Abstract
Introduction
Club cell protein 16 (CC16) is the most abundant protein in bronchoalveolar lavage fluid. CC16 has anti-inflammatory properties in smoke-exposed lungs, and chronic obstructive pulmonary disease (COPD) is associated with CC16 deficiency. Herein, we explored whether CC16 is a therapeutic target for COPD.
Areas Covered
We reviewed the literature on the factors that regulate airway CC16 expression, its biologic functions and its protective activities in smoke-exposed lungs using PUBMED searches. We generated hypotheses on the mechanisms by which CC16 limits COPD development, and discuss its potential as a new therapeutic approach for COPD.
Expert Opinion
CC16 plasma and lung levels are reduced in smokers without airflow obstruction and COPD patients. In COPD patients, airway CC16 expression is inversely correlated with severity of airflow obstruction. CC16 deficiency increases smoke-induced lung pathologies in mice by its effects on epithelial cells, leukocytes, and fibroblasts. Experimental augmentation of CC16 levels using recombinant CC16 in cell culture systems, plasmid and adenoviral-mediated over-expression of CC16 in epithelial cells or smoke-exposed murine airways reduces inflammation and cellular injury. Additional studies are necessary to assess the efficacy of therapies aimed at restoring airway CC16 levels as a new disease-modifying therapy for COPD patients.
Keywords: Bronchoalveolar lavage fluid, CC10, CC16, chronic bronchitis, Club cells, COPD, emphysema, formyl peptide receptor-2, lipoxin A4, lung inflammation, nuclear factor kappa B, phospholipase A2, recombinant therapy, serum amyloid A, secretoglobin 1A1, small airway remodeling, toll-like receptor, uteroglobin
1. Introduction
1.1. COPD Overview
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide, and its incidence continues to increase. COPD is expected to become the third leading cause of death in the adult population by 20201. Interestingly, only a relatively small proportion of smokers develop clinical COPD2. Likely, exposure to environmental factors (such as cigarette smoke [CS] and pollutants) coupled with genetic factors affect an individual's susceptibility to develop the disease. Although COPD was first described more than two centuries ago, the pathways that contribute to this variable susceptibility to develop COPD remain unclear.
1.2. COPD therapies
Current medical therapies for COPD (inhaled long-acting muscarinic receptor antagonists, β2-agonist, corticosteroids, and oral phosphodiesterase-4 inhibitors) reduce symptoms but modify minimally the course of COPD3. Giving the limited effectiveness of these therapies, several potential new medications targeting pathways involved in the pathogenesis of COPD have been evaluated recently with negative results3;4;5. Thus, there is a huge unmet need to develop disease-modifying therapies for COPD patients. One molecule that has recently been linked to COPD is Club cell protein-16 (CC16) which is also known as CC10, uteroglobin, secretoglobin-1A1, Club cell secretory protein [CCSP], urine protein-1, and human protein-1. Biomarker studies of large human COPD cohorts and studies of CC16-gene targeted mice in models of experimental COPD have provided strong evidence that CS-induced reductions in CC16 expression in the airways promote COPD development and progression. Below, we will review the literature on CC16, outline the evidence supporting CC16 as a novel disease-modifying therapy for COPD, and the challenges associated with targeting CC16 as a novel therapy for COPD patients.
2. Club cell protein-16 (CC16)
2.1. Sources of CC16
Airway Club cells
The main source of CC16 is the Club cell (formerly called Clara cell) which is a non-ciliated, non-mucous secreting club-shaped cell present throughout the respiratory tract epithelium from the nose to the respiratory bronchioles. The density of Club cells throughout the respiratory tract varies substantially between species. In humans, Club cells represent 1% of all airway epithelial cells in the bronchioles and 5% in the respiratory bronchioles6. In mice and rats, Club cells are also distributed throughout the tracheo-bronchial tree increasing in density from proximal to distal airways7. The main difference between rodents and humans is that Club cells are found more frequently in the trachea and bronchi of rodents, while they are less abundant in the large airways of humans. Other organs that contain CC16-secreting cells are the prostate, ovaries, pancreas, mammary glands, and uterine endometrium8;9. However, there are conflicting reports on whether CC16 is expressed in the kidney8;10. Club cells are associated with important functional activities such as cellular proliferation, and the biosynthesis, storage, and release of various secretory products including CC1611. Club cells have unique characteristics that allow their identification in the epithelial lining, such as a dome-shaped luminal surface projecting beyond that of other airway epithelial cells. Club cells also have electron dense cytoplasmic granules containing their principal product, CC1612. Club cells have the highest levels of cytochrome P450 oxidases, and play a crucial role in detoxifying xenobiotics in the human lung13. However, this property also renders the cells very susceptible to the cytotoxic effects of naphthalene and other toxins found in CS14.
Other CC16-expressing epithelial cells
Although the Club cells are the main producer of CC16, other lung cells also express this protein. Variants of CC16-expressing cells are localized in neuro-epithelial bodies15 and broncho-alveolar duct junctions16. These cells are either airway progenitor stem cells, or cells that contribute to the maintenance of stem cells, and are necessary for epithelial renewal15-17.
CC16-expressing leukocytes
A subset of stem cells has been identified in human and murine bone marrow cells (BMC) that express CC16 (CC16+ BMC)18;19. These cells also express CD45 (the common leukocyte antigen), and mesenchymal markers (e.g., CD73, CD90, and CD105), and are involved in alveologenesis and lung epithelial renewal. A recent study showed that CC16+ BMC regulate epithelial repair and lung inflammatory responses to injury20. In the latter study, CC16+ BMC were depleted in mice by transplanting their bone marrow with bone marrow from transgenic mice expressing a thymidine kinase suicide gene under the control of the CC16 promoter. Control mice received bone marrow from wild-type (WT) instead. When mice were treated with ganciclovir, this treatment selectively depleted all CC16-expressing BMC cells expressing thymidine kinase by generating an intracellular toxic metabolite of thymidine kinase. When challenged with naphthalene to induce acute lung injury, recipients that had CC16+ BMC depleted with ganciclovir had a slower rate of recovery of airway epithelial cell proliferation, higher leukocyte counts in bronchoalveolar lavage (BAL) samples, and lower arterial blood oxygen saturation levels than mice receiving WT BMC20. Interestingly, delivering recombinant murine CC16 by the intra-tracheal route to recipients lacking CC16+ BMC reduced these naphthalene-induced changes20.
There are conflicting reports on whether CC16 is expressed by inflammatory cells recruited to the lungs of different species. Willey et al. measured CC16 gene expression in human alveolar macrophages using real-time PCR and detected CC16 transcripts in all subjects21. Acute exposure of murine macrophages to CS increased their expression of CC16 as assessed by immuno-staining methods22. However, studies of cultured rat and rabbit alveolar macrophages did not detect either CC16 mRNA transcripts using real-time PCR, or synthesis or secretion of CC16 protein by the cells using 35S methionine-labeling and pulse-chase methods23,24.
2.2. CC16 gene structure and regulation of expression
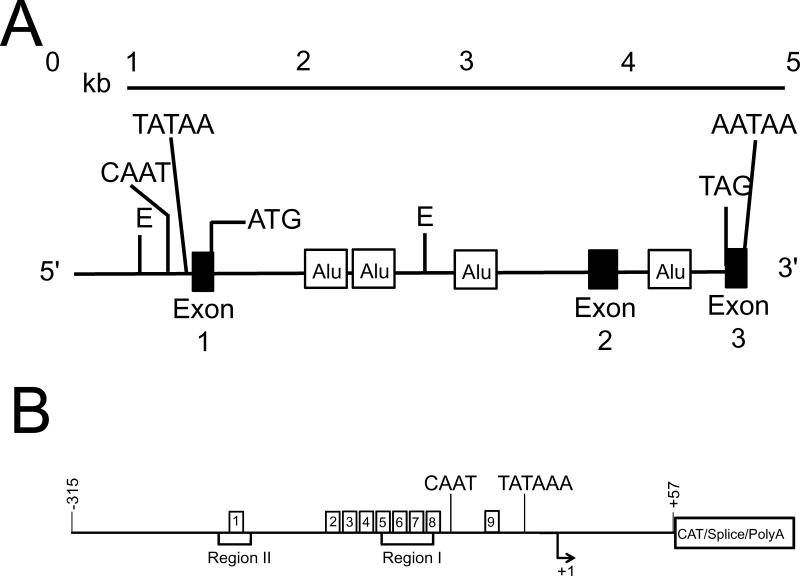
In humans, the gene (SCGB1A1) that encodes CC16 is located on chromosome 11q12.3-13.125. In mice, the CC16 gene (Scgb1a1) is located on chromosome 1826. The CC16 gene (Figure 1A) contains 18,108 base pairs (bp) in humans and 4,323 bp in mice, but the human and murine genes have the same structure (three exons and two introns)27. Two transcription stimulatory regions localized between +49 and −320 bp upstream from the transcriptional start site in the DNA have been identified (Figure 1B) which are named region-I and region-II. In the human CC16 gene, region-I is centered around −110 bp, and region-II around −220 bp27.
Figure 1. A diagram of the human SCGB1A1 gene.
In A, the human CC16 (SCGB1A1) gene is localized in chromosome 11q12.3. The gene has 18,108 bases pairs and contains three exons and two introns. There are four Alu repeats including three in intron 1 and one in intron 2. B shows the promoter regions of the human SCGB1A1 gene which contains two binding regions. Region I (I) is located ~110 bp upstream and region II (II) is located around −220 bp upstream of the transcription initiation site. The region-I is the binding site for several transcription factors that are known to regulate CC16 expression: hepatocyte nuclear factors α and β (HNF-α/β), activation protein-1 (AP-1), and the transcription factor Octamer (Oct) and also for transcription factors that inhibit CC16 expression: chicken ovoalbumin upstream promoter transcription factors (COUP-TFs). Region-II binds transcription factors that enhance transcriptional activation of the gene when region I is activated, and also contains binding sites for HNFs.
Transcriptional regulation of CC16 expression
Transcription factors that modulate CC16 expression in mice and rats include the forkhead transcription factors28, hepatocyte nuclear factor-3α (HNF-3α), and HNF-3β29, thyroid-specific enhancer binding protein (T/EBP)/NKX226, and the homeodomain factor thyroid transcription factor-1 [TTF-1]23. Members of the C/EBP family of transcription factors also bind to proximal sites in the rat and murine CC16 promoter. Interestingly, the onset of C/EBP-α expression in Club cells correlates with strong increases in CC16 expression by the cells indicating that C/EBP-α binding to the promoter increases differentiation-dependent CC16 gene expression in Club cells. C/EBP −α and TTF-1 act synergistically to increase CC16 gene expression in mice23. In humans, both HNF-3α and HNF-3β bind to the CC16 promoter to increase CC16 expression. Whether C/EBP regulates the expression of the human CC16 gene is not clear30;31, but this activation may depend on the presence of specificity protein-1 (Sp1) and Sp3 transcription factors23. Other transcriptional factors that regulate CC16 expression include activation protein-1 (AP-1) and the octamer family of transcription factors32. All these factor bind to region-I. Region-II has a positive effect on the SCGB1A1 promoter, but is not as important as region-I for regulating the transcription of CC1632. Less is known about transcription factors that down-regulate CC16 expression. However, chicken ovalbumin upstream promoter transcription factors (COUP-TFs) may repress CC16 expression in cells other than Club cells in vivo33.
Glucocortocoids (GCs) and hormones
GCs increase the synthesis of CC16 in the airways34, but regulation of CC16 expression by GCs, varies between species. In healthy human subjects, inhaled LPS induces leakage of CC16 from the airways into the circulation that can be blocked by GCs35. In rats, GCs do not increase CC16 expression in Club cells following severe airway epithelial injury36. Tissue-specific expression of the CC16 gene is regulated by several steroid hormones including estrogen and progesterone in lung, esophagus, and uterus, and the non-steroid hormone, prolactin, further augments CC16 expression in the uterus37.
Mediators of inflammation
Cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) increase CC16 expression by Club cell and human bronchial epithelial cell lines in vitro38;39. Interleukin-10 (IL-10), a cytokine with anti-inflammatory properties, increases CC16 expression in a lung adenocarcinoma cell line40. After intranasal treatment of pregnant mice with IL-10, CC16 mRNA levels increase in embryonic lung epithelial cells30. IL-10 increases CC16 gene expression at the steady state mRNA level (as assessed by RT-PCR) and this is mediated by IL-10 signaling through the IL-10 receptor to activate the homeodomain T/EBP transcription factor30. Exposing airway epithelium to IL-13 induces Club cell differentiation into goblet cells associated with loss of CC16 expression and increases in mucin gene expression41.
2.3. CC16 protein structure
CC16 has a molecular mass of 16,000 daltons, and belongs to the secretoglobin subfamily of proteins. CC16 is a homodimeric protein with identical 70-amino acid subunits linked in an anti-parallel orientation by two disulfide bonds that are formed between Cys3 and Cys69’, and Cys3’ and Cys69. Each of the subunits forms three α-helices with one β-turn between the second and third α-helices42. The two disulfide bridges stabilize the CC16 dimer and contribute to the formation of a central hydrophobic cavity which contains Tyr’21 and Tyr21’. This cavity is sufficiently large in volume to accommodate small hydrophobic molecules such as progesterone, polychlorinated biphenyls, or retinol43;44;45. Each of the CC16 monomers also forms minor hydrophobic cavities42;46;47. Although the biologic function of these minor hydrophobic cavities is not clearly understood, hydrophobic ligands such as prostaglandin D2 (PGD2) and PGF2α can be sequestered into these cavities reducing the activities of these prostaglandins48;49.
CC16 is expressed by humans, rodents, non-human primates, dogs, cats, horses, cattle, sheep, pigs, and lagomorphs, and is thought to be present in all mammals. The CC16 amino acid sequence is almost fifty percent identical in mammals, suggesting that CC16 has important roles in lung biology50. In healthy volunteers, CC16 is the most abundant protein in the bronchoalveolar lavage fluid (BALF) accounting for nearly 2-3% of its total protein content in concentrations that range from 1-5 μg/mL51. Given that epithelial lining fluid (ELF) is usually diluted ~100-fold in BALF, this suggests that undiluted ELF has CC16 concentrations ~100-500 μg/mL52. CC16 produced by airway Club cells diffuses from the ELF into the serum, and then it is excreted by the kidneys in humans and rodents7. Mean plasma CC16 concentrations in healthy human non-smokers are reported to be ~10-15 ng/mL. However, a recent study reported 6.4 ng/mL as the median serum CC16 concentration in a larger cohort of 201 healthy non-smokers53. It should be noted that measurements of CC16 concentrations using ELISAs can be highly variable depending on the assay used 54.
CC16 isoforms
Several isoforms of CC16 have been identified in airway fluids from patients with severe airway inflammation, including premature infants in respiratory distress55;56 and adults exposed to inhaled industrial chemicals with respiratory complications57. One isoform found in tracheal aspirate fluids of premature infants was associated with subsequent development of neonatal bronchopulmonary dysplasia (BPD)55. These isoforms were primarily identified through their altered isoelectric points in two-dimensional gels, indicating shifts in total surface charge and overall conformation. At present there are no published studies that characterize the chemical modifications present in these isoforms, or their potential alterations to CC16 protein structure and/or biological activities.
2.4. CC16 expression during lung development
During a normal pregnancy, CC16 is detectable in amniotic fluid samples at 14-16 weeks of gestational age. Thereafter, CC16 levels increase exponentially until gestational weeks 30-34, and CC16 levels in amniotic fluid samples are highest just before birth58;59. In preterm infants, CC16 lung levels are correlated directly with lung maturity and reduced during systemic inflammatory responses in the fetus60;61. Low lung CC16 levels occur in premature infants with the acute respiratory distress syndrome of prematurity62, and low lung levels are linked to the subsequent development of BDP63. The mechanisms by which low CC16 lung levels in premature infants lead to subsequent abnormal lung development are not clear. However, it is noteworthy that infants that develop BDP and survive to adulthood have changes in their lungs that resemble some of those in COPD lungs including airspace enlargement and gas trapping64;65 and reduced FEV166. A randomized, placebo-controlled clinical trial of a single dose of rCC16 delivered by the intra-tracheal route to premature infants with respiratory distress syndrome having low CC16 levels in tracheal aspirate fluid (TAF) samples showed that this therapy was well tolerated, and resulted in a significant reduction in leukocyte counts and a strong trend towards reduced IL-6 levels in TAF samples62. Interestingly, prenatal exposure to CS is associated with decreased expression of CC16 in fetal rat lungs67, but this correlation has not been confirmed in human neonates68.
2.5. Regulation of CC16 protein levels by inhalation of pollutants
Acute environmental exposures generally cause transient increases in CC16 BALF and plasma levels, but repeated exposures usually result in lower plasma and BALF CC16 levels in humans and other animals. Toxic compounds that reduce BALF and serum CC16 levels include oxidant-rich air pollutants such as CS69;70;71, nitrogen dioxide72, ozone73, chlorination products74;75, and toxic particles such as silica76 and wood smoke77 which is an important risk factor for COPD in developing countries. Pollutants likely reduce Club cell CC16 expression via their toxic effects on the cells. Club cells express cytochrome P450 at very high levels and are responsible for metabolizing most of the pollutants that are inhaled into the lung14. However, their immediate exposure to pollutants and their generation of toxic metabolites of pollutants can render Club cells very susceptible to toxin-induced injury.
2.6. CC16 expression in COPD patients
Blood and BALF CC16 levels
CC16 levels have been measured in plasma, serum, and BALF samples in several COPD cohort studies. In a small COPD cohort (mean forced expiratory volume in the first second [FEV1] 55± [SD] 7% of predicted), Bernard et al were the first to report that serum and BALF CC16 levels were significantly lower in COPD patients than non-smoker controls54. This group was also the first to report that serum levels were lower in smokers versus healthy non-smokers78. In the large Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort, significantly reduced serum CC16 levels were detected in healthy smokers and COPD patients compared with non-smokers controls79. However, no correlation was found between serum CC16 levels and the severity of COPD. In the same study, when the COPD patient were divided by active versus former smoker status, the median serum CC16 levels were lower in current than in former smokers in Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II-III but not in GOLD stage IV COPD patients.
In the Lung Health Study (which studied a cohort of predominantly mild to moderately-severe COPD patients) reduced serum CC16 levels were associated with accelerated decline in FEV1 over 9 years80. In this cohort, active smokers had the lowest serum CC16 levels, sustained quitters had the highest levels, and intermittent quitters had intermediate levels. These investigators suggested that low serum CC16 levels could serve as a useful predictive biomarker of rapid decline in FEV1 in COPD patients that are intermittent quitters. Studies of the Lovelace Smokers Cohort revealed that plasma CC16 levels are associated with chronic bronchitis and decline in lung function even before subjects develop COPD81.
Early life events have been linked to the pathogenesis of COPD82. A recent study83 assessed longitudinal data on serum CC16 concentrations, and assessed their relationship to decline in FEV1 and incidence of airflow limitation in adults who were free from COPD at baseline in the population-based Tucson Epidemiological Study of Airway Obstructive Disease ([TESAOD], the European Community Respiratory Health Survey ([ECRHS-Sp], and the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults ([SAPALDIA]. This study also measured CC16 serum levels in samples from children aged 4-6 years in the Tucson Children's Respiratory Study, the United Kingdom Manchester Asthma and Allergy Study, and the Swedish Barn/Children, Allergy, Milieu, Stockholm, Epidemiological survey birth cohorts to assess whether low serum CC16 levels in childhood predict subsequent lung function83. This study found that low serum CC16 levels are associated with reduced lung function in childhood, accelerated lung function decline in adulthood, and development of moderate airflow limitation in the general adult population83.
Biphasic changes in serum CC16 levels in CS-exposed mammals
Studies of smaller cohorts of humans show that inhaling smoke induces a biphasic response in CC16 serum levels. In firefighters, acute smoke exposures induce transient increases in serum CC16 levels that return to baseline within 10 days69;54 which is likely to be due to smoke-induced lung epithelial cell injury, increased epithelial cell permeability, and increased diffusion of CC16 from the epithelial lining fluid into the circulation84. However, more chronic smoke exposures reduce CC16 levels at different rates in various biologic samples. For example, a decrease in CC16 levels in nasal lavage fluids (but not in serum samples) is a very early effect of inhaling CS which can be detected in adolescent humans with a cumulative smoking history of < 1 pack-years85. Longer pack-year smoking histories are needed to reduce serum CC16 levels86;87;88, and one study reported a linear dose-response relationship between smoking history and serum CC16, the latter decreasing on average by about 15% for each 10 pack-year increase in smoking history86;87;88. Similarly, in rats exposed acutely to CS, serum CC16 levels increase 2-4 h later (likely due to CS-induced increased lung epithelial permeability), and return to baseline within 24 h89. Chronic CS exposure leads to reduced airway (and likely serum) CC16 expression90;91.
Other factors that affect serum CC16 serum levels
Serum CC16 levels are affected by factors other than COPD and smoke exposures. CC16 is excreted by the kidney and serum CC16 levels are increased in patients with impaired renal function92. Serum CC16 levels are lower in healthy males than healthy females due to higher glomerular filtration rates in males versus females92. Serum CC16 levels are also altered in lung diseases other than COPD including other diseases linked to smoking cigarettes such as lung cancer and idiopathic pulmonary fibrosis (IPF). As is the case for COPD, lung cancer is associated with lower serum CC16 levels93;94. Patients with asthma also have lower serum CC16 levels than healthy controls95;96. In marked contrast, serum CC16 levels are higher in patients that have both emphysema and IPF than controls without lung disease, and levels are higher still in patients that have IPF but not COPD97.
Airway CC16 expression
Few studies have evaluated airway CC16 expression in COPD patients. Pilette et al. found that airway CC16 expression is inversely correlated with the severity of airflow obstruction (measured as reductions in FEV1) in COPD patients98. Laucho-Contreras et al. confirmed these findings and showed that patients with GOLD stages III-IV COPD have much lower airway CC16 immunostaining than GOLD stages I-II COPD patients90. Chen et al. found that increased CC16 levels in bronchoalveolar lavage fluid (and plasma) samples correlated with improvement in bronchial dysplasia following smoking cessation99.
2.7. Genetic and epigenetic regulation of CC16
A genome-wide association study (GWAS) performed on the ECLIPSE cohort identified significant associations between the presence of eleven single nucleotide polymorphisms (SNPs) and serum CC16 levels in COPD patients100. However, these findings were not replicated in other COPD cohorts (the International COPD Genomics Network [ICGN] and COPD-Gene), probably because of smaller sample sizes in the latter cohorts and differences in the cohort inclusion criteria100. In the Lovelace Smokers cohort, one SNP within the CC16 promoter was associated with chronic bronchitis, two different SNPs were linked to rapid decline in lung function, and all three SNPs are associated with altered plasma CC16 levels81. Thus, plasma CC16 levels and SNPs in the CC16 locus may be useful for identifying subjects at increased risk of developing COPD and targeting them for earlier intervention.
CC16 expression can also be regulated by epigenetic mechanisms. A genome-wide DNA methylation analysis found that the CC16 locus is hyper-methylated in the bronchial epithelial samples obtained from the small airways of healthy smokers compared with samples obtained from healthy non-smokers. These results suggest that epigenetic silencing of CC16 expression might contribute to the low CC16 airway expression and reduced plasma and BALF CC16 levels that have been reported in smokers and COPD patients101. Whether smokers and COPD patients have other epigenetic modifications of the CC16 locus (e.g., increased histone acetylation) or express micro RNAs that could regulate CC16 expression is not known.
2.8. CC16 function in animal models with COPD
Several animal models have been used to evaluate the effects of CS exposure on CC16 expression in vivo and the activities of CC16 in regulating the development of COPD.
Expression of CC16 in CS-exposed animals
Murine studies
Miert et al. reported that serum CC16 levels were increased in mice exposed acutely to CS and that levels were directly related to the CS concentration89. Transient increases in CC16 expression also occurred in alveolar macrophages and alveolar ductal cells in mice exposed acutely to CS. In the same study, BALF CC16 levels were reduced and were lowest in mice exposed to the highest CS concentrations, suggesting that CS exposure depletes airway Club cells of CC16. In chronic CS exposure models, Laucho-Contreras et al. detected decreased CC16 expression in the airway epithelium of WT mice exposed to CS for up to 6 months along with an indirect relationship between CC16 expression and the duration of the CS exposure90. A second study showed that 4 months of CS exposure reduced airway CC16 expression in WT mice91.
Studies of other animals
CC16 expression also has been evaluated in non-human primates (NHPs) exposed to CS. Zhu et al91 found that chronic CS exposure decreased airway epithelial CC16 expression by rhesus monkeys (Macaca mulatta). Polverino et al demonstrated that 4 weeks of CS-exposure was sufficient to decrease airway CC16 expression in Macaca fascicularis NHPs102.
CC16 function in CS-exposed mice
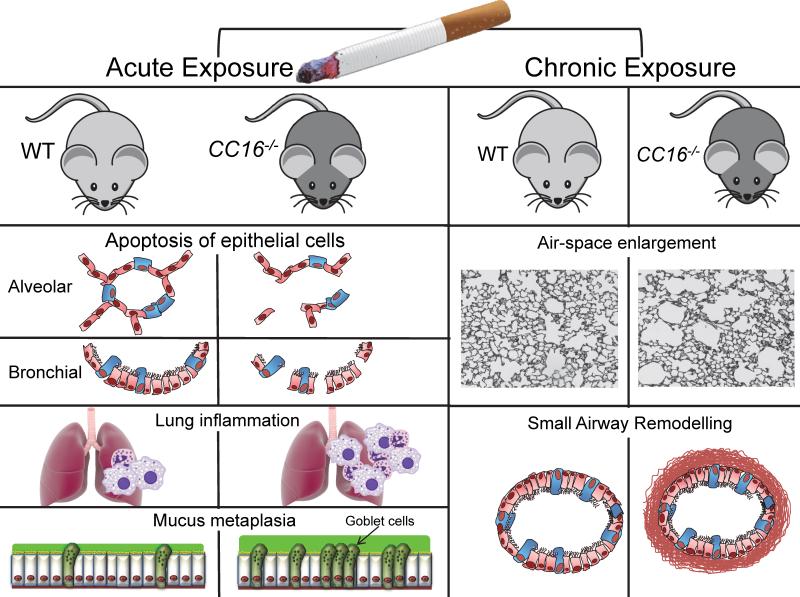
Two different strains of CC16 deficient (CC16−/−) mice have been generated using different gene-targeting constructs103;104. However, only one of these strains has been studied in COPD models103. Park et al. exposed wild-type (WT) and CC16-deficient (CC16−/−) mice to CS or air for 6 months and found similar increases in airspace enlargement, small airway remodeling, and lung macrophage and polymorphonuclear neutrophil (PMN) counts80. Using the same strain of CC16−/− mice, Laucho-Contreras et al. found that CC16−/− mice exposed to CS for 6 months had greater airspace enlargement, small airway remodeling, mucus metaplasia, and rates of apoptosis of alveolar and bronchial epithelial cells than CS-exposed WT mice (Figure 2)90. In the latter study, CS-exposed CC16−/− mice also had greater increases in lung macrophage and PMN counts, higher lung levels of some pro-inflammatory mediators (CCL5 and matrix metalloproteinase-9 [MMP-9]) and one pro-fibrotic mediator (active transforming growth factor β1 [TGF-β1]), but lower lung levels of anti-inflammatory IL-10 than CS-exposed WT mice. Zhu et al also reported that CS-exposed CC16−/− had greater airspace enlargement and lung inflammatory cell counts than CS-exposed WT mice91. It is not clear why Park et al did not find differences in emphysema development in CS-exposed WT and CC16−/− mice, but this could be due (in part) to differences in the CS exposure methods used (nose-only exposures in the Park study versus longer-duration whole-body exposures in the Laucho-Contreras and Zhu studies).
Figure 2. CC16 protects murine lungs from developing several lung pathologies when exposed to cigarette smoke.
Left panel: In acute CS exposure models (up to 12 week of CS exposure) CC16−/− mice develop greater increased bronchial and alveolar epithelial apoptosis (second and third panel), lung inflammation (increases in lung macrophage and PMN counts and lung levels of pro-inflammatory cytokines) and greater mucus metaplasia (increased numbers of Muc5ac positive airway epithelial cells) than WT mice. Right panel: In mice exposed chronically to CS (24 weeks) CC16−/− mice had greater increases in lung compliance, and greater airspace enlargement (a readout of emphysema) and greater fibrosis around small airways (with increased deposition of type-I collagen and fibronectin) than WT mice.
CC16's potential functions in organs other than the lung in COPD
One line of the two CC16−/− murine lines that have been generated thus far spontaneously develops glomerular disease. CC16 binds with high-affinity to fibronectin and thereby prevents the self-aggregation of fibronectin monomers104. In the CC16−/− lines of mice that develops renal lesions, these are due to abnormal deposition of multimeric fibronectin in the glomeruli in the absence of CC16104. However, the other line of CC16−/− mice that were studied in the CS-induced COPD model by all three laboratories (vide supra) do not spontaneously develop renal lesions for reasons that are not clear. However, it is noteworthy that delivering rCC16 to WT mice with experimental crescentic glomerulonephritis attenuates the progression of this disease105;106. As COPD is associated with systemic inflammation, these results suggest that rCC16 therapy has potential to reduce inflammation and associated tissue injury in organs other than the lung in COPD patients.
2.9. Mechanisms underlying CC16's protective activities in COPD
CC16 may limit the development and progression of COPD, in part, by reducing CS-induced pulmonary inflammation to limit emphysema development. CC16 has anti-inflammatory activities in various models of pulmonary inflammatory diseases other than COPD such as allergen-induced airway inflammation107;108, bleomycin-induced pulmonary inflammation109;110, and airway respiratory syncytial viral infections and inflammation in mice111. It is noteworthy that macrophages are required for emphysema development in mice112, and CC16 reduced lung macrophage counts in CS-exposed mice90. CC16 also has anti-fibrotic activities in the CS-exposed lung by limiting the development of small airway fibrosis.
Anti-inflammatory effects of CC16 on different cells implicated in the pathogenesis of COPD
CC16 is reported to have anti-inflammatory effects on various cells implicated in the pathogenesis of COPD including lung epithelial cells and inflammatory cells.
Airway epithelial cells
CC16 limits airway epithelial cell production of IL-8, a key pro-inflammatory molecule produced in COPD lungs. CC16−/− nasal epithelial cells produce greater amounts of IL-8 when activated with IL-1β than cells from WT mice both in vitro and in vivo113. Moreover, plasmid-mediated over-expression of CC16 in the nasal epithelium of CC16−/− mice reduces their increased release of IL-8 when IL-1β is administered113. Gamez et al reported that epithelial cells isolated from COPD airways produce more IL-8 compared with cells isolated from healthy smoker and non-smoker airways, and this increased release of IL-8 was reduced by adding rCC16 to the COPD cell cultures114. Also, rCC16 reduces LPS- or IL-13-stimulated production of IL-8 (and also MUC5AC) by human bronchial epithelial cells115.
PMNs
Purified native CC16 inhibits chemotaxis of human PMNs leukocytes towards N-formyl-methionyl-leucyl-phenylalanine (fMLP) in a dose-dependent manner (Figure 3)116. Studies of horses with recurrent airway obstruction (which is similar to human asthma) found that: 1) equine CC16 was present in airway PMNs in animals that developed neutrophilic airway inflammation; and 2) incubating blood and BAL PMNs with CC16 significantly reduced the respiratory oxidative burst and increased the phagocytic activity of PMNs117. During LPS-induced acute lung injury, lung PMN counts were higher in CC16−/− vs. WT mice118. However, to our knowledge, there have been no studies of the effects of CC16 on the function of PMNs from COPD patients or animals with experimental COPD.
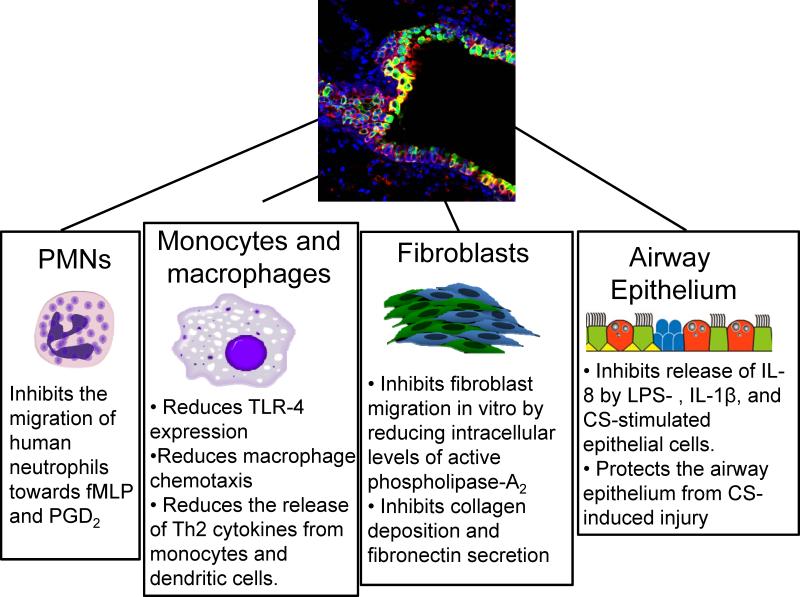
Figure 3. Effects of CC16 on the function of different cellular types implicated in the pathogenesis of COPD.
The figure illustrates the known effects of CC16 on different cells involved in the pathogenesis of COPD including PMNs, monocytes, macrophages, fibroblast and airway epithelial cells.
Mononuclear phagocytes
Adenoviral-mediated over-expression of CC16 decreases the release of two Th2 cytokines involved in COPD pathogenesis (TGF-β and IL-10) from stimulated with supernatant from human non-small cell lung carcinoma cells40. CC16 protein also reduces the migration of macrophages to chemoattractants119. During bleomycin-induced pulmonary fibrosis in mice, CC16−/− alveolar macrophages express more TGF-β than WT macrophages109. In CC16−/− mice, peritoneal and alveolar macrophages have a post-translational modification of annexin-1 (ANXA1 or lipocortin-1) which is an anti-inflammatory protein, converting it to a more acidic form120. The CC16-dependent ANXA1 modifications in peritoneal macrophages indicate that airway epithelial alterations can induce changes in immune cells at other mucosal sites120. During experimentally-induced BPD in WT mice121, decreases in airway CC16 expression occur and are associated with increases in lung levels of the acidic isoform of annexin-1. Interestingly, annexin-1 has a region of homology with CC16122, and both annexin-1 and CC16 mediate some of the anti-inflammatory properties of glucocorticoids.
Anti-fibrotic activities of CC16
Small airway fibrosis contributes significantly to the fixed airflow obstruction that is characteristic of COPD. There is evidence that CC16 limits small airway fibrosis CS-exposed mice as CS-exposed CC16−/− mice had greater small airway fibrosis than CS-exposed WT mice90. It is noteworthy that CC16 has anti-fibrotic activities in other models of lung injury. CC16−/− mice developed more severe pulmonary fibrosis when exposed to bleomycin96 and more severe airway remodeling in a model of bronchiolitis obliterans which was attenuated by treating the animals with rCC16123.
The mechanisms underlying CC16's anti-fibrotic activities in CS-exposed small airways are not clear. However, CC16 regulates several pathways that are linked to the development of small airway fibrosis including CS-induced lung inflammation. It is noteworthy in this respect, that products of PMNs [myeloperoxidase124] and macrophages [MMP-7, -9, and -12] promote small airway fibrosis in CS-exposed animals125;126. CC16 also reduces lung levels of active TGF-β (a potent pro-fibrotic cytokine that stimulates fibroblasts to produce interstitial collagens) in CS-exposed mice90 and bleomycin-treated mice109. The mechanism by which CC16 reduces lung levels of active TGF-β in CS-exposed lungs is not known. However, CC16 reduces MMP-7 activity in a preterm lamb model of infant respiratory distress syndrome (RDS)125, and reduces lung levels of MMP-9 in CS-exposed mice90. MMPs including MMP-9 can activate latent TGF-β127;128. Thus, it is possible that CC16 reduces lung levels of MMPs and other proteinases produced by inflammatory cells that activate TGF-β. In addition, CC16 may have direct activities on fibroblasts to promote small airway fibrosis as it inhibits agonist-induced activation of fibroblasts and fibroblast chemotaxis in vitro129;130;131. Furthermore, rCC16 also inhibits angiogenesis (which can contribute to tissue remodeling) by reducing the binding of endothelial cells to fibronectin, and their subsequent proliferative and migratory responses in vitro132.
Molecular pathways involved in CC16's protective activities in the CS-exposed lung
CC16 inhibits activation of several pro-inflammatory signaling pathways, the most well-characterized of which are inhibition of phospholipase A2 (PLA2) enzyme activity (thereby suppressing the arachidonic acid cascade) and suppression of nuclear factor kappa B (NFκB) activation.
Inhibition of PLA2 enzyme activity
In vitro experiments first demonstrated that CC16 inhibits the sPLA2 pro-inflammatory pathway. CC16 inhibits multiple PLA2 enzymes including secretory PLA2-type 1b (s PLA2-type 1b), sPLA2-type 2a45;133, and cPLA2-type IV134. CC16 binds to calcium135 and phosphatidylcholine43 which serve as a co-factor or substrate for sPLA2, respectively. Calcium is required for binding of CC16 or sPLA2 to cationic micelles. CC16 also interferes with the interaction of sPLA2 and the aqueous lipid interface which is a necessary step for the initial activation of this enzyme136. One study demonstrated that CC16 inhibits the migration of fibroblasts by reducing sPLA2 activity in these cells129;137.
Inhibition of NFκB activation
CC16 inhibits activation of NFκB in lung cancer cells138, uterine endometrial cells139 and airway epithelial cells113; 48 in vitro. Plasmid-mediated over-expression of CC16 in nasal epithelial cells inhibits NFκB activation by blocking the phosphorylation of the inhibitor of NFκB subunit-α (IκB-α) thereby reducing degradation of this inhibitor113. To our knowledge, there is only one study that assessed whether CC16 regulates the sPLA2 and/or NFκB pro-inflammatory pathways during the development of COPD90. This study found that CC16−/− mice have higher lung levels of sPLA2 enzyme activity at baseline than WT mice, but CC16−/− and WT mice had similar lung levels of sPLA2 enzyme activity when exposed to CS suggesting that CC16 does not limit CS-induced lung inflammation and injury in mice by inhibiting sPLA2 activity. However, additional studies are needed determine whether CC16 inhibits the activity of cPLA2 (the cytosolic Type IV enzyme) in CS-exposed lungs. The same study reported that CS-exposed CC16−/− mice had higher NFκB activation in their lungs than CS-exposed WT mice90. Furthermore, adenoviral-mediated over-expression of CC16 in airway epithelial cells of both WT and CC16−/− mice that were exposed acutely to CS led to reductions in lung macrophage counts, alveolar epithelial cell apoptosis, and airway mucin expression and these changes were associated with reduced CS-induced NFκB activation in the lungs90. However, additional studies are needed to determine whether CC16's protective activities in the lung are mediated by this protein inhibiting activation of NFκB in the lung and the mechanisms involved140.
Putative receptors for CC16
Only a few studies have assessed whether CC16 mediates its effects on cells by binding to a receptor, and no single receptor identified thus far explains all of the observed effects of CC16. Listed below are several receptors that have been implicated in transducing CC16 signaling in vitro (vide infra). However, it is important to note that the exact mechanisms by which CC16 mediates its anti-inflammatory activities in the CS-exposed lung (including whether or not it binds to a receptor) have not been determined.
a) fMLP receptors (FPRs)
FPRs are a family of three different seven-spanning domain transmembrane, G-protein couple receptors (FPR1-3). FPR1 and FPR2 are expressed on PMN, monocyte, and macrophage surfaces. FPR3 is expressed by monocytes, macrophages, and dendritic cells but not PMNs. All three receptors are activated by the chemoattractant N-formyl methionyl-leucyl-phenylalanine (fMLP). FPR2 is also a receptor for annexin-A1, lipoxin-A4 (ALX receptor), and serum amyloid A (SAA)141 which is a pro-inflammatory mediator that promotes PMN migration142. Lipoxin-A4 inhibits the actions of SAA in vitro by binding non-competitively to FPR2/ALX. In COPD patients, there is evidence that lung levels of pro-inflammatory SAA are high and overwhelm the protective signaling of anti-inflammatory lipoxin-A4 through FPR2/ALX143. CC16 also interacts with FPR2 and thereby reduces FPR2's response to SAA on human primary fibroblast-like synoviocytes141. CC16 binding to FPR2 also inhibits PMN migration towards fMLP in vitro (Figure 3)144;145. Whether this occurs in CS-exposed lungs is not clear, but by inhibiting PMN migration into the CS-exposed lung, CC16 could reduce CS-induced lung inflammation and destruction mediated by PMN proteinases and other products.
b) Toll-like receptor-4 (TLR4) expression
During LPS-mediated acute lung injury, surface expression of TLR4 was higher on lung macrophages from CC16−/− than WT mice118 suggesting that CC16 reduces the expression of TLR4 on activated lung macrophages. In the latter study, lung macrophage TLR4 expression levels were correlated with higher lung inflammatory responses (higher lung levels of IL-6 and TNF-α) and increased release of TNF-α from alveolar macrophages in CC16−/− mice. One of the many functions of TLR4 is to activate the NFκB pathway and CC16-mediated reductions in TLR4 expression on macrophages (Figure 3) could also contribute to the anti-inflammatory effects of CC16 in experimental COPD118.
c) Other receptors
Other molecules have been implicated as receptors for CC16, but additional studies are needed to better define their role. For example, cubilin146, which is mainly localized in the kidney, is involved in the excretion of CC16147. Cubilin is also expressed by lung epithelial cells and contributes to high-density lipoprotein uptake by alveolar epithelium148 and lung cancer cells149. CC16 was recently identified as a ligand of the lipocalin-1 receptor, and CC16 binding to lipocalin-1 reduces the migration and invasion of carcinoma cells in vitro150.
2.10. Therapeutic approaches for CC16 in COPD
Several human cohort and animal studies have linked CS-induced loss of CC16 airway expression to the genesis and progression of COPD80;90;91;53;81. These results suggest that CC16 augmentation approaches might have therapeutic efficacy in COPD. This hypothesis is supported by several experimental findings outlined below.
In vitro studies
The efficacy of recombinant CC16 (rCC16) has been examined in cell culture systems. Tokita et al115 activated human bronchial epithelial cells with IL-13 or LPS and incubated them with or without rCC16 in concentrations similar to serum levels found in healthy adult humans (20 ng/mL). The rCC16 treatments inhibited MUC5AC and IL-8 expression (Figure 3) and reduced activation of NFκB and extracellular signal-regulated kinase (ERK1/2) in the IL-13- or LPS-activated cells. Gamez et al. showed that rCC16 inhibited the CSE-induced release of IL-8 from bronchial epithelial cells isolated from COPD patients114.
Augmenting CC16 expression in epithelial cells and cell lines using recombinant adenoviral vectors inhibits the development of cellular phenotypes linked to COPD. For example, plasmid-mediated over-expression of CC16 in human bronchial epithelial cells reduced IL-1β-induced release of IL-8 (Figure 3), suppressed the proliferation of human lung cancer cells in vitro and increased their rates of apoptosis151;152, and reduced NFκB activation in the cells113. Treating cells involved in the pathogenesis of COPD (e.g., fibroblasts129, PMNs153, and macrophages118) with recombinant CC16 also inhibited their migration to chemoattractants in vitro (Figure 3).
In vivo studies
Adenoviral vectors have been used to over-express CC16 in mice with inflammatory lung disease. Adenoviral-mediated over-expression of CC16 in the airways of mice with allergen-induced airway inflammation reduced airway hyper-responsiveness and airway inflammation154. Adenoviral-mediated over-expression of CC16 in airway epithelial cells in mice exposed acutely to CS reduced CS-induced lung inflammation and alveolar septal cell apoptosis (which both contribute to emphysema development), and also reduced airway mucus metaplasia90. These changes were associated with reduced activation of NFκB in the lung90. As gene therapy using viral vectors for other diseases such as cystic fibrosis has been associated with challenges (including the development of effective viral vectors, host immune responses to the vectors, and the possibility that insertional mutagenesis will occur)155, it is not clear whether this approach will be feasible for increasing CC16 levels in COPD airways.
Recombinant CC16 protein could be delivered to the lungs of COPD patients as clinical trials for rCC16 in premature human infants with RDS at high risk of developing BPD are ongoing. Initial results indicate that a single dose of rCC16 can be delivered safely to infants by the intra-tracheal route, and that it reduces indicators of acute lung inflammation and injury62. Also, another biologic protein (α1-antitrypsin; AAT) is being used to treat AAT-deficient emphysema patients in the USA. Thus, rCC16 therapy might be feasible as a therapeutic approach for COPD patients having low CC16 blood and lung levels. Thus far, rCC16 therapy has only been tested in naphthylene-treated mice lacking CC16+ BMCs20. Delivering 30 μg of rmCC16 to these mice daily by the intra-tracheal route beginning 24 h after naphthalene delivery reduced lung leukocyte counts, improved blood oxygen saturation levels, and surprisingly increased airway CC16 expression in the animals, suggesting that rCC16 limits Club cell injury and stabilizes endogenous production of CC16 by airway cells.
Limitations of rCC16 protein replacement as a therapeutic strategy for COPD
Delivering exogenous CC16 to CS-exposed animals or COPD patients may have some limitations as a new therapeutic approach for COPD. First, in order for rCC16 protein to be considered as a viable approach for ambulatory COPD patients, CC16 would need to be delivered by the inhaled route in high enough doses with a dosing frequency sufficient to effectively increase lung CC16 levels. This approach can be challenging to accomplish for proteins156. Also, rCC16 protein would need to be resistant to oxidative inactivation as CS contains high concentrations of reactive oxygen and nitrogen species, and these oxidants are generated by CS-activated lung cells. The development of antibodies to rCC16 could limit the potential of this approach if these antibodies are produced and cross-react with endogenous CC16, thereby reducing lung levels of active endogenous CC16. The potential to suppress expression of native CC16 in the lungs was identified and may also limit the utility of rCC1662. However, the dose and dosing frequency of rCC16 could be optimized to reduce the development of this potential problem.
Small molecule approaches
Orally-available small molecules that boost CC16 levels in COPD lungs would be a very attractive approach for ambulatory CC16-deficient COPD patients. However, to date, there is only one report in the literature showing that a small molecule increases CC16 levels in cells. Shiyu et al found that polydatin (a precursor of resveratrol) induced CC16 expression in LPS-treated BEAS-2B cells and also in the lungs of rats with acute lung injury157. However, there have not been any studies of polydatin or other small molecules that increase CC16 expression by airway epithelial cells in CSE-treated cell culture systems or animal models of COPD.
3. Conclusions
CC16 levels in blood and lung samples are reduced when humans and experimental animals are exposed to CS. There are conflicting reports on whether CS-induced reductions in CC16 directly contribute to the genesis and progression of COPD in mice. One of three published studies indicated that CC16 has no role in COPD genesis or progression80, but two subsequent studies90;91 reported that CC16 robustly limits the development of several CS-induced lung pathologies (illustrated in Figure 2). Our review of the literature strongly supports the notion that CC16 limits COPD development, but whether CC16 mediates its protective activities by inhibiting NFκB activation in the CS-exposed lung has not been conclusively proven. It is possible that CC16 has other yet-to-be-identified protective activities in lungs exposed to CS.
There is growing evidence that CC16 augmentation approaches may have efficacy in limiting CS-induced inflammation based upon results in cell culture systems and a murine model of toxin-induced airway injury. So far, only one study using an acute (one month) CS exposure of mice, has shown that CC16 augmentation using adenoviral vectors limits CS-induced lung inflammation and airway mucus metaplasia90. Whether this approach would also reduce emphysema progression, small airway fibrosis, and chronic bronchitis phenotypes that contribute to symptoms in COPD patients is not clear. While delivering rCC16 to CC16-deficient COPD lungs represents an alternative approach, it is not clear whether adequate amounts can be delivered safely to the lung to achieve a therapeutic effect. However, this may be the only option for patients with advanced airway remodeling and few functional CC16-secreting airway epithelial cells. On the other hand, the most attractive approach would be to develop orally-available small molecules that boost CC16 expression by airway epithelial cells through activation of CC16 transcription or by switching off epigenetic silencing of CC16. Only one small molecule has been identified that increases CC16 expression in airway epithelial cells (polydatin)157, but its mechanism of action is not clear. There is a need for additional studies to increase our knowledge about CC16's mechanism of action, identify potential receptors and signaling pathways, and effects other than anti-inflammatory actions in COPD lungs.
4. Expert Opinion
The key findings of the research done in this field thus far include the overwhelming evidence that inhaling CS and the development of COPD are associated with CC16 deficiency in blood and lung samples. CC16 deficiency is independently and significantly linked to rapid rate of decline in lung function in COPD patients and the chronic bronchitis COPD phenotype80;81. There is evidence that CC16 limits several acute and chronic lung pathologies in CS-exposed mice (see Figure 2)90;91. Also, CC16 augmentation using adenoviral vector approaches reduces CS-induced lung inflammation and mucus metaplasia in mice90, and treating lung epithelial cells (including cells from COPD patients) with rCC16 limits their pro-inflammatory responses to signals that are present in COPD lungs115;114.
The main weaknesses of the research conducted on CC16 in COPD to date include the failure to fill a number of key knowledge gaps in this field. For example, the mechanisms by which CC16 protects the lung from CS-induced inflammation and injury and the key cell types that are protected by this protein in the CS-exposed lung are not known. It is also not known whether delivering rCC16 to the lungs has efficacy in limiting the progression of some or all of the key lung pathologies that develop in CS-exposed animals. In addition, previous research studies have not determined whether CC16 deficiency is linked to COPD phenotypes other than rapid decline in FEV1 and chronic bronchitis. It is also not known whether CC16 deficiency increases the systemic inflammatory response in COPD patients.
The potential that future research in this field holds is that it may: 1) identify the receptors or signaling pathways by which CC16 protects the lung from CS-induced injury; 2) show that delivering rCC16 to the lungs limits the progression of COPD in CS-exposed experimental animals; 3) identify the lung pathologies that are ameliorated by rCC16 in animal models; and 4) identify the subgroups of COPD patients that are most likely to respond to therapy.
The ultimate goal of this research area is to determine whether CC16 augmentation approaches are as a first-in-class disease-modifying therapy for COPD patients having both low plasma CC16 levels and the specific phenotypes that are likely to respond to rCC16 therapy such as a rapid decline in FEV1, chronic bronchitis80;81;158 and other yet-to-be identified COPD phenotypes such as patients with small airway-predominant disease. Another goal is to determine whether plasma CC16 levels can be used to select patients likely to respond to therapy and also to monitor responses to therapy.
The additional research studies needed to achieve these goals include studies that will test the therapeutic efficacy of rCC16 in pre-clinical models of COPD such as CS-exposed mice. If efficacy of rCC16 is demonstrated in these pre-clinical models, rCC16 could then be advanced to clinical trials in subgroups of COPD patients having low serum CC16 levels that are identified as having phenotypes that are the most likely to respond to this therapy. Additional studies could then be done to measure CC16 serum levels in treated and untreated patients to determine whether serum CC16 levels predict responses to therapy. To advance rCC16 augmentation therapy into human COPD patients, it will also be necessary to better understand CC16's protective mechanism of action in the COPD lung. Studies could definitely determine whether CC16 mediates its protective activities by inhibiting NFκB activation in the lung and identify the receptors (if any) through which CC16 signals using gene silencing and over expression studies, and pharmacologic inhibitors and agonists in cell culture systems.
There are a number of challenges that will be encountered in achieving these goals. These include the identification of the human COPD phenotypes that are most likely to respond to therapy which will require studies of serum CC16 levels in subgroups of carefully phenotyped COPD patients (e.g. patients with emphysema- versus small airway disease-predominant disease). It will also be necessary to determine the optimal method to deliver rCC16 to the lungs (e.g., intranasal versus aerosolized delivery), and optimize protein formulation for delivery to the lung and the dose and dosing frequency to induce a therapeutic effect while maintaining a good safety profile. Additional challenges include the possibility that rCC16 therapy may have side effects. In particular, it will be important to determine whether rCC16 therapy elicits an immune response leading to the generation of antibodies that could cross-react with endogenous protein thereby exacerbating CC16 deficiency and possibly disease progression in COPD patients. Another hurdle will be determining whether or not serum CC16 levels can be used to monitor responses to therapy, as both COPD and renal impairment both increase with age, and renal impairment reduces excretion of CC16 leading to higher serum CC16 serum levels.
In short term, future studies will likely test the efficacy of rCC16 augmentation therapy in pre-clinical studies aimed at evaluating the tolerance, safety, and efficacy of the protein. If rCC16 has efficacy in animal models of COPD, thereafter, the safety profile of rCC16 therapy will be tested in COPD patients with low plasma CC16 levels and/or the COPD phenotypes that would be most likely to response to the therapy, followed by larger clinical trials evaluating efficacy of rCC16 therapy in COPD patients. In addition, efforts will be made to develop orally-available small molecules that are shown to augment CC16 expression in cell culture systems and CS-exposed animals. Orally-available small molecules that augment CC16 expression, while expensive to develop, would be ideal for treating stable COPD patients in a community setting. In the longer term, studies would test the efficacy of these small molecules in randomized clinical trials in stable COPD patients.
It is not known whether COPD patients having low serum CC16 levels have increased rates of acute exacerbations of COPD (AECOPD) or more severity AECOPD. If this is the case, future studies could test the efficacy of rCC16 therapy in patients with AECOPD140 which are associated with increased lung and systemic inflammation, high morbidity and mortality, and high healthcare costs159. It is noteworthy that CC16 has anti-viral actions160;161 and viral upper respiratory tract infections [URTIs] are common causes of AECOPD162;163. Thus, future studies could test the efficacy of rCC16 delivered during the winter months (when viral URTIs are more common) in reducing the AECOPD rates in COPD subjects who have frequent AECOPD.
In the coming years, we predict that studies will provide additional insights into the factors that regulate CC16 expression in the airways including genetic and epigenetic regulation of CC16 expression. If the CC16 locus is shown to be hypermethylated in COPD airways leading to reduced CC16 expression, future approaches could be developed to demethylate the CC16 locus and thereby provide alternative therapies that augment CC16 expression in COPD airways. In addition, as SNPs in the CC16 locus have been identified that are associated with altered CC16 levels in lung samples100, it may be possible to use CC16 genotype to select individuals that might response to CC16 augmentation therapy.
Areas of research in this area that are of particular interest to us include identifying the mechanisms by which CC16 mediates its protective effects in the CS-exposed lung including identifying the key cell types that are protected from CS-induced injury by CC16, and the receptors and signaling pathways that are involved. We are also interested in determining whether rCC16 augmentation approaches can reduce COPD-like lung disease in CS-exposed mice and, if so, in human COPD patients with low serum CC16 levels and the phenotypes that will respond to this therapy. We are also interested in performing studies to validate serum CC16 levels as a prognostic biomarker for COPD or a biomarker that predict responses to rCC16 therapy, and to determine whether CC16 deficiency is linked to increased systemic inflammation in COPD patients.
Article highlights box.
CC16 is a major product of Club cells and it is a marker of epithelial airway injury.
COPD is associated with CC16 deficiency as CC16 BALF and serum levels are lower in COPD patients versus smokers without COPD, and lower in smokers without COPD than healthy non-smoker controls.
Low serum CC16 levels are linked to rapid rate of decline in lung function in COPD patients and to the chronic bronchitis COPD phenotype.
CC16 protects lungs from cigarette smoke-induced pulmonary inflammation and injury. CC16 deficiency in mice leads to greater cigarette smoke-induced pulmonary inflammation, bronchial and alveolar epithelial cell apoptosis, mucus metaplasia, increases in lung compliance, airspace enlargement, and small airway fibrosis.
Adenoviral vector-mediated over-expression of CC16 reduces release of pro-inflammatory mediators by activated epithelial cell cultures in vitro, and reduces pulmonary inflammation, lung epithelial cell apoptosis, and mucus metaplasia in the lungs of smoke-exposed mice.
Adding recombinant CC16 (rCC16) to activated epithelial cell cultures decreases their release of pro-inflammatory mediators and expression of mucins.
The beneficial effects of delivering or over-expressing CC16 in cells or smoke-exposed murine lungs are associated with reduced nuclear translocation of the pro-inflammatory transcription factor, NFκB.
Currently, there are no published studies determining whether chronic delivery of rCC16 in COPD patients is safe and reduces cigarette smoke-induced lung inflammation or injury.
CC16 augmentation approaches including delivering rCC16 to the lungs of COPD patients could represent a first-in-class disease modifying therapy for COPD by boosting key anti-inflammatory pathways that are down-regulated in COPD lungs.
Acknowledgments
The authors were supported by the United States Public Health Service; the National Institutes of Health under National Heart, Lung, and Blood Institute (grant numbers HL063137, HL086814, HL111835, PO1 HL105339, P01 HL114501, and AI111475-01); the Flight Attendants Medical Research Institute (grant numbers CIA123046 and YFEL141004); and the Brigham and Women's Hospital-Lovelace Respiratory Research Institute Consortium. A Pilon is co-founder, CEO and a major shareholder of Therabron Therapeutics, a biotechnology company dedicated to developing human recombinant CC16 protein as a drug for treating human lung diseases. A also Pilon has several patents issued (publication numbers: US6255281 B1; US7122344 B2; US7846899 B2; US8470767 B2) and pending (application numbers: US 13/501,908; US 12/945,622; US 12/637,573) in this area.
List of Abbreviations
- AAT
α1-antitrypsin
- AECOPD
Acute exacerbations of COPD
- ALX receptor
Receptor for annexin-A1, lipoxin-A4
- ANXA1
Annexin-1
- AP-1
Activation protein-1
- BALF
Bronchoalveolar lavage fluid
- BMC
Bone marrow cells
- bp
Base pairs
- BPD
bronchopulmonary dysplasia
- CC10
Club cell protein 10
- CC16−/−
CC16-deficient mice
- CC16
Club cell protein 16
- CCSP
Club cell secretory protein
- COPD
Chronic obstructive pulmonary disease
- COUP-TFs
Chicken ovalbumin upstream promoter transcription factors
- cPLA2
Cytosolic phospholipase A2
- CS
Cigarette smoke
- ECLIPSE
Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints
- ELF
Epithelial lining fluid
- ERK1/2
Extracellular signal-regulated kinase 1/2
- FEV1
Forced expiratory volume in the first second
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- FPRs
fMLP receptors
- GCs
Glucocorticoids
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- GWAS
Genome-wide association study
- HFN
Hepatocyte nuclear factor
- ICGN
International COPD Genomics Network
- IFN-γ
Interferon-γ
- IL
Interleukin
- IκB-α
Inhibitor of NFκB subunit-α
- LPS
Lipopolysaccharide
- MMPs
Metalloproteinases
- NFκB
Nuclear factor kappa B
- NHPs
Non-human primates
- PGD2
Prostaglandin D2
- PGF2α
Platelet grown factor 2α
- PLA2
Phospholipase A2
- PMN
Polymorphonuclear neutrophil
- rCC16
Recombinant CC16
- RT-PCR
Real Time PCR
- SAA
Serum amyloid A
- SAR
Small airway remodeling
- SNPs
Single nucleotide polymorphisms
- Sp1 and Sp3
Specificity protein-1 and specificity protein-3
- sPLA2
Secretory phospholipase A2
- TAF
Tracheal aspirate fluids
- T/EBP
Thyroid-specific enhancer binding protein
- TGF-β1
Tumor grown factor-β1
- TLR4
Toll-like receptor-4
- TNF-α
Tumor Necrosis factor-α
- TTF-1
Thyroid transcription factor-1
- URTIs
Viral upper respiratory tract infections
- WT
Wild-type
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Reference annotations
* Of interest
** Of considerable interest
- 1.Khakban A, Sin DD, FitzGerald JM, Ng R, Zafari Z, McManus B, Hollander Z, Marra CA, Sadatsafavi M. 10-Year Trends in Direct Costs of COPD: A Population Based Study. Chest. 2015 doi: 10.1378/chest.15-0721. [DOI] [PubMed] [Google Scholar]
- 2.Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am.J.Respir.Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 4.Rennard SI, Fogarty C, Kelsen S, Long W, Ramsdell J, Allison J, Mahler D, Saadeh C, Siler T, Snell P, Korenblat P, Smith W, Kaye M, Mandel M, Andrews C, Prabhu R, Donohue JF, Watt R, Lo KH, Schlenker-Herceg R, Barnathan ES, Murray J. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am.J.Respir.Crit Care Med. 2007;175:926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 5.Mahler DA, Huang S, Tabrizi M, Bell GM. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 2004;126:926–934. doi: 10.1378/chest.126.3.926. [DOI] [PubMed] [Google Scholar]
- 6.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of clara cells in normal human airway epithelium. Am.J.Respir.Crit Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- 7.Halatek T, Hermans C, Broeckaert F, Wattiez R, Wiedig M, Toubeau G, Falmagne P, Bernard A. Quantification of Clara cell protein in rat and mouse biological fluids using a sensitive immunoassay. Eur.Respir.J. 1998;11:726–733. [PubMed] [Google Scholar]
- 8.Peri A, Cordella-Miele E, Miele L, Mukherjee AB. Tissue-specific expression of the gene coding for human Clara cell 10-kD protein, a phospholipase A2-inhibitory protein. J.Clin.Invest. 1993;92:2099–2109. doi: 10.1172/JCI116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller-Schottle F, Classen-Linke I, Beier-Hellwig K, Sterzik K, Beier HM. Uteroglobin expression and release in the human endometrium. Ann.N.Y.Acad.Sci. 2000;923:332–335. doi: 10.1111/j.1749-6632.2000.tb05544.x. [DOI] [PubMed] [Google Scholar]
- 10.Geick A, Redecker P, Ehrhardt A, Klocke R, Paul D, Halter R. Uteroglobin promoter-targeted c-MYC expression in transgenic mice cause hyperplasia of Clara cells and malignant transformation of T-lymphoblasts and tubular epithelial cells. Transgenic Res. 2001;10:501–511. doi: 10.1023/a:1013085228119. [DOI] [PubMed] [Google Scholar]
- 11.Massaro GD, Singh G, Mason R, Plopper CG, Malkinson AM, Gail DB. Biology of the Clara cell. Am.J.Physiol. 1994;266:L101–L106. doi: 10.1152/ajplung.1994.266.1.L101. [DOI] [PubMed] [Google Scholar]
- 12.Bedetti CD, Singh J, Singh G, Katyal SL, Wong-Chong ML. Ultrastructural localization of rat Clara cell 10 KD secretory protein by the immunogold technique using polyclonal and monoclonal antibodies. J.Histochem.Cytochem. 1987;35:789–794. doi: 10.1177/35.7.2438324. [DOI] [PubMed] [Google Scholar]
- 13.Tong SS, Hirokata Y, Trush MA, Mimnaugh EG, Ginsburg E, Lowe MC, Gram TE. Clara cell damage and inhibition of pulmonary mixed-function oxidase activity by naphthalene. Biochem.Biophys.Res.Commun. 1981;100:944–950. doi: 10.1016/0006-291x(81)91914-8. [DOI] [PubMed] [Google Scholar]
- 14.Buckpitt A, Chang AM, Weir A, Van, Winkle L, Duan X, Philpot R, Plopper C. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol.Pharmacol. 1995;47:74–81. [PubMed] [Google Scholar]
- 15.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am.J.Respir.Cell Mol.Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 16.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am.J.Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, Stripp BR. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J.Clin.Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Londhe VA, Maisonet TM, Lopez B, Jeng JM, Li C, Minoo P. A subset of epithelial cells with CCSP promoter activity participates in alveolar development. Am.J.Respir.Cell Mol.Biol. 2011;44:804–812. doi: 10.1165/rcmb.2009-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bustos ML, Mura M, Hwang D, Ludkovski O, Wong AP, Keating A, Waddell TK. Depletion of bone marrow CCSP-expressing cells delays airway regeneration. Mol.Ther. 2015;23:561–569. doi: 10.1038/mt.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willey JC, Coy E, Brolly C, Utell MJ, Frampton MW, Hammersley J, Thilly WG, Olson D, Cairns K. Xenobiotic metabolism enzyme gene expression in human bronchial epithelial and alveolar macrophage cells. Am.J.Respir.Cell Mol.Biol. 1996;14:262–271. doi: 10.1165/ajrcmb.14.3.8845177. [DOI] [PubMed] [Google Scholar]
- 22.Cuzic S, Bosnar M, Kramaric MD, Ferencic Z, Markovic D, Glojnaric I, Erakovic Haber. V Claudin-3 and Clara cell 10 kDa protein as early signals of cigarette smoke-induced epithelial injury along alveolar ducts. Toxicol.Pathol. 2012;40:1169–1187. doi: 10.1177/0192623312448937. [DOI] [PubMed] [Google Scholar]
- 23.Nord M, Lag M, Cassel TN, Randmark M, Becher R, Barnes HJ, Schwarze PE, Gustafsson JA, Lund J. Regulation of CCSP (PCB-BP/uteroglobin) expression in primary cultures of lung cells: involvement of C/EBP. DNA Cell Biol. 1998;17:481–492. doi: 10.1089/dna.1998.17.481. [DOI] [PubMed] [Google Scholar]
- 24.Patton SE, Gupta RP, Nishio S, Eddy EM, Jetten AM, Plopper CG, Nettesheim P, Hook GE. Ultrastructural immunohistochemical localization of Clara cell secretory protein in pulmonary epithelium of rabbits. Environ.Health Perspect. 1991;93:225–232. doi: 10.1289/ehp.9193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf M, Klug J, Hackenberg R, Gessler M, Grzeschik KH, Beato M, Suske G. Human CC10, the homologue of rabbit uteroglobin: genomic cloning, chromosomal localization and expression in endometrial cell lines. Hum.Mol.Genet. 1992;1:371–378. doi: 10.1093/hmg/1.6.371. [DOI] [PubMed] [Google Scholar]
- 26.Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, Kimura S. UGRP1, a uteroglobin/Clara cell secretory protein-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor. Mol.Endocrinol. 2001;15:2021–2036. doi: 10.1210/mend.15.11.0728. [DOI] [PubMed] [Google Scholar]
- 27.Stripp BR, Huffman JA, Bohinski RJ. Structure and regulation of the murine Clara cell secretory protein gene. Genomics. 1994;20:27–35. doi: 10.1006/geno.1994.1123. [DOI] [PubMed] [Google Scholar]
- 28.Ustiyan V, Wert SE, Ikegami M, Wang IC, Kalin TV, Whitsett JA, Kalinichenko VV. Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways. Dev.Biol. 2012;370:198–212. doi: 10.1016/j.ydbio.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingle CD, Hackett BP, Moxley M, Longmore W, Gitlin JD. Role of hepatocyte nuclear factor-3 alpha and hepatocyte nuclear factor-3 beta in Clara cell secretory protein gene expression in the bronchiolar epithelium. Biochem.J. 1995;308(Pt 1):197–202. doi: 10.1042/bj3080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srisodsai A, Kurotani R, Chiba Y, Sheikh F, Young HA, Donnelly RP, Kimura S. Interleukin-10 induces uteroglobin-related protein (UGRP) 1 gene expression in lung epithelial cells through homeodomain transcription factor T/EBP/NKX2.1. J.Biol.Chem. 2004;279:54358–54368. doi: 10.1074/jbc.M405331200. [DOI] [PubMed] [Google Scholar]
- 31.Stahlman MT, Gray ME, Whitsett JA. Expression of thyroid transcription factor-1(TTF-1) in fetal and neonatal human lung. J.Histochem.Cytochem. 1996;44:673–678. doi: 10.1177/44.7.8675988. [DOI] [PubMed] [Google Scholar]
- 32.Sawaya PL, Stripp BR, Whitsett JA, Luse DS. The lung-specific CC10 gene is regulated by transcription factors from the AP-1, octamer, and hepatocyte nuclear factor 3 families. Mol.Cell Biol. 1993;13:3860–3871. doi: 10.1128/mcb.13.7.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navab R, Wang Y, Chow YH, Wang A, Jankov RP, Takamoto N, Tsai SY, Tsai MJ, Tanswell AK, Hu J. Regulation of human Clara cell 10 kD protein expression by chicken ovalbumin upstream promoter transcription factors (COUP-TFs). Am.J.Respir.Cell Mol.Biol. 2002;27:273–285. doi: 10.1165/rcmb.2002-0014OC. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Renau D, Lombardero M, Nieto A. Glucocorticoid-dependent uteroglobin synthesis and uteroglobulin mRNA levels in rabbit lung explants cultured in vitro. Eur.J.Biochem. 1984;144:523–527. doi: 10.1111/j.1432-1033.1984.tb08497.x. [DOI] [PubMed] [Google Scholar]
- 35.Michel O, Murdoch R, Bernard A. Inhaled LPS induces blood release of Clara cell specific protein (CC16) in human beings. J.Allergy Clin.Immunol. 2005;115:1143–1147. doi: 10.1016/j.jaci.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 36.Elia J, Aoki A, Maldonado CA. Regulation of uteroglobin/Clara cell protein expression after acute lung exposure to an organophosphoreted insecticide. Histochem.Cell Biol. 2003;120:33–39. doi: 10.1007/s00418-003-0546-z. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr.Rev. 2007;28:707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 38.Magdaleno SM, Wang G, Jackson KJ, Ray MK, Welty S, Costa RH, DeMayo FJ. Interferon-gamma regulation of Clara cell gene expression: in vivo and in vitro. Am.J.Physiol. 1997;272:L1142–L1151. doi: 10.1152/ajplung.1997.272.6.L1142. [DOI] [PubMed] [Google Scholar]
- 39.Yao XL, Ikezono T, Cowan M, Logun C, Angus CW, Shelhamer JH. Interferon-gamma stimulates human Clara cell secretory protein production by human airway epithelial cells. Am.J.Physiol. 1998;274:L864–L869. doi: 10.1152/ajplung.1998.274.5.L864. [DOI] [PubMed] [Google Scholar]
- 40.Yoon JM, Lee KH, Lee SM, Lim JJ, Yang SC, Yoo CG, Lee CT, Han SK, Shim YS, Kim YW. The immune modulation of Clara cell-10 in human peripheral monocytes and dendritic cells. Int.J.Mol.Med. 2010;26:415–423. [PubMed] [Google Scholar]
- 41.Kim S, Shim JJ, Burgel PR, Ueki IF, Dao-Pick T, Tam DC, Nadel JA. IL-13-induced Clara cell secretory protein expression in airway epithelium: role of EGFR signaling pathway. Am.J.Physiol Lung Cell Mol.Physiol. 2002;283:L67–L75. doi: 10.1152/ajplung.00404.2001. [DOI] [PubMed] [Google Scholar]
- 42.Callebaut I, Poupon A, Bally R, Demaret JP, Housset D, Delettre J, Hossenlopp P, Mornon JP. The uteroglobin fold. Ann.N.Y.Acad.Sci. 2000;923:90–112. doi: 10.1111/j.1749-6632.2000.tb05522.x. [DOI] [PubMed] [Google Scholar]
- 43.Umland TC, Swaminathan S, Singh G, Warty V, Furey W, Pletcher J, Sax M. Structure of a human Clara cell phospholipid-binding protein-ligand complex at 1.9 A resolution. Nat.Struct.Biol. 1994;1:538–545. doi: 10.1038/nsb0894-538. [DOI] [PubMed] [Google Scholar]
- 44.Barnes HJ, Nordlund-Moller L, Nord M, Gustafsson J, Lund J, Gillner M. Structural basis for calcium binding by uteroglobins. J.Mol.Biol. 1996;256:392–404. doi: 10.1006/jmbi.1996.0094. [DOI] [PubMed] [Google Scholar]
- 45.Miele L, Cordella-Miele E, Mukherjee AB. Uteroglobin: structure, molecular biology, and new perspectives on its function as a phospholipase A2 inhibitor. Endocr.Rev. 1987;8:474–490. doi: 10.1210/edrv-8-4-474. [DOI] [PubMed] [Google Scholar]
- 46.Matthews JH, Pattabiraman N, Ward KB, Mantile G, Miele L, Mukherjee AB. Crystallization and characterization of the recombinant human Clara cell 10-kDa protein. Proteins. 1994;20:191–196. doi: 10.1002/prot.340200209. [DOI] [PubMed] [Google Scholar]
- 47.Pattabiraman N, Matthews JH, Ward KB, Mantile-Selvaggi G, Miele L, Mukherjee AB. Crystal structure analysis of recombinant human uteroglobin and molecular modeling of ligand binding. Ann.N.Y.Acad.Sci. 2000;923:113–127. doi: 10.1111/j.1749-6632.2000.tb05523.x. [DOI] [PubMed] [Google Scholar]
- 48.Mandal AK, Zhang Z, Ray R, Choi MS, Chowdhury B, Pattabiraman N, Mukherjee AB. Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J.Exp.Med. 2004;199:1317–1330. doi: 10.1084/jem.20031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nieto A, Ponstingl H, Beato M. Purification and quaternary structure of the hormonally induced protein uteroglobin. Arch.Biochem.Biophys. 1977;180:82–92. doi: 10.1016/0003-9861(77)90011-x. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto S, Nakagawa K, Sueishi K. Monkey Clara cell 10 kDa protein (CC10): a characterization of the amino acid sequence with an evolutional comparison with humans, rabbits, rats, and mice. Am.J.Respir.Cell Mol.Biol. 1996;15:361–366. doi: 10.1165/ajrcmb.15.3.8810640. [DOI] [PubMed] [Google Scholar]
- 51.Bernard A, Dumont X, Roels H, Lauwerys R, Dierynck I, De, Ley M, Stroobant V, de, Hoffmann E. The molecular mass and concentrations of protein 1 or Clara cell protein in biological fluids: a reappraisal. Clin.Chim.Acta. 1993;223:189–191. doi: 10.1016/0009-8981(93)90077-h. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds HY, Newball HH. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J.Lab Clin.Med. 1974;84:559–573. [PubMed] [Google Scholar]
- 53.Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer R. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax. 2008;63:1058–1063. doi: 10.1136/thx.2008.102574. [DOI] [PubMed] [Google Scholar]
- 54*.Bernard A, Marchandise FX, Depelchin S, Lauwerys R, Sibille Y. Clara cell protein in serum and bronchoalveolar lavage. Eur.Respir.J. 1992;5:1231–1238. [This paper was the first to show that healthy smokers controls and COPD patients have lower levels of CC16 in serum and plasma than non-smokers controls.] [PubMed] [Google Scholar]
- 55.Ramsay PL, DeMayo FJ, Hegemier SE, Wearden ME, Smith CV, Welty SE. Clara cell secretory protein oxidation and expression in premature infants who develop bronchopulmonary dysplasia. Am.J.Respir.Crit Care Med. 2001;164:155–161. doi: 10.1164/ajrccm.164.1.2008022. [DOI] [PubMed] [Google Scholar]
- 56.Arias-Martinez J, Palacios-Sanchez M, Delgado-Franco D, Guzman-Barcenas J, Garcia-Latorre E, Zhang L, Irles C. Clara cell protein expression in human neonates during respiratory distress syndrome. Cell Physiol Biochem. 2012;29:753–760. doi: 10.1159/000264417. [DOI] [PubMed] [Google Scholar]
- 57.Lindahl M, Stahlbom B, Tagesson C. Newly identified proteins in human nasal and bronchoalveolar lavage fluids: potential biomedical and clinical applications. Electrophoresis. 1999;20:3670–3676. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3670::AID-ELPS3670>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 58.Maturana A, Bernard A, Germain AM, Chau VL, Moya FR. Amniotic fluid Clara cell protein concentration in normal pregnancy, a marker of fetal airway growth or fetal lung maturation? J.Perinatol. 2001;21:516–520. doi: 10.1038/sj.jp.7210578. [DOI] [PubMed] [Google Scholar]
- 59.Bernard A, Thielemans N, Lauwerys R, Langhendries JP, Van, Lierde M, Freund MM. Clara cell protein in human amniotic fluid: a potential marker of fetal lung growth. Pediatr.Res. 1994;36:771–775. doi: 10.1203/00006450-199412000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Maturana A, Bernard A, Germain AM, Chau VL, Moya FR. Amniotic fluid Clara cell protein concentration in normal pregnancy, a marker of fetal airway growth or fetal lung maturation? J.Perinatol. 2001;21:516–520. doi: 10.1038/sj.jp.7210578. [DOI] [PubMed] [Google Scholar]
- 61.Thomas W, Seidenspinner S, Kawczynska-Leda N, Chmielnicka-Kopaczyk M, Marx A, Wirbelauer J, Szymankiewicz M, Speer CP. Clara cell secretory protein in tracheobronchial aspirates and umbilical cord serum of extremely premature infants with systemic inflammation. Neonatology. 2010;97:228–234. doi: 10.1159/000253152. [DOI] [PubMed] [Google Scholar]
- 62.Levine CR, Gewolb IH, Allen K, Welch RW, Melby JM, Pollack S, Shaffer T, Pilon AL, Davis JM. The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr.Res. 2005;58:15–21. doi: 10.1203/01.PDR.0000156371.89952.35. [DOI] [PubMed] [Google Scholar]
- 63.Ramsay PL, DeMayo FJ, Hegemier SE, Wearden ME, Smith CV, Welty SE. Clara cell secretory protein oxidation and expression in premature infants who develop bronchopulmonary dysplasia. Am.J.Respir.Crit Care Med. 2001;164:155–161. doi: 10.1164/ajrccm.164.1.2008022. [DOI] [PubMed] [Google Scholar]
- 64.Wong P, Murray C, Louw J, French N, Chambers D. Adult bronchopulmonary dysplasia: computed tomography pulmonary findings. J.Med.Imaging Radiat.Oncol. 2011;55:373–378. doi: 10.1111/j.1754-9485.2011.02285.x. [DOI] [PubMed] [Google Scholar]
- 65.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur.Respir.J. 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 66.Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, Kotecha S. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013;68:760–766. doi: 10.1136/thoraxjnl-2012-203079. [DOI] [PubMed] [Google Scholar]
- 67.Ji CM, Royce FH, Truong U, Plopper CG, Singh G, Pinkerton KE. Maternal exposure to environmental tobacco smoke alters Clara cell secretory protein expression in fetal rat lung. Am.J.Physiol. 1998;275:L870–L876. doi: 10.1152/ajplung.1998.275.5.L870. [DOI] [PubMed] [Google Scholar]
- 68.Hermans C, Libotte V, Robin M, Clippe A, Wattiez R, Falmagne P, Langhendries JP, Bernard A. Maternal tobacco smoking and lung epithelium-specific proteins in amniotic fluid. Pediatr.Res. 2001;50:487–494. doi: 10.1203/00006450-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 69.Bernard A, Hermans C, Van, Houte G. Transient increase of serum Clara cell protein (CC16) after exposure to smoke. Occup.Environ.Med. 1997;54:63–65. doi: 10.1136/oem.54.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robin M, Dong P, Hermans C, Bernard A, Bersten AD, Doyle IR. Serum levels of CC16, SP-A and SP-B reflect tobacco-smoke exposure in asymptomatic subjects. Eur.Respir.J. 2002;20:1152–1161. doi: 10.1183/09031936.02.02042001. [DOI] [PubMed] [Google Scholar]
- 71.Yu M, Zheng X, Witschi H, Pinkerton KE. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicol.Sci. 2002;68:488–497. doi: 10.1093/toxsci/68.2.488. [DOI] [PubMed] [Google Scholar]
- 72.Barth PJ, Muller B. Effects of nitrogen dioxide exposure on Clara cell proliferation and morphology. Pathol.Res.Pract. 1999;195:487–493. doi: 10.1016/S0344-0338(99)80052-1. [DOI] [PubMed] [Google Scholar]
- 73.Royce FH, Plopper CG. Effect of chronic daily ozone exposure on Clara cell secretory protein mRNA expression in the adult rat lung. Exp.Lung Res. 1997;23:51–64. doi: 10.3109/01902149709046047. [DOI] [PubMed] [Google Scholar]
- 74.Lagerkvist BJ, Bernard A, Blomberg A, Bergstrom E, Forsberg B, Holmstrom K, Karp K, Lundstrom NG, Segerstedt B, Svensson M, Nordberg G. Pulmonary epithelial integrity in children: relationship to ambient ozone exposure and swimming pool attendance. Environ.Health Perspect. 2004;112:1768–1771. doi: 10.1289/ehp.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernard A, Nickmilder M, Dumont X. Chlorinated pool attendance, airway epithelium defects and the risks of allergic diseases in adolescents: Interrelationships revealed by circulating biomarkers. Environ.Res. 2015;140:119–126. doi: 10.1016/j.envres.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 76.Bernard AM, Gonzalez-Lorenzo JM, Siles E, Trujillano G, Lauwerys R. Early decrease of serum Clara cell protein in silica-exposed workers. Eur.Respir.J. 1994;7:1932–1937. [PubMed] [Google Scholar]
- 77.Van, Miert E, Sardella A, Nickmilder M, Bernard A. Respiratory effects associated with wood fuel use: a cross-sectional biomarker study among adolescents. Pediatr.Pulmonol. 2012;47:358–366. doi: 10.1002/ppul.21554. [DOI] [PubMed] [Google Scholar]
- 78.Bernard A, Roels H, Buchet JP, Lauwerys R. Decrease of serum Clara cell protein in smokers. Lancet. 1992;339:1620. doi: 10.1016/0140-6736(92)91891-b. [DOI] [PubMed] [Google Scholar]
- 79.Lomas DA, Silverman EK, Edwards LD, Locantore NW, Miller BE, Horstman DH, Tal-Singer R. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur.Respir.J. 2009;34:95–102. doi: 10.1183/09031936.00156508. [DOI] [PubMed] [Google Scholar]
- 80.Park HY, Churg A, Wright JL, Li Y, Tam S, Man SF, Tashkin D, Wise RA, Connett JE, Sin DD. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am.J.Respir.Crit Care Med. 2013;188:1413–1419. doi: 10.1164/rccm.201305-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petersen H, Leng S, Belinsky SA, Miller BE, Tal-Singer R, Owen CA, Celli B, Tesfaigzi Y. Low plasma CC16 levels in smokers are associated with a higher risk for chronic bronchitis. Eur.Respir.J. 2015 doi: 10.1183/13993003.00682-2015. [DOI] [PubMed] [Google Scholar]
- 82.Lange P, Celli B, Agusti A, Boje, Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor P, Meek P, Owen CA, Petersen H, Pinto-Plata V, Schnohr P, Sood A, Soriano JB, Tesfaigzi Y, Vestbo J. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N.Engl.J.Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 83.Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, Wright AL, Lavi I, Tares L, Carsin AE, Dobano C, Barreiro E, Zock JP, Martinez-Moratalla J, Urrutia I, Sunyer J, Keidel D, Imboden M, Probst-Hensch N, Hallberg J, Melen E, Wickman M, Bousquet J, Belgrave DC, Simpson A, Custovic A, Anto JM, Martinez FD. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir.Med. 2015;3:613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bernard A, Hermans C, Van, Houte G. Transient increase of serum Clara cell protein (CC16) after exposure to smoke. Occup.Environ.Med. 1997;54:63–65. doi: 10.1136/oem.54.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van, Miert E, Sardella A, Bernard A. Biomarkers of early respiratory effects in smoking adolescents. Eur.Respir.J. 2011;38:1287–1293. doi: 10.1183/09031936.00000911. [DOI] [PubMed] [Google Scholar]
- 86.Bernard AM, Roels HA, Buchet JP, Lauwerys RR. Serum Clara cell protein: an indicator of bronchial cell dysfunction caused by tobacco smoking. Environ.Res. 1994;66:96–104. doi: 10.1006/enrs.1994.1047. [DOI] [PubMed] [Google Scholar]
- 87.Dell'Omo M, Hermans C, Muzi G, Haufroid V, Bernard A, Carrieri P, Abbritti G. Serum Clara cell protein (CC16) in healthy young smokers. Biomarkers. 2000;5:158–164. doi: 10.1080/135475000230479. [DOI] [PubMed] [Google Scholar]
- 88.Robin M, Dong P, Hermans C, Bernard A, Bersten AD, Doyle IR. Serum levels of CC16, SP-A and SP-B reflect tobacco-smoke exposure in asymptomatic subjects. Eur.Respir.J. 2002;20:1152–1161. doi: 10.1183/09031936.02.02042001. [DOI] [PubMed] [Google Scholar]
- 89.Van, Miert E, Dumont X, Bernard A. CC16 as a marker of lung epithelial hyperpermeability in an acute model of rats exposed to mainstream cigarette smoke. Toxicol.Lett. 2005;159:115–123. doi: 10.1016/j.toxlet.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 90**.Laucho-Contreras ME, Polverino F, Gupta K, Taylor KL, Kelly E, Pinto-Plata V, Divo M, Ashfaq N, Petersen H, Stripp B, Pilon AL, Tesfaigzi Y, Celli BR, Owen CA. Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur.Respir.J. 2015;45:1544–1556. doi: 10.1183/09031936.00134214. [This paper was the first to show that CC16−/− mice have greater increases in CS-induced pulmonary inflammation, mucus metaplasia, airspace enlargement, and fibrosis of the small airways fibrosis than WT mice and to correlate these findings with activation of two main pro-inflammatory pathways that CC16 is known to regulate in CS-exposed CC16−/− lungs. The investigators demonstrated that CC16 deficiency is associated with increased activation of NFκB (but not increased lung sPLA2 activity levels) in the CS-exposed lung. This study also showed that CS-induced lung pathologies and activation of NFκB are reversed by adenoviral-mediated over-expression of CC16 in murine airway epithelial cells in both WT and CC16−/− mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu L, Di PY, Wu R, Pinkerton KE, Chen Y. Repression of CC16 by cigarette smoke (CS) exposure. PLoS.One. 2015;10:e0116159. doi: 10.1371/journal.pone.0116159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hermans C, Dong P, Robin M, Jadoul M, Bernard A, Bersten AD, Doyle IR. Determinants of serum levels of surfactant proteins A and B and Clara cell protein CC16. Biomarkers. 2003;8:461–471. doi: 10.1080/13547500310001647021. [DOI] [PubMed] [Google Scholar]
- 93.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martinez FD. Serum concentrations of club cell secretory protein (Clara) and cancer mortality in adults: a population-based, prospective cohort study. Lancet Respir.Med. 2013;1:779–785. doi: 10.1016/S2213-2600(13)70220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Celli BR, Owen CA. The club cell and its protein, CC16: time to shine. Lancet Respir.Med. 2013;1:757–759. doi: 10.1016/S2213-2600(13)70247-9. [DOI] [PubMed] [Google Scholar]
- 95.Laing IA, Hermans C, Bernard A, Burton PR, Goldblatt J, Le Souef PN. Association between plasma CC16 levels, the A38G polymorphism, and asthma. Am.J.Respir.Crit Care Med. 2000;161:124–127. doi: 10.1164/ajrccm.161.1.9904073. [DOI] [PubMed] [Google Scholar]
- 96.Lee YC, Zhang Z, Mukherjee AB. Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis. FEBS Lett. 2006;580:4515–4520. doi: 10.1016/j.febslet.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 97.Kokuho N, Ishii T, Kamio K, Hayashi H, Kurahara M, Hattori K, Motegi T, Azuma A, Gemma A, Kida K. Diagnostic Values For Club Cell Secretory Protein (CC16) in Serum of Patients of Combined Pulmonary Fibrosis and Emphysema. COPD. 2015;12:347–354. doi: 10.3109/15412555.2014.948994. [DOI] [PubMed] [Google Scholar]
- 98.Pilette C, Godding V, Kiss R, Delos M, Verbeken E, Decaestecker C, De, Paepe K, Vaerman JP, Decramer M, Sibille Y. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am.J.Respir.Crit Care Med. 2001;163:185–194. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 99.Chen J, Lam S, Pilon A, McWilliams A, Macaulay C, Szabo E. Higher levels of the anti-inflammatory protein CC10 are associated with improvement in bronchial dysplasia and sputum cytometric assessment in individuals at high risk for lung cancer. Clin.Cancer Res. 2008;14:1590–1597. doi: 10.1158/1078-0432.CCR-07-4066. [DOI] [PubMed] [Google Scholar]
- 100**.Kim DK, Cho MH, Hersh CP, Lomas DA, Miller BE, Kong X, Bakke P, Gulsvik A, Agusti A, Wouters E, Celli B, Coxson H, Vestbo J, MacNee W, Yates JC, Rennard S, Litonjua A, Qiu W, Beaty TH, Crapo JD, Riley JH, Tal-Singer R, Silverman EK. Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am.J.Respir.Crit Care Med. 2012;186:1238–1247. doi: 10.1164/rccm.201206-1013OC. [This paper studied the ECLIPSE cohort and found significant associations between the presence of eleven SNPs and serum CC16 levels in COPD patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buro-Auriemma LJ, Salit J, Hackett NR, Walters MS, Strulovici-Barel Y, Staudt MR, Fuller J, Mahmoud M, Stevenson CS, Hilton H, Ho MW, Crystal RG. Cigarette smoking induces small airway epithelial epigenetic changes with corresponding modulation of gene expression. Hum.Mol.Genet. 2013;22:4726–4738. doi: 10.1093/hmg/ddt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polverino F, Doyle-Eisele M, McDonald J, Kelly EM, Wilder J, Mauderly J, Divo M, Pinto-Plata V, Celli BR, Tesfaigzi Y, Owen CA. A Novel Non-Human Primate Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease. Eur.Respir.J. 2014;44(Suppl. 58) [Google Scholar]
- 103.Stripp BR, Lund J, Mango GW, Doyen KC, Johnston C, Hultenby K, Nord M, Whitsett JA. Clara cell secretory protein: a determinant of PCB bioaccumulation in mammals. Am.J.Physiol. 1996;271:L656–L664. doi: 10.1152/ajplung.1996.271.4.L656. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Z, Kundu GC, Yuan CJ, Ward JM, Lee EJ, DeMayo F, Westphal H, Mukherjee AB. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science. 1997;276:1408–1412. doi: 10.1126/science.276.5317.1408. [DOI] [PubMed] [Google Scholar]
- 105.Yang SH, Shin SJ, Oh JE, Jin JZ, Chung NH, Lim CS, Kim S, Kim YS. The protective role of uteroglobin through the modulation of tissue transglutaminase in the experimental crescentic glomerulonephritis. Nephrol.Dial.Transplant. 2008;23:3437–3445. doi: 10.1093/ndt/gfn268. [DOI] [PubMed] [Google Scholar]
- 106.Lee DS, Yang SH, Kim HL, Joo KW, Lim CS, Chae DW, Kim S, Lee JS, Kim YS. Recombinant uteroglobin prevents the experimental crescentic glomerulonephritis. Kidney Int. 2004;66:1061–1067. doi: 10.1111/j.1523-1755.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- 107.Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J.Immunol. 2001;167:3025–3028. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- 108.Wang SZ, Rosenberger CL, Espindola TM, Barrett EG, Tesfaigzi Y, Bice DE, Harrod KS. CCSP modulates airway dysfunction and host responses in an Ova-challenged mouse model. Am.J.Physiol Lung Cell Mol.Physiol. 2001;281:L1303–L1311. doi: 10.1152/ajplung.2001.281.5.L1303. [DOI] [PubMed] [Google Scholar]
- 109.Lee YC, Zhang Z, Mukherjee AB. Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis. FEBS Lett. 2006;580:4515–4520. doi: 10.1016/j.febslet.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 110.Liu W, Wan J, Han JZ, Li C, Feng DD, Yue SJ, Huang YH, Chen Y, Cheng QM, Li Y, Luo ZQ. Antiflammin-1 attenuates bleomycin-induced pulmonary fibrosis in mice. Respir.Res. 2013;14:101. doi: 10.1186/1465-9921-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J.Immunol. 2003;171:1051–1060. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- 112.Ofulue AF, Ko M. Effects of depletion of neutrophils or macrophages on development of cigarette smoke-induced emphysema. Am.J.Physiol. 1999;277:L97–105. doi: 10.1152/ajplung.1999.277.1.L97. [DOI] [PubMed] [Google Scholar]
- 113.Long XB, Hu S, Wang N, Zhen HT, Cui YH, Liu Z. Clara cell 10-kDa protein gene transfection inhibits NF-kappaB activity in airway epithelial cells. PLoS.One. 2012;7:e35960. doi: 10.1371/journal.pone.0035960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114**.Gamez AS, Gras D, Petit A, Knabe L, Molinari N, Vachier I, Chanez P, Bourdin A. Supplementing Defect in Club Cell Secretory Protein Attenuates Airway Inflammation in COPD. Chest. 2015;147:1467–1476. doi: 10.1378/chest.14-1174. [This paper demonstrated that the use of recombinant CC16 reduced mucus metaplasia and the release of IL-8 induced by CSE in airway epithelial cells from COPD patients.] [DOI] [PubMed] [Google Scholar]
- 115.Tokita E, Tanabe T, Asano K, Suzaki H, Rubin BK. Club cell 10-kDa protein attenuates airway mucus hypersecretion and inflammation. Eur.Respir.J. 2014;44:1002–1010. doi: 10.1183/09031936.00080913. [DOI] [PubMed] [Google Scholar]
- 116.Vasanthakumar G, Manjunath R, Mukherjee AB, Warabi H, Schiffmann E. Inhibition of phagocyte chemotaxis by uteroglobin, an inhibitor of blastocyst rejection. Biochem.Pharmacol. 1988;37:389–394. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 117.Katavolos P, Ackerley CA, Clark ME, Bienzle D. Clara cell secretory protein increases phagocytic and decreases oxidative activity of neutrophils. Vet.Immunol.Immunopathol. 2011;139:1–9. doi: 10.1016/j.vetimm.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 118.Snyder JC, Reynolds SD, Hollingsworth JW, Li Z, Kaminski N, Stripp BR. Clara cells attenuate the inflammatory response through regulation of macrophage behavior. Am.J.Respir.Cell Mol.Biol. 2010;42:161–171. doi: 10.1165/rcmb.2008-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vasanthakumar G, Manjunath R, Mukherjee AB, Warabi H, Schiffmann E. Inhibition of phagocyte chemotaxis by uteroglobin, an inhibitor of blastocyst rejection. Biochem.Pharmacol. 1988;37:389–394. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 120.Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR. CCSP regulates cross talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am.J.Physiol Lung Cell Mol.Physiol. 2007;293:L114–L123. doi: 10.1152/ajplung.00014.2007. [DOI] [PubMed] [Google Scholar]
- 121.Raffay TM, Locy ML, Hill CL, Jindal NS, Rogers LK, Welty SE, Tipple TE. Neonatal hyperoxic exposure persistently alters lung secretoglobins and annexin A1. Biomed.Res.Int. 2013;2013:408485. doi: 10.1155/2013/408485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miele L, Cordella-Miele E, Facchiano A, Mukherjee AB. Novel anti-inflammatory peptides from the region of highest similarity between uteroglobin and lipocortin I. Nature. 1988;335:726–730. doi: 10.1038/335726a0. [DOI] [PubMed] [Google Scholar]
- 123.Wendt C, Tram K, Price A, England K, Stiehm A, Panoskaltsis-Mortari A. Club cell secretory protein improves survival in a murine obliterative bronchiolitis model. Am.J.Physiol Lung Cell Mol.Physiol. 2013;305:L642–L650. doi: 10.1152/ajplung.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Churg A, Marshall CV, Sin DD, Bolton S, Zhou S, Thain K, Cadogan EB, Maltby J, Soars MG, Mallinder PR, Wright JL. Late intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary disease. Am.J.Respir.Crit Care Med. 2012;185:34–43. doi: 10.1164/rccm.201103-0468OC. [DOI] [PubMed] [Google Scholar]
- 125.Miller TL, Shashikant BN, Pilon AL, Pierce RA, Shaffer TH, Wolfson MR. Effects of recombinant Clara cell secretory protein (rhCC10) on inflammatory-related matrix metalloproteinase activity in a preterm lamb model of neonatal respiratory distress. Pediatr.Crit Care Med. 2007;8:40–46. doi: 10.1097/01.PCC.0000253022.10607.61. [DOI] [PubMed] [Google Scholar]
- 126.Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;62:706–713. doi: 10.1136/thx.2006.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix Metalloproteinases As Therapeutic Targets For Idiopathic Pulmonary Fibrosis. Am.J.Respir.Cell Mol.Biol. 2015 doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Owen CA. Leukocyte cell surface proteinases: regulation of expression, functions, and mechanisms of surface localization. Int.J.Biochem.Cell Biol. 2008;40:1246–1272. doi: 10.1016/j.biocel.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lesur O, Bernard A, Arsalane K, Lauwerys R, Begin R, Cantin A, Lane D. Clara cell protein (CC-16) induces a phospholipase A2-mediated inhibition of fibroblast migration in vitro. Am.J.Respir.Crit Care Med. 1995;152:290–297. doi: 10.1164/ajrccm.152.1.7541278. [DOI] [PubMed] [Google Scholar]
- 130.Kundu GC, Mantile G, Miele L, Cordella-Miele E, Mukherjee AB. Recombinant human uteroglobin suppresses cellular invasiveness via a novel class of high-affinity cell surface binding site. Proc.Natl.Acad.Sci.U.S.A. 1996;93:2915–2919. doi: 10.1073/pnas.93.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mandal AK, Ray R, Zhang Z, Chowdhury B, Pattabiraman N, Mukherjee AB. Uteroglobin inhibits prostaglandin F2alpha receptor-mediated expression of genes critical for the production of pro-inflammatory lipid mediators. J.Biol.Chem. 2005;280:32897–32904. doi: 10.1074/jbc.M502375200. [DOI] [PubMed] [Google Scholar]
- 132.Antico G, Lingen MW, Sassano A, Melby J, Welch RW, Fiore S, Pilon AL, Miele L. Recombinant human uteroglobin/CC10 inhibits the adhesion and migration of primary human endothelial cells via specific and saturable binding to fibronectin. J.Cell Physiol. 2006;207:553–561. doi: 10.1002/jcp.20604. [DOI] [PubMed] [Google Scholar]
- 133.Mantile G, Miele L, Cordella-Miele E, Singh G, Katyal SL, Mukherjee AB. Human Clara cell 10-kDa protein is the counterpart of rabbit uteroglobin. J.Biol.Chem. 1993;268:20343–20351. [PubMed] [Google Scholar]
- 134.Yoshikawa S, Miyahara T, Reynolds SD, Stripp BR, Anghelescu M, Eyal FG, Parker JC. Clara cell secretory protein and phospholipase A2 activity modulate acute ventilator-induced lung injury in mice. J.Appl.Physiol (1985.) 2005;98:1264–1271. doi: 10.1152/japplphysiol.01150.2004. [DOI] [PubMed] [Google Scholar]
- 135.Andersson O, Nordlund-Moller L, Barnes HJ, Lund J. Heterologous expression of human uteroglobin/polychlorinated biphenyl-binding protein. Determination of ligand binding parameters and mechanism of phospholipase A2 inhibition in vitro. J.Biol.Chem. 1994;269:19081–19087. [PubMed] [Google Scholar]
- 136.Facchiano A, Cordella-Miele E, Miele L, Mukherjee AB. Inhibition of pancreatic phospholipase A2 activity by uteroglobin and antiflammin peptides: possible mechanism of action. Life Sci. 1991;48:453–464. doi: 10.1016/0024-3205(91)90501-2. [DOI] [PubMed] [Google Scholar]
- 137.De, Luca D, Minucci A, Tripodi D, Piastra M, Pietrini D, Zuppi C, Conti G, Carnielli VP, Capoluongo E. Role of distinct phospholipases A2 and their modulators in meconium aspiration syndrome in human neonates. Intensive Care Med. 2011;37:1158–1165. doi: 10.1007/s00134-011-2243-z. [DOI] [PubMed] [Google Scholar]
- 138.Yoon JM, Lim JJ, Yoo CG, Lee CT, Bang YJ, Han SK, Shim YS, Kim YW. Adenovirus-uteroglobin suppresses COX-2 expression via inhibition of NF-kappaB activity in lung cancer cells. Lung Cancer. 2005;48:201–209. doi: 10.1016/j.lungcan.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 139.Mandal AK, Ray R, Zhang Z, Chowdhury B, Pattabiraman N, Mukherjee AB. Uteroglobin inhibits prostaglandin F2alpha receptor-mediated expression of genes critical for the production of pro-inflammatory lipid mediators. J.Biol.Chem. 2005;280:32897–32904. doi: 10.1074/jbc.M502375200. [DOI] [PubMed] [Google Scholar]
- 140.Knabe L, Fort A, Chanez P, Bourdin A. Club cells and CC16: another “smoking gun”? (With potential bullets against COPD). Eur.Respir.J. 2015;45:1519–1520. doi: 10.1183/09031936.00010515. [DOI] [PubMed] [Google Scholar]
- 141.Antico G, Aloman M, Lakota K, Miele L, Fiore S, Sodin-Semrl S. Uteroglobin, a possible ligand of the lipoxin receptor inhibits serum amyloid A-driven inflammation. Mediators.Inflamm. 2014;2014:876395. doi: 10.1155/2014/876395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.El, Kebir D, Jozsef L, Filep JG. Opposing regulation of neutrophil apoptosis through the formyl peptide receptor-like 1/lipoxin A4 receptor: implications for resolution of inflammation. J.Leukoc.Biol. 2008;84:600–606. doi: 10.1189/jlb.1107765. [DOI] [PubMed] [Google Scholar]
- 143.Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, Anderson GP. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc.Natl.Acad.Sci.U.S.A. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johansson S, Andersson K, Wennergren G, Wenneras C, Rudin A. CC16 inhibits the migration of eosinophils towards the formyl peptide fMLF but not towards PGD2. Inflammation. 2009;32:65–69. doi: 10.1007/s10753-008-9103-1. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y, Yu HJ, Wang N, Zhang YN, Huang SK, Cui YH, Liu Z. Clara cell 10-kDa protein inhibits T(H)17 responses through modulating dendritic cells in the setting of allergic rhinitis. J.Allergy Clin.Immunol. 2013;131:387–394. doi: 10.1016/j.jaci.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sugiyama Y, Tomoda K, Tanaka T, Arata Y, Yoneda-Kato N, Kato J. Direct binding of the signal-transducing adaptor Grb2 facilitates down-regulation of the cyclin-dependent kinase inhibitor p27Kip1. J.Biol.Chem. 2001;276:12084–12090. doi: 10.1074/jbc.M010811200. [DOI] [PubMed] [Google Scholar]
- 147.Burmeister R, Boe IM, Nykjaer A, Jacobsen C, Moestrup SK, Verroust P, Christensen EI, Lund J, Willnow TE. A two-receptor pathway for catabolism of Clara cell secretory protein in the kidney. J.Biol.Chem. 2001;276:13295–13301. doi: 10.1074/jbc.M010679200. [DOI] [PubMed] [Google Scholar]
- 148.Kolleck I, Wissel H, Guthmann F, Schlame M, Sinha P, Rustow B. HDL-holoparticle uptake by alveolar type II cells: effect of vitamin E status. Am.J.Respir.Cell Mol.Biol. 2002;27:57–63. doi: 10.1165/ajrcmb.27.1.4774. [DOI] [PubMed] [Google Scholar]
- 149.Vortherms AR, Kahkoska AR, Rabideau AE, Zubieta J, Andersen LL, Madsen M, Doyle RP. A water soluble vitamin B12-ReI fluorescent conjugate for cell uptake screens: use in the confirmation of cubilin in the lung cancer line A549. Chem.Commun.(Camb.) 2011;47:9792–9794. doi: 10.1039/c1cc13615a. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Z, Kim SJ, Chowdhury B, Wang J, Lee YC, Tsai PC, Choi M, Mukherjee AB. Interaction of uteroglobin with lipocalin-1 receptor suppresses cancer cell motility and invasion. Gene. 2006;369:66–71. doi: 10.1016/j.gene.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 151.Lee JC, Park KH, Han SJ, Yoo CG, Lee CT, Han SK, Shim YS, Kim YW. Inhibitory effect of adenovirus-uteroglobin transduction on the growth of lung cancer cell lines. Cancer Gene Ther. 2003;10:287–293. doi: 10.1038/sj.cgt.7700569. [DOI] [PubMed] [Google Scholar]
- 152.Szabo E, Goheer A, Witschi H, Linnoila RI. Overexpression of CC10 modifies neoplastic potential in lung cancer cells. Cell Growth Differ. 1998;9:475–485. [PubMed] [Google Scholar]
- 153.Cote O, Clark ME, Viel L, Labbe G, Seah SY, Khan MA, Douda DN, Palaniyar N, Bienzle D. Secretoglobin 1A1 and 1A1A differentially regulate neutrophil reactive oxygen species production, phagocytosis and extracellular trap formation. PLoS.One. 2014;9:e96217. doi: 10.1371/journal.pone.0096217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu CJ, Chen LC, Huang WC, Chuang CL, Kuo ML. Alleviation of lung inflammatory responses by adeno-associated virus 2/9 vector carrying CC10 in OVA-sensitized mice. Hum.Gene Ther. 2013;24:48–57. doi: 10.1089/hum.2012.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin.Microbiol.Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kane C, O'Neil K, Conk M, Picha K. Inhalation delivery of protein therapeutics. Inflamm.Allergy Drug Targets. 2013;12:81–87. doi: 10.2174/1871528111312020002. [DOI] [PubMed] [Google Scholar]
- 157.Shiyu S, Zhiyu L, Mao Y, Lin B, Lijia W, Tianbao Z, Jie C, Tingyu L. Polydatin up-regulates Clara cell secretory protein to suppress phospholipase A2 of lung induced by LPS in vivo and in vitro. BMC.Cell Biol. 2011;12:31. doi: 10.1186/1471-2121-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, Miller BE, Silverman EK, Tal-Singer R, Wouters E, Rennard SI. Changes in forced expiratory volume in 1 second over time in COPD. N.Engl.J.Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 159.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 160.Kurowski M, Jurczyk J, Jarzebska M, Moskwa S, Makowska JS, Krysztofiak H, Kowalski ML. Association of serum Clara cell protein CC16 with respiratory infections and immune response to respiratory pathogens in elite athletes. Respir.Res. 2014;15:45. doi: 10.1186/1465-9921-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pilon AL. Rationale for the development of recombinant human CC10 as a therapeutic for inflammatory and fibrotic disease. Ann.N.Y.Acad.Sci. 2000;923:280–299. doi: 10.1111/j.1749-6632.2000.tb05536.x. [DOI] [PubMed] [Google Scholar]
- 162.Kokturk N, Bozdayi G, Yilmaz S, Dogan B, Gulbahar O, Rota S, Tatlicioglu T. Detection of adenovirus and respiratory syncytial virus in patients with chronic obstructive pulmonary disease: Exacerbation versus stable condition. Mol.Med.Rep. 2015;12:3039–3046. doi: 10.3892/mmr.2015.3681. [DOI] [PubMed] [Google Scholar]
- 163.Rohde G, Wiethege A, Borg I, Kauth M, Bauer TT, Gillissen A, Bufe A, Schultze-Werninghaus G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]