SUMMARY
Several therapeutic options are used for treatment of early stage glottic carcinoma (Tis/T1/T2): open partial laryngectomy (OPL), radiotherapy and CO2 laser-assisted endoscopic surgery. Laser surgery has gradually gained approval in the management of laryngeal cancer. We present our experience in endoscopic laser surgery for early stage glottic carcinomas. This was a retrospective analysis of 72 patients with T1-T2 glottic cancer treated with laser cordectomy between 2006 and 2012. All patients had at least a 36-month follow-up period. Percentages for disease-specific survival, disease-free survival (DFS) and laryngeal preservation rates were 98.6%, 84.7% and 97.2% respectively. Considering neoplastic features that could predict long-term oncological outcome, tumoural involvement of anterior commissure and pathological staging (pT) significantly correlate with local recurrence (p = 0.021 and p = 0.035) and with a lowered DFS (p = 0.017 and p = 0.023). Other variables such as clinical staging, type of cordectomy, involvement of other structures and surgical margin status showed no significant impact on oncological endpoints. CO2 laser surgery is a reliable technique for T1-T2 glottic cancer considering oncological outcomes. The recurrence rate seems to be affected by involvement of anterior commissure and pT stage.
KEY WORDS: Larynx, Glottic carcinoma, Laryngeal cancer, CO2 laser-surgery, Early stage, Endoscopic cordectomy
RIASSUNTO
Esistono numerose strategie terapeutiche per il trattamento del carcinoma glottico in stadio iniziale (Tis/T1/T2): la laringectomia parziale a cielo aperto, la radioterapia e la chirurgia endoscopica condotta mediante laser CO2. In particolare quest'ultimo approccio ha gradualmente, ma inesorabilmente, acquisito un ruolo sempre più centrale nel management del cancro laringeo. In questo lavoro presentiamo la nostra esperienza in materia di chirurgia endoscopica laser-assistita delle neoplasie glottiche in stadio iniziale. è stata realizzata un'analisi retrospettiva su un campione di 72 pazienti affetti da carcinoma glottico in classe T1-T2 trattati con cordectomia laser endoscopica nel periodo compreso tra il 2006 e il 2012. Tutti i pazienti avevano almeno 36 mesi di follow-up. La disease-specific survival, la disease-free survival (DFS) e il tasso di preservazione laringea rilevati con il presente studio sono stati rispettivamente del 98,6%, 84,7% e 97,2%. Analizzando l'influenza sull'outcome oncologico a lungo termine di alcune tra le principali caratteristiche della malattia o del trattamento eseguito, abbiamo riscontrato come il coinvolgimento da parte del tumore della commissura anteriore e lo staging patologico della neoplasia (pT) correlino significativamente con un aumentato tasso di recidiva locale (p = 0,021 e p = 0,035) e con una ridotta DFS (p = 0,017 e p = 0,023). Gli altri parametri presi in esame, come staging clinico, tipo di cordectomia, coinvolgimento di altre specifiche sottosedi laringee e stato dei margini di resezione, non si sono dimostrati, invece, correlare significativamente con gli endpoint oncologici stabiliti. La chirurgia endoscopica laser-assistita è quindi una tecnica estremamente affidabile per il trattamento dei tumori glottici in stadio iniziale in termini di outcome oncologico. Il tasso di recidiva risulta significativamente influenzato dal coinvolgimento della commissura anteriore e dal pT.
Introduction
Larynx carcinoma accounts for 4.5% of malignant neoplasms and glottic cancer makes up approximately 50% of laryngeal tumours 1. Different options are available for treatment, especially when the tumour is identified at an early stage: transoral laser microsurgery (TLM), open partial laryngectomy (OPL) and radiotherapy (RT) 2 3.
In the last years the use of TLM has greatly expanded. For this reason, it has been debated whether the oncological and functional results of this method are comparable to those of other techniques. OPL allows obtaining larger free margins and better oncological results; it also gives the possibility to treat the neck simultaneously, when required. On the other hand, hospitalisation times are longer, the rate of complications is higher and functional results are worse 4.
RT is associated with good oncological outcome, but higher costs and longer time of care; the voice is generally good at the end of treatment 5.
TLM can be used to manage Tis, T1, T2 and selected T3 glottic cancers 6 7. Hospitalisation time is reduced to 1-3 days on average (depending on the type of cordectomy); oncological and functional results are usually good if an experienced surgical team performs the procedure.
In this paper, we analyse our experience in TLM in terms of oncological outcomes. Different prognostic factors were analysed to identify specific tumour features that are related to poor prognosis.
Materials and methods
The study was carried out retrospectively on a series of 72 patients with T1-T2 glottic cancer treated with CO2 laser-assisted endoscopic cordectomy (TLM) between February 2006 and February 2012 at the ENT department of San Raffaele Hospital in Milan, Italy. The original cohort included 120 cases, but we excluded patients who did not reach a minimum 36-month follow-up period (57.4 ± 20.2 months). Forty-eight patients had shorter follow-up period or, alternatively, were missed during the follow-up. The cohort included 66 men (91.7%) and 6 women (8.3%) with a mean age of 65 (range: 35-87) at the time of surgery. No patient had received previous treatment for laryngeal cancer.
All patients underwent pre-operative white light and NBI video-laryngoscopy with flexible endoscope and videolaryngo- stroboscopy. Patients with manifest involvement of anterior commissure, Morgagni's ventricle, subglottis, arytenoids, or with impaired vocal fold mobility underwent contrast-enhanced neck computed tomography (CT) that helped rule out paraglottic space invasion or cartilage infiltration, which would contraindicate TLM. They were also evaluated with angled telescopes (0°, 30°, 70°) immediately before surgery to better explore those critical subsites. According to TNM classification of the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) (7th edition) 8, clinical staging of primary laryngeal lesions (cT) was cT1a in 61 cases (84.7%), cT1b in 3 cases (4.2%) and cT2 in 8 cases (11.1%). No regional lymph node metastases (N) were detected at diagnosis, either clinically or with CT.
All surgical procedures were performed under general anaesthesia after oro-tracheal intubation. Tumour excision under microlaryngoscopy was always performed with a Lumenis AcuPulse 30ST CO2 laser (Lumenis Ltd. Yokneam, Israel) with super pulse beam delivery and power tailored on the target structures (4 W for vestibulectomy, 1.5-2 W for cordectomy). Endoscopic resections were graded according to the European Laryngological Society Classification 9 and its more recent revision 10, including 6 types of cordectomy: subepithelial (type I), subligamental (type II), transmuscular (type III), total (type IV), extended (type V) and the latest anterior commissurectomy with bilateral anterior cordectomy (type VI). As far as type III cordectomy was concerned, excision had both diagnostic and therapeutic aim. When deeper resections were indicated, patients achieved pathological diagnosis of lesions with bioptic microlaryngoscopy under general anaesthesia and all available therapeutic options were discussed, focusing on oncological and functional outcomes linked with each procedure.
Endoscopic resections were carried out with an en-bloc or piece-meal technique according to tumour location, extension on glottic surface and ease of laryngeal exposure. No elective neck dissection was performed. In order to evaluate both deep and superficial resection margins, surgical specimens were sent to a dedicated pathologist with a clearly inked margin. A detailed diagram of the surgical procedure was sent together with the specimen in case of piece-meal excision with critical margins specifically indicated.
Resection margins were classified as free, positive (superficial or deep), or unassessable (due to surgical artefacts). In case of a single superficial positive margin, our policy was to assign patients to a careful follow-up since resection margins were approached by photovaporisation. In particular, patients were followed up with monthly endoscopic controls and periodical neck CT evaluation. Patients with deep positive margin underwent revision TLM. Patients with free margins were assigned to 3-month endoscopic follow-up during the first two years, which was increased to 6 months during the third year. No patients underwent RT because of involved resection margins.
Statistical analysis was performed with the SPSS statistical package. The log-rank test and the Kaplan-Meier survival function were applied to assess different diseasefree survival (DFS) rates for patients stratified according to variables of interest. Same variables were assessed with regards to recurrence rates with a chi-squared test. A p < 0.05 was considered statistically significant.
Results
Nine patients underwent type I cordectomy (12.5%), 14 patients type II (19.4%), 20 patients type III (27.8%), 12 patients type IV (16.7%) and 17 patients type V (23.6%). No type VI cordectomy was performed in our sample since no tumour originating from the anterior commissure was detected. En-bloc resection was preferred in 69 cases (95.8%), while a piece-meal technique was performed in only 3 cases (4.2%).
Median hospitalisation time was 3 days (range: 1-20 days). In particular, patients who underwent type I, II and III cordectomy were discharged on the first postoperative day, while deeper resections generally required hospitalization for 3 days. Longer hospital stay was associated with postsurgical complications.
Tumour staging according to pathological examination (pT) was pTis in 8 cases (11.1%), pT1a in 53 cases (73.6%), pT1b in 2 cases (2.8%) and pT2 in 9 cases (12.5%). Anterior commissure was involved in 19 patients (26.4%), vocal muscle in 17 patients (23.6%) and supraglottic structures (Morgagni's ventricle, false vocal cord) in 9 patients (12.5%). Resection margin status showed 38 cases with free margins (52.8%), 13 cases with a single superficial positive margin (18.1%), 7 cases with deep positive margin (9.7%) and 14 cases with unassessable margins due to surgical artefacts (19.4%). No surgical specimens with multiple superficial positive margins were detected.
Major and minor procedure-related complications are common in this kind of surgery. Major complications are defined as those that need extended medical therapies, blood transfusions, early surgical revision, or intensive care unit recovery. Two patients (2.8%) required nasogastric tube insertion to avoid inhalation, while 5 cases (6.9%) underwent prophylactic tracheostomy. All tracheostomies were closed before discharge. No intra-operative complications were reported. Four patients (5.6%) experienced early complications related to the surgical procedure: one case of limited post-operative laryngeal bleeding not requiring surgical haemostasis (1.4%), 2 cases of aspiration pneumonia (2.8%) and one subcutaneous cervical emphysema (1.4%). None required early surgical intervention. Late complications affected 8 patients in our cohort (11.9%): 3 cases of anterior glottic synechia (4.5%) and 5 cases of vocal cord granulomas (7.5%). The latter reported to chronically suffer from reflux-induced laryngitis: at first they were treated with proton-pump inhibitors and logopaedic therapy but later endoscopically excised, since none resolvedwith conservative treatment.
Recurrence is conventionally defined as a biopsy-proven neoplastic lesion in patients treated with TLM less than 60 months prior 11. Since our study evaluated the first three years after surgical procedure, relapse was defined within the period considered. Recurrence occurred in 11 patients (15.3%) after a mean of 16.8 ± 8.9 months. Ten patients experienced a local recurrence (13.9%): 5 cases underwent salvage TLM (one type II cordectomy, one type III and three type V), 2 patients were treated with RT, 1 with supracricoid OPL and 2 with total laryngectomy followed by adjuvant RT. Two of these patients experienced a second recurrence: both were treated with concomitant chemo-RT. One patient developed cervical lymph node involvement (1.4%) demonstrated by positive fine-needle aspiration cytology (FNAC) and underwent selective ipsilateral neck dissection (Robbins level II-IV 12. One patient died of disease (1.4%), while 8 patients died for unrelated causes (11.1%).
Table I shows the recurrence rate and DFS stratified according to common variables of interest: cT or pT staging, involvement of anterior commissure, supraglottis or vocal muscle, type of cordectomy and resection margin status. Statistical analysis highlighted substantial correlation between pT staging or anterior commissure involvement and recurrence (p = 0.035 and p = 0.021, respectively), as displayed in Figures 1 and 2. Moreover, neoplastic extension to the anterior commissure appears to adversely influence DFS (p = 0.017, Fig. 3) as does pT staging (p = 0.023, Fig. 4). None of the other parameters reached statistical significance.
Table I.
Recurrence rates and disease-free survival (DFS; mean ± standard deviation) stratified according to variables of interest.
| Variable | No. of cases | Recurrence rates | p value a | DFS in months (mean ± SD) | p value b | |
|---|---|---|---|---|---|---|
| cT | cT1a | 61 | 8/61 (13.1%) | 0.230 | 33.7 ± 6.8 | 0.198 |
| cT1b-cT2 | 11 | 3/11 (27.3%) | 29.7 ± 11.4 | |||
| pT | pTis-pT1a | 61 | 7/61 (11.5%) | 0.035 | 34.0 ± 6.3 | 0.023 |
| pT1b-pT2 | 11 | 4/11 (36.4%) | 27.7 ± 12.1 | |||
| Anterior commissure | Free | 53 | 5/53 (9.4%) | 0.021 | 34.3 ± 6.2 | 0.017 |
| Involved | 19 | 6/19 (31.6%) | 29.7 ± 10.4 | |||
| Supraglottis | Free | 63 | 8/63 (12.7%) | 0.108 | 33.6 ± 7.0 | 0.094 |
| Involved | 9 | 3/9 (33.3%) | 29.0 ± 11.2 | |||
| Vocal muscle | Free | 55 | 8/55 (14.5%) | 0.756 | 33.2 ± 7.7 | 0.760 |
| Involved | 17 | 3/17 (17.6%) | 32.6 ± 8.0 | |||
| Cordectomy | Type I | 9 | 2/9 (22.2%) | 0.561 | 34.3 ± 4.3 | 0.600 |
| Type II | 14 | 3/14 (21.4%) | 31.3 ± 10.2 | |||
| Type III | 20 | 3/20 (15.0%) | 32.7 ± 8.3 | |||
| Type IV | 12 | 0/12 (0%) | 36.0 ± 0 | |||
| Type V | 17 | 3/17 (17.6%) | 32.2 ± 8.8 | |||
| Superficial (type I, II, III) | 43 | 8/43 (18.6%) | 0.339 | 32.6 ± 8.3 | 0.353 | |
| Deep (type IV, V) | 29 | 3/29 (10.3%) | 33.8 ± 6.9 | |||
| Resection margin status | Free | 38 | 4/38 (10.5%) | 0.250 | 34.8 ± 3.8 | 0.193 |
| Single superficial positive | 13 | 1/13 (7.7%) | 33.9 ± 7.5 | |||
| Deep positive | 7 | 2/7 (28.6%) | 28.0 ± 13.7 | |||
| Unassessable (surgical artifacts) | 14 | 4/14 (28.6%) | 30.0 ± 10.6 |
Chi-squared test;
Log-rank test;
cT = clinical staging; pT = pathological staging
Fig. 1.
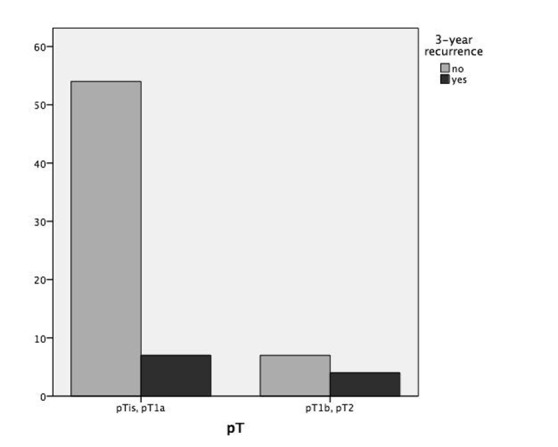
Bar chart showing 3-year recurrence rates according to pT stage (p = 0.035).
Fig. 2.
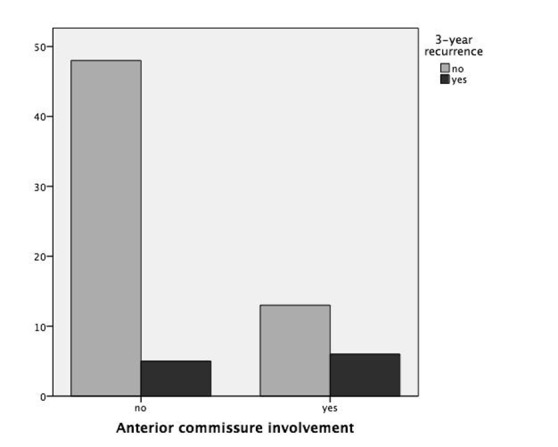
Bar chart showing 3-year recurrence rates according to anterior commissure involvement (p = 0.021).
Fig. 3.
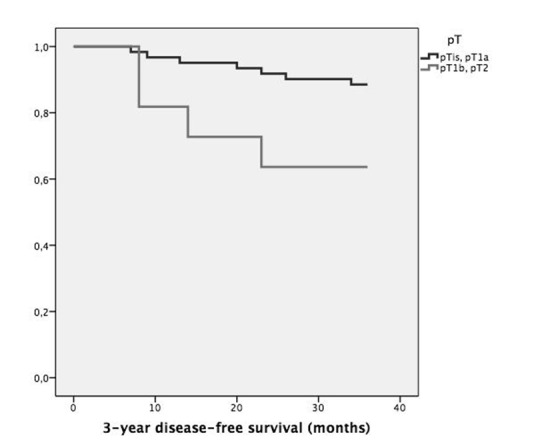
Kaplan-Meier survival curves stratified according to pT stage (p = 0.023).
Fig. 4.
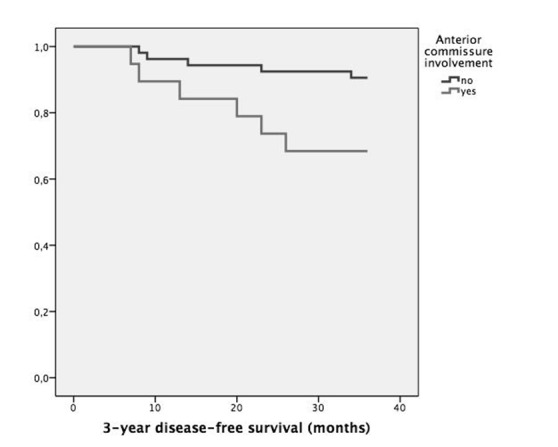
Kaplan-Meier survival curves stratified according to anterior commissure involvement (p = 0.017).
Discussion
Early stage glottic cancer can be managed by OPL, RT or TLM. All these methods are associated with good oncological results. For this reason, the choice of treatment should be related to costs, time of hospitalisation, rate of complications and functional results, although the patients' will must be considered first. In fact, there are no prospective randomised clinical trials assessing long-term outcomes associated with these different techniques.
As a general rule, early stage laryngeal tumours should be approached by a single modality of treatment 13 14. This allows preserving the chance of a second, different treatment in case of relapse and also provides better functional results, since combined approaches (TLM followed by RT) are always associated with worse functional outcome. In our cohort, laryngeal tumour was more common in men than in women (male-female ratio 8:1), as is well known in the ENT literature 15. This can be largely explained by higher rates of smoking and alcohol assumption in men. Mean age at the time of surgery (65 years) was also similar to those described by other authors 16. Hospital stay was influenced by type of cordectomy and postoperative complications, as discussed above. Day surgery was generally enough for patients who underwent type I, II and III cordectomy, while deeper resections often required longer stays. Three days was the mean hospitalisation time in type IV and V cordectomy, even though post-surgical complications usually increased hospital stay.
Early postoperative complications generally include subcutaneous emphysema, bleeding, dyspnoea, dysphagia, aspiration pneumonia and laryngo-cutaneous fistula. Subcutaneous emphysema is mainly associated with cricothyroid membrane perforation. This can occur when glottic tumours extend beyond the inferior margin of thyroid cartilage or in commissural lesions in which oncologically- safe resection need to overcome that border 17. Only 1 patient (1.4%) suffered from subcutaneous emphysema in our cohort; conservative management allowed its resolution before hospital discharge, as stated by Steiner in his assessment of postoperative complications after laser surgery of tumours of the upper aerodigestive tract 18. In a previous work, Steiner and Ambrosch reported the incidence of post-surgical bleeding to be 7% in supraglottic partial laser-assisted endoscopic resections, 3% in hypopharyngeal ones and 0% in glottic ones 19. One case in our cohort experienced this postoperative complication, and the haemorrhage stopped without surgical haemostasis. Aspiration pneumonia occurs most frequently in surgical treatment of supraglottic (5.8%) or hypopharyngeal cancers (12.9%) than in glottic tumours (1.7%) 20. Two patients suffered from this complication within our cohort (2.8%), which is consistent with most of the ENT literature. The rate of postoperative dysphagia is obviously influenced by extension of surgical resection: only 2 patients (2.8%) required nasogastric tube insertion to avoid inhalation.
One of the greatest advantages of TLM compared to OPL is the reduced need for prophylactic tracheostomy 21. The study by Preuss reported a rate of prophylactic tracheostomy among patients who underwent TLM for laryngeal cancer of 1.9% 22. As a general rule, our policy is to resort to tracheostomy in patients with a high risk of post-surgical endolaryngeal oedema or bleeding (patients taking anticoagulants or with significant intraoperative haemorrhage). In our cohort, 5 cases (6.9%) underwent prophylactic tracheostomy and all were closed before hospital discharge. However, dividing the temporal range (2006-2012), 4 tracheostomies were performed in the first half (2006-2009), compared to just 1 in the second interval (2009-2012), suggesting a clear decreasing trend. This can be explained with an improved intra- and postoperative management of patients associated with the physiological, surgical learning curve and the low rate of postoperative complications. This is highlighted by Vilaseca: in his investigation the post-surgical complication rate drastically reduced with increasing surgical expertise 23.
Late postoperative complications normally consist of anterior glottic synechia, vocal cord granulomas and arytenoid oedema. Anterior glottic synechiae are clinically relevant since they shrink the laryngeal respiratory space, although they can ease post-surgical glottic adduction and improve vocal outcome. That is why excision is considered only in case of substantial dyspnoea. In our cohort, 3 patients experienced anterior glottic synechia (4.2%) and none underwent surgical intervention. Vocal cord granulomas arise when endoscopic resection reaches inner perichondrium, especially in patients with a history of gastro-oesophageal reflux disease. Five of our patients developed granulomas (6.9%) and all referred to chronically suffer from reflux-induced laryngitis. Proton-pump inhibitor therapy combined with logopaedic rehabilitation did not achieve resolution in any of the affected patients, and they underwent endoscopic excision of granulomas. Arytenoid oedema can cause dyspnoea and dysphagia. In these cases, photovaporisation of the oedematous mucosa by CO2 laser can be useful, and was required by two patients in our cohort (2.8%).
The incidence of laryngeal tumour relapse after TLM was studied for the first time by Steiner and Ambrosch 19. They reported a relapse rate of 9.5% in stage I and II and 19.5% in stage III and IV. Herein, the recurrence rate was 15.3% (11 cases) and a disease-specific survival rate of 98.6% was seen, with a preservation rate of 97.2%. These results are similar to those described by Lucioni 24.
Accurate preoperative staging allows good correspondence between clinical and postoperative definition of the tumour and can help to predict recurrence. Pre-operative white light and NBI video-laryngoscopy with flexible endoscope, video-laryngo-stroboscopy, imaging assessment (CT, MRI) and intraoperative evaluation with angled telescopes (0°, 30°, 70°) are all tools for such a purpose. Recently, even direct autofluorescence during intraoperative work-up has been shown to be useful 25. In our study, we found no statistical significance in the association between relapse and clinical staging (p = 0.230), while pathological staging showed a clear correlation with the incidence of recurrence (p =†0.035). The same evidence was noted with regards to DFS shrinkage (p = 0.198 and p = 0.023 for cTNM and pTNM, respectively). This difference can be easily explained by the pathological upstaging that occurred in some cases: Ansarin has already highlighted this possibility 26.
We also found that anterior commissure involvement was related to a higher rate of recurrence (p = 0.021) with a lower DFS (p = 0.017). Since the recent introduction of type VI cordectomy 10, a pure commissural tumour can be treated in a single session, offering better disease control compared to two-stage bilateral cordectomy. However, this latter technique can be still adopted in case of T1b glottic tumours. Considering T1b tumours, indeed, Roedel found reduced local control with TLM, evidence that was not confirmed in T2 neoplasms with anterior commissure involvement (transcommissural tumours). Moreover, in his experience T1b cancers and anterior commissure involvement in T2 transcommissural tumours did not show a substantial reduction in disease-specific survival 27. On the other hand, Blanch observed that anterior commissure involvement influenced both relapse rate and disease-specific survival 28.
No significant difference was found between vocal muscle or supraglottic involvement and rate of recurrence (p = 0.756 and p = 0.108, respectively), as these subsites can be easily controlled by TLM.
Although no cases of arytenoid or subglottic region involvement were detected, a higher recurrence rate is associated with tumour extension to those laryngeal subsites 24; different distribution of submucosal lymphatic drainage within the larynx largely justifies this finding.
A margin of 1 mm is considered oncologically safe in glottic TLM 26. However, the relationship between resection margin status and relapse rate is still controversial 29-31. As a general rule, we recommend strict follow-up when a superficial margin is given as involved by pathologists. Instead, when a deep margin is involved we usually perform a revision TLM within a few weeks.
Conclusions
TLM in early stage glottic cancer shows several advantages compared to RT and to OPL, such as reduced intra- and postoperative morbidity and shorter time of care. To date, we consider TLM as the treatment of choice in T1a, T1b and selected T2 and T3 tumours. In fact, it elicits no changes in swallowing and allows preservation of physiological respiration. Early complications are usually manageable without surgery and decrease with increasing surgical experience; on the other hand, late complications frequently need revision procedures, in most cases endoscopically.
TLM shows good results in terms of DFS and OS, comparable to those of RT. The relapse rate is higher (p = 0.021 and p = 0.035) and DFS is lower (p = 0.017 and p = 0.023) when anterior commissure is involved or pT is concerned, respectively. Type of cordectomy, cT, supraglottic or vocal muscle involvement and resection margin status do not significantly impact the recurrence rate.
References
- 1.Schultz P. Vocal fold cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:301–308. doi: 10.1016/j.anorl.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786–1792. doi: 10.1002/cncr.20181. [DOI] [PubMed] [Google Scholar]
- 3.Hartl DM. Evidence-based practice: management of glottic cancer. Otolaryngol Clin North Am. 2012;45:1143–1161. doi: 10.1016/j.otc.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Succo G, Crosetti E, Bertolin A, et al. Benefits and drawbacks of open partial horizontal laryngectomies, Part A: Early-intermediate stage glottic carcinoma. Nead Neck. 2016;38(Suppl 1):E333–E340. doi: 10.1002/hed.23997. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall WM, Amdur RJ, Morris CG, et al. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19:4029–4036. doi: 10.1200/JCO.2001.19.20.4029. [DOI] [PubMed] [Google Scholar]
- 6.Peretti G, Piazza C, Cocco D, et al. Transoral CO(2) laser treatment for T(is)-T(3) glottic cancer: the University of Brescia experience on 595 patients. Nead Neck. 2010;32:977–983. doi: 10.1002/hed.21278. [DOI] [PubMed] [Google Scholar]
- 7.Bocciolini C, Presutti L, Laudadio P. Oncological outcome after CO2 laser cordectomy for early-stage glottic carcinoma . Acta Otorhinolaryngol Ital. 2005;25:86–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Sobin LH, Gospodarowicz MK, Wittekindt C, et al. TNM Classification of Malignant Tumours. 7th ed. London: Wiley-Blackwell; 2009. [Google Scholar]
- 9.Remacle M, Eckel HE, Antonelli A, et al. Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. Eur Arch Otorhinolaryngol. 2000;257:227–231. doi: 10.1007/s004050050228. [DOI] [PubMed] [Google Scholar]
- 10.Remacle M, Haverbeke C, Eckel H, et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies . Eur Arch Otorhinolaryngol. 2007;264:499–504. doi: 10.1007/s00405-007-0279-z. [DOI] [PubMed] [Google Scholar]
- 11.Peretti G, Piazza C, Bolzoni A, et al. Analysis of recurrences in 322 Tis, T1, or T2 glottic carcinomas treated by carbon dioxide laser. Ann Otol Rhinol Laryngol. 2004;113:853–858. doi: 10.1177/000348940411301101. [DOI] [PubMed] [Google Scholar]
- 12.Robbins KT, Medina JE, Wolfe GT, et al. Standardizing neck dissection terminology. Official report of the Academy's Committee for Head and Neck Surgery and Oncology. Arch Otolaryngol Head Neck Surg. 1991;117:601–605. doi: 10.1001/archotol.1991.01870180037007. [DOI] [PubMed] [Google Scholar]
- 13.Pfister DG, Laurie SA, Weinstein GS, et al. American Society of Clinical Oncology Clinical Practice Guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer . J Clin Oncol. 2007:735–736. [Google Scholar]
- 14.Lee HS, Chun BG, Kim SW, et al. Transoral laser microsurgery for early glottic cancer as one-stage single-modality therapy . Laryngoscope. 2013;123:2670–2674. doi: 10.1002/lary.24080. [DOI] [PubMed] [Google Scholar]
- 15.Dijk BA, Gatta G, Capocaccia R, et al. Rare cancers of the head and neck area in Europe. Eur J Cancer. 2012;48:783–796. doi: 10.1016/j.ejca.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment . Mayo Clinic Proceed. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 17.Motta G, Esposito E, Motta S, et al. CO2 laser surgery in the treatment of glottic cancer. Nead Neck. 2005;27:733–733. doi: 10.1002/hed.20266. [DOI] [PubMed] [Google Scholar]
- 18.Ellies M, Steiner W. Peri- and postoperative complications after laser surgery of tumors of the upper aerodigestive tract. Am J Otolaryngol. 2007;28:168–172. doi: 10.1016/j.amjoto.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Steiner W, Ambrosch P. Endoscopic laser surgery of the upper aerodigestive tract. New York: Thieme; 2000. pp. 112–113. [Google Scholar]
- 20.Bernal-Sprekelsen M, Vilaseca-Gonzalez I, Blanch-Alejandro JL. Predictive values for aspiration after endoscopic laser resections of malignant tumors of the hypopharynx and larynx . Nead Neck. 2004;26:103–110. doi: 10.1002/hed.10363. [DOI] [PubMed] [Google Scholar]
- 21.Cabanillas R, Rodrigo JP, Llorente JL, et al. Functional outcomes of transoral laser surgery of supraglottic carcinoma compared with a transcervical approach . Nead Neck. 2004;26:653–659. doi: 10.1002/hed.20063. [DOI] [PubMed] [Google Scholar]
- 22.Preuss SF, Cramer K, Klussmann JP, et al. Transoral laser surgery for laryngeal cancer: outcome, complications and prognostic factors in 275 patients . Eur J Surg Oncol. 2009;35:235–240. doi: 10.1016/j.ejso.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Vilaseca-Gonzalez I, Bernal-Sprekelsen M, Blanch-Alejandro JL, et al. Complications in transoral CO2 laser surgery for carcinoma of the larynx and hypopharynx . Nead Neck. 2003;25:382–388. doi: 10.1002/hed.10207. [DOI] [PubMed] [Google Scholar]
- 24.Lucioni M, Marioni G, Bertolin A, et al. Glottic laser surgery: outcomes according to 2007 ELS classification . Eur Arch Otorhinolaryngol. 2011;268:1771–1778. doi: 10.1007/s00405-011-1695-7. [DOI] [PubMed] [Google Scholar]
- 25.Succo G, Garofalo P, Fantini M, et al. Direct autofluorescence during CO2 laser surgery of the larynx: can it really help the surgeon? . Acta Otorhinolaryngol Ital. 2014;34:174–183. [PMC free article] [PubMed] [Google Scholar]
- 26.Ansarin M, Santoro L, Cattaneo A, et al. Laser surgery for early glottic cancer: impact of margin status on local control and organ preservation . Arch Otolaryngol Head Neck Surg. 2009;135:385–390. doi: 10.1001/archoto.2009.10. [DOI] [PubMed] [Google Scholar]
- 27.Rodel RM, Steiner W, Muller RM, et al. Endoscopic laser surgery of early glottic cancer: involvement of the anterior commissure . Nead Neck. 2009;31:583–592. doi: 10.1002/hed.20993. [DOI] [PubMed] [Google Scholar]
- 28.Blanch JL, Vilaseca I, Caballero M, et al. Outcome of transoral laser microsurgery for T2-T3 tumors growing in the laryngeal anterior commissure. Nead Neck. 2011;33:1252–1259. doi: 10.1002/hed.21605. [DOI] [PubMed] [Google Scholar]
- 29.Sigston E, Mones E, Babin E, et al. Early-stage glottic cancer: oncological results and margins in laser cordectomy . Arch Otolaryngol Head Neck Surg. 2006;132:147–152. doi: 10.1001/archotol.132.2.147. [DOI] [PubMed] [Google Scholar]
- 30.Hartl DM, Mones E, Hans S, et al. Treatment of earlystage glottic cancer by transoral laser resection. Ann Otol Rhinol Laryngol. 2007;116:832–836. doi: 10.1177/000348940711601107. [DOI] [PubMed] [Google Scholar]
- 31.Brondbo K, Fridrich K, Boysen M. Laser surgery of T1a glottic carcinomas; significance of resection margins. Eur Arch Oto-rhino-laryngol. 2007;264:627–630. doi: 10.1007/s00405-006-0233-5. [DOI] [PubMed] [Google Scholar]


