Abstract
Background and aims
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been reported in irritable bowel syndrome (IBS). Enhanced HPA axis response has been associated with reduced glucocorticoid receptor (GR) mediated negative feedback inhibition. We aimed to study the effects of IBS status, sex, or presence of early adverse life events (EAL) on the cortisol response to corticotropin-releasing factor (CRF) and adrenocorticotropic hormone (ACTH), and on GR mRNA expression in peripheral blood mononuclear cells (PBMCs).
Methods
Rome III+ IBS patients and healthy controls underwent CRF (1 μg/kg ovine) and ACTH (250 μg) stimulation tests with serial plasma ACTH and cortisol levels measured (n = 116). GR mRNA levels were measured using quantitative PCR (n = 143). Area under the curve (AUC) and linear mixed effects models were used to compare ACTH and cortisol response measured across time between groups.
Results
There were divergent effects of IBS on the cortisol response to ACTH by sex. In men, IBS was associated with an increased AUC (p = 0.009), but in women AUC was blunted in IBS (p = 0.006). Men also had reduced GR mRNA expression (p = 0.007). Cumulative exposure to EALs was associated with an increased HPA response. Lower GR mRNA was associated with increased pituitary HPA response and increased severity of overall symptoms and abdominal pain in IBS.
Conclusion
This study highlights the importance of considering sex in studies of IBS and the stress response in general. Our findings also provide support for PBMC GR mRNA expression as a peripheral marker of central HPA response.
Keywords: Irritable bowel syndrome, Hypothalamic pituitary-regulating, hormones, Adrenal cortex hormones, Glucocorticoids receptors, Sex differences
1. Introduction
Irritable bowel syndrome (IBS) is a stress-sensitive disorder. Perceived current stress is associated with first symptom onset and exacerbation in the majority of patients, (Whitehead et al., 1992) stress is a risk factor for the development of post-infectious IBS, (Gwee et al., 1999) and IBS patients report a higher prevalence of early adverse life events (EALs). (Bradford et al., 2012) The response to a stressor is in part mediated by the hypothalamic-pituitary-adrenal (HPA) axis. Activation of the HPA axis results in release of CRF from the paraventricular nucleus of the hypothalamus, stimulating release of adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH stimulates the adrenal cortex to release cortisol. Cortisol attenuates the HPA axis response via negative feedback, primarily via glucocorticoid receptors (GRs), in the hypothalamus and the pituitary.
1.1. HPA axis augmentation in IBS—current evidence in humans in inconclusive
In animal models, augmentation of the HPA axis has been linked to increased visceral sensitivity, (Myers and Greenwood-Van Meerveld, 2010) a cardinal feature of IBS. HPA axis studies in IBS have yielded inconsistent results (summarized with references in Appendix C (see Supplementary material)). Most studies supporting augmentation of the HPA axis in IBS have measured the response to a psychological stressor. The HPA response to visceral stressors such as rectal distension or flexible sigmoidoscopy has been similar between IBS patients and healthy controls (HCs) in most studies. Hormone challenge allows an isolated assessment of the HPA axis. To date, three studies have assessed the response to CRF in IBS vs. HCs, with two showing an increased and one showing a blunted HPA response in IBS vs. HCs. (Bohmelt et al., 2005; Dinan et al., 2006; Fukudo et al., 1998) In the largest of these studies, the patient group included only 10 with IBS alone, with 15 having IBS and non-ulcer dyspepsia and 5 with non-ulcer dyspepsia. In a well-characterized and sufficiently large sample without psychiatric comorbidity, we aim to test the hypothesis that the HPA response at both the pituitary and adrenal level is augmented in IBS vs. HCs by comparing the area under the curve (AUC) of the ACTH and cortisol responses to hormone stimulation, as well as the rate of rise from baseline to peak as a secondary measure.
1.2. HPA axis dysregulation may be different in men and women
Like other stress-related disorders, IBS is more prevalent among women. There are sex differences in the HPA response among healthy individuals, and dysregulation of the HPA axis in psychiatric disease differs by sex (e.g. lower cortisol in post traumatic stress disorder is not seen in men, and in depression, cortisol is elevated to a higher degree in women). (Bangasser and Valentino, 2014) Sex differences in the autonomic system have been described in IBS, with increased sympathetic tone and decreased vagal tone in men vs. women with IBS, (Chang and Heitkemper, 2002), but sex differences in the HPA axis in IBS have not been systematically evaluated. We planned to compare the HPA response to hormone challenge separately in men and women to test the hypotheses that a) among HCs, HPA response will be increased in women vs. men, and b) among IBS patients, HPA response will be increased in men vs. women.
1.3. Peripheral glucocorticoid receptor expression
The response of the HPA axis is attenuated via negative feedback though binding of cortisol to glucocorticoid receptors in the hypothalamus and pituitary, and one mechanism of HPA axis dysregulation is impairment of negative feedback by changes in GR expression, signaling or trafficking. There is evidence for a role of altered central GR signaling in IBS from preclinical studies. For example, central knockdown of GRs resulted in increased visceromotor response to colorectal distension. (Johnson and Greenwood-Van Meerveld, 2015) In humans, decreased central expression of GRs has been associated with depression in findings from autopsy specimens, (Webster et al., 2002) and impaired negative feedback in depression is also supported by the consistent response to dexamethasone suppression or dexamethasone-CRF testing. Though negative feedback is mediated by GRs in the central nervous system, changes in peripheral blood mononuclear cell (PBMC) GR number, (de Kloet et al., 2007) sensitivity, (Yehuda et al., 2004) promoter methylation status, (Yehuda et al., 2015) and mRNA expression (Gola et al., 2014; Hepgul et al., 2013) have been reported in psychiatric disease associated with negative feedback of the HPA axis. While studies in IBS have not supported impaired negative feedback based on dexamethasone suppression, (Bohmelt et al., 2005; Dinan et al., 2006) PBMC GR mRNA expression has not been studied. We aimed to test the hypothesis that PBMC GR mRNA is decreased in IBS vs. HCs which could support altered negative feedback as a mechanism for IBS-associated augmentation of the HPA axis. We also aimed to test the hypothesis that PBMC GR mRNA expression is a peripheral biomarker reflecting the “activity” of the HPA axis by determining whether GR mRNA expression is correlated with basal or stimulated hormone levels.
1.4. Early adverse life events
Exposure to EALs is associated with GR expression, HPA response, and IBS. (Bradford et al., 2012; McGowan et al., 2009; Videlock et al., 2009) We aimed to test the hypothesis that both IBS patients and HCs with EALs will have increased HPA response and decreased GR mRNA.
To our knowledge, this is the largest study to evaluate the independent and interaction effects of sex, IBS status and history of EALs on the HPA axis. It is also the largest study evaluating the response to CRF in IBS, and the first study to evaluate: 1) the response to ACTH in IBS; 2) PBMC GR mRNA expression in IBS; and 3) GR mRNA together with HPA axis response to both CRF and ACTH within the same sample of participants.
2. Methods
All references in the methods section are in Appendix A.1 (see Supplementary material).
2.1. Study participants
Rome III+ IBS patients and HCs ages 18–55 were recruited primarily by community advertisement. Bowel habit subtypes (IBS with diarrhea (IBS-D), constipation (IBS-C) and mixed pattern (IBS-M)) were based on the Rome III criteria. (A.1.a) The diagnosis was confirmed by a clinician with expertise in IBS. HCs had no personal or family history of IBS or other chronic pain conditions. Additional exclusion criteria for all subjects included: infectious or inflammatory disorders, active psychiatric illness over the past 6 months as assessed by structured clinical interview for the DSM-IV (MINI), (A.1.b) use of corticosteroids in the past six months, use of narcotics, antidepressants or other medications that could affect neuroendocrine function in the past two months, or current tobacco or alcohol abuse. Participants were compensated. Our goal was to evaluate women during the follicular phase, or days 4–14 of their menstrual cycle if on oral contraceptive pills. Phase was determined by date of last menstrual period and progesterone levels. The study was approved by the UCLA Institutional Review Board, and all subjects signed a written informed consent prior to start of study.
2.2. Symptom measures
IBS symptom severity over the prior week was assessed with a numeric rating scale (0–20). (A.1.c) Current anxiety and depression symptoms were measured with the Hospital Anxiety and Depression (HAD) scale. (A.1.d) The presence of EALs before age 18 was determined using the Trauma History Questionnaire. (A.1.e) In addition, the Early Trauma Inventory Self Report Short Form (ETI-SR) determined the number of EAL events experienced. (A.1.f) The symptom measures for correlation with hormone response and GR mRNA expression were obtained on the days of the screening visit.
2.3. Hormone challenge
CRF stimulation test: An intravenous (IV) catheter was placed at 1300 h. Blood samples for ACTH and cortisol levels were collected from 1500 h (−60 min) to 1600 h (0 min) in 30-min intervals. At 1600 h, 1 μg/kg ovine CRF (Acthrel, Ferring, New York) was given IV. Blood samples were collected at 5, 15, 30, 60, 90, and 120 min after the administration of CRF. Participants were fasting from 1000 h and were instructed to eat a low glycemic index breakfast. Waking times of participants were not assessed.
ACTH stimulation test: The ACTH stimulation test was performed at least one week after the CRF stimulation test. IV catheters were placed into a forearm vein at 0800 h. Blood samples for baseline ACTH and cortisol were collected at 0830 h and 0900 h. 250 μg Cortrosyn (Organon, West Orange, N.J.) was administered IV at 0900 h. Blood samples for cortisol were collected at 30, 60, 90, 120, 150, and 180 min after ACTH was given. Participants were fasting.
Samples were collected, processed and assays were performed according to standard operating procedures of the UCLA Clinical and Translational Research Center. The assays for ACTH and cortisol were performed with the IMMULITE system (Siemens Healthcare Diagnostics Inc).
Baseline values were calculated from the average of values prior to CRF/ACTH administration. Area under the curve with respect to minimum value (AUCi) was calculated with the trapezoidal method. We also calculated rise and decline slope from the slope of the regression line from baseline to peak, and peak to lowest value after the peak, respectively. (A.1.g)
2.4. Glucocorticoid receptor mRNA
PBMCs were isolated from blood collected on a date prior to the hormone challenge assays. This was part of a separate research protocol but the inclusion and exclusion criteria were the same. Participants were not required to fast. Quantitative PCR (qPCR) determined the expression levels of GRα (active isoform) and GRβ (inactive isoform). Details of RNA extraction and qPCR are in Appendix A.3 (see Supplementary material).
2.5. Statistical analysis
2.5.1. Sample size
For our sample size calculation, we used an estimated mean difference in AUCi of 570 (ng/L)•h based on the data from the study by Dinan et al. and a standard deviation of 500 based on values for healthy controls in the literature. Using the pwr package for R, we would have an 80% power to dectect this difference with an alpha of 0.05 with a sample size of 13 per group.
2.5.2. Statistical analysis
For each outcome of the three hormone challenge experiments (CRF-stimulated ACTH, CRF-stimulated cortisol, ACTH-stimulated cortisol), we calculated average baseline values, AUCi, rise slope, and decline slope (absolute value) for each subject. We compared each outcome (IBS, sex, and EAL), and tested an IBS × sex interaction using linear regression, controlling for age and body mass index (BMI). For AUCi, rise, and decline we additionally controlled for baseline hormone levels. A similar analysis strategy was used for the GR mRNA outcome. If needed, outcomes were transformed to achieve approximate normality using natural logarithm or square root (Appendix A.4 (see Supplementary material)). Due to the presence of different hormone responses in men and women, we performed post-hoc pairwise comparisons in the linear regression models even in cases where the IBS × sex interaction was not statistically significant. For the post-hoc comparisons (IBS-Men vs. IBS-Women, HC-Men vs. HC-Women, IBS-Men vs. HC-Men, IBS-Women vs. HC-Women), we used a Bonferroni-adjusted significance threshold of p = 0.0125.
We also evaluated the change of hormone levels over time by group: IBS vs. HCs (IBS × time), men vs. women (sex × time), or +EAL vs. −EAL (EAL × time) by piecewise mixed models with a knot at the group maximum (CRF-stimulated ACTH at 90 min, CRF-stimulated cortisol at 60 min, ACTH-stimulated cortisol at 30 min). We also evaluated the effect of the three-way interaction IBS × sex × time which assesses the statistical significance of the difference of differences (i.e. whether the difference in response over time between IBS men and HC men is significantly different from the difference between IBS women and HC women). P-values were from a likelihood ratio test evaluating the model with and without the group*time interaction term, with <0.05 considered significant.
The associations of measures of hormone response with anxiety and depression symptoms and IBS overall symptom and abdominal pain severity were tested with linear regression. Correlation of GR mRNA with AUCi and baseline for each hormone challenge experiment was determined with partial correlations controlling for time interval (days) between the tests. The effect of EAL was also evaluated by the effect of Total ETI-SR score on measures of hormone response (AUCi, rise slope, decline slope) and on GR mRNA with linear regression models.
As a post-hoc comparison, we evaluated bowel habit subtype groups: HCs vs. IBS with constipation (IBS-C), diarrhea (IBS-D), and mixed (IBS-M).
3. Results
3.1. Participant characteristics
All partipants in the sample were recruited as described in Section 2.1 and completed measures described in Section 2.2. This manuscript reports results from three subsets of the sample (see diagram in Appendix B.1 (see Supplementary material)). Results of the hormone challenge are reported for the subset that completed this experiment (Table 1). Comparison of GR mRNA includes the subset for which GR mRNA was measured (Table 1). Finally, comparison of HPA measures with GR mRNA levels includes only participants who completed both experiments (Appendix B.3 (see Supplementary material)).
Table 1.
Participant characteristics.
| Variable: Mean (SD) | Hormone Challengea HC (n = 56) |
IBS (n = 60) | GR mRNA HC (n = 69) |
IBS (n = 74) |
|---|---|---|---|---|
| Women | 29 (52%) | 39 (65%) | 36 (52%) | 48 (65%) |
| Age (years) | 30.9 (10.9) | 33.4 (11.8) | 33.4 (11.8) | 32.7 (11.7) |
| BMI | 26.9 (5.3) | 25.8 (5.9) | 25.7 (4.6) | 26.2 (5.7) |
| Ethnicity | ||||
| Hispanic | 14 (25.5%) | 12 (21.1%) | 18 (26.5%) | 14 (19.7%) |
| Race | ||||
| Asian | 9 (17.0%) | 13 (23.2%) | 16 (24.2%) | 13 (18.1) |
| Black/African American | 13 (24.5%) | 10 (17.9%) | 10 (15.2%) | 8 (11.1%) |
| White | 21 (39.6%) | 24 (42.9%) | 25 (37.9%) | 34 (47.2%) |
| Other/Mixed | 10 (18.9%) | 9 (16.1%) | 15 (22.7%) | 17 (23.2%) |
| Bowel Habit Subtype | ||||
| IBS-C | 16 (26.7%) | 28 (38%) | ||
| IBS-D | 19 (16.4%) | 26 (35%) | ||
| IBS-M | 23 (38.3) | 20 (27%) | ||
| IBS-U | 2 (3.3%) | – | ||
| Presence of EALs (<age 18) | 27 (48.2%) | 34 (59.7%)* | 34 (54%) | 37 (56%) |
| HAD Anxiety score (0–21) | 3.7 (2.9) | 5.4 (4.2)* | 3.7 (3.1) | 6.4 (5.3)* |
| HAD Depression score (0–21) | 1.3 (1.7) | 2.4 (2.9) | 1.2 (1.2) | 2.9 (3.5)* |
| Overall IBS Severity (0–20) | 10.7 (4.2) | 10.0 (4.7) | ||
| Abdominal Pain (0–20) | 10.1 (4.4) | 9.5 (4.4) |
see Appendix B.1 (see Supplementary material) for N with complete data for each analysis.
p < 0.05 between IBS and HC; SD, standard deviation; HC, healthy control; IBS, irritable bowel syndrome; GR, glucocorticoid receptor; BMI, body mass index; HAD, Hospital Anxiety and Depression; EAL, early adverse life events; IBS-C, IBS with constipation; IBS-D, IBS with diarrhea; IBS-M, mixed IBS; IBS-U, unsubtyped IBS.
Hormone challenge participants (Table 1) were 56 HCs and 60 IBS patients (52% and 65% women). Of these 116 subjects, 102 and 109 completed the CRF and ACTH stimulation tests. The number of participants by IBS and sex for each experiment is shown in Appendix B.1 (see Supplementary material). Among women, 67% and 39% (CRF and ACTH stimulation, respectively) were evaluated during the follicular phase of the menstrual cycle or days 4–14 if on oral contraceptive pills. Menstrual cycle details are shown in Appendix B.2 (see Supplementary material). There was no effect of menstrual cycle on HPA axis response among women. GR mRNA participants (Table 1) were 69 HCs and 74 IBS patients (52% and 65% women). Participants in the GR mRNA/hormone challenge overlap group (GR mRNA within 90 days of the hormone challenge) were 24 IBS patients and 23 HCs (79% and 61% women). Mean (SD) time interval between hormone challenge and mRNA was 27.0 (31.8) days and was similar in IBS and HCs (p = 0.62). Clinical characteristics were similar to those of both the hormone challenge and the GR mRNA groups and are shown in Appendix B.3 (see Supplementary material).
3.2. HPA axis in IBS vs. HCs
There were no significant IBS vs. HC differences in baseline, AUCi, rise slope or decline slope for any of the hormone tests. Using mixed models, there was an effect of IBS × time on the CRF-stimulated ACTH response (p = 0.049), characterized by a slower decline following peak in IBS, but this became non-significant when controlling for sex. There were no significant effects of IBS × time on CRF- or ACTH-stimulated cortisol response (p = 0.33, p = 0.37). HPA axis measures did not correlate with symptoms or pain severity in IBS.
3.3. HPA axis in men vs. women
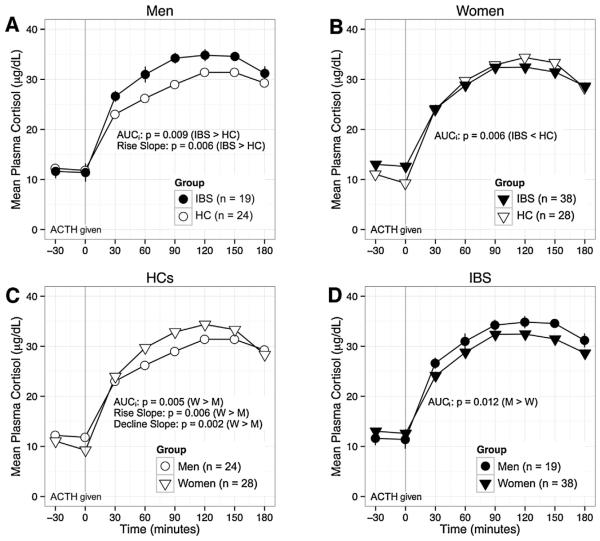
There was an overall effect of sex on baseline ACTH (men > women, p < 0.001) but no difference in AUCi for CRF-stimulated ACTH. Using mixed models, there was a significant effect of sex × time on CRF-stimulated ACTH response (p < 0.001, likely due to a larger rise from baseline to peak among women), and CRF-stimulated cortisol (p < 0.001, likely due to a slower decline from peak in women). For ACTH-stimulated cortisol, there was a significant effect of sex on decline slope with a faster decline in women vs. men (p = 0.012). This was mainly accounted for by a difference within HCs (p = 0.002). There was a non-significant sex × time effect on ACTH-stimulated cortisol (p = 0.06). Among HCs, AUCi was greater in women than men (p = 0.005), but the opposite was true in IBS (see below).
3.4. HPA axis: IBS × sex
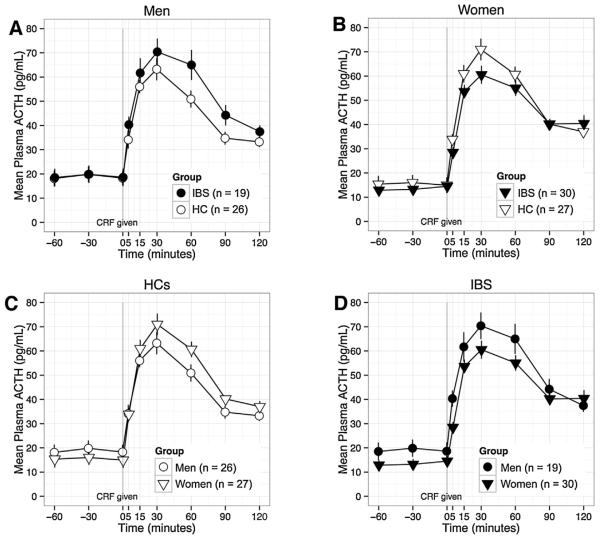
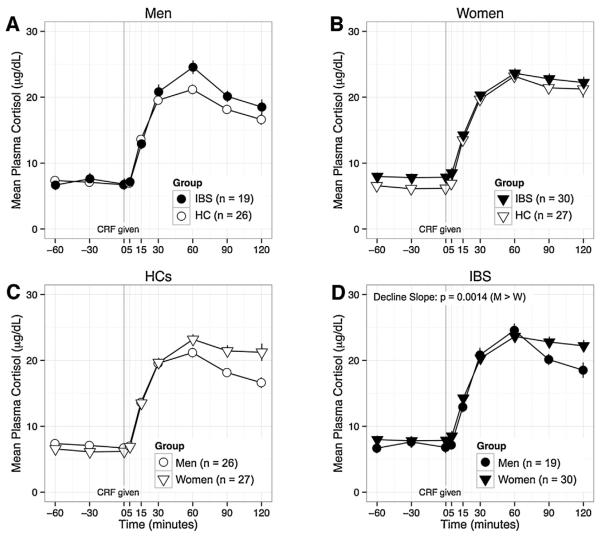
For CRF-stimulated ACTH (Fig. 1) and CRF-stimulated cortisol (Fig. 2) there were no significant IBS × sex interaction effects. For CRF-stimulated cortisol, there was a weak effect for a faster decline (greater absolute value of decline slope) in women vs. men within the IBS group (p = 0.014). For ACTH-stimulated cortisol (Fig. 3), there was a significant IBS × sex interaction effect on AUCi (p < 0.001). Among men, AUCi was higher in IBS vs. HCs (p = 0.009), but among women, AUCi was lower in IBS vs. HCs (p = 0.006). Like-wise, within IBS, AUCi was higher in men vs. women (p = 0.012), but the opposite was true within HCs (see above). There was also a significant IBS × sex interaction effect on rise slope (p = 0.008), which was greater in IBS men vs. HC men (p = 0.006) and in HC women vs. HC men (p = 0.006). Using mixed models, there was a significant IBS × sex × time interaction for ACTH-stimulated cortisol (p = 0.0002). The differences between men and women were in the opposite direction in IBS vs. HCs. Although the interactions were not significant for CRF-stimulated ACTH or cortisol (p = 0.35, p = 0.11), visualization of the plotted responses (Figs. 1–3) showed similarly divergent effects of IBS in men and women.
Fig. 1.
The ACTH response to CRF is shown by subgroup to highlight the following comparisons: A) men with IBS vs. HC men; B) women with IBS vs. HC women; C) HC men vs. HC women; and D) women with IBS vs. men with IBS. IBS × sex interaction for AUCi was not statistically significant. Baseline ACTH was increased in men vs. women (p < 0.001, for women vs. men overall: C + D). CRF, corticotropin-releasing factor; ACTH, adrenocorticotrophic hormone; pg, picograms; mL, milliliter; HC, healthy control, IBS, irritable bowel syndrome.
Fig. 2.
The cortisol response to CRF is shown by subgroup to highlight the following comparisons: A) men with IBS vs. HC men; B) women with IBS vs. HC women; C) HC men vs. HC women; and D) women with IBS vs. men with IBS. IBS × sex interaction for AUCi was not statistically significant. Within the IBS group (D), decline was faster in men vs. women (p = 0.014). CRF, corticotropin-releasing factor; mL, milliliter; HC, healthy control, IBS, irritable bowel syndrome; M, men; W, women; μg, micrograms; dL deciliter.
Fig. 3.
The cortisol response to ACTH is shown by subgroup to highlight the following comparisons: A) men with IBS vs. HC men; B) women with IBS vs. HC women; C) HC men vs. HC women; and D) women with IBS vs. men with IBS. IBS × sex for AUCi: p < 0.001. Among men IBS had greater AUCi (A, p = 0.009), but AUCi was higher in HCs among women (B, p = 0.006). IBS × sex interaction was also significant for rise slope (p = 0.008) with both IBS men(A) and HC women(C) > HC men (p = 0.006 for both). A). Decline was also faster in women vs. men (C, p = 0.012). ACTH, adrenocorticotrophic hormone; mL, milliliter; HC, healthy control, IBS, irritable bowel syndrome; M, men; W, women; μg, micrograms; dL deciliter.
3.5. GR mRNA
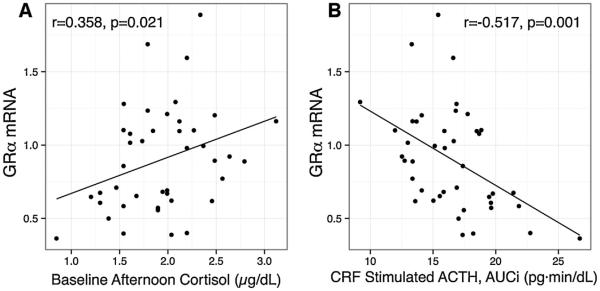
There was a significant effect of IBS on GRα mRNA (Fig. 4, IBS < HC, p = 0.013) but not GRβ. This was mainly due to lower GRα mRNA in men (IBS vs. HC, p = 0.007). There was no effect of sex on GRα or GRβ. Increased severity of overall IBS symptoms was associated with lower GRα mRNA (β = −5.1, p = 0.046). There was a weak effect for a similar association with abdominal pain severity (β = −4.8, p = 0.051). Within the 48 participants comprising the hormone challenge/GR mRNA overlap subset, GRα mRNA was positively correlated with cortisol at 1500 h (CRF-stimulation test; r = 0.358, p = 0.021, Fig. 5A), and negatively correlated with CRF-stimulated ACTH AUCi (r = −0.517, p = 0.001, Fig. 5B).
Fig. 4.
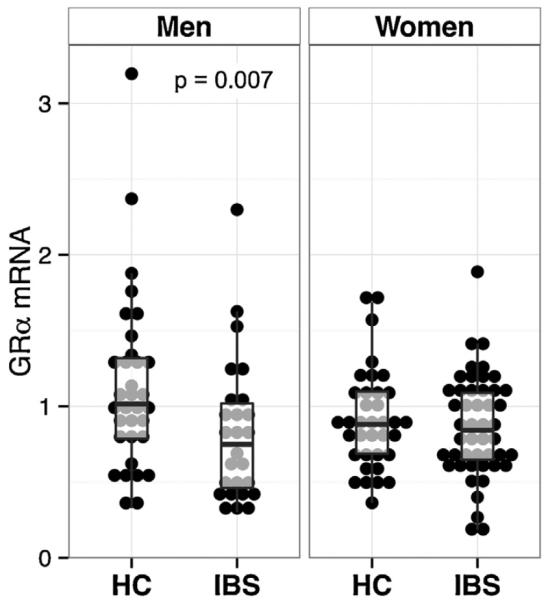
Glucocorticoid receptor α mRNA expression was lower in IBS vs. HCs overall (p = 0.013); however, this difference was mainly accounted for by a difference among men (p = 0.007). HC, healthy controls; IBS, irritable bowel syndrome; GR, glucocorticoid receptor.
Fig. 5.
Glucocorticoid receptor α mRNA is associated with baseline cortisol (A) and the ACTH response to CRF (AUCi). N = 47 (GR mRNA/hormone challenge overlap group). The value for r is the partial correlation controlling for time in days between collection (mean(SD): 27.0 (31.8)) of each measure. Baseline is at 1500 h on the day of the CRF-stimulation test. GR, glucocorticoid receptor; CRF, corticotrophin releasing factor; ACTH, adrenocorticotrophic hormone; AUCi, area under the curve with respect to increase; pg, picrograms; dL deciliter; min, minute.
3.6. Early adverse life events
There were no effects of EAL or interaction effects with IBS on baseline, AUCi, rise slope or decline slope (p > 0.1 for all). However, a higher total ETI-SR score (i.e., total number of EAL items) was associated with increased CRF-stimulated cortisol AUCi (p = 0.019) and rise slope (p = 0.007) and a slower decline in ACTH-stimulated cortisol (p = 0.014). Using mixed models, a history of EAL (EAL × time interaction) and EAL × IBS × time had non-significant effects on all hormone tests (for CRF-stimulated ACTH and cortisol and ACTH-stimulated cortisol, respectively, EAL × time: p = 0.095, p = 0.30, p = 0.12; EAL × IBS × time: p = 0.22, p = 0.12, p = 0.82). There was no effect of EAL on GRα or GRβ.
3.7. Bowel habit
The hormone responses over time by bowel habit are shown in Appendix B.5 (see Supplementary material). There was a Group × time (IBS-C, IBS-D, IBS-M, HC) effect for CRF-stimulated ACTH (p = 0.008) and ACTH-stimulated cortisol (p = 0.005). In both cases, the primary difference was due to the IBS-C group, which had a slower rise and decline than the other three groups. The IBS-C group also had a higher baseline AM cortisol (ACTH stimulation test; p < 0.001) compared to each of the other groups.
4. Discussion
The main findings of this study include: 1) similar ACTH and cortisol response to CRF in IBS vs. HCs; 2) sex-specific disease-related changes in the cortisol response to ACTH with an increased response in men with IBS vs. HC men and a blunted response in women with IBS vs. HC women; 3) disease-specific sex-related changes in the cortisol response to ACTH with an increased response in women vs. men among HCs and the opposite among IBS; 4) reduced expression of PBMC GRα mRNA in IBS vs. HC (primarily in men); 5) an inverse relationship between GRα mRNA in PBMCs and the pituitary response to CRF as well as symptom severity in IBS; and 6) a positive association between the number of EAL events and the cortisol response to CRF. We also found an effect of bowel habit subtupe on HPA axis response with an increased baseline and blunted response in CRF-stimulated ACTH and ACTH-stimulated cortisol in the IBS-C group. To our knowledge, this is the largest and most comprehensive study characterizing the HPA axis in IBS patients, taking the contributions of sex and history of EALs into account.
4.1. HPA axis response in IBS vs. HCs
Our findings do not support the hypothesis that IBS is associated with an augmented cortisol or ACTH response to CRF stimulation. In two of three previous CRF stimulation studies in IBS, HPA axis response was greater in IBS vs. HCs. (Dinan et al., 2006; Fukudo et al., 1998) Differences may be related to the use of human and not ovine CRF in one study, (Fukudo et al., 1998) and differences in the sample of IBS patients (IBS-M and IBS-D were the majority in both). (Dinan et al., 2006; Fukudo et al., 1998) In contrast, the third study (Bohmelt et al., 2005) showed a blunted HPA response in IBS. In this latter study, there was a higher prevalence of psychiatric comorbidity, and IBS patients (bowel habit not specified) had a blunted waking cortisol, which contrasts with findings of most studies. (Eriksson et al., 2008; Patacchioli et al., 2001; Suarez-Hitz et al., 2012)
4.2. HPA axis response in men vs. women
We found that among HCs, HPA response was increased in women vs. men. This is in agreement with other studies showing an increased HPA response to CRF in women following stress, (Young and Altemus, 2004) or dexamethasone. (Kunugi et al., 2006) Sex differences may be due to opposing effects of ovarian hormones and androgens on CRF expression and GR expression (ovarian hormones increase CRF and decrease GR). Human studies in healthy individuals have demonstrated increased cortisol response in post-pubertal females compared to males. (Panagiotakopoulos and Neigh, 2014) Animal studies have also found decreased expression and glucocorticoid binding of GRs in the hypothalamus and pituitary in females and sex-differences in CRF1 receptor signaling and trafficking. (Bangasser and Valentino, 2014)
4.3. Divergent IBS-related changes in men vs. women
At the adrenal level, we found divergent IBS-related changes in men vs. women. While the cortisol response to ACTH stimulation was greater in IBS vs. HCs within men, it was blunted within women. Although not statistically significant, the divergent HPA responses to between IBS and HCs within men and women were similar with CRF stimulation. Although the response to a psychological stressor is often increased in men vs. women, (Kudielka and Kirschbaum, 2005) there is good evidence that HPA response varies by type of challenge. Even among similar stressors, cortisol responses were greater in men vs. women to achievement challenges, but were greater in women to social rejection challenges. (Stroud et al., 2002) Women have also been shown to have increased brain activation to negative emotions in several regions, including the amygdala. (Stevens and Hamann, 2012)
The difference between a hormonal vs. a contextual stressor may be particularly relevant in IBS, as IBS has been associated with changes in several components of the integrated response to a stressor that may result in changes in the HPA axis response. Increased brain activity in emotional arousal circuits that include the amygdala and decreased activity of pain modulatory circuits that include the prefrontal cortex have been associated with IBS (Mayer and Tillisch, 2011). Both increased activity in the amygdala and decreased activity in the prefrontal cortex could result in an increased HPA axis response (Smith and Vale, 2006). In addition, IBS patients have increased cardiosympathetic tone, which may also result in an increased HPA response, and this was seen primarily in men (Tillisch et al., 2005).
4.4. PBMC GR mRNA expression is reduced in IBS and is negatively correlated with the ACTH response to CRF
IBS was associated with decreased GRα mRNA expression. This difference was mainly due to a difference among men. GR is a transcription factor of the nuclear receptor family (Official gene symbol: NR3C1). When bound to a ligand, it translocates to the nucleus and binds to glucocorticoid response elements to regulate downstream expression of genes primarily involved in the inflammatory response. The α isoform is the active isoform and the β isoform does not bind ligand and has a dominant negative effect on the transcriptional activity of GRα (Lewis-Tuffin and Cidlowski, 2006). Expression of the inactive GRβ, which was not different in IBS vs. HCs, has been associated with glucocorticoid resistance in several diseases including ulcerative colitis (there was no difference in GRα mRNA in this study) (Honda et al., 2000). While expression of the inactive GRβ plays an important role in inflammatory disorders, expression of GRα has been associated with stress-related and psychiatric disorders. There are no other published studies of GR mRNA in IBS, but lower PBMC GRα mRNA was also found in association with fibromyalgia (Macedo et al., 2008), which has shared pathophysiology with IBS, and in post-traumatic stress disorder (Gola et al., 2014). Limited evidence for a link between peripheral and central GR expression exists (Hepgul et al., 2013). In a study in rats, chronic treatment with corticosterone decreased GR in both lymphocytes and in the hippocampus (Lowy, 1991). In humans, PBMC GR density was decreased in patients with depression pre-treatment and increased following successful treatment with antidepressants (Calfa et al., 2003), and increased suppression of cortisol following dexamethasone was associated with lower methylation of the GR promoter in PBMCs (Yehuda et al., 2015).
Our findings of concordant group differences in HPA response and GRα expression among men with IBS vs. HC men (increased HPA axis response and decreased GR mRNA expression) as well as a negative correlation between GRα expression and CRF stimulated ACTH response provides additional evidence that PBMC GRα expression may be a peripheral marker of central HPA activity, and could support impaired negative feedback as a mechanism of HPA axis dysregulation in men with IBS. Alternatively, changes in peripheral GRα mRNA expression may occur as a result of increased HPA activation rather than reflecting a causative mechanism (alterations in central gene expression). It should be noted that mRNA expression does not necessarily reflect receptor function; there are post-transcriptional and post-translational regulatory mechanisms that can regulate GR signaling, and in one study, trauma-associated changes in PBMC GR number as assessed by dexamethasone binding capacity, were not reflected in mRNA expression. (van Zuiden et al., 2011)
In summary, our results support the hypothesis that PBMC GR mRNA expression is a peripheral marker of central HPA axis activity. While the findings are consistent with impaired negative feedback, they are inconclusive for the reasons described above. Other existing, albeit limited, data have not supported impaired negative feedback including normal dexamethasone suppression (Bohmelt et al., 2005; Dinan et al., 2006), and in a pilot study (n = 27), we evaluated the response to dexamethasone-CRF and found it to be similar in IBS vs. HCs (Videlock et al., 2015). As described in Section 4.3, the fact that an augmented HPA axis reponse has been seen in IBS in reponse to psychological stressors in the context of our findings of similar reponses at the pituitary level in HCs and IBS, also supports increased neural regulation or “CRF-hyperdrive” in IBS rather than impaired negative feedback. Interestingly, Ehlert et al. found that when they divided their sample by level of awakening cortisol, the group with high cortisol had higher levels of depression symptoms and lower GI symptom scores than the other groups, suggesting different effects of psychological symptoms and IBS on the HPA axis (Ehlert et al., 2005).
4.5. Influence of early adverse life events
We did not find that the presence or absence of EALs affected the HPA response to hormone challenge; however, among all participants, a higher number of EAL events was associated with an increased CRF-stimulated cortisol response. We previously demonstrated an increased salivary cortisol response to flexible sigmoidoscopy associated with EAL, predominantly in men (Videlock et al., 2009). The smaller effect of EAL on hormone challenge vs. a visceral stressor is likely related to the differences in the types of provocation; flexible sigmoidoscopy is a physical and likely psychological stressor.
4.6. Bowel habit subtype and symptoms
HPA response and baseline cortisol were affected by bowel habit subtype. The most prominent difference was a higher baseline and blunted response in CRF-stimulated ACTH and ACTH-stimulated cortisol in IBS-C. Other studies have also shown differences in measures of both the HPA axis and the autonomic nervous system by bowel habit subtype (Suarez-Hitz et al., 2012). For example, Burr et al. found significantly higher cortisol levels in women with IBS-C during sleep compared to both IBS-D and HCs (Burr et al., 2009).
We did not find an association between HPA response and overall IBS symptom or abdominal pain severity. Interestingly, studies have not consistently shown differences in the HPA response between IBS patients and HCs to perturbations of the gut, which the brain may perceive as stressful events. This includes stimulation by a meal (Elsenbruch et al., 2004), flexible sigmoidoscopy (Chang et al., 2009), and rectal distention (Walter et al., 2006). However, there is one study in which increased HPA suppression (lower post dexamethasone cortisol) was associated with increased IBS-like symptoms in HCs (Karling et al., 2007). In addition, higher doses of CRF have been shown to affect motility and symptoms in IBS patients (Fukudo et al., 1998) and HCs. (Pritchard et al., 2015) We did find an association between increased symptom severity and decreased GR mRNA expression. If decreased GR expression is a response to HPA axis activation as hypothesized (discussed above), this would support an association between symptom severity and activation of the HPA axis.
4.7. Limitations
This study has limitations. Differences in cortisol may have been obscured by our decision to measure total plasma cortisol and not salivary or serum free cortisol. In addition, not all women were in the follicular phase of the menstrual cycle. This was unlikely to have impacted the results as we did not find differences associated with menstrual cycle. Additionally, women in the luteal phase would likely introduce a conservative bias, if any, as luteal phase women have salivary cortisol (but not total plasma cortisol) responses to stressors that are similar to men (Kudielka and Kirschbaum, 2005).
5. Conclusions and clinical implications
In conclusion, we provide further evidence that IBS is associated with a dysregulated HPA response to hormone challenge, with divergent IBS-related changes in men vs. women. IBS men vs. HC men had an enhanced HPA response and this was associated with a concordant reduction in GRα mRNA expression in PBMCs. Hormone challenge tests the endocrine regulation of the HPA response to a weight-based dose of CRF, whereas the HPA response to a stressor depends on the neural regulation which is affected by the salience of the stressor and the “wiring” of brain circuits with inputs into the hypothalamus. An increased HPA response to stressors (Kennedy et al., 2014) but not to hormone challenge in IBS women, in combination with both increased emotional response (Chang et al., 2006) and increased connectivity of emotional-arousal circuits (Labus et al., 2008) in response to visceral distension, supports higher order cognitive and emotional processes as key factors in the relationship of stress and symptoms in women with IBS. The ability to better understand endophenotypes within IBS that may have divergent dysregulation of the HPA axis will be important in developing treatments targeted at different subgroups.
Supplementary Material
Acknowledgements
We thank the study participants and the staff of the Oppenheimer Center for Neurobiology of Stress and Resilience as well as the Clinical and Translational Research Center at UCLA. We specifically want to thank Cathy Liu for management of the database and Arlene Licudine and Stephanie Yee for their work as study coordinators.
Role of funding source
This project was supported by NIH grants P50 DK64539 (EAM, LC), R01 AR46122 (LC).
Core services utilized receive funding from NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Study personnel are also supported NIH grant P30 DK41301 (CURE: Digestive Disease Research Center)
EJV is supported by T32 DK07180 and the NIH loan repayment program (L30 DK106759).
Footnotes
Contributors
LC designed and implemented the trial, monitored data collection, wrote the statistical analysis plan, analyzed data, drafted and revised the paper, and provided funding and is the guarantor of the paper. EJV wrote the data analysis plan, analyzed data and drafted and revised the paper. WS and APP wrote the data analysis plan, analyzed the data and revised the drafted paper. M Adeyemo analyzed the data and revised the drafted paper. SM performed the mRNA extraction and quantitative PCR, analyzed the data and revised the drafted paper. CP and DI designed and performed the mRNA extraction and PCR. M Alberto monitored data collection throughout the study. EAM initiated the collaborative project, revised the drafted paper and provided funding.
Conflict of interest statement
All authors have no conflicts of interest to report.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2016.03.016.
References
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmelt AH, Nater UM, Franke S, Hellhammer DH, Ehlert U. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom. Med. 2005;67:288–294. doi: 10.1097/01.psy.0000157064.72831.ba. [DOI] [PubMed] [Google Scholar]
- Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, Chang L. Association between early adverse life events and irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2012;10:385–390. e381–383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterol. Motil. 2009;21:1148–e1197. doi: 10.1111/j.1365-2982.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G, Kademian S, Ceschin D, Vega G, Rabinovich GA, Volosin M. Characterization and functional significance of glucocorticoid receptors in patients with major depression: modulation by antidepressant treatment. Psychoneuroendocrinology. 2003;28:687–701. doi: 10.1016/s0306-4530(02)00051-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- Chang L, Mayer EA, Labus JS, Schmulson M, Lee OY, Olivas TI, Stains J, Naliboff BD. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R277–284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalmic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential marker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Nater UM, Bohmelt A. High and low unstimulated salivary cortisol levels correspond to different symptoms of functional gastrointestinal disorders. J. Psychosom. Res. 2005;59:7–10. doi: 10.1016/j.jpsychores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Holtmann G, Oezcan D, Lysson A, Janssen O, Goebel MU, Schedlowski M. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? Am. J. Gastroenterol. 2004;99:703–710. doi: 10.1111/j.1572-0241.2004.04138.x. [DOI] [PubMed] [Google Scholar]
- Eriksson EM, Andren KI, Eriksson HT, Kurlberg GK. Irritable bowel syndrome subtypes differ in body awareness, psychological symptoms and biochemical stress markers. World J. Gastroenterol. 2008;14:4889–4896. doi: 10.3748/wjg.14.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola H, Engler A, Morath J, Adenauer H, Elbert T, Kolassa IT, Engler H. Reduced peripheral expression of the glucocorticoid receptor alpha isoform in individuals with posttraumatic stress disorder: a cumulative effect of trauma burden. PLoS One. 2014;9:e86333. doi: 10.1371/journal.pone.0086333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepgul N, Cattaneo A, Zunszain PA, Pariante CM. Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med. 2013;11:28. doi: 10.1186/1741-7015-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Orii F, Ayabe T, Imai S, Ashida T, Obara T, Kohgo Y. Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 2000;118:859–866. doi: 10.1016/s0016-5085(00)70172-7. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Greenwood-Van Meerveld B. Knockdown of steroid receptors in the central nucleus of the amygdala induces heightened pain behaviors in the rat. Neuropharmacology. 2015;93:116–123. doi: 10.1016/j.neuropharm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karling P, Norrback KF, Adolfsson R, Danielsson A. Gastrointestinal symptoms are associated with hypothalamic-pituitary-adrenal axis suppression in healthy individuals. Scand. J. Gastroenterol. 2007;42:1294–1301. doi: 10.1080/00365520701395945. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Cryan JF, Quigley EM, Dinan TG, Clarke G. A sustained hypothalamic-pituitary-adrenal axis response to acute psychosocial stress in irritable bowel syndrome. Psychol. Med. 2014;44:3123–3134. doi: 10.1017/S003329171400052X. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Ida I, Owashi T, Kimura M, Inoue Y, Nakagawa S, Yabana T, Urushibara T, Kanai R, Aihara M, Yuuki N, Otsubo T, Oshima A, Kudo K, Inoue T, Kitaichi Y, Shirakawa O, Isogawa K, Nagayama H, Kamijima K, Nanko S, Kanba S, Higuchi T, Mikuni M. Assessment of the dexamethasone/CRH test as a state-dependent marker for hypothalamic-pituitary-adrenal (HPA) axis abnormalities in major depressive episode: a multicenter study. Neuropsychopharmacology. 2006;31:212–220. doi: 10.1038/sj.npp.1300868. [DOI] [PubMed] [Google Scholar]
- Labus J, Naliboff B, Fallon J, Berman S, Suyenobu B, Bueller J, Mandelkern M, Mayer E. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. NeuroImage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Cidlowski JA. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Ann. N. Y. Acad. Sci. 2006;1069:1–9. doi: 10.1196/annals.1351.001. [DOI] [PubMed] [Google Scholar]
- Lowy MT. Corticosterone regulation of brain and lymphoid corticosteroid receptors. J. Steroid Biochem. Mol. Biol. 1991;39:147–154. doi: 10.1016/0960-0760(91)90055-a. [DOI] [PubMed] [Google Scholar]
- Macedo JA, Hesse J, Turner JD, Meyer J, Hellhammer DH, Muller CP. Glucocorticoid sensitivity in fibromyalgia patients: decreased expression of corticosteroid receptors and glucocorticoid-induced leucine zipper. Psychoneuroendocrinology. 2008;33:799–809. doi: 10.1016/j.psyneuen.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behav. Brain Res. 2010;214:465–469. doi: 10.1016/j.bbr.2010.05.049. [DOI] [PubMed] [Google Scholar]
- Panagiotakopoulos L, Neigh GN. Development of the HPA axis: where and when do sex differences manifest? Front. Neuroendocrinol. 2014;35:285–302. doi: 10.1016/j.yfrne.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Patacchioli FR, Angelucci L, Dellerba G, Monnazzi P, Leri O. Actual stress, psychopathology and salivary cortisol levels in the irritable bowel syndrome (IBS) J. Endocrinol. Invest. 2001;24:173–177. doi: 10.1007/BF03343838. [DOI] [PubMed] [Google Scholar]
- Pritchard SE, Garsed KC, Hoad CL, Lingaya M, Banwait R, Thongborisute W, Roberts E, Costigan C, Marciani L, Gowland PA, Spiller RC. Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterol. Motil. 2015;27:542–549. doi: 10.1111/nmo.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Suarez-Hitz KA, Otto B, Bidlingmaier M, Schwizer W, Fried M, Ehlert U. Altered psychobiological responsiveness in women with irritable bowel syndrome. Psychosom. Med. 2012;74:221–231. doi: 10.1097/PSY.0b013e318244fb82. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videlock EJ, Adeyemo M, Licudine A, Hirano M, Ohning G, Mayer M, Mayer EA, Chang L. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videlock EJ, Shih W, Mayer EA, Chang L. Negative feedback of the hypothalamic-pituitary-adrenal (HPA) axis as assessed by the dexamethasone-corticotropin-releasing factor (CRF) test in irritable bowel syndrome (IBS) Am. J. Gastroenterol. 2015;110:S755. [Google Scholar]
- Walter SA, Aardal-Eriksson E, Thorell LH, Bodemar G, Hallbook O. Pre-experimental stress in patients with irritable bowel syndrome: high cortisol values already before symptom provocation with rectal distensions. Neurogastroenterol. Motil. 2006;18:1069–1077. doi: 10.1111/j.1365-2982.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry. 2002;7:985–994. 924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biol. Psychiatry. 2004;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Bierer LM, Henn-Haase C, Lehrner A, Desarnaud F, Makotkine I, Daskalakis NP, Marmar CR, Meaney MJ. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol. Psychiatry. 2015;77:356–364. doi: 10.1016/j.biopsych.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann. N. Y. Acad. Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Bikker A, Meulman E, Geuze E, Kavelaars A, Westenberg HG, Heijnen CJ. Leukocyte glucocorticoid receptor expression and immunoregulation in veterans with and without post-traumatic stress disorder. Mol. Psychiatry. 2007;12:443–453. doi: 10.1038/sj.mp.4001934. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Heijnen CJ, Kavelaars A. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am. J. Psychiatry. 2011;168:89–96. doi: 10.1176/appi.ajp.2010.10050706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.