SUMMARY
Hemifacial spasm is a condition that may severely reduce patients' quality of life. Microvascular decompression is the neurosurgical treatment of choice. The objective of this work was to describe the efficacy and morbidity of microvascular decompression for hemifacial spasm, evaluate the long-term efficacy on the quality of life and investigate prognostic factors for failure of the procedure. A retrospective study of 446 cases of hemifacial spasm treated by 511 retrosigmoid microvascular decompression over 22 years was conducted. Epidemiological, clinical and imaging findings, treatment modalities and outcomes of patients with pre- and postoperative HSF-8 quality of life questionnaire were studied. Success rate was 82% after first surgery and 91.6% after revision surgery. A low rate of perioperative morbidity was found. Facial palsy was mostly transient (5.5% transient and 0.2% permanent) and cochleovestibular deficit was seen in 4.8% of patients. Revision surgery increased nervous lesions (10.6% to 20.7%). Mean quality of life scores were significantly improved from 18 to 2 over 32, evaluated 7.3 years after surgery. Predictive factors of surgical failure were single conflicts (p = 0.041), atypical vasculo-nervous conflicts involving other vessel than postero-inferior cerebellar artery (p = 0.036), such as vein (p = 0.045), and other compression sites than root exit zone (p = 0.027). Retrosigmoid microvascular decompression is a safe and effective treatment of hemifacial spasm. Revision surgery is not to be excluded in case of failure, but does place patients at risk for more complications. Quality of life is improved in the long-term, indicating objective and subjective satisfaction.
KEY WORDS: Hemifacial spasm, HFS-8 score, Microvascular decompression, Neurovascular conflict, Quality of life
RIASSUNTO
L'emispasmo facciale è una condizione clinica che può seriamente compromettere la qualità di vita del paziente. In questi casi la decompressione microvascolare rappresenta il trattamento neurochirurgico di scelta. L'obiettivo del presente lavoro è stato quello di descrivere sia l'efficacia che la morbilità della decompressione microvascolare nel trattamento dell'emispasmo facciale, di valutare l'outcome della procedura in termini di qualità di vita e di individuare eventuale fattori prognostici predittivi dell'eventuale fallimento della procedura. È stata revisionata la nostra casistica di 446 casi di emispasmo facciale trattati complessivamente nell'arco di 22 anni con 511 procedure di decompressione microvascolare con approccio retrosigmoideo. Abbiamo quindi analizzato i reperti epidemiologici, clinici e radiologici, le modalità di trattamento e gli outcome mediante la somministrazione pre e post operatoria del questionario HSF-8. Il rateo di successo è stato dell'82% dopo la prima procedura chirurgica e del 91,6 dopo la seconda procedura. Abbiamo registrato una bassa morbilità perioperatoria. La paralisi del facciale è stato per lo più un fenomeno transitorio (5,5% dei casi, permanente nello 0,2%). Nel 4,8% dei casi si è avuto invece un deficit cocleovestibolare. La chirurgia di revisione è stata invece gravata da un aumentato rateo di lesioni nervose (10.6 -20.7%). La qualità di vita a seguito della chirurgia valutata mediante HSF-8 è risultata migliore con uno score ridotto in media da 18 a 2 su 32. I fattori predittivi di fallimento chirurgico individuate sono stati I conflitti singoli (p = 0,041), conflitti atipici non coinvolgenti la PICA (p = 0,036), come quelli venosi (p = 0,045) e zone di compressione alternative all'emergenza radicolare (p = 0,027). In conclusione, la decompressione microvascolare con accesso retrosigmoideo si è rivelata essere una tecnica sicura ed efficace nel trattamento dell'emispasmo facciale. La revisione chirurgica è un opzione percorribile, ma espone a un maggior rischio di complicanze. La qualità di vita è risultata accresciuta a nel lungo termine, dimostrando un elevato grado di soddisfazione e beneficio oggettivo e soggettivo.
Introduction
Hemifacial spasm (HFS) is characterised by tonic and/or clonic contraction of the facial muscles of one side of the face. The symptoms usually progress in frequency and severity, starting with intermittent twitches in the orbicularis oculi muscle, and spreading downward to the ipsilateral facial muscles.
The most frequent cause of HFS is neurovascular conflict due to a focal compression of the root exit zone (REZ) of the facial nerve by an aberrant vascular structure 1. In cases identified by magnetic resonance imaging (MRI), retrosigmoid microvascular decompression (MVD) is indicated after unsatisfactory or decreasing effect of botulinum toxin treatment. It consists in a neurosurgical intervention in the cerebellopontine angle, and therefore exposes patients to rare morbidity such as nervous lesion (facial palsy, hearing loss, vestibular dysfunction), neurosurgical (cerebrospinal fluid (CSF) leak, infection, bleeding), scarring and general complications (anesthesia complications) 2. Therefore, long-term efficacy of MVD must be documented in order to strengthen the benefit-risk ratio.
HFS frequently leads to social anxiety, but can also affect vision, sleep and mental concentration 3. Such problems have a strong impact on the patient's perception and satisfaction in various aspects of their life 4 5. Quality of life (QOL) is an important outcome measure in chronic diseases such as HFS, where patients expect to find relief in subjective complains such as social embarrassment. The HemiFacial Spasm 8 Quality of Life Scale (HFS-8) is the reference parameter to investigate efficacy of post-operative results of MVD 6.
This study focused on long-term surgical results in retrosigmoid MVD for HFS. Based on our experience and a review of the literature, our study aimed: (i) to describe the morbidity of this therapeutic approach; (ii) to evaluate the long-term efficacy on QOL; (iii) to investigate prognostic factors for failure of the procedure.
Materials and methods
This work reports on a retrospective study of consecutive cases of HFS treated by MVD in a university tertiary referral centre over 22 years (1990-2012). Patients with non-vascular aetiologies of facial nerve irritation were excluded.
Workup
All HFS patients presented unsatisfactory or decreasing effect of botulinum toxin treatment. HFS onset was reported. All patients were clinically investigated for otological and neurological symptoms. Audiometry for pure tones and speech was performed, and recommendations from the Committee on Hearing and Equilibrium were followed for reporting hearing test results 7. MRI, focusing on the facial nerve trajectory, was performed to diagnose and localise the neurovascular conflict, and to exclude facial nerve tumour or other differential diagnoses.
Failure or recurrence of HFS after MVD was defined by: no change in spasms, worsening of spasms and improvement but persistence of spasms. Preoperative MRI, intraoperative videos, or pictures were reviewed to ensure a missed compression (multiple conflicts). Before revision surgery, a new MRI was performed to identify a missed compression, adhesions around REZ and/or Teflon pledget migration.
Microvascular decompression procedure using a minimal invasive retrosigmoid approach
Surgical strategy was as previously described with microneurosurgical instruments, powered instrumentation and intraoperative facial nerve integrity monitoring system (NIM II®; Medtronic Inc, Fridley, Minnesota) 8 9.
A limited craniotomy behind and close to the sigmoid sinus was performed (2-1.5 cm in diameter). After hyperventilation to diminish CSF pressure, the dura was U-shaped opened. Under binocular microscopy, the cerebellum was depressed spontaneously and progressively helped by CSF aspiration of the cerebellopontine angle cistern. By an endoscopic procedure, the cerebellopontine angle was explored without the use of fixed retraction, allowing clear visualisation of the facial nerve and precise location of the neurovascular conflict. The site of the disorder was exposed safely and non-traumatically with a 30°- or 45° endoscope with a diameter of 4 mm and length of 11 cm. Conflict was treated by interposition of Teflon pledget and/or liberation vessel or nerve from fibrosis or retraction. Multiple offending vessels were searched in multiple possible affected sites in addition to the REZ of the facial nerve.
Post-operative management
A compression bandage was kept for 5 days. No antibiotic prophylaxis was given. Pain was handled with appropriate analgesics. During hospitalisation, computed tomography (CT) was carried out in case of headache or neurological disorder. Audiometry for pure tones was performed at day 7. The patient was discharged after 7 post-operative days.
Facial palsy was treated with high-dose corticosteroids, long-term eye care and facial physical therapy. Vestibular deficit was managed with high-dose corticosteroids and vestibular rehabilitation. Sensorineural hearing loss (SNHL) was initially treated with high-dose corticosteroids. In the event of CSF leak, conservative measures (compression bandage, bed rest, head elevation, stool softeners and acetazolamid) were combined with anti-pneumococcal vaccination. If the leak persisted after 3 days, surgical closure of the breach was indicated.
Follow-up
Resolution of symptoms after MVD was evaluated at a minimum of 2 months post-operatively with otological and neurological assessment. Complete symptoms resolution was considered as immediate or delayed if resolution took less or more than 2 weeks. Audiometry for pure tones and speech was performed at the same time. Followup was based on clinical control visits with audiometry at 6 months post-operatively.
HFS-8 quality of life self-questionnaire
This QOL short self-rating scale investigates 8 validated items for severity quotation from 0 (normal) to 4 (severely incapacitated): difficulty driving, reading, watching television/ movie, feels depressed, avoids eye contact, feels embarrassed about having the condition, feels worried about other's reaction and sleep disturbance 6. The HSF-8 score ranged from 0 to 32, the former indicating an excellent QOL and the latter a mediocre QOL. Two HFS-8 questionnaires were submitted to patients by mail or by phone: a retrospective one evaluating preoperative QOL, and a prospective one evaluating presently postoperative QOL (assessment from 2012 to 2014).
Statistical analysis
Continuous data were expressed as mean ± standard deviation or median [25th-75th percentiles]. Symptom prevalence rates were expressed as percentages. The clinical follow-up interval was calculated by year, from the date of surgery to the date of last consultation. Comparison of individual pre- and post-operative QOL scores was carried out by paired Wilcoxon test. The prognostic factors for surgical failure were investigated using independent Student t-tests for continuous factors and chi-square tests for categorical factors. A p value less than 0.05 was considered statistically significant. All analyses were carried out using IBM SPSS Statistics 20.0 (IBM Inc., New York, USA).
Results
Population
Over 22 years, 446 patients underwent retrosigmoid MVD, and 511 procedures were performed. Twelve patients were operated for a failure or a relapse after a first MVD in another institution. Median clinical follow-up was 24 months [3-75].
Among the 446 patients, 160 (35.9%) were male and 286 (64.1%) were female, for a sex ratio of 0.6. Age of HFS onset and at first surgery was, respectively, 49 ± 12 years and 57 ± 12 years. Surgery was single for 384 patients. In 62 cases of failure or relapse after first surgery (including 12 cases with first procedure in another center), revision procedure was performed 25 ± 43 months after first MVD. A total of 77 revision procedures were performed in 62 patients: 52 patients (11.6%) had 2 surgeries, 7 patients (1.6%) had 3 surgeries, 1 patient (0.2%) had 4 surgeries and 2 patients (0.4%) had 5 surgeries.
Imaging and endoscopic findings
Pre-operative MRI was performed in 99.9% of 511 procedures (3 claustrophobics and 1 patient with pacemaker were explored by CT with contrast infusion). Before first surgery, 4 patients (1%) did not exhibit neurovascular conflict on MRI and the nature of vessel was notified in 406 MRI (91%) and 424 procedures (95%) in our data (Table I). The postero-inferior cerebellar artery (PICA) was involved in 77% of per-operative conflicts, vertebral artery (VA) in 38% and antero-inferior cerebellar artery (AICA) in 38%. Among the 74 conflicts classified as "other", 35 (8% of all conflicts) were triple, involving the PICA, VA and AICA. Multiple vessel conflict was 40% in MRI evaluation and 55% in endoscopic evaluation.
Table I.
Imaging vs endoscopic findings discordance for neurovascular conflicts.
| PICA | VA + PICA | VA | AICA | PICA + AICA | Other | Total | |
|---|---|---|---|---|---|---|---|
| MRI | 37% (n = 151) |
30% (n =121) |
11% (n = 45) |
10% (n = 40) |
7% (n = 28) |
5% (n = 21) |
N = 406 |
| Endoscopy | 30% (n = 127) |
23% (n = 98) |
3% (n = 13) |
11% (n =46) |
16% (n = 66) |
17% (n = 74) |
N = 424 |
| Discordance | 36% | 34% | 46% | 58% | 78% | 93% |
Discordance was calculated for each surgical conflict as the rate of patients that exhibited a different conflict in MRI.
AICA: Antero-Inferior Cerebellar Artery; MRI: Magnetic Resonance Imaging; PICA: Postero-Inferior Cerebellar Artery; VA: Vertebral Artery
Revision surgery for recurrence or failure let to finding incomplete nervous decompression, retraction and fibrosis around REZ and/or Teflon pledget migration.
Surgical efficacy
Resolution of symptoms after first hand MVD was complete and immediate in 54% of cases, complete and delayed in 23.6%, partial in 4.7%, and 4.1% were improved before relapsing. Efficacy was 82% after first surgery and 91.6% after revision surgery.
Complications
At least one complication was seen in 83 patients (19%) after first surgery, and in 23 patients (30%) after revision surgery. Complications are detailed in Table II. No patient died.
Table II.
Complications of retrosigmoid microvascular decompression for hemifacial spasm.
| First surgery (n=434) No. of patients (%) |
Revision surgery (n=77) No. of patients (%) |
|
|---|---|---|
| General complications | 6 (1.4) | 1 (1.3) |
| Neurosurgical complications | ||
| Infection | 7 (1.6) | 1 (1.3) |
| Bleeding | 3 (0.7) | 0 |
| CSF leak | 9 (2) | 6 (7.8) |
| Scarring complications | 24 (5.5) | 7 (9) |
| Nervous complications | 46 (10.6) | 16 (20.7) |
| Facial nerve | ||
| Transient | 24 (5.5) | 5 (6.5) |
| Permanent | 1 (0.2) | 1 (1.3) |
| Cochleo-vestibular nerve | ||
| Cochlear nerve | ||
| Transient | 9 (2) | 5 (6.5) |
| Permanent | 12 (2.7) | 3 (3.9) |
| Vestibular nerve | 3 (0.7) | 1 (1.3) |
| Lower cranial nerves (IX, X, XI, XII) | ||
| Transient | 4 (0.9) | 0 |
CSF: cerebrospinal fluid
Quality of life
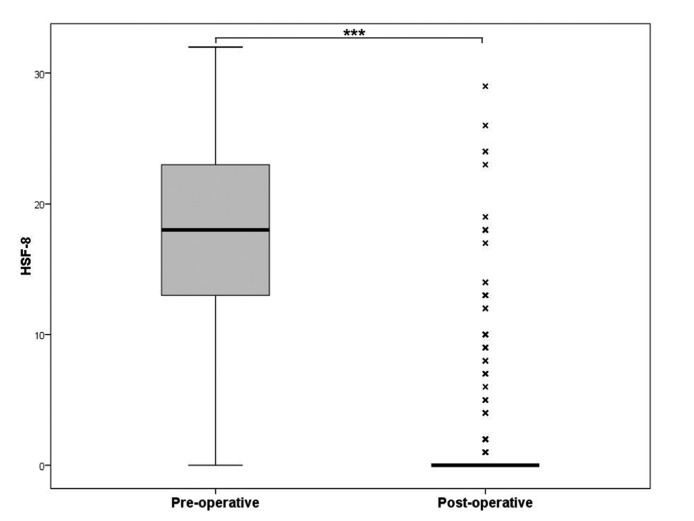
Three hundred and three patients (68%) underwent longterm assessment with the HFS-8 questionnaire; 143 patients were lost to QOL evaluation. Mean (median [25th- 75th percentiles]) time between first surgery and evaluation was 7.3 years (6 [3.9-9.7] years). The HSF-8 score dramatically improved after surgery from 18 (18 [13-23]) to 2 (0 [0-0]) over 32 (p < 0.001) (Fig. 1).
Fig. 1.
Pre- and post-operative HFS-8 score. The median is indicated by a thick black line inside the box. The top and bottom of the box are the upper and lower quartile [25th-75th percentiles] and indicate the inter-quartile range (IQR). The whiskers indicate the lowest/highest datum still within 1.5 IQR of the lower/upper quartile. Outliers are marked with a cross. HSF-8 score was significantly improved after surgery (*** p < 0.001).
Prognosis factors
Pre-, peri- and postoperative data were studied as prognostic factors for surgical failure after first MVD (Table III). Failure was significantly associated with single conflict (p = 0.041), venous conflict (p = 0.045), absence of involvement of PICA in the neurovascular conflict (p = 0.036) and absence of involvement of REZ in the neurovascular conflict (p = 0.027).
Table III.
Prognostic factors for surgical failure after first microvascular decompression for hemifacial spasm.
| Variable tested | Surgical failure if the variable is present | Surgical failure if the variable is not present | p |
|---|---|---|---|
| Imaging findings | |||
| Multiple conflict | 3.5% | 6.5% | 0.402 |
| PICA | 5.4% | 4.7% | 0.999 |
| VA | 3.6% | 6.4% | 0.311 |
| AICA | 6.3% | 5% | 0.722 |
| REZ conflict | 4.8% | 5.6% | 0.604 |
| Endoscopic findings | |||
| Multiple conflict | 2.6% | 7.9% | 0.041 |
| PICA | 3.6% | 10.9% | 0.036 |
| VA | 2.9% | 6.3% | 0.203 |
| AICA | 3.1% | 6.5% | 0.218 |
| Vein | 18.8% | 4.5% | 0.045 |
| REZ conflict | 3.9% | 16% | 0.027 |
| Post-operative findings | |||
| Nervous complication | 7.1% | 5.4% | 0.230 |
| Mixed nerve palsy | 50% | 5.3% | 0.109 |
AICA: Antero-Inferior Cerebellar Artery; PICA: Postero-Inferior Cerebellar Artery; REZ: Root Exit Zone; VA: Vertebral Artery
Discussion
HFS is an incapacitating condition whose therapeutic approach warrants due consideration. It is now generally accepted that HFS patients with unsatisfactory or decreasing effects of botulinum toxin treatment should be referred to surgery. We aimed to investigate the results of MVD for HFS, and to evaluate the morbidity of this therapeutic approach and long-term impact on QOL. We retrospectively studied 446 patients who underwent 511 retrosigmoid MVD over 22 years. QOL was quantified by HSF-8 score 7.3 years after MVD. The major findings of this work were a low rate of perioperative morbidity and a significant improvement of QOL after surgery.
MRI is the gold standard for neurovascular conflicts diagnosis
MRI, focusing on the facial nerve trajectory, was performed to diagnose and localise the neurovascular conflict, and to exclude a facial nerve tumour or other differential diagnoses. In our study, only 4 patients (1%) did not exhibit neurovascular conflict on MRI, suggesting a good specificity of imagery. However, the surgeon, helped by endoscopy, must look for any possible conflict since MRI description was discordant from endoscopy qualitatively (vessel involved) and quantitatively (number of vessels and conflicts involved). Therefore, fully trusting imaging risks partial resolution of symptoms. Conflicts involving the PICA and VA were over-described by radiologists, whereas AICA (more difficult to visualise on MRI) conflicts were underestimated. Multiple conflicts were met in 55% of interventions versus 40% on MRI. Multiple conflicts must be systematically investigated by MRI. Pre-operative MRI enables to predict vascular pathology 10. Major changes in MRI protocols or imaging techniques for evaluation of patients were observed between 1990 and 2012. Improvement in imaging modalities since 1990 would result in overestimation of discordance. Recently, for better information, neurovascular conflicts are documented by MRI with three-dimensional constructive interference in steady-state, alone or together with threedimensional time-of-flight MR angiography 11 12. In fact, the progress in medical imaging techniques has reduced discordance. However, during an endoscopic surgical procedure, the cerebellopontine angle is explored, allowing clear visualisation of the facial nerve and precise location of the neurovascular conflict, and multiple conflicts must be systematically investigated.
Complications of MVD are mostly few, benign and transient
No death was seen in our study. Extra-nervous complications were few (10%) and always transient. Among cranial nerve injuries, facial palsy was less frequent than literature, either transient (5.5% versus 9.5%) or permanent (0.2% versus 0.9%), whereas SNHL, vestibular deficit, or lower cranial nerve palsy (dysphagia, hoarseness, dysphonia) were as rare as in other studies 13. Revision surgery increased permanent facial palsy and SNHL from 0.2% to 1.3% and from 2.7% to 3.9%, respectively.
Because MVD for HFS is effective in the long-term, nervous complications must be handled with maximum care. Neuromonitoring of the facial nerve and muscles enables, respectively, to minimise the risk of facial lesion and improve the efficacy of MVD (modification of lateral spread responses) 14. Considering that auditory damage is the first permanent nervous complication, monitoring of brainstem auditory evoked potentials (BAEP) can be helpful in warning the surgeon of auditory damage. Eighth cranial nerve directly compounds the action potential, and auditory brainstem evoked response showed correlation between per-operative electrophysiologic alterations and clinical post-operative hypoacousia. Intraoperative BAEP change and poorer recovery, especially persistent decreases in amplitude greater than 50% in wave V and persistent absolute latency increase of the peak of Wave V, which equals or exceeds 0.5 msec, was a strong indicator for a worse outcome of the hearing capacity. Vigilant intraoperative monitoring of the BAEP and adequate steps for recovery of the BAEP change could prevent hearing loss after MVD for HFS 15-17.
During MVD for HFS, fixed cerebellar retraction has historically been used to expose the cranial nerves. In recent years, some surgeons have attempted to reduce the use of fixed retraction to reduce the incidence of iatrogenic microtrauma and ischaemia within brain parenchyma. Additionally, fixed cerebellar retraction may also lead to operative manipulation and stretching of the cochleovestibular nerve, leading to iatrogenic SNHL and balance issues. Retraction of the cerebellum by a spatula could be the major cause of surgical complications. In our study, the surgical procedure without the use of fixed retraction may greatly reduce the occurrence of SNHL following MVD for HFS 18 19. Fixed retraction of the cerebellum with selfretaining retractor was never used during any step of the procedure in our institution since the beginning of our use of the minimal invasive retrosigmoid approach 9. Additionally, endoscopic visualisation by a 30° or 45° endoscope decreases cerebellar retraction compared to microscopic visualisation to prevent postoperative SNHL in MVD for HFS 20.
Revision surgery was indicated in case of recurrence or failure of procedure
MVD for HFS is a highly effective treatment, but residual spasms or reappearance of spasms after surgery are not uncommon. Revision surgery for recurrence or failure is associated with incomplete nervous decompression, retraction and fibrosis around REZ and/or Teflon pledget migration. When revision surgery is considered, pre- and postoperative MRI, intraoperative videos, or pictures should be reviewed to ensure a missed compression (multiple conflicts), adhesions around REZ and/or Teflon pledget migration. MRI after MVD contribute to the decision for repeat MVD 21. We did not find any study incriminating Teflon pledget dysfunction (granuloma or compression) in recurrence or failure of MVD for HFS. However, Haidar et al. showed in a retrospective study a 8-fold decreased rate of recurrence in MVD for trigeminal neuralgia with use of autologous muscle interposition in comparison to Teflon (5.2% vs 40%) 22. We ought to consider the nature of interposition material as a possible cause of failure or recurrence for HFS as it has been suspected for trigeminal neuralgia. Further investigations are needed on this point, and use of autologous muscle interposition should be considered.
Efficacy was increased when revision surgeries were taken into account (from 82% to 91.6%) and comparable to that reported in the literature 13 23 24. Repeat MVD of the facial nerve may be sufficient to resolve symptoms in selected patients with persistent or recurrent HFS 25. Hence, the benefit-risk ratio should be explained to the patient: in our study, most patients accepted second surgery (69% versus 13.1-50% in literature) 26. No consensus has been reached on the timing of re-operation. In 62 cases of failure or relapse after first surgery in our study, revision procedure was performed at 25 ± 43 months after first MVD. Predictive factors of surgical failure were single conflicts, atypical vasculo-nervous conflicts involving other vessels than PICA such as veins and compression sites other than REZ. In these cases, other conflicts of the facial nerve must be systematically investigated and risk of failure must be explained to the patient. Multiple compression sites were met in 55% of patients, but underdescribed by imaging (40%), thus reinforcing the utility of peri-operative extensive endoscopy investigation of the facial nerve. In fact, during endoscopic surgical procedures, the cerebellopontine angle is explored, allowing clear visualisation of the facial nerve and a precise location of the neurovascular conflict, and multiple conflicts must be systematically investigated. Peri-operative neurophysiological data might predict and prevent surgical failure. In fact, residual lateral spread response after seemingly adequate decompression for HFS could be a prognostic factor of outcome of MVD. Adequate decompression in patients with residual lateral spread response improved long-term spasm relief, although the amplitude of residual lateral spread response after adequate decompression does not significantly affect long-term spasm relief 27.
MVD is an effective and durable treatment for HFS
QOL research provides fundamental information about the impact of HFS, its treatment and side effects or complications. HFS patients suffer mainly from emotional distress and fear of social stigmatisation 3. Therefore, surgical effectiveness cannot be only presumed by partial or total disappearance of spasm. In order to consider patients' subjectivity in various aspects of their life, the HFS-8 questionnaire is the most solid tool for HFS 28.
The HSF-7 scale had been described by Tan et al. as a short, pratical, validated quality of life scale specific to HSF patients 3. The HSF-7 scale has been found to correlate well with the SF-36 health survey generic quality of life scale. The disease-specific HSF-7 form was extended with the item "sleep disturbance due to spasms". Findings from adding additional items into an existing validated instrument should be interpreted with caution as these items have not undergone the same rigorous validation procedures. Nevertheless, the results from the HFS-8 measurement are consistent with the conclusions from the validated HFS-7 instrument 6 28.
Long-term QOL was significantly increased by MVD in our study. QOL was evaluated in a retrospective way, and introducing a recall bias for pre-operative QOL status: first patients filled in their retrospective questionnaires 15 years after MVD. Subjective perception of previous QOL was inevitably altered. These results should, however, be confirmed in a prospective study.
The retrospective nature over a long period of data collection suffers recording and recollection bias amongst many other limitations of these types of analyses. The accuracy and exact criteria for selection of each patient, uniformity of MR diagnostic technique over 22 years of study and comprehensiveness of recording for complications are some of these issues. The outcome assessment for both treatment and complications are done by the surgeon, and not by an independent neurologist, making the result prone to bias.
Reporting results in a long-time scale highlights that MVD is an effective and durable treatment for HFS. Our mean time of clinical follow-up was 4 ± 4.7 years, but 167 patients (37%) were lost to follow-up less than 1 year after surgery, thus increasing the standard deviation of follow-up. Median time of clinical follow-up was shorter (2 vs 2.9 years) than studies in the literature 13. To our knowledge, the latest follow-up studies performed with HFS-8 questionnaire do not exceed 3 years post-operative (prospective studies) or 4 years post-operative (retrospective studies) 6 28. In our study, QOL was evaluated 7.3 years after MVD, which seems reasonable to consider its effectiveness. We found no cases of HFS recurrence in either our study or in the literature after a delay of 5 years with no symptoms. In their study, Payner et al. documented an efficacy of 94% after 6 years of follow-up; 86% of recurrences occurred within 2 years post-operative and no patient relapsed after 2 years without having some degree of recurrent spasm 24. In addition, Miller et al. reported no significant differences in symptom cure rates at 1 year postoperatively compared to a maximum follow-up period of 5 years postoperatively 13. Therefore, MVD is an effective and durable treatment for HFS, with a significant improvement of long-term postoperative QOL.
Conclusions
This retrospective study included 511 retrosigmoid decompressions for HFS performed over 22 years. MVD is a safe and effective treatment for HFS. Imaging helps the surgeon to find conflicts, but endoscopy prevails in finding and treating any possible source of conflict. Revision surgery is not to be excluded in case of failure or recurrence, but place put patients at risk for more complications. QOL is improved in the long-term, indicating objective and subjective satisfaction of this surgical procedure.
References
- 1.Lu AY, Yeung JT, Gerrard JL, et al. Hemifacial spasm and neurovascular compression . Sci World J. 2014;2014:349319–349319. doi: 10.1155/2014/349319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huh R, Han IB, Moon JY, et al. Microvascular decompression for hemifacial spasm: analyses of operative complications in 1582 consecutive patients . Surg Neurol. 2008;69:153–157. doi: 10.1016/j.surneu.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Tan EK, Fook-Chong S, Lum SY, et al. Validation of a short disease specific quality of life scale for hemifacial spasm: correlation with SF-36 . J Neurol Neurosurg Psychiatry. 2005;76:1707–1710. doi: 10.1136/jnnp.2005.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers AR, Gage H, Hendricks A. Health-related quality of life in neurology . Arch Neurol. 2000;57:1224–1227. doi: 10.1001/archneur.57.8.1224. [DOI] [PubMed] [Google Scholar]
- 5.Marinus J, Ramaker C, Hilten JJ, et al. Health related quality of life in Parkinson's disease: a systematic review of disease specific instruments . J Neurol Neurosurg Psychiatry. 2002;72:241–248. doi: 10.1136/jnnp.72.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuser K, Kerty E, Eide PK, et al. Microvascular decompression for hemifacial spasm: postoperative neurologic follow-up and evaluation of life quality . Eur J Neurol. 2007;14:335–340. doi: 10.1111/j.1468-1331.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- 7.Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium . Otolaryngol Head Neck Surg. 1995;113:176–178. doi: 10.1016/S0194-5998(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki H, Deveze A, Magnan J. Neuro-otologic surgery through minimally invasive retrosigmoid approach: endoscope assisted microvascular decompression, vestibular neurotomy, and tumor removal . Laryngoscope. 2005;115:1612–1617. doi: 10.1097/01.mlg.0000172038.22929.63. [DOI] [PubMed] [Google Scholar]
- 9.Magnan J, Caces F, Locatelli P, et al. Hemifacial spasm: endoscopic vascular decompression . Otolaryngol Head Neck Surg. 1997;117:308–314. doi: 10.1016/S0194-5998(97)70118-9. [DOI] [PubMed] [Google Scholar]
- 10.Sekula RF, Jr, Frederickson AM, Branstetter BF, 4th, et al. Thin-slice T2 MRI imaging predicts vascular pathology in hemifacial spasm: a case-control study . Mov Disord. 2014;29:1299–1303. doi: 10.1002/mds.25947. [DOI] [PubMed] [Google Scholar]
- 11.Iijima K, Horiguchi K, Yoshimoto Y. Microvascular decompression of the root emerging zone for hemifacial spasm: evaluation by fusion magnetic resonance imaging and technical considerations . Acta Neurochir (Wien) 2013;155:855–862. doi: 10.1007/s00701-013-1671-7. [DOI] [PubMed] [Google Scholar]
- 12.El Refaee E, Langner S, Baldauf J, et al. Value of 3-dimensional high-resolution magnetic resonance imaging in detecting the offending vessel in hemifacial spasm: comparison with intraoperative high definition endoscopic visualization . Neurosurgery. 2013;73:58–67. doi: 10.1227/01.neu.0000429838.38342.e2. [DOI] [PubMed] [Google Scholar]
- 13.Miller LE, Miller VM. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: a systematic review . Br J Neurosurg. 2012;26:438–444. doi: 10.3109/02688697.2011.641613. [DOI] [PubMed] [Google Scholar]
- 14.Hale T, Hoffman SN, Dehdashti AR. Intra-operative monitoring of two facial muscles in hemifacial spasm surgery . Neurochirurgie. 2015;61:266–270. doi: 10.1016/j.neuchi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Polo G, Fischer C. Intraoperative monitoring of brainstem auditory evoked potentials during microvascular decompression of cranial nerves in cerebellopontine angle . Neurochirurgie. 2009;55:152–157. doi: 10.1016/j.neuchi.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Jo KW, Kim JW, Kong DS, et al. The patterns and risk factors of hearing loss following microvascular decompression for hemifacial spasm . Acta Neurochir (Wien) 2011;153:1023–1030. doi: 10.1007/s00701-010-0935-8. [DOI] [PubMed] [Google Scholar]
- 17.Thirumala PD, Krishnaiah B, Crammond DJ, et al. Analysis of wave III of brain stem auditory evoked potential waveforms during microvascular decompression of cranial nerve VII for hemifacial spasm . J Clin Neurophysiol. 2014;31:127–132. doi: 10.1097/WNP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu K, Matsumoto M, Wada A, et al. Supine no-retractor method in microvascular decompression for hemifacial spasm: results of 100 consecutive operations . J Neurol Surg B Skull Base. 2015;76:202–207. doi: 10.1055/s-0034-1396660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thirumala P, Frederickson AM, Balzer J, et al. Reduction in high-frequency hearing loss following technical modifications to microvascular decompression for hemifacial spasm . Reduction in high-frequency hearing loss following technical modifications to microvascular decompression for hemifacial spasm . 2015;123:1059–1064. doi: 10.3171/2014.12.JNS141699. [DOI] [PubMed] [Google Scholar]
- 20.Lee MH, Lee HS, Jee TK, et al. Cerebellar retraction and hearing loss after microvascular decompression for hemifacial spasm . Acta Neurochir (Wien) 2015;157:337–343. doi: 10.1007/s00701-014-2301-8. [DOI] [PubMed] [Google Scholar]
- 21.Hughes MA, Branstetter BF, Taylor CT, et al. MRI findings in patients with a history of failed prior microvascular decompression for hemifacial spasm: how to image and where to look . AJNR Am J Neuroradiol. 2015;36:768–773. doi: 10.3174/ajnr.A4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haidar H, Montava M, Collin M, et al. Endoscopy-assisted microvascular decompression for trigeminal neuralgia: the prognostic impact of interposing material . Int Adv Otol. 2014;10:107–112. [Google Scholar]
- 23.Shibahashi K, Morita A, Kimura T. Surgical results of microvascular decompression procedures and patient's postoperative quality of life: review of 139 cases . Neurol Med Chir (Tokyo) 2013;53:360–364. doi: 10.2176/nmc.53.360. [DOI] [PubMed] [Google Scholar]
- 24.Payner TD, Tew JM., Jr Recurrence of hemifacial spasm after microvascular decompression . Neurosurgery. 1996;38:686–691. [PubMed] [Google Scholar]
- 25.Park YS, Chang JH, Cho J, et al. Reoperation for persistent or recurrent hemifacial spasm after microvascular decompression . Neurosurgery. 2006;58:1162–1167. doi: 10.1227/01.NEU.0000215954.97948.B3. [DOI] [PubMed] [Google Scholar]
- 26.Xia L, Zhong J, Zhu J, et al. Delayed relief of hemifacial spasm after microvascular decompression . J Craniofac Surg. 2015;26:408–410. doi: 10.1097/SCS.0000000000001406. [DOI] [PubMed] [Google Scholar]
- 27.Thirumala PD, Wang X, Shah A, et al. Clinical impact of residual lateral spread response after adequate microvascular decompression for hemifacial spasm: a retrospective analysis . Br J Neurosurg. 2015;29:818–822. doi: 10.3109/02688697.2015.1054351. [DOI] [PubMed] [Google Scholar]
- 28.Ray DK, Bahgat D, McCartney S, et al. Surgical outcome and improvement in quality of life after microvascular decompression for hemifacial spasms: a case series assessment using a validated disease-specific scale . Stereotact Funct Neurosurg. 2010;88:383–389. doi: 10.1159/000319883. [DOI] [PubMed] [Google Scholar]