Abstract
Background:
Fetal arrhythmias can lead to fetal congestive heart failure and hydrops fetalis. Digoxin (the first-line treatment) has low transplacental permeability and high risk of maternal side effects. Biodegradable digoxin-loaded PEGylated poly(lactic-co-glycolic acid) nanoparticles may increase digoxin transport across BeWo b30 cell monolayers (an in vitro model of trophoblast in human placenta) by reducing the drug's interaction with P-gp.
Results/methodology:
The nanoparticles showed high encapsulation efficiency and sustained release over 48 h. Transport studies revealed significantly increased permeability across BeWo cell layers of digoxin-loaded nanoparticles when compared with free digoxin. P-gp inhibition also increased the permeability of digoxin, but not digoxin-loaded nanoparticles.
Conclusion:
This represents a novel treatment strategy for fetal cardiovascular disease which may improve maternal and fetal outcomes.
Background
Fetal cardiac arrhythmias, which occur in 1% of pregnancies, can result in fetal heart failure [1–3]. Arrhythmias also represent a leading cause of fetal hydrops (effusions in more than one fetal compartment), which has an incidence of one in 2500 pregnancies [1,4]. Hydrops fetalis is associated with up to 72% mortality, and accounts for 3% of all fetal mortality [4,5]. Digoxin is the drug of choice for treating fetal tachyarrhythmias and fetal congestive heart failure [6]. Maternally administered digoxin, as well as sotalol and flecainide, have been shown to be effective in terms of conversion of supraventricular fetal tachycardias to normal sinus rhythm [1]. Still, fetal death occurs in about 10% of fetal tachyarrhythmia cases, and even more in hydropic fetuses. Transplacental digoxin treatment is 71% effective in cases involving nonhydropic fetuses and 10% effective in hydropic fetuses [7]. Transplacental transfer of digoxin to the fetus is limited because digoxin is a substrate for the efflux transporter P-gp, which is highly expressed in human placenta [8], and is also expressed in BeWo cells [9]. Therefore, higher and more frequent doses of digoxin are required during pregnancy to maintain therapeutic concentrations [10]. Prenatal digoxin therapy can lead to undesirable side effects for the mother, because the majority of the dose remains in the maternal circulation [11]. These maternal side effects include palpitations, second degree atrioventricular block and hypotension [11]. There is a great need to improve the delivery of digoxin to the fetus and simultaneously minimize maternal drug exposure.
Previous work has demonstrated that polymeric nanoparticles with diameters around 100 nm can cross the placental barrier [12]. The hypothesis driving this project is that an innovative, nanomedicine-based approach could improve the transplacental delivery of digoxin to the fetus. Such an approach may improve fetal digoxin therapy by means of reduced interactions of nanoencapsulated digoxin with P-gp, resulting in more efficient transplacental transfer of digoxin to the fetus. This strategy could have major implications in future therapy for fetal arrhythmias. Targeted delivery of digoxin to the fetus may lower the risk of maternal complications during pregnancy as well. Thus, the studies described herein are intended to fill a critical void in fetal cardiovascular therapy during pregnancy and improve cardioversion to normal sinus rhythm. Biocompatible and biodegradable polymeric nanoparticles containing digoxin have been prepared and characterized in terms of particle size, encapsulation efficiency and drug release. These nanoparticles are advantageous because they are composed of poly(lactic-co-glycolic acid) (PLGA), a material already present in many formulations approved by the US FDA and the EMA [13]. In addition, PLGA is known for its excellent controlled release characteristics [14]. The transport of digoxin as free drug or encapsulated in nanoparticles was compared using the BeWo b30 cell line, an in vitro model of human placental trophoblast cells separating the maternal and fetal circulations [15]. Finally, the effect of nanoencapsulation on the interaction of digoxin with P-gp was investigated by means of transport studies in the presence of verapamil, a P-gp inhibitor [16].
Materials & methods
Preparation of nanoparticles
Nanoparticles were prepared by a modified solvent displacement method, as described elsewhere [17]. Briefly, solutions of PEGylated PLGA polymer (Resomer® RGPd50105 and RGPd5055, Boehringer Ingelheim, Ingelheim am Rhein, Germany) were prepared at concentrations of 3 mg/ml in tetrahydrofuran (THF, Acros Organics, Geel, Belgium), along with digoxin (MP Biomedicals, LLC, CA, USA) at 5 and 10% theoretical drug loading, and allowed to mix overnight to ensure that both the drug and the polymer were completely dissolved in the solvent. The use of PEGylated polymers is expected to reduce the clearance of nanoparticles by the reticuloendothelial system in future in vivo experiments. The solutions (1 ml) were nanoprecipitated in 5 ml purified water by injection through a 22-gauge needle (0.7 × 40 mm, PrecisionGlide Needle®, BD, NJ, USA) with a variable-speed peristaltic pump (Traceable® Calibration Control Company, TX, USA) at a rate of 6 ml/min. During the injection, the suspension was stirred at 500 rpm on a magnetic stir place. Following precipitation, suspensions were allowed to stir at 850 rpm until tetrahydrofuran had completely evaporated (at least 8 h). Volumes were then completed to 5 ml using purified water [18].
HPLC
Digoxin concentrations were determined using HPLC, similar to the method of Hu et al. [19]. Flow was established using a binary pump (1500-series HPLC pump, Waters, MA, USA) at 1 ml/min in isocratic conditions of 30:70 (v/v) acetonitrile to purified water, with a C18 Symmetry® column (5 μm, 4.6 × 150 mm, Waters), at room temperature. Detection was at a wavelength of 230 nm using a Waters 2998 photodiode array detector, and the method was validated (see Supplementary Table 1).
Nanoparticle characterization
Suspensions of the nanoparticles were characterized using a high performance particle sizer (HPPS, Malvern Instruments, Malvern, UK) to determine particle size and polydispersity index. ζ-potential was determined using a Zetasizer 2000 (Malvern Instruments).
Thermal properties of nanoparticles were determined with a Q200 differential scanning calorimeter (TA Instruments, DE, USA). Nanoparticle suspensions were lyophilized using a FreeZone 2.5L benchtop freeze dry system (Labconco, MO, USA). Following lyophilization, nanoparticle samples were centrifuged for collection, samples (1–8 mg) were placed in sealed aluminum hermetic sample pans, and scanned from 0 to 300°C at a rate of 10°C/min.
Encapsulation efficiency was determined by HPLC. Nonencapsulated digoxin was separated from the nanoparticles by placing 500 μl of the suspensions into Amicon® Ultra-0.5 centrifugal filters (100,000 MWCO, Tullagreen, Ireland) spun at 10,000 × g for 15 min using an Eppendorf® 5430 centrifuge (Hamburg, Germany). Nanoparticles in the aqueous nanosuspension do not pass through the filters. The filtrate, containing the unencapsulated drug in the nanosuspension, was analyzed by HPLC, and encapsulation efficiency was calculated as described previously [20], with correction for any nonspecific binding of digoxin to the filter membranes. Details regarding the equations relating drug loading and encapsulation efficiency have been published previously [21].
Drug release
Release of digoxin from the nanoparticles was studied under sink conditions in phosphate buffered saline (PBS) at 37°C as described previously [21,22]. In vitro drug release profiles in PBS have been previously shown to correlate well with the kinetics of in vivo therapeutic effects [18]. Briefly, 1.5 ml of aqueous nanosuspension (nanoparticles prepared at 10% drug loading) was added to 13.5 ml of 1.11X PBS (so that the final mixture was 1X PBS) at time zero. The mixture was rotated at 75 rpm (Junior Orbit Shaker, Lab-Line, IL, USA) in a mini-incubator (Boekel, PA, USA). Samples were taken at the specified time points and placed in centrifugal filters, which were spun at 4000 × g rpm for 10 min at 25°C in an Eppendorf® 5810R centrifuge. The filtrates of samples taken in triplicate were analyzed by HPLC to quantify the amount of drug released from the nanoparticles over time.
Cell culture
BeWo cells retain trophoblastic transport characteristics and phenotype. It has been previously shown that the BeWo cells utilized in this study (b30 clone) express functional levels of P-gp, including reports of uptake and transport studies of P-gp substrates [9,23,24]. BeWo b30 cells (obtained from Lisbeth Knudsen, University of Copenhagen, Denmark) were cultured in DMEM/F-12 media (Dulbecco's Modified Eagle's Medium/Ham's F-12 50/50 mixture, Mediatech, VA, USA) containing 10% fetal bovine serum (Atlanta Biologicals, GA, USA), antibiotic/antimycotic (containing 10,000 units/ml penicillin, 10,000 μg/ml streptomycin and 25 μg/ml amphotericin B, Gibco®, Life Technologies, CA, USA), 100X MEM nonessential amino acid solution (Sigma, St. Louis, MO, USA) and 200 mM L-glutamine (Cellgro, NY, USA). Cells were revived from an aliquot stored in liquid nitrogen (passage number 29) and grown in a 95% humidified incubator with 5% CO2 at 37°C. At confluence (80–90%), cells were seeded at a density of 224,000 cells/ml in polycarbonate Transwell® inserts (pore size 3 μm, growth area 1.12 cm2, apical volume 0.5 ml, basolateral volume 1.5 ml) coated with human placental collagen (Type IV, Sigma) in preparation for transport studies as described previously [25]. Cell culture media was changed daily. No endotoxin contamination was expected as all studies were conducted under sterile conditions and the cell culture medium components were already tested for endotoxin contamination. A continuous trophoblast layer is important in the protective function of the placenta [26], and therefore, transepithelial electrical resistance (TEER) measurements were used to measure the integrity of cell monolayers [20]. TEER values were measured using an EndOhm® voltohmmeter (World Precision Instruments, FL, USA), at 25°C starting on day 4 post seeding to ensure monolayer formation. TEER values between 30 and 60 Ω cm2 (corrected for resistance of blank Transwell inserts) were considered acceptable for performing transport studies (which were performed on day 6 post seeding). Each set of cells grown on the Transwell inserts had similar TEER values. Acceptable TEER values for BeWo cell monolayers have been reported previously for the b30 clone [27,28]. These ranges of TEER values are the same which resulted in strong comparisons of transplacental permeability measured by both BeWo cell transport studies and ex vivo placental perfusions [15,29]. Ali et al. utilized BeWo b30 cells with similar TEER values and demonstrated an inverse relationship between TEER values and the permeability of the paracellular marker Lucifer yellow, which provides confidence of monolayer formation appropriate for transport studies when TEER values are in this range [20].
Cell viability
The viability of BeWo cells after exposure to blank (not loaded with drug) PEGylated PLGA nanoparticles for 4, 24 and 48 h was investigated in order to determine whether the nanoparticles might affect trophoblast monolayer integrity (previous digoxin permeability studies across BeWo cell monolayers did not demonstrate any concern for the toxicity of digoxin itself to BeWo cells [22]). This was assessed by a well-established colorimetric cell proliferation, cell viability and cytotoxicity assay based on a water-soluble tetrazolium salt (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate, WST-1) (Roche Diagnostics, IN, USA). Cells were seeded in 96-well plates at a density of 4 × 105 cells/well (well surface area = 0.32 cm2) and allowed to grow for 48 h. Blank nanoparticle treatments were then added to appropriate wells and allowed to incubate for either 4, 24 or 48 h before aspiration of the treatment solutions and immediate addition of 10 µl of WST-1 reagent premixed with 100 µl of fresh cell culture medium (a total of 110 µl was added to each well). Cells were then allowed to incubate for an additional 2 h at 37°C, and absorbance of the WST-1 metabolite was measured using a VMax microplate reader (Molecular Devices, CA, USA) at λ = 450 nm and corrected for background at λ = 650 nm. Cells were treated with blank cell culture medium as a positive control and 0.1% (v/v) Triton X-100 (Integra Chemical, WA, USA) as the negative control.
Transport studies
Transport studies were conducted using BeWo b30 cells following the protocol of Cartwright et al. with slight modifications [12]. After ensuring the formation of monolayers in each Transwell, 0.5 ml of cell culture media containing each digoxin formulation to be compared was placed in the apical side of the respective Transwell inserts. The inserts were maintained at 37°C in cell culture conditions (95% relative humidity, 5% CO2) and stirred at 50 rpm with an orbital shaker. Samples (200 μl) were collected at the specified time points from the basolateral chamber and analyzed by HPLC. Cell culture media (200 μl) was replaced immediately following each sample from the basolateral chamber to maintain volume. Mass transfer and the apparent permeability (Pe) of digoxin were calculated and corrected for mass removed during sampling as described previously [12].
Statistical analysis
Averages and standard deviations were calculated for each experimental group. Data were analyzed by one-way ANOVA and p-values <0.05 were considered significant.
Results
HPLC
An HPLC method was developed and validated for the detection of digoxin at a wavelength of 230 nm (see Supplementary Table 1). The method was checked for accuracy and precision, and the limit of detection was determined to be 24 ng/ml. Short term stability of samples was also assessed. The method was validated using both 50:50 F12:DMEM cell culture media (applicable to the transport studies) and purified water (applicable to the determination of encapsulation efficiency).
Nanoparticle characterization
Digoxin-loaded polymeric nanoparticles were prepared by a modified solvent displacement method. Two different polymers and various percentages of theoretical drug loading were screened (see Table 1 & Supplementary Table 2). Table 1 shows that >90% encapsulation efficiency was observed for both polymers at drug loading values of 5 and 10%. Increased PEG content of the polymer resulted in less negative ζ-potential values, and the polydispersity index values were all less than or equal to 0.25, indicating monodisperse nanosuspensions. Differential scanning calorimetry studies confirmed encapsulation of the drug within the nanoparticles. The decrease in glass transition temperature with increased drug loading shown in Table 2 suggests a solid dispersion of digoxin in the polymer matrix [30]. Because the glass transition temperature of the polymeric nanoparticles is below 37°C, the polymers would be in an elastomeric, rubbery state at physiological temperature, rather than in a rigid, glassy state. The kinetics of drug release from these nanoparticles at 37°C is described below.
Table 1. . Physicochemical characterization and encapsulation efficiency of polyethylene glycol–poly(lactic-co-glycolic acid) nanoparticles loaded with digoxin (n = 3).
| Formulation: polymer (drug loading) | Z-average particle size (nm) | Polydispersity index | ζ-potential (mV) | Encapsulation efficiency (%) |
|---|---|---|---|---|
| RGPd50105 (5%)† |
64 ± 0.4 |
0.19 ± 0.00 |
-14 ± 14 |
>97.4 |
| RGPd50105 (10%) |
84 ± 0.4 |
0.25 ± 0.02 |
-9 ± 11 |
98.4 ± 0.2 |
| RGPd5055 (5%)‡ |
123 ± 0.7 |
0.22 ± 0.01 |
-48 ± 2 |
>97.4 |
| RGPd5055 (10%) | 124 ± 1 | 0.22 ± 0.01 | -47 ± 3 | 94.9 ± 0.7 |
†Polymer is 10% polyethylene glycol and 90% poly(lactic-co-glycolic acid) by weight.
‡Polymer is 5% polyethylene glycol and 95% poly(lactic-co-glycolic acid) by weight.
PLGA: Poly(lactic-co-glycolic acid).
Table 2. . Glass transition temperatures (Tg) of blank nanoparticles (no drug loading) and digoxin-loaded polyethylene glycol–poly(lactic-co-glycolic acid) (RGPd50105) nanoparticles, as determined by differential scanning calorimetry.
| Sample | Tg (°C) |
|---|---|
| Blank nanoparticles |
33.3 |
| Digoxin-loaded nanoparticles (5% drug loading) |
32.3 |
| Digoxin-loaded nanoparticles (10% drug loading) | 28.6 |
Release study
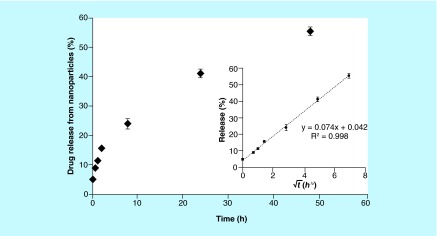
The release study for digoxin-loaded nanoparticles was conducted in PBS at 37°C under sink conditions, using centrifugal filters to separate nanoparticles from released digoxin. Figure 1 demonstrates sustained release over the 48-h study period, with no initial burst release. The classical Higuchi model for drug release correlates the percentage of drug released to the square root of time, as follows: Mt/M∞ = K(t)½, where Mt is the mass of drug released at time t, M∞ is the total cumulative mass of drug released at infinite time, and the constant K is dependent on experimental variables [31]. The data from the release study were fit to the Higuchi model for drug release. The inset to Figure 1 shows that for this nanoparticle formulation, K = 0.074 (R2 = 0.998). The value of the intercept of the fitted line (4.2%) is similar to the amount of unencapsulated drug determined experimentally (100% – the encapsulation efficiency), providing further evidence of inconsequential burst release.
Figure 1. . In vitro drug release of digoxin from polyethylene glycol–poly(lactic-co-glycolic acid) nanoparticles (RGPd50105, 10% drug loading) over 48 h under sink conditions at 37°C in phosphate-buffered saline.
The inset shows the fit of the data to the Higuchi equation (as described in the ‘Release study’ subsection). Error bars represent the standard deviation (n = 3).
PLGA: Poly(lactic-co-glycolic acid).
Cell viability
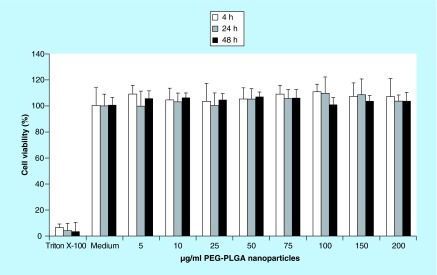
The viability of BeWo cells after exposure to blank nanoparticles was measured using the well-established WST-1 colorimetric assay. 0.1% (v/v) Triton X-100 was used as a negative control, and cell culture medium was used as a positive control (i.e., 100% cell viability). Figure 2 shows that no significant cytotoxicity was observed after 4, 24 or 48 h of cell exposure to the nanoparticles for any of the concentrations of blank nanoparticles tested. This indicates that any permeability differences in transport studies are not attributed to any cytotoxic effects of nanoparticles on the cells.
Figure 2. . Biocompatibility of blank polyethylene glycol–poly(lactic-co-glycolic acid) nanoparticles in BeWo cells at various concentrations after 4, 24 and 48 h of exposure, as assessed by the WST-1 colorimetric assay.
Triton X-100 was used as a negative control, and blank cell culture medium without nanoparticles was used as the positive control (set to 100% cell viability). The error bars represent the standard deviation (n = 6).
PLGA: Poly(lactic-co-glycolic acid).
Transport studies
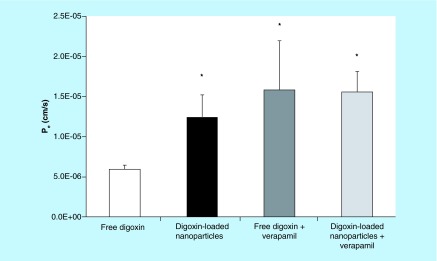
Transport studies were conducted by dosing treatments to the apical (maternal) side of the BeWo cells on Transwell inserts and measuring the basolateral (fetal side) concentrations of digoxin by HPLC at prespecified time points. Treatments were administered at a concentration of 7.2 μg/ml digoxin for all transport studies. Apparent Pe at 2 h (a time point at which the mass flux was linear) was calculated from the concentration of drug in the basolateral chamber [12]. Figure 3 shows that encapsulation of digoxin in nanoparticles resulted in an increase in the apparent permeability of the drug in the apical (maternal) to basolateral (fetal) direction by 2.5-fold (p < 0.05). The transport studies were repeated in the presence of 100 μM verapamil, a P-gp inhibitor [16]. The concentration of verapamil selected for this study matches the concentration used by Polachek et al. as an inhibitor of P-gp in BeWo cells [32]. A significant difference was also seen between permeability values of free digoxin and free digoxin in the presence of inhibitor.
Figure 3. . Apparent permeability (Pe) for the transport of free digoxin (white bar) and digoxin-loaded polyethylene glycol–poly(lactic-co-glycolic acid) nanoparticles (RGPd50105, 10% drug loading, black bar) across BeWo cell monolayers in the apical (maternal) to basolateral (fetal) direction at the 2-h time point.
The transport studies were carried out at 37°C under cell culture conditions with stirring. Pe was also determined for both formulations in the presence of 100 μM verapamil, a P-gp inhibitor. Asterisks indicate significant differences from the permeability of free digoxin (p < 0.05). There were no significant differences between the Pe values of the digoxin-loaded nanoparticles, free digoxin in the presence of verapamil (dark gray bar) or digoxin-loaded nanoparticles in the presence of verapamil (light gray bar). Error bars indicate standard deviation (n = 3). PLGA: Poly(lactic-co-glycolic acid).
Discussion
Fetal cardiac arrhythmia is not easily managed, and treatment often causes severe and unintentional maternal effects. Although digoxin can be used to treat fetal arrhythmias, the placenta as an anatomical barrier between the mother and the fetus limits the maternal-to-fetal transfer of this particular drug, such that a substantial fraction of the dose remains in the maternal circulation. Efflux mechanisms in the placenta (such as P-gp) restrict the passage of digoxin to the fetal circulation. This study was conducted to show that polymeric nanoparticles may present an alternative treatment strategy to deliver digoxin and improve therapy in fetal cardiac arrhythmia. It is widely known that PLGA and PEG are biocompatible polymers and have been previously approved by the FDA for delivery applications [13,33]. PEGylation improves nanoparticle pharmacokinetics by reducing opsonization [34]. Our experiments have also confirmed that at concentrations up to 200 µg/ml, PEGylated PLGA nanoparticles are not cytotoxic to trophoblast cells, and are therefore an ideal drug delivery platform.
It has previously been shown that polymeric particles approximately 100 nm in diameter can cross the placenta [12,35]. In this work, digoxin-loaded nanoparticles were synthesized by a modified solvent displacement method, which resulted in high encapsulation efficiency, narrow polydispersity and appropriate particle size to promote transplacental drug delivery.
One advantage of nanoparticles for drug delivery is controlled release of the drug from the nanocarrier [36]. Sustained release of the drug over time can reduce the required dosing frequency, which improves patient convenience. We observed a sustained release of the digoxin from the polymeric nanoparticles (Figure 1). The lack of substantial burst release is also advantageous, as the drug release kinetics may be used to predict the pharmacokinetics of the drug when delivered as a nanoformulation.
Nanoparticles may also address the challenge of reduced transplacental transfer of digoxin due to P-gp-mediated efflux of the drug within trophoblast cells, which represent the rate-limiting layer of the maternal–fetal interface. BeWo cells express functional P-gp, and therefore serve as an appropriate in vitro placental trophoblast model [9]. It must be noted that the present study does not differentiate between the MDR1 (ABCB1) and MDR3 (ABCB4) isoforms of P-gp. Digoxin is a substrate for both efflux transporters, and verapamil inhibits both transporters [37]. Although the MDR3 P-gp homolog may be more abundant in BeWo cells than MDR1, its predominant expression on the basolateral side of human placental trophoblast cells suggests that it may efflux substrates in the opposite direction, in other words, toward the fetal side [37]. Therefore, MDR1 may maintain appreciable responsibility in limiting the transplacental transfer of digoxin. Until additional mechanistic studies are undertaken to delineate the roles of MDR1 and MDR3 in BeWo cells, we will use the term P-gp broadly in the discussion that follows to include potential contributions of both MDR1 and MDR3.
Figure 3 shows that the encapsulation of digoxin in PEG–PLGA nanoparticles resulted in a 2.5-fold increase in the apparent Pe of digoxin across BeWo cells in the maternal-to-fetal direction. We hypothesized that the nanoparticles may result in increased transfer of digoxin across trophoblast cells by reducing the interaction of the drug from the efflux transporter P-gp. Each nanoparticle carries multiple drug molecules into and across the trophoblast cell barrier (the translocation of fluorescent PLGA nanoparticles across BeWo cell monolayers has been demonstrated previously [20]). Drug molecules encapsulated within the nanoparticles could be shielded from interaction with P-gp as they are delivered across the trophoblast cells. In line with this hypothesis, we observed significantly increased permeability of digoxin upon nanoencapsulation that was similar to the magnitude of increased permeability of free digoxin in the presence of an efflux transporter inhibitor.
Efflux transporters in the placental trophoblast reduce the accumulation of digoxin into the cells, thereby limiting the permeability of the drug across the cells and toward the fetal circulation. Although Figure 3 also demonstrates that the permeability of digoxin across trophoblast cells increases in the presence of verapamil, an efflux transporter inhibitor, this is unlikely to represent an acceptable clinical approach. The coadministration of P-gp inhibitors in order to increase the delivery of a drug that is a substrate of P-gp may lead to serious side effects associated with off-target tissue accumulation, such as neurotoxicity due to inhibition of P-gp in the brain [38].
It should also be noted that the fraction of drug molecules already released from the nanoparticles may still be susceptible to P-gp-mediated efflux. For example, Figure 1 shows that after 2 h – the time point at which Pe was determined in the transport studies – approximately 16% of the drug had been released. This is represented experimentally as the small (but not statistically significant) difference in the apparent permeability of digoxin when encapsulated in nanoparticles administered in the presence of verapamil (the light gray bar in Figure 3) minus the permeability of the nanoencapsulated drug in the absence of verapamil (the black bar in Figure 3). It should also be noted that differences in permeability may also be due to differences in transport mechanisms for nanoparticles, such as endocytosis [39]. Future work will examine these mechanisms in greater detail.
Conclusion
In conclusion, this study provides a novel approach to the treatment of fetal cardiac arrhythmia. This is a life-threatening condition for the fetus, and although treatment with digoxin represents standard treatment and is often successful, it can cause severe side effects in the mother [11]. We have demonstrated that polymeric (PEGylated PLGA) nanoparticles can be successfully loaded with digoxin with high encapsulation efficiency using a modified solvent displacement method. These nanoparticles exhibit sustained drug release kinetics, and nanoencapsulation can protect digoxin from P-gp-mediated efflux in the placental trophoblast layer, thereby increasing the maternal-to-fetal transfer of the drug, which is desired to optimize fetal drug therapy. Increased delivery of digoxin to the fetus will result in lower levels of the drug in the maternal circulation, which should result in reduced risks for the aforementioned maternal side effects. The next step in the development of this strategy is to functionalize nanoparticles with ligands that can further induce targeted uptake of the nanoparticles in the placenta. The use of polymeric nanoparticles encapsulating digoxin to treat fetal cardiac arrhythmia may significantly improve outcomes for both the mother and her fetus.
Future perspective
This study represents the first steps toward specialized nanomedicine for fetal cardiac arrhythmias. Current treatments for fetal arrhythmias have the potential for serious adverse effects for the mother. Strategies that circumvent placental efflux mechanisms to increase the delivery of digoxin to the fetus may reduce the dangers presently associated with the treatment of fetal tachyarrhythmia. Future steps for the development of this novel approach include the incorporation of placenta-specific targeting moieties on the particle surface, as well as in vivo experiments to verify the safety and efficacy of these nanoparticles for treating fetal tachyarrhythmia. In the age of smart drug formulation design, problems such as toxic side effect profiles of systemically delivered drugs can be overcome.
Key terms.
Hydrops fetalis: Fetal condition in which effusions occur in multiple fetal compartments.
Second degree atrioventricular block: One of three degrees of conduction block in the heart, characterized by periodic dropping of impulses traveling from atria to ventricles.
Trophoblast: Placental cellular layer across which nutrient, gas and xenobiotic transport between mother and fetus occur.
ζ-potential: The electric potential at the interface of the electric double layer surrounding a nanoparticle in solution; this measurement is used to assess colloidal stability.
Transepithelial electrical resistance: Measurement of the electrical resistance per unit area between apical and basolateral sides of a cellular layer, used to evaluate tight junction integrity.
Glass transition temperature: The temperature at which an amorphous polymer shifts from a glassy, rigid state to a flexible, rubber-like state.
Executive summary.
Background
Digoxin-loaded polymeric nanoparticles may represent a safer and more effective therapy strategy for the treatment of fetal tachyarrhythmias.
Results & discussion
High encapsulation efficiency (up to 98.4%) was observed for digoxin loading in PEG–PLGA nanoparticles.
Small size (84 ± 0.4 nm), narrow size distribution and sufficiently negative ζ-potential were observed, which represent ideal properties for this particular drug delivery application.
Sustained drug release was observed; the release kinetics fit the Higuchi model, i.e., a linear correlation between the square root of time and the percent of drug released.
Significantly higher drug permeability across trophoblast cells was observed when digoxin was encapsulated in the nanoparticles compared with free digoxin. The nanoparticles effectively shield digoxin from P-gp-mediated efflux.
Conclusion
Digoxin-loaded polymeric nanoparticles are able to deliver digoxin across placental trophoblast cells and avoid placental P-gp-mediated efflux. These properties are amenable to future therapeutic strategies for fetal tachyarrhythmia.
Supplementary Material
Acknowledgements
A portion of this work was presented at the 40th Annual Meeting & Exposition of the Controlled Release Society (CRS) and subsequently highlighted in the 31st volume of the CRS Newsletter.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NICHD, OD or the NIH.
Financial & competing interests disclosure
Support from the Saudi Cultural Mission is gratefully acknowledged. This work was also supported in part by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women's Health Program, BIRCWH) from the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the Office of the Director (OD), NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Thakur V, Fouron JC, Mertens L, Jaeggi ET. Diagnosis and management of fetal heart failure. Can. J. Cardiol. 2013;29:759–767. doi: 10.1016/j.cjca.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg AA, Galan HL. Fetal drug therapy. Pediatr. Clin. North Am. 1997;44:113–135. doi: 10.1016/s0031-3955(05)70466-1. [DOI] [PubMed] [Google Scholar]
- 3.Huhta JC. Fetal congestive heart failure. Semin. Fetal Neonatal Med. 2005;10:542–552. doi: 10.1016/j.siny.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Parker LA. Hydrops Fetalis. Newborn Infant Nurs. Rev. 2006;6:e1–e8. [Google Scholar]
- 5.Hofstaetter C, Hansmann M, Eik-Nes SH, Huhta JC, Luther SL. A cardiovascular profile score in the surveillance of fetal hydrops. J. Matern. Fetal Neonatal Med. 2006;19:407–413. doi: 10.1080/14767050600682446. [DOI] [PubMed] [Google Scholar]
- 6.Mongiovì M, Fesslova V, Fazio G, Barbaro G, Pipitone S. Diagnosis and prognosis of fetal cardiomyopathies: a review. Curr. Pharm. Des. 2010;16:2929–2934. doi: 10.2174/138161210793176428. [DOI] [PubMed] [Google Scholar]
- 7.McElhinney DB, Tworetzky W, Lock JE. Current status of fetal cardiac intervention. Circulation. 2010;121:1256–1263. doi: 10.1161/CIRCULATIONAHA.109.870246. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the treatment options of fetal arrhythmias and their poor outcomes. There is a critical need for safer alternative treatment options.
- 8.Petropoulos S, Gibb W, Matthews SG. Developmental expression of multidrug resistance phosphoglycoprotein (P-gp) in the mouse fetal brain and glucocorticoid regulation. Brain Res. 2010;1357:9–18. doi: 10.1016/j.brainres.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Utoguchi N, Chandorkar GA, Avery M, Audus KL. Functional expression of P-glycoprotein in primary cultures of human cytotrophoblasts and BeWo cells. Reprod. Toxicol. 2000;14:217–224. doi: 10.1016/s0890-6238(00)00071-x. [DOI] [PubMed] [Google Scholar]; •• Demonstration of functional P-gp in BeWo cells, important for BeWo cell transport study design.
- 10.Kleinman CS, Nehgme RA. Cardiac arrhythmias in the human fetus. Pediatr. Cardiol. 2004;25:234–251. doi: 10.1007/s00246-003-0589-x. [DOI] [PubMed] [Google Scholar]
- 11.Ward RM. Pharmacology of the maternal-placental-fetal-unit and fetal therapy. Prog. Pediatr. Cardiol. 1996;5:79–89. [Google Scholar]; • Describes the dangers of maternal administration of digoxin for transplacental delivery.
- 12.Cartwright L, Poulsen MS, Nielsen HM, et al. In vitro placental model optimization for nanoparticle transport studies. Int. J. Nanomedicine. 2012;7:497–510. doi: 10.2147/IJN.S26601. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Illustrates the use of BeWo cells in modeling the transplacental transport of nanoparticles, and the cell culture methods used therein.
- 13.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Campolongo MJ, Luo D. Drug delivery: old polymer learns new tracts. Nat. Mater. 2009;8:447–448. doi: 10.1038/nmat2456. [DOI] [PubMed] [Google Scholar]
- 15.Poulsen MS, Rytting E, Mose T, Knudsen LE. Modeling placental transport: correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol. In Vitro. 2009;23:1380–1386. doi: 10.1016/j.tiv.2009.07.028. [DOI] [PubMed] [Google Scholar]; •• Discusses the relevance and limitations of using BeWo cells for in vitro modeling of placental transport of drugs.
- 16.Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem. Pharmacol. 2009;78:1272–1278. doi: 10.1016/j.bcp.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck-Broichsitter M, Rytting E, Lebhardt T, Wang X, Kissel T. Preparation of nanoparticles by solvent displacement for drug delivery: a shift in the ‘ouzo region’ upon drug loading. Eur. J. Pharm. Sci. 2010;41:244–253. doi: 10.1016/j.ejps.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Rytting E, Bur M, Cartier R, et al. In vitro and in vivo performance of biocompatible negatively-charged salbutamol-loaded nanoparticles. J. Control. Release. 2010;141:101–107. doi: 10.1016/j.jconrel.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Hu L, Jia H, Luo Z, Liu C, Xing Q. Improvement of digoxin oral absorption in rabbits by incorporation into solid lipid nanoparticles. Pharmazie. 2010;65:110–113. [PubMed] [Google Scholar]
- 20.Ali H, Kalashnikova I, White MA, Sherman M, Rytting E. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int. J. Pharm. 2013;454:149–157. doi: 10.1016/j.ijpharm.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses the importance of size and other physicochemical characteristics of nanoparticles in transplacental delivery.
- 21.Kalashnikova I, Albekairi N, Al-Enazy S, Rytting E. Characterization of drug-loaded nanoparticles. In: Naik J, editor. Nano Based Drug Delivery. IAPC Publishing; Zagreb, Croatia: 2015. pp. 147–164. [Google Scholar]
- 22.Ali H, Kilic G, Vincent K, Motamedi M, Rytting E. Nanomedicine for uterine leiomyoma therapy. Ther. Deliv. 2013;4:161–175. doi: 10.4155/tde.12.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ushigome F, Takanaga H, Matsuo H, et al. Human placental transport of vinblastine, vincristine, digoxin and progesterone: contribution of P-glycoprotein. Eur. J. Pharmacol. 2000;408:1–10. doi: 10.1016/s0014-2999(00)00743-3. [DOI] [PubMed] [Google Scholar]
- 24.Nanovskaya T, Nekhayeva I, Karunaratne N, Audus K, Hankins GD, Ahmed MS. Role of P-glycoprotein in transplacental transfer of methadone. Biochem. Pharmacol. 2005;69:1869–1878. doi: 10.1016/j.bcp.2005.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL. In vitro models for studying trophoblast transcellular transport. Methods Mol. Med. 2006;122:225–239. doi: 10.1385/1-59259-989-3:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Q, Li J, Wang F, et al. Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition. Cell. Signal. 2013;25:1027–1035. doi: 10.1016/j.cellsig.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am. J. Physiol. 1997;273:C1596–C1604. doi: 10.1152/ajpcell.1997.273.5.C1596. [DOI] [PubMed] [Google Scholar]
- 28.Mørck TJ, Sorda G, Bechi N, et al. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010;30:131–137. doi: 10.1016/j.reprotox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Li H, van Ravenzwaay B, Rietjens IM, Louisse J. Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch. Toxicol. 2013;87:1661–1669. doi: 10.1007/s00204-013-1074-9. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Dai WG. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta. Pharm. Sin. B. 2014;4:18–25. doi: 10.1016/j.apsb.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv. Drug Deliv. Rev. 2001;48:139–157. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 32.Polachek H, Holcberg G, Polachek J, et al. Carrier-mediated uptake of Levofloxacin by BeWo cells, a human trophoblast cell line. Arch. Gynecol. Obstet. 2010;281:833–838. doi: 10.1007/s00404-009-1177-y. [DOI] [PubMed] [Google Scholar]
- 33.Ning YM, He K, Dagher R, et al. Liposomal doxorubicin in combination with bortezomib for relapsed or refractory multiple myeloma. Oncology (Williston Park) 2007;21:1503–1508. [PubMed] [Google Scholar]
- 34.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Wick P, Malek A, Manser P, et al. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010;118:432–436. doi: 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rytting E, Nguyen J, Wang X, Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008;5:629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- 37.Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1357–R1365. doi: 10.1152/ajpregu.00630.2005. [DOI] [PubMed] [Google Scholar]
- 38.Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin. Pharmacokinet. 2003;42:59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 39.Iversen TG, Skotland T, Sandvig K. Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today. 2011;6:176–185. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.