SUMMARY
Age-related hearing loss (ARHL) has a multifactorial pathogenesis and it is an inevitable hearing impairment associated with reduction of communicative skills related to ageing. Increasing evidence has linked ARHL to more rapid progression of cognitive decline and incidental dementia. Many aspects of daily living of elderly people have been associated to hearing abilities, showing that hearing loss (HL) affects the quality of life, social relationships, motor skills, psychological aspects and function and morphology in specific brain areas. Epidemiological and clinical studies confirm the assumption of a relationship between these conditions. However, the mechanisms are still unclear and are reviewed herein. Long-term hearing deprivation of auditory inputs can impact cognitive performance by decreasing the quality of communication leading to social isolation and depression and facilitate dementia. On the contrary, the limited cognitive skills may reduce the cognitive resources available for auditory perception, increasing the effects of HL. In addition, hearing loss and cognitive decline may reflect a 'common cause' on the auditory pathway and brain. In fact, some pathogenetic factors are recongised in common microvascular disease factors such as diabetes, atherosclerosis and hypertension. Interdisciplinary efforts to investigate and address HL in the context of brain and cognitive ageing are needed. Surprisingly, few studies have been adressed on the effectiveness of hearing aids in changing the natural history of cognitive decline. Effective interventions with hearing aids or cochlear implant may improve social and emotional function, communication, cognitive function and positively impact quality of life. The aim of this review is to overview new insights on this challenging topic and provide new ideas for future research.
KEY WORDS: Hearing loss, Dementia, Elderly, Cochlear implant, Cognitive impairment
RIASSUNTO
La perdita dell'udito legata all'età o presbiacusia è un deficit correlato al processo irreversibile di invecchiamento che riconosce una patogenesi multifattoriale. Crescenti osservazioni hanno collegato la presbiacusia a una rapida progressione del declino cognitivo e incidentalmente con la demenza. Molti aspetti della vita quotidiana degli anziani sono stati collegati alle loro capacità uditive, mostrando che la perdita uditiva incide sulla qualità della vita, i rapporti sociali, le capacità motorie, gli aspetti psicologici, la funzione e la morfologia di specifiche aree cerebrali. Studi epidemiologici e clinici confermano l'ipotesi di un legame tra queste condizioni e questo lavoro ha lo scopo di fare il punto sui meccanismi patogenetici che sostengono tale associazione. Lo sforzo di un lavoro congiunto tra otorinolaringoiatri, audiologi, neurologi e cognitivisti è quello di chiarire gli aspetti comuni, le possibilità di diagnosi e di intervento precoce al fine di ridurre gli effetti dell'uno sull'altro di questi processi degenerativi. Le osservazioni sperimentali e cliniche si concentrano su differenti aspetti: in primo luogo la deprivazione uditiva per lungo tempo può avere un impatto negativo sulle prestazioni cognitive diminuendo la qualità della comunicazione che porta all'isolamento sociale e la depressione e quindi facilitare la demenza. Al contrario, le capacità cognitive limitate possono ridurre le risorse cognitive disponibili per la percezione uditiva, aumentando così gli effetti della perdita dell'udito. Inoltre, questa associazione può rappresentare la conseguenza di una 'causa comune' nella patogenesi del deficit uditivo e del sistema nervoso centrale. Infatti, molti dei fattori eziopatogenetici sono comuni, quali le cause microvascolari della malattia (es. diabete, aterosclerosi, ipertensione). La sfida di questi anni è quella di aumentare le conoscenze sui rapporti tra invecchiamento cerebrale e cognitivo ed ipoacusia, grazie anche ai progressi del neuroimaging. Sorprendentemente pochi dati sono stati pubblicati sull'utilità delle protesi acustiche nel cambiare la storia naturale di declino cognitivo. La protesizzazione e gli impianti cocleari possono migliorare le attività sociali e la sfera emotiva, la comunicazione e quindi più in generale la funzione cognitiva, con un globale impatto positivo sulla qualità della vita. Lo scopo di questo lavoro è quello di fornire le informazioni attualmente disponibili in letteratura su rapporto tra declino cognitivo e deficit uditivo nell'anziano, fornendo nuovi spunti di ricerca per il futuro.
Introduction
It is well known that both hearing loss (HL) and cognitive impairment are associated with ageing. The first report on the independent relationship between hearing impairment and cognitive dysfunction appeared about 30 years ago 1, suggesting the hypothesis that age-related hearing loss (ARHL) may contribute to dementia. Probably the lack of interaction among ENT specialists, audiologists, neurologists, epidemiologists and cognitive scientists has limited the possibility to better recognise their correlation and impact on elderly people. More recently, growing epidemiological, neurobiological and neuroimaging evidence opened a new interest in this field and an increasing number of reports have focused on the relationship and effects of both HL and cognitive decline on the quality of life and rehabilitative perspectives 2. ARHL can be defined as a progressive, bilateral, symmetrical HL that reduces an individual's communicative skills due to age and can be considered a multifactorial complex disorder, with both environmental and genetic factors contributing to the aetiology of the disease 3. Cognitive impairment generically refers to a wide range of conditions ranging from mild cognitive impairment to severe dementia, while different degrees of hearing loss can impact the communicative impairment and quality of life. The increasing prevalence of cognitive decline 4 5 and the devastating impact of dementia on affected individuals and the burden imposed on families and society has made prevention and treatment of dementia a public health priority. Many aspects of daily living of elderly people have been linked to hearing abilities, showing that ARHL affects the quality of life, social relations, motor skills, psychological aspects, function and morphology in specific brain areas. On the basis of clinical evidence, it has been suggested that ARHL is linked with more rapid progression of dementia. The potential public health impact of ARHL in the context of dementia is substantial given the high worldwide prevalence of HL in older adults and the ready availability of existing hearing rehabilitative interventions, which remain risk free and underutilised. Until now, in the literature there are no studies demonstrating the utility of hearing rehabilitation in changing the natural history of dementia. Interdisciplinary efforts to investigate and address ARHL in the context of brain and cognitive impairment in older subjects are challenging. Despite the increasing attention, the relationship between cognitive status and HL is still controversial, and in particular it remains to be investigated whether HL is involved in the causal mechanisms of dementia or whether there is an independent relationship in which ARHL might enhance the effects of dementia. The aim of this paper is to focus on the new insights on epidemiological aspects, prevention, assessment and intervention strategies for older adults with HL who are at risk of developing dementia.
Epidemiology of hearing loss and dementia
Hearing loss affects approximately one-third of adults from 61 to 70 years of age and more than 80 percent of those older than 85 years. After hypertension and arthritis, it is the most common health disorder in older patients. More than 90% of HL in older patients can be classified as ARHL, while few patients are effected by conductive or mixed hearing loss. The impact of ARHL will increase due to the ageing of baby boomers and it is reasonable to assume a further escalation because of the constant growth of average lifespan in industrialised countries. In the US, 26.7 million adults older than 50 years suffer from ARHL and only 3.8 million use hearing aids 6, while, in UK 8.1 million suffer from HL, of whom 1.4 million use hearing aids 4-7. According to the United Nations, the global population will grow from 6.9 billion in 2010 to 9.3 billion in 2050. The proportion of the population aged 60 or older will nearly double in the same period, reaching 21% of the total population in 2050, or nearly 2 billion of people in 2050. Males demonstrate a higher incidence of presbycusis with earlier onset compared to women. Among European Countries, in Italy, 1 in 6 of people suffer some form of HL, in Finland 1 of 7, while in Sweden and Denmark 1 of 10 people are affected by ARHL. For the WHO, in Europe about 70 million people are affected by ARHL, even if the statistics include also slight hearing levels with threshold greater than 25 dB. Only about 20% of people 65 years or older with moderate to profound ARHL perceive themselves as hearing impaired and about 70% of people with ARHL refuse hearing aids (www.actiononhearingloss.org.uk/yourhearing/ aboutdeafness-and-hearing-loss/statistics.aspx). Interestingly, costs for hearing aids are lower than the cost of the untreated HL with an expense cost of € 213 billion per year in Italy and France, about € 22 billion in UK and € 30.2 billion in Germany 5 6. In developed countries, hearing loss (HL) is very prevalent; although in African and South East Asian regions preventable causes of hearing impairment such as otitis media, sensorineural damage due to nutritional deficiencies, noise-induced hearing loss, ototoxicity and genetic hearing loss from consanguinity are more commonly reported in the literature than ARHL even if they can contribute to this condition 8. Epidemiological evidence 7-9 supports the association between ARHL and late-life cognitive disorders suggesting that hearing impairment is a modifiable factor that, with appropriate treatment, could facilitate activities of daily living, decrease isolation and loneliness of aged subjects and slow down cognitive decline.
Similar to ARHL, there are gradual and age-related losses in cognitive processing including speed of information processing, memory and attention. Beyond normal agerelated cognitive changes, clinically mild cognitive impairment (MCI) increases with age and about one fifth of people have some degree of cognitive loss by the age of 70 years 10. The prevalence of dementia increases from 5% in those 71 to 79 years to 37% in those 90 years and older, with an overall prevalence of approximately 14% for those over 70 years of age 9. Moreover, a continuum between MCI and dementia has been recognised 12 13 and patients with MCI are at an intermediary stage that often, but not always, progresses to Alzheimer's disease (AD), which is the most common form of dementia. It is suggested that the rate of conversion from MCI to AD is about 10% to 15% per year, which increases to 80% after 6 years, and it is higher than the rate of 1-2% per year observed in the general population 14. Numbers of dementia are impressive: in 2005, 24.3 million people were estimated to have dementia, with 4.6 million new cases of dementia every year (one new case every 7 patient). This number is expected to double every 20 years to 8.1 million people by 2040 4 5.
Altogether, given the high prevalence of both hearing loss and cognitive decline in older adults that increases in prevalence with age 8-15, it is reasonable to assume that cognitive disorders are common in many of the oldest adults who have ARHL. Therefore, epidemiological evidence supports the hypothesis that there is a link between ARHL and dementia.
The hypotheses on the relationship between age-related hearing loss and cognitive decline
Even if epidemiological, audiological and auditory central testing corroborate the association between HL and incidental dementia 16 17, different relationship are debated. Major evidence supports the hypothesis that cognitive decline can reduce the cognitive resources available for auditory perception manifesting as hearing loss and reduced understanding of speech, also indicated as "cognitive load on perception hypothesis". In contrast, it has been shown that the risk of developing dementia is higher in individuals affected by ARHL 11-13 17 suggesting that hearing loss leads to cognitive decline because of degradation of inputs to brain (Fig. 1). Lin et al. 11 demonstrated that for every 10 dB increase in HL over 25 dB HL there was a 20% increased risk of developing dementia. More recently, Gurcel et al. 18 showed that in adults over 65 years of age the mean time for developing dementia was 10.3 years in those with hearing loss at baseline versus 11.9 years for counterparts with normal hearing. Thus, ARHL has been found to be independently associated with poorer cognitive function and incident dementia; in particular, normal hearing individuals, compared to mild, moderate, and severe hearing loss patients had a two, three, and five-fold increased risk of incident all-cause dementia, respectively 13 19. It has also been demonstrated that ARHL impacts several domains of healthy aging including social engagement, physical mobility and activity, falls, vitality and even dementia, in addition to cognitive dysfunction 1 15 20. Moreover, it has been demonstrated that scores from several cognitive tests generally declined linearly with increasing levels of HL 12,17. A strong association has been observed between HL and measures of memory and executive function. Furthermore, a significant association between severe HL and poorer cognitive function has been found administering both verbal and non-verbal cognitive tests to older patients 21-25. More recently, Dupuis and colleagues 26 confirmed Lin's data, but focused attention on the influence of HL or other sensory deficits on the results of cognitive tests. Nevertheless, this datum is still debated, and previous studies have been prone to exclude this relationship 24 25.
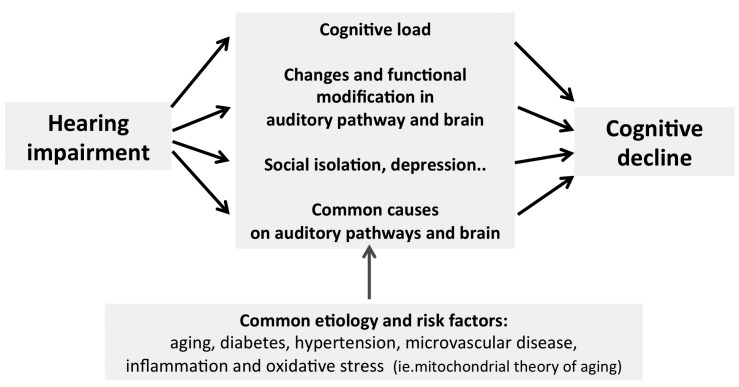
Fig. 1.
Conceptual model of the association of hearing loss with cognitive decline (adapted from Lin 2).
All efforts should be made to establish the relationship between HL and cognition in older patients undergoing to clinical evaluation. In some cases, cognitive losses may be misdiagnosed or conversely over-diagnosed when the sensory abilities of patients are not considered. This matter is especially important when a diagnosis of dementia is based on orally administered evaluation using tests environment in which there may be varying levels of ambient noise. A recent study by Jorgensen et al. 27 indicates that in only 13% of patients in a primary care clinic who were affected by memory loss was hearing status investigated. In summary, the association between ARHL and cognitive impairment is now well established by several cross-sectional and longitudinal studies, and it is unquestionable that hearing loss is more common in patients affected by dementia that in healthy older adults.
Interestingly, it has been postulated that ARHL may act as a ''second hit'' on the brain, thus adversely affecting cognitive performance and increasing the risk of dementia by adding to brain injuries derived from other disorders (e.g., amyloid-beta accumulation, neurofibrillary tangles and microvascular disease). For example, cross-sectional neuroimaging studies have demonstrated that peripheral hearing impairment is associated with reduced cortical volumes in the primary auditory cortex and variation in the integrity of central auditory white matter tracksas described in the following paragraph 28-32 37-40. Longitudinal data from animal models 33 34 also demonstrated that cochlear impairment may precipitate changes in cortical reorganisation and brain morphometry. Accordingly, confirmation for a link from HL and cognitive impairment is also derived by the evidence that both conditions are sequelae of an underlying pathology such as hypertension, diabetes and/or atherosclerosis (Fig. 1). As previously mentioned, ARHL is a complex multifactorial disorder with both environmental (i.e. noise, ototoxic drugs, atherosclerosis, diabetes, hypertension) and genetic factors (i.e. genetic susceptibility) that contribute to its aetiology. As experimentally shown 3 33 34, the aged cochlea shows degeneration of stria vascularis, sensorineural epithelium and neurons in the spiral ganglion and auditory cortex of the central auditory pathways related to exogenous factors (e.g. noise and ototoxic drugs, vascular risk factors) inducing oxidative stress pathways (including the "mitochondrial theory of ageing") and inflammation; some of these mechanisms are common to neurodegenerative diseases including AD. Experimentally, the role of microvascular damage in the pathogenesis of AHRL has been clearly demonstrated together with the evidence that spiral ganglion deafferentation is associated with altered dendritic architecture of auditory pyramidal neurons 3 33. Thus, common factors could underlie a simple correlation between hearing and cognition including age, vascular risk factors and social factors (e.g. education) 2 11-13.
Alternatively, mechanistic hypotheses have been proposed that argue for a causal association between ARHL and cognitive decline, including increased social isolation and loneliness, increased cognitive load and changes in brain structure 35. Further studies will be needed to clarify this association.
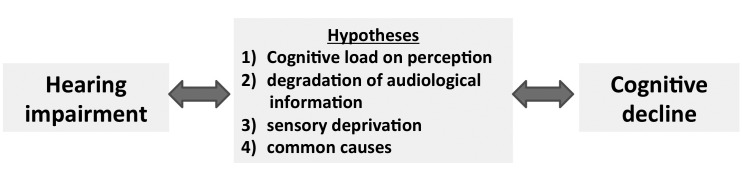
All epidemiological and clinical evidence has been organized in the following four hypotheses (Fig. 2): first, cognitive decline may reduce the cognitive resources that are available for auditory perception, increasing the effects of hearing loss, also referred as "cognitive load on perception hypothesis". When the inputs are poor, either through degraded stimuli or impaired perception, additional cognitive resources are required to understand the signal. For the "information degradation hypothesis", an additional effort is required either because the stimoli are degraded, for example in noisy enviromenment, because the perception is decreased, and therefore cognitive resources used for the signal codification are not available for cognitive roles 28. In contrast, as previouly mentioned, the "sensory-deprivation hypothesis" described by Lin and colleagues 11-13, suggested that hearing loss causes cognitive decline that is permanent or potentially remediable after rehabilitation. According to this hearing impairment increases cognitive effort in patients with cognitive defects and depressive symptoms 35. A plausible mechanism may be that impaired perception could lead to worsening cognition over time and social isolation which in turn leads to cognitive decline. Finally, a fourth mechanism takes inspiration by the evidence that some common factors cause both declines, also called the "common cause hypothesis", in facts the clinical demonstration is that multiple sensory modalities and cognition appear to decline concurrently in the older patients. As summarised by Lin et al. 2 in a convincing model (Figg. 1, 2), there is a common aetiology for ARHL and cognitive decline, even dementia, that is based firstly, on microvascular and ageing risk factors, and secondly hearing impairment affects different domains such as social isolation, loneliness, increased cognitive load and changes in brain structure that may contribute to the onset of cognitive decline and dementia 2.
Fig. 2.
The epidemiological and clinical evidence is summarised in the following hypotheses on the relationship between HL and cognitive decline.
Peripheral and central origin of ARHL: a link between central auditory processing disorders and cognitive decline
Traditionally, cochlear damage, including hair cell loss and damage of stria vascularis and spiral ganglion neurons, was considered to be the main cause for ARHL according to Schuknecht topology, which correlated the patterns of hearing loss with the location of the hearing defect. Temporal bone studies suggested three main types of presbycusis: sensory presbycusis with high frequencies HL caused by hair cell loss and subsequent neural degeneration, neural presbycusis characterised by the loss of word discrimination caused by the primary degeneration of cochlear neurons and metabolic/strial presbycusis characterised by a flat pure-tone audiometry caused by the atrophy of stria vascularis 36. Additional types have been described: mechanical (cochlear/conductive hypothetical) with changes in the basilar membrane affecting its properties and function, and mixed and indeterminate when multiple influences interact 37. However, sound perception depends not only on normal cochlea but also on the function of the auditory pathway, which may explain the fall of discrimination, in particular in noisy environments, and increasing evidence supports a pivotal role of central auditory processes (CAP) in presbycusis. The role of CAP is well established in several behavioural phenomena such as sound localisation and lateralisation, auditory discrimination, temporal aspects of audition (temporal resolution, masking, integration and ordering), auditory performance with competing acoustic signals and auditory performance with degraded signals. Even if the simplest auditory tasks are influenced by higher-level, non modality-specific factors as attention, learning, motivation, memory and decision processes and higher level contextual information influence the perceptual analysis of the acoustic signal; while various knowledge sources interact and support the auditory processing of spoken language and other complex acoustic signals. Briefly, central auditory process disorders (CAPDs) concern an auditory perceptual dysfunction that cannot be explained on the basis of peripheral hearing loss and refer to impairment in central auditory pathways, such as neural transmission, feature extraction deficit, or information processing problems that lead to impaired speech understanding. CAPD can affect all people at any age (even children or young adults) as a consequence of brain focal injures, or neurological and genetic disorders 38-42.
The existence of ARHL decline in CAPD is well established, but the mechanism and effects are still controversial. While the presence of a pure CAPD seems to be uncommon in older patients, the evaluation of comorbidity between central and peripheral damage using central auditory testing in these patients may be challenging. The prevalence of ARHL and CAPD in a population older than 65 years was 64.1 and 14.3%, respectively 43. Furthermore, studies on the association between CAP dysfunction and MCI or AD are limited 44. Many authors identified these disorders in the auditory portions of the central nervous system without clear lesions 39. The pathophysiology of CAPD is not fully understood, probably involves interhemispheric interaction and corpus callosum function. CAPD tests typically require extracting auditory signals in noise or competing signals and the diagnosis of a CAPD can be very tricky in older patients even affected by cognitive decline 39 41. Gates et al. 16 45 investigated the relationship between CAPD and dementia. In a first study they found that CAPD was evident in subjects with mild AD, whereas peripheral auditory function was not different from control subjects. A link between CAPD and cognitive dysfunction is also explained by the observation that older patients with CAPD seem to be more prone to experience dementia than those without CAPD. Furthermore, in older people with mild, amnestic, single domain cognitive impairment (MCI), severe CAPD is more prevalent than in people with normal cognitive status and the presence of CAD is more likely to be associated with an increased risk of dementia diagnosis in the follow-up period 46 47. Gates et al. 45 in a 3-year follow-up study showed that severe CAPD was predictive for the risk of subsequent diagnosis of AD. Moreover, as recently reviewed by Panza et al. 44, longitudinal studies also confirmed that the peripheral ARHL is associated with decline of several cognitive domains and accelerated cognitive decline.
As previously mentioned, diagnosis of CAPD is clinically argue and results of auditory central testing controversial. From 2009 to 2011, the America Academy of Audiology Task Force on Central Presbycusis reviewed 145 papers to understand the evidence on age-related changes in auditory portions of the central nervous system and the impact of such changes on everyday communication and function. Based on this review of the literature, the authors concluded that the evidence for the existence of central presbycusis in the isolated entity is insufficient 48. On the other hand, recent findings support the existence of central presbycusis as a multifactorial condition that involves age- and/or disease-related changes in the auditory system and brain 44.
Age-related changes in the human central auditory system: morphological and neuroimaging evidence
In the last years, increasing interest has been focused on neuroplasticity of the brain which indicates the changes of its structure and function in response to environment and experience occurring in both synaptic, network and anatomical levels. It is common sense that an "active" life is beneficial for the mind and brain. In fact, it is well known that both physical and intellectual activity have a positive influence on the incidence of neurodegenerative disorders and cognitive decline. Recently, it has been described that enriched environment improves learning, enhances neurogenesis and branching, synapse formation and activity of neurotrophic factors 33 34.
In principle, ageing causes cortical atrophy, which is accompanied by shrinkage of grey and white matter volumes and enlargement of the cerebrospinal fluid space 49. Post-mortem analyses have shown a decline in the number of dendrites, synapses and neuronal fibres without direct loss of neurons 50. Thus, as also discussed in the previous paragraph, lifestyle and environment can modulate neuroplasticity even in aged adults, who can be affected by neurobiological and anatomical changes. Major modifications include white matter de-myelination and grey matter shrinkage, altered neurotransmission and neural atrophy that can be enhanced by visual and hearing deprivation involving neural connectivity and brain organisation. In this field, studies are still ongoing. However, growing contributions by neuroimaging and neurobiological findings suggest a cortical and neuronal reorganisation after hearing loss in consequence of adaptive or maladaptive plasticity observed in neurodegenerative diseases and HL induced by exogenous factors or ageing 33 34.
It has been recently demonstrated by MRI that subjects with hearing impairment have accelerated rates of whole brain atrophy as well as specific volume declines in the right superior, middle and inferior temporal gyri over a mean 6.4 years of follow-up 17. These findings extend the discussion on whether peripheral hearing impairment has broader implications for brain structure and function. Prior cross-sectional neuroimaging studies demonstrated that greater audiometric hearing impairment is associated with reduced volumes in the primary auditory cortex and temporal lobe 28-30. Other studies, using diffusion-tensor imaging of central auditory pathways, demonstrated decreased fractional anisotropy in the lateral lemniscus and inferior colliculus in individuals with hearing impairment versus those with normal hearing 31 50. The temporal regions are intriguing because they are important not only for spoken language processing, but also for semantic memory and sensory integration, and are involved in the early stages of mild cognitive impairment or early AD 52. A shared neuropathological or intrinsic cellular ageing process leading to both cochlear and brain ageing is a mechanistic option. Hearing impairment may also be potentially associated with brain volume changes through reduced neural stimulation of the auditory cortex by impoverished auditory signals 28. New insights on the biochemical changes in the auditory cortex have been detected by MR spectroscopy, which is an interesting tool for studying cortical mechanisms. In a recent report 53 it was demonstrated that ARHL is accompanied by the reduction of the excitatory activity in the auditory cortex. By using MR spectroscopy, the authors examined metabolite levels in the auditory cortex of subjects older than 65 years either with mild or severe presbycusis, demonstrating significant lower concentrations of glutamate and N-acetylaspartate in aged subjects with increased levels of lactate. Significant differences were not found in other metabolites, including GABA, which is the most important inhibitory neurotransmitter. In summary, the older brain suffers not only from atrophy but also from changes in the content of some metabolites affecting both grey and white matter even if the morphological findings in neuroimaging are still controversial. We expect that in the future robust evidence will be obtained by improvements in neuroimaging techniques. In principle, there is substantial evidence supporting the hypothesis that the modifications observed in the brain in patients affected by ARHL depend more on ageing than on hearing impairment.
Impact of ageing and hearing loss on linguistic abilities
Evidence on the relationship among ageing, hearing loss and linguistic abilities is scarce, however the question of how hearing impairment affects linguistic abilities due to the consequences on the quality of life (i.e. social isolation, depression, etc.) remains an interesting feature. In principle, linguistic abilities do not seem to be affected by age. Although there is little loss of word knowledge, word retrieval during speaking becomes slower and more difficult 54. This can lead to more frequent occurrences of "tipof- the-tongue" status, where a desired word or person's name is known, but there is difficulty in its retrieval 55 56.
On the other hand, spoken language comprehension tends to be preserved, despite atrophy in the neural regions involved. Some functional MRI studies identified a twocomponent model of sentence comprehension: a core sentence-processing area located in the perisylvian region of the left cerebral hemisphere and an associated network of brain regions that support working memory and other resources needed for comprehension of long or syntactically complex sentences 57-59. In fact, working memory is known to constrain the comprehension of sentences with complex syntactic structures 60 that result in adults in the alteration in producing syntactically complex utterances. The syntactic organisation represents a special burden on working memory 61.
Although, there is a question of whether all aspects of language processing are constrained by a single working memory resource or by a complex of specialised resources 62, there is no doubt that working memory limitations affect cognition in aging adulthood. It might be predicted by two observations: firstly, complex syntax and rapid speech rates operate in a multiplicative fashion in affecting sentence comprehension 63. Secondly, in spoken language comprehension, it is well known that recognition is superior for words heard within a meaningful sentence than for words heard in isolation. This longstanding observation reflects the general principle that the amount of sensory information needed for correct recognition of any stimulus will be inversely proportional to its probability within a constraining context 64.
This generality, although correct, overlooks the potential importance of the cognitive effort required for comprehension of speech that is syntactically complex 65.
This is important for word recognition because understanding the meaning of a sentence is the force that constrains the probability of a particular word in that context. The comprehension of syntactically complex speech may draw on working memory resources that are already limited in normal ageing 65; many studies have shown differential effects of syntactic complexity on older adults' comprehension relative to that of younger adults. This latter aspect has been investigated in older adults with hearing loss, because the decreased activation of specialised processing regions of brain, and limited ability to coordinate activity between regions, contribute to older adults' difficulty with sentence comprehension under difficult listening condition 66. In fact, it would be expected that when older adults with already limited working memory resources are further strained by perceptual effort attendant to even a mild hearing loss, the negative effects on sentence comprehension of age and syntactic complexity can be further multiplied 59.
Stewart and Wingfield 67 confirmed the common findings of better report accuracy for meaningful sentences than for words heard in isolation without a sentence context, but for older adults there was also a significant effect of syntactic complexity of sentence stimuli. This effect was further increased by HL. These results are interpreted in terms of age-limited working memory resources that are impacted both by the resource demands required for comprehension of syntactically complex sentences and by effortful listening attendant to hearing.
An interesting study analysed the role of hearing acuity, age and verbal and cognitive ability in word recognition when words are heard in the absence of a linguistic context, or when heard proceeded by varying degrees of contextual constraint. Results emphasise the importance of cognitive function in auditory performance 57 58, showing in addition, that as the degree of contextual support from a linguistic context increases, the relative contributions of cognitive ability and hearing acuity are reversed. Specifically, in a neutral context there is a large role for hearing acuity on word recognition and a modest role for cognitive ability. By contrast, in the highest context condition, hearing acuity was no longer a significant predictor, but general cognitive ability played a significant role. These findings underscore the need to take into account the relationship of individual differences in cognitive ability and constraints of linguistic context, as well as hearing acuity.
Impact of hearing loss on physical activity and quality of life in the elderly
As previoulsy adduced, maintaining an optimal level of physical functioning is a critical aspect of healthy aging, however, longitudinal studies on the association of hearing impairment with incident functional are not conclusive, with some studies demonstrating a positive association 11-13, and other contributions denying 68. This heterogeneity on results is likely explained by differences in how hearing (e.g. subjective self-report3 vs objective clinical audiometry) and physical functioning (e.g. activities of daily living, walking difficulty, falls) or other selfreported measures were quantified 68-72.
Chen and colleagues 73 reported that subjects with average greater hearing impairment had poorer short physical performance battery scores and slower gait speeds at two time points 10 years apart, which were also reflected in an increased risk of incident disability and requirement for nursing care in women. Moreover, Gispen et al. 74 showed that moderate or severe hearing impairment in older adults was independently associated with less physical activity as measured subjectively by the self-report and objectively according to accelerometry. Patients with moderate or severe hearing impairment had a 59% greater possibility of having lower levels of self-reported physical activity and 70% greater odds of having lower levels of accelerometermeasured physical activity than those with normal hearing. Interestingly, several studies have demonstrated associations between HL and poor physical functioning 75-80, poor cardiorespiratory fitness, sedentary behaviour and slow gait speed in older adults 81. In contrast, other reports have indicated that there is no significant association between HL and physical functioning and activity 82. Subjective measurement 83 or varying definitions 84 of hearing may explain the reported heterogeneity in study results.
Different mechanisms can explain the observed association between hearing and physical activity. Individuals with moderate or greater hearing impairment may perform less physical activity because they are socially isolated (and thus have less likelihood of exercise in a social setting) than those with normal hearing. Studies have also demonstrated that impaired hearing can contribute to cognitive load and therefore affect attentional and cognitive resources 11 17 that are important for maintaining posture and balance 85. Impaired hearing can restrict the ability to monitor the auditory environment effectively (e.g., hearing footfalls and other auditory cues that provide orientation to the physical environment), thereby affecting the likelihood of performing physical activities. Alternatively, common pathological processes may underlie impairments in hearing and physical activity. Accordingly with the evidence for a link between presbycusis and cognitive impairment as described above, cardiovascular disease may contribute to HL 83 and poorer health and physical activity. In fact, HL in older patients is often associated with cardiovascular disease (e.g., congestive heart failure, coronary artery disease, angina pectoris, myocardial infarction, hypertension, smoking status, BMI). Alternatively, common neural degeneration affecting not only the cochlea but also the vestibular organ, involved in the balance control, can explain the relationship between HL and poor physical activity.
Growing findings indicate that in older patients frailty represents a clinical syndrome characterised by decreased physiologic reserve and weakness that causes an increased vulnerability to stressors 86. The prevalence of frailty is increased in the institutionalised population. Epidemiologic studies investigating the association of HL with frailty and physical functioning suggest that moderate to severe HL seems to be associated with increased risk of developing frailty, independently of age, demographic characteristics and cardiovascular risk factors 2. Further studies are needed to determine the pathogenesis of this association. It is interesting also to take into consideration the effects of dual sensorial impairment (i.e. hearing and visual loss, DSI) in older patients and their relationship with cognitive decline. Obviously the effects of late-onset DSI depend by the severity of sensorial impairments in both ears and eyes, however it is demonstrated that DSI affects physical, psychological, and psychosocial well-being 87. Furthermore, Lin et al. 11 reported that, patients affects by DSI had the greatest odds of cognitive and functional decline, although the risk was not different in patients with visual impairment alone suggesting that the presence of HL does not further decreased the effects on cognition. Nevertheless, more studies are needed to better understand the interaction of visual and hearing impairment in older adults. Finally, as a consequence of all the aspects interfering with the good health of older patients, Genther et al. 84 found that HL was associated with a 34% increased risk of mortality compared with normal hearing in community-dwelling older adult aged 70-79. After adjustment for other demographic characteristics (sex, education, and study site) and cardiovascular risk factors (diabetes, stroke, and smoking), HL was associated with a 20% increased risk of mortality compared with normal hearing 84. Two studies examined the association of audiometric HL with mortality. The first paper performed in adults aged 70 and older found an association between HL and mortality after adjusting for demographic characteristics, but this association disappeared after adjusting for various health factors 88. A second study conducted in adults aged 50 and older used structural equation modeling and found an association between HL and mortality that was mediated through cognitive impairment and walking disability89. Finally, in adults aged 67 and older an association between objective HL and increased cardiovascular mortality has been found, but not with all-cause mortality 90. In contrast with these findings , in others studies this association seems to be inconsistent, so we agree that additional studies are needed in proving the relationship between HL and overall mortality 91-94.
The impact of hearing aids and cochlear implant on eldery deaf patients
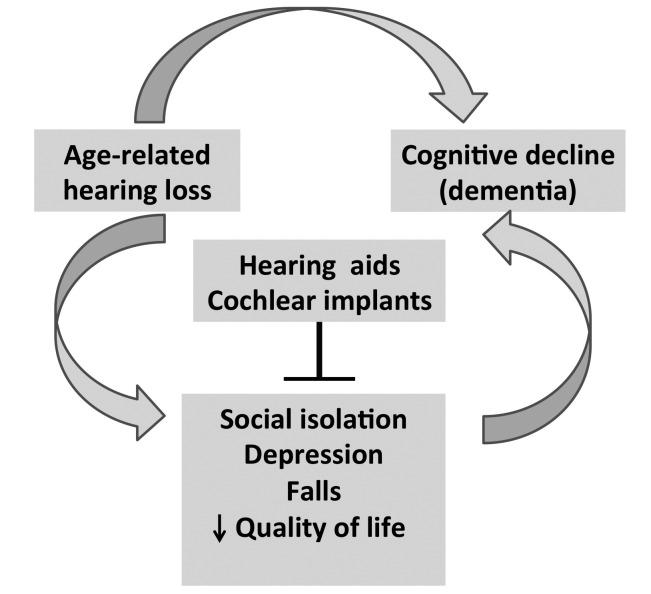
On the basis of "a cascade hypothesis" between HL and cognitive decline the natural consequence is that the use of hearing aids (HA) or cochlear implant should be associated with better cognitive performance. It is evident that older adults with untreated moderate to profound hearing loss may develop a cascade of conditions including communication difficulties, social isolation, depression, an association with falls and declines in physical functioning, decreased quality of life and even cognitive decline that could be counteracted by HA (Fig. 3). However, few studies investigated whether auditory amplification can reduce the risk of cognitive decline and dementia. A recent paper addressed the positive association of HA use on cognition that was independent of any positive association of hearing aid use on social isolation and depression. Therefore, the authors suggested that the effects of HA on cognition was not correlated to the reduction of the adverse effects of hearing loss on social isolation or depression and probably related to the direct impact on the hearing amplification on daily life 95. How HA impact on cognitive performance through a reduction of depression or social isolation or, as a direct consequence, remains controversal. Furthermore, the question how hearing aids might interfere with cognitive decline remains unexplained. Accordingly with the hypothesis suggested by the Dawes et al. 95 untreated hearing loss may increase the effect of auditory deprivation on the brain resulting in increased cognitive decline. Moreover, cognitive decline can reduce social participation, increase isolation and depression thus reducing the interest for hearing rehabilitation. A randomised clinical trial of HL treatment 96 that examined outcomes beyond measures of speech perception and quality of life, demonstrated improved social and emotional function, communicative abilities and cognitive function in the treatment group. Unfortunately, the reported prevalence of HA users in older adults varies from 21.5% in UK, 11.0% in Australia and approximately 14% in the US with a level of satisfaction that is lower than in young and adult patients 6 97. Possible explanations for the no-use of HA are the poor quality of amplified sound, the availability of technological solutions and the cost for hearing aids. Further research is needed to determine whether HA independently improves the quality of life of these patients.
Fig. 3.
Untreated moderate to profound hearing loss may develop a cascade of conditions including communication difficulties, social isolation, depression, falls and decreased quality of life that could be counteracted by hearing aids and cochlear implants.
An interdisciplinary approach and collaboration between otolaryngologists and neuro-psychologists is mandatory to investigate and address hearing loss in the context of brain and cognitive aging for a correct management of older patients and the decision for hearing aids or cochlear implant (CI).
In the last two decades, the literature has been enriched by several studies based on speech perception outcome, comparing younger and older adult CI recipients. Older adults underwent to CI achieve significant improvement in speech perception performance scores over preoperative performance with conventional amplification 98. Furthermore, older adults can continue to benefit from cochlear implantation with long-term use although psychosocial changes, hearing deprivation length, age at implantation, and reduced cognitive and learning abilities influence outcomes in elderly patients. A recent paper from Lin's Group 2 addresses the positive impact of both HA and CI on mental health quality of life. Interestingly, the authors found that patients who received CI had twice the gain in the mental component summary score compared to HA recipients at 6 and 12 months after amplification. Di Nardo et al. 98 compared auditory performances and several quality of life outcomes between under 60 years CI users and over 60 years recipients. They found a significant benefit on speech recognition tests compared to preimplantation condition, even if younger CI users scored significantly better in both bisyllabic words and sentences recognition test. No significant difference was found between the study and control group in physical and mental health status, conversation with an outsider, or use of TV and phone. Overall satisfaction derived from CI was higher in the older than in the younger patients. These findings indicate a high level of satisfaction and a dramatic improvement in quality of life and communication abilities after cochlear implantation in elderly people with postlingually bilateral severe-to-profound hearing loss that cannot be explained only by enhancements to auditory perception. More studies that demonstrate a link between CI rehabilitation and cognitive level will be challenging. In 2015, Miller et al. 99 reviewed 5057 articles and concluded that only 3 studies met the full criteria for this topic. The overall results were inconclusive in terms of cognitive benefit provided by cochlear implantation. Mosnier et al. 100 conduced a prospective study on impact of cochlear implant in old people and concluded that hearing rehabilitation using cochlear implantation is associated with an improvement in function in all cognitive domains as early as 6 months after implantation in elderly patients who had abnormal test scores at baseline. More than 80% of patients who had the poorest cognitive scores before implantation improved their cognitive function at the 1-year post implantation interval. In contrast, patients with the best cognitive performance before implantation demonstrated stable results. These preliminary results have never been confirmed in a trial with a larger and more representative cohort. Further research is thus needed to evaluate the long-term influence of hearing restoration on cognitive decline and its effect on public health.
Conclusions
Robust evidence suggests that HL in the elderly is independently associated with development of cognitive decline and dementia. Several hypotheses on the pathogenic relationship between HL and cognitive decline have been postulated and summarized in a conceptual model in which the hearing impairment impacts cognitive load, changes in brain structure and function, leads to social isolation and depression related with a common aetiology (i.e. genetic and environmental factors).
HL and their putative effects on cognition are highly prevalent in older patients and their effects may be preventable and treatable with rehabilitative devices (i.e. hearing aids and cochlear implants) that remain widely underutilised. Further research is needed to understand if and how hearing aids and cochlear implants can change the natural history of these conditions and improve quality of life in the elderly.
References
- 1.Uhlmann RF, Larson EB, Rees TS, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. [PubMed] [Google Scholar]
- 2.Lin FR, Albert M. Hearing loss and dementia –who is listening? Aging Ment Health. 2014;18:671–673. doi: 10.1080/13607863.2014.915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fetoni AR, Picciotti PM, Paludetti G, et al. Pathogenesis of presbycusis in animal models: a review. Exp Gerontol. 2011;46:413–425. doi: 10.1016/j.exger.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernabei R, Bonuccelli U, Maggi S, et al. Hearing loss and cognitive decline in older adults: questions and answers. Aging Clin Exp Res. 2014;26:567–577. doi: 10.1007/s40520-014-0266-3. [DOI] [PubMed] [Google Scholar]
- 6.Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Arch Int Med. 2012;172:292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RS, Paisley S, Davis A. Systematic review of the clinical and cost effectiveness of digital hearing aids. Br J Audiol. 2001;35:271–288. doi: 10.1080/00305364.2001.11745246. [DOI] [PubMed] [Google Scholar]
- 8.Girotto G, Mezzavilla M, Abdulhadi K, et al. Consanguinity and hereditary hearing loss in Qatar. Hum Hered. 2014;77:175–182. doi: 10.1159/000360475. [DOI] [PubMed] [Google Scholar]
- 9.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yesavage JA, O'Hara R, Kraemer H, et al. Modeling the prevalence and incidence of Alzheimer's disease and mild cognitive impairment. J Psychiatr Res. 2002;36:281–286. doi: 10.1016/s0022-3956(02)00020-1. [DOI] [PubMed] [Google Scholar]
- 11.Lin FR, Metter EJ, O'Brien RJ,, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. Health ABC Study Group JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen NK, Jørgensen AW, Ovesen T. Prevalence of various etiologies of hearing loss among cochlear implant recipients: Systematic review and meta-analysis. Int J Audiol. 2015;54:924–932. doi: 10.3109/14992027.2015.1091094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cacciatore F, Napoli C, Abete P, et al. Quality of life determinants and hearing function in an elderly population: Osservatorio Geriatrico Campano Study Group. Gerontology. 1999;45:323–328. doi: 10.1159/000022113. [DOI] [PubMed] [Google Scholar]
- 16.Gates B. Contemporary issues in intellectual disability practice policy and research. J Intellect Disabil. 2011;15:226–228. doi: 10.1177/1744629511433256. [DOI] [PubMed] [Google Scholar]
- 17.Lin FR, Ferrucci L, Metter EJ, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25:763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurgel RK1, Ward PD, Schwartz S,, et al. Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol. 2014;35:775–781. doi: 10.1097/MAO.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster SM, Davis HP, Kisley MA. Brain responses to emotional images related to cognitive ability in older adults. Psychol Aging. 2013;28:179–190. doi: 10.1037/a0030928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ives DG, Bonino P, Traven ND, et al. Characteristics and comorbidities of rural older adults with hearing impairment. J Am Geriatr Soc. 1995;43:803–806. doi: 10.1111/j.1532-5415.1995.tb07056.x. [DOI] [PubMed] [Google Scholar]
- 21.Ohta RJ, Carlin MF, Harmon BM. Auditory acuity and performance on the mental status questionnaire in the elderly. J Am Geriatr Soc. 1981;29:476–478. doi: 10.1111/j.1532-5415.1981.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 22.Tay T, Wang JJ, Kifley A, et al. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. 2006;52:386–394. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- 23.Thomas PD, Hunt WC, Garry PJ, et al. Hearing acuity in a healthy elderly population: effects on emotional, cognitive, and social status. J Gerontol. 1983;38:321–325. doi: 10.1093/geronj/38.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 2001;47:289–293. doi: 10.1159/000052814. [DOI] [PubMed] [Google Scholar]
- 25.Gennis V, Garry PJ, Haaland KY, et al. Hearing and cognition in the elderly. New findings and a review of the literature. Arch Intern Med. 1991;151:2259–2264. [PubMed] [Google Scholar]
- 26.Dupuis K, Pichora-Fuller MK, et al. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22:413–437. doi: 10.1080/13825585.2014.968084. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen L, Palmer C, Fischer G. Evaluation of hearing status at the time of dementia diagnosis. Audiology Today. 2014;26:38–45. [Google Scholar]
- 28.Peelle JE, Troiani V, Grossman M, et al. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 2011;31:12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckert MA, Cute SL, Vaden KI, Jr, et al. Auditory cortex signs of age-related hearing loss. J Assoc Res Otolaryngol. 2012;13:703–713. doi: 10.1007/s10162-012-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain FT, Medina RE, Davis CW, et al. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 2010;1369:74–88. doi: 10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang Y, Lee SH, Lee YJ, et al. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport. 2004;15:1699–1703. doi: 10.1097/01.wnr.0000134584.10207.1a. [DOI] [PubMed] [Google Scholar]
- 32.Kakigi A, Hirakawa H, Harel N, et al. Tonotopic mapping in auditory cortex of the adult chinchilla with amikacin-induced cochlear lesions. Audiology. 2010;39:153–160. doi: 10.3109/00206090009073068. [DOI] [PubMed] [Google Scholar]
- 33.Fetoni AR, Bartolo P, Eramo SL, et al. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci. 2013;33:4011–4023. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fetoni AR, Troiani D, Petrosini L, et al. Cochlear injury and adaptive plasticity of the auditory cortex. Front Aging Neurosci. 2015;7:8–8. doi: 10.3389/fnagi.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pichora-Fuller MK. Cognitive aging and auditory information processing. Int J Audiol. 2003;42(Suppl 2):2S26–2S32. [PubMed] [Google Scholar]
- 36.Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102(1 Pt 2):1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- 37.Nelson EG, Hinojosa R. Presbycusis. A human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 2006;116(9 Pt 3 Suppl 112):1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- 38.Bamiou DE, Musiek FE, Luxon LM. Aetiology and clinical presentations of auditory processing disorders – a review. Arch Dis Child. 2001;85:361–365. doi: 10.1136/adc.85.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimes AM, Grady CL, Foster NL, et al. Central auditory function in Alzheimer's disease. Neurology. 1985;35:352–358. doi: 10.1212/wnl.35.3.352. [DOI] [PubMed] [Google Scholar]
- 40.Humes LE, Dubno JR, Gordon-Salant S, et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012;23:635–666. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jäncke L, Shah NJ, et al. Does dichotic listening probe temporal lobe functions? Neurology. 2002;58:736–743. doi: 10.1212/wnl.58.5.736. [DOI] [PubMed] [Google Scholar]
- 42.Paludetti G, Conti G, Nardo W, et al. Infant hearing loss: from diagnosis to therapy Official Report of XXI Conference of Italian Society of Pediatric Otorhinolaryngology. Acta Otorhinolaryngol Ital. 2012;32:347–370. [PMC free article] [PubMed] [Google Scholar]
- 43.Quaranta N, Coppola F, Casulli M, et al. The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol Neurootol. 2014;19(Suppl 1):10–14. doi: 10.1159/000371597. [DOI] [PubMed] [Google Scholar]
- 44.Panza F, Seripa D, Solfrizzi V, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47:793–813. doi: 10.3233/JAD-150358. [DOI] [PubMed] [Google Scholar]
- 45.Gates GA, Anderson ML, McCurry SM, et al. Central auditory dysfunction as a harbinger of Alzheimer dementia . Arch Otolaryngol Head Neck Surg. 2011;137:390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gates GA, Karzon RK, Garcia P, et al. Auditory dysfunction in aging and senile dementia of the Alzheimer's type. Arch Neurol. 1995;52:626–634. doi: 10.1001/archneur.1995.00540300108020. [DOI] [PubMed] [Google Scholar]
- 47.Gates GA, Anderson ML, Feeney MP, et al. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2008;134:771–777. doi: 10.1001/archotol.134.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Profant O, Tintìra J, Balogová Z, et al. Functional changes in the human auditory cortex in ageing. PLoS One. 2015;10:e0116692–e0116692. doi: 10.1371/journal.pone.0116692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemaitre H, Goldman AL, Sambataro F, et al. Normal agerelated brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33:e1–e9. doi: 10.1016/j.neurobiolaging.2010.07.013. 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouda L, Profant O, Syka J, et al. Age-related changes in the central auditory system. Cell Tissue Res. 2015;361:337–358. doi: 10.1007/s00441-014-2107-2. [DOI] [PubMed] [Google Scholar]
- 51.Lin Y, Wang J, Wu C, et al. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: changes in radial diffusivity and diffusion anisotropy. J Magn Reson Imaging. 2008;28:598–603. doi: 10.1002/jmri.21464. [DOI] [PubMed] [Google Scholar]
- 52.Kantarci K, Jack CR, Jr, et al. Quantitative magnetic resonance techniques as surrogate markers of Alzheimer's disease. NeuroRx. 2004;1:196–205. doi: 10.1602/neurorx.1.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Profant O, Škoch A, Balogová Z, et al. Diffusion tensor imaging and MR morphometry of the central auditory pathway and auditory cortex in aging. Neuroscience. 2014;260:87–97. doi: 10.1016/j.neuroscience.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Verhaeghen P. Aging and vocabulary scores: a meta-analysis. Psychol Aging. 2003;18:332–339. doi: 10.1037/0882-7974.18.2.332. [DOI] [PubMed] [Google Scholar]
- 55.Burke DM, MacKay DG, Worthley JB, et al. On the tip of the tongue: what causes word-finding failure in young and older adults. J Mem Lang. 1991;30:237–246. [Google Scholar]
- 56.Tyler LK, Shafto MA, Randall B, et al. Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb Cortex. 2010;20:352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humes LE. Do 'auditory processing' tests measure auditory processing in the elderly? Ear Hear. 2005;26:109–119. doi: 10.1097/00003446-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Rooij JCGM, Plomp R. Auditive and cognitive factors in speech perception by elderly listeners. II. Multivariate analyses J Acoust Soc Am. 1990;88:2611–2624. doi: 10.1121/1.399981. [DOI] [PubMed] [Google Scholar]
- 59.Wingfield A, McCoy SL, Peelle JE, et al. Effects of adult aging and hearing loss on comprehension of rapid speech varying in syntactic complexity. J Am Acad Audiol. 2006;17:487–497. doi: 10.3766/jaaa.17.7.4. [DOI] [PubMed] [Google Scholar]
- 60.Wingfield A, Stine-Morrow EAL. Language and speech. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. 2nd ed. Mahwah, NJ: Erlbaum; 2000. pp. 359–416. [Google Scholar]
- 61.Kemper S. Language and aging. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 213–270. [Google Scholar]
- 62.Wingfield A, Peelle JE, Grossman M. Speech rate and syntactic complexity as multiplicative factors in speech comprehension by young and older adults. J Aging Neuropsychol Cogn. 2003;10:310–322. [Google Scholar]
- 63.Morton J. Interaction of information in word recognition. Psychol Rev. 1969;76:165–178. [Google Scholar]
- 64.Carpenter PA, Miyaki A, Just MA. Working memory constraints in comprehension: evidence from individual differences, aphasia, and aging. In: Gernsbacher M, editor. Handbook of Psycholinguistics. San Diego: Academic Press; 1994. pp. 1075–1122. [Google Scholar]
- 65.Salthouse TA. The aging of working memory. Neuropsychology. 1994;8:535–543. [Google Scholar]
- 66.Peelle JE, Troiani V, Wingfield A, et al. Neural processing during older adults' comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb Cortex. 2010;20:773–782. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart R, Wingfield A. Hearing loss and cognitive effort in older adults' report accuracy for verbal materials. J Am Acad Audiol. 2009;20:147–154. doi: 10.3766/jaaa.20.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24:761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viljanen A, Kaprio J, Pyykko I, et al. Hearing as a predictor of falls and postural balance in older female twins. J Gerontol A Biol Sci Med Sci. 2009;64A:312–317. doi: 10.1093/gerona/gln015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gopinath B, Schneider J, McMahon CM, et al. Severity of age-related hearing impairment is associated with impaired activities of daily living. Age Ageing. 2012;41:195–200. doi: 10.1093/ageing/afr155. [DOI] [PubMed] [Google Scholar]
- 71.Reuben DB, Mui S, Damesyn M, et al. The prognostic value of sensory impairment in older persons. J Am Geriatr Soc. 1999;47:930–935. doi: 10.1111/j.1532-5415.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 72.Laforge RGSW, Sternberg J. The relationship of vision and hearing impairment to one-year mortality and functional decline. J Aging Health. 1992;4:126–148. [Google Scholar]
- 73.Chen DS, Genther DJ, Betz J, et al. Association between hearing impairment and self-reported difficulty in physical functioning. Am Geriatr Soc. 2014;62:850–856. doi: 10.1111/jgs.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gispen FE, Chen DS, Genther DJ, et al. Association between hearing impairment and lower levels of physical activity in older adults. J Am Geriatr Soc. 2014;62:1427–1433. doi: 10.1111/jgs.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reuben DB, Mui S, Damesyn M, et al. The prognostic value of sensory impairment in older persons. J Am Geriatr Soc. 1999;47:930–935. doi: 10.1111/j.1532-5415.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 76.Strawbridge WJ, Wallhagen MI, Shema SJ, et al. Negative consequences of hearing impairment in old age: A longitudinal analysis. Gerontologist. 2000;40:320–326. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- 77.Loprinzi PD. Association between accelerometer-assessed sedentary behavior and objectively-measured hearing sensitivity in older US adults. Prev Med. 2013;57:143–145. doi: 10.1016/j.ypmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Chen DS, Betz J, Yaffe K, et al. Health ABC study. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:654–661. doi: 10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2012;38:25–29. doi: 10.1016/j.gaitpost.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 81.Curhan SG, Eavey R, Wang M, et al. Body mass index, waist circumference, physical activity, and risk of hearing loss in women . Am J Med. 2013;126(1142):e1–e1142. doi: 10.1016/j.amjmed.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research . Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 83.Gates GA, Cobb JL, D'Agostino RB, et al. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors . Arch Otolaryngol Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- 84.Genther DJ, Betz J, Pratt S. Association of hearing impairment and mortality in older adults . J Gerontol A Biol Sci Med Sci. 2015;70:85–90. doi: 10.1093/gerona/glu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shumway-Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci. 2000;55A:M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 86.Kamil RJ, Betz J, Powers BB, et al. Health ABC study. Association of hearing impairment with incident frailty and falls in older adults. J Aging Health. 2015 Oct 05; doi: 10.1177/0898264315608730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lam BL, Lee DJ, Gomez-Marin O, et al. Concurrent visual and hearing impairment and risk of mortality: the National Health Interview Survey. Arch Ophthalmol. 2006;124:95–101. doi: 10.1001/archopht.124.1.95. [DOI] [PubMed] [Google Scholar]
- 88.Anstey KJ, Luszcz MA, Giles LC, et al. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging. 2001;16:3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- 89.Karpa MJ, Gopinath B, Beath K, et al. Associations between hearing impairment and mortality risk in older persons: the Blue Mountains Hearing Study. Ann Epidemiol. 2010;20:452–459. doi: 10.1016/j.annepidem.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Fisher D, Li CM, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: the AGESReykjavik Study. Age Ageing. 2013;43:69–76. doi: 10.1093/ageing/aft122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Appollonio I, Carabellese C, Magni E, et al. Sensory impairments and mortality in an elderly community population: a six-year follow-up study. Age Ageing. 1995;24:30–36. doi: 10.1093/ageing/24.1.30. [DOI] [PubMed] [Google Scholar]
- 92.Barnett S, Franks P. Deafness and mortality: analyses of linked data from the National Health Interview Survey and National Death Index. Public Health Rep. 1999;114:330–336. doi: 10.1093/phr/114.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ostbye T, Steenhuis R, Wolfson C, et al. Predictors of fiveyear mortality in older Canadians: the Canadian Study of Health and Aging. J Am Geriatr Soc. 1999;47:1249–1254. doi: 10.1111/j.1532-5415.1999.tb05207.x. [DOI] [PubMed] [Google Scholar]
- 94.Sarampalis A, Kalluri S, Edwards B, et al. Objective measures of listening effort: effects of background noise and noise reduction. J Speech Lang Hear Res. 2009;52:1230–1240. doi: 10.1044/1092-4388(2009/08-0111). [DOI] [PubMed] [Google Scholar]
- 95.Dawes P, Emsley R, Cruickshanks KJ, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One. 2015;10:e0119616–e0119616. doi: 10.1371/journal.pone.0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med. 1990;113:188–194. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]
- 97.Hartley D, Rochtchina E, Newall P, et al. Use of hearing AIDS and assistive listening devices in an older Australian population. J Am Acad Audiol. 2010;21:642–653. doi: 10.3766/jaaa.21.10.4. [DOI] [PubMed] [Google Scholar]
- 98.Nardo W, Anzivino R, Giannantonio S, et al. The effects of cochlear implantation on quality of life in the elderly. Eur Arch Otorhinolaryngol. 2014;271:65–73. doi: 10.1007/s00405-013-2396-1. [DOI] [PubMed] [Google Scholar]
- 99.Miller G, Miller C, Marrone N, et al. The impact of cochlear implantation on cognition in older adults: a systematic review of clinical evidence. BMC Geriatr. 2015;15:16–16. doi: 10.1186/s12877-015-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mosnier I, Bebear JP, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg. 2015;141:442–450. doi: 10.1001/jamaoto.2015.129. [DOI] [PubMed] [Google Scholar]