Caparelli E, Wadghiri Y, Sitharaman B. Graphene based MRI contrast agents: synthesis, characterization and in-vitro MRI. Proc. Int. Soc. Mag. Reson. Med. 2011;19:1678. [Google Scholar]
- 126.Lalwani G, Sundararaj JL, Schaefer K, Button T, Sitharaman B. Synthesis, characterization, in vitro phantom imaging, and cytotoxicity of a novel graphene-based multimodal magnetic resonance imaging-X-ray computed tomography contrast agent. J. Mater. Chem. 2014;2(22):3519–3530. doi: 10.1039/C4TB00326H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lalwani G, Cai X, Nie L, Wang LV, Sitharaman B. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics. 2013;1(3–4):62–67. doi: 10.1016/j.pacs.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang J, Zong C, Shen H, Cao Y, Ren B, Zhang Z. Tracking the intracellular drug release from graphene oxide using surface-enhanced Raman spectroscopy. Nanoscale. 2013;5(21):10591–10598. doi: 10.1039/c3nr03264g. [DOI] [PubMed] [Google Scholar]
- 129.Podila R, Brown JM. Toxicity of engineered nanomaterials: a physicochemical perspective. J. Biochem. Mol. Toxic. 2013;27(1):50–55. doi: 10.1002/jbt.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu C, Wang C, Han T, Zhou X, Guo S, Zhang J. Insight into the cellular internalization and cytotoxicity of graphene quantum dots. Adv. Health Mater. 2013;2(12):1613–1619. doi: 10.1002/adhm.201300066. [DOI] [PubMed] [Google Scholar]
- 131.Teo WZ, Chng EL, Sofer Z, Pumera M. Cytotoxicity of halogenated graphenes. Nanoscale. 2014;6(2):1173–1180. doi: 10.1039/c3nr05275c. [DOI] [PubMed] [Google Scholar]
- 132.Akhavan O, Ghaderi E, Emamy H, Akhavan F. Genotoxicity of graphene nanoribbons in human mesenchymal stem cells. Carbon. 2013;54:419–431. [Google Scholar]
- 133.Hashemi E, Akhavan O, Shamsara M, Rahighi R, Esfandiar A, Tayefeh AR. Cyto and genotoxicities of graphene oxide and reduced graphene oxide sheets on spermatozoa. RSC Adv. 2014;4(52):27213–27223. [Google Scholar]
- 134.Kanakia S, Toussaint JD, Mullick Chowdhury S, et al. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials. 2014;35(25):7022–7031. doi: 10.1016/j.biomaterials.2014.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]