Abstract
Background:
Exposure to some perfluoroalkyl substances (PFAS), such as perfluorohexane sulfonate (PFHxS), perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), and perfluorononanoic acid (PFNA), may alter levels of sex hormones and insulin-like growth factor-1 (IGF-1) in animals. Human studies on this topic are scarce, and none have been conducted in young children.
Objectives:
We investigated the relationship between levels of PFAS and estradiol, total testosterone, and IGF-1 in 2,292 children (6–9 years of age) from the C8 Health Project who lived near a chemical plant in the Mid-Ohio Valley (USA) with local contamination from PFOA.
Methods:
Serum samples were collected in 2005–2006 and analyzed for PFAS, sex hormones, and IGF-1. Results from regression models were expressed as the adjusted percentage difference (95% CI) per sex-specific interquartile range (IQR) increment of each PFAS serum concentration. Analyses by PFAS quartiles were also conducted.
Results:
Median concentrations of PFHxS, PFOA, PFOS, and PFNA were 8, 35, 22, and 1.7 ng/mL in boys and 7, 30, 21, and 1.7 ng/mL in girls. In boys, PFOA concentrations were significantly associated with testosterone levels (–4.9%; 95% CI: –8.7, –0.8%); PFOS with estradiol (–4.0%; 95% CI: –7.7, –0.1%), testosterone (–5.8%; 95% CI: –9.4, –2.0%), and IGF-1 (–5.9%; 95% CI: –8.3, –3.3%); and PFNA with IGF-1 (–3.5%; 95% CI: –6.0, –1.0%). In girls, significant associations were found between PFOS and testosterone (–6.6%; 95% CI: –10.1, –2.8%) and IGF-1 (–5.6%; –8.2, –2.9%); and PFNA and IGF-1 (–3.8%; 95% CI: –6.4, –1.2%). In both sexes, the magnitudes of the associations decreased monotonically across quartiles for both testosterone and IGF-1 in relation to PFOS, and for IGF-1 and PFNA in girls.
Conclusions:
To our knowledge, this is the first study suggesting that PFAS are associated with lower levels of IGF-1 and sex hormones in young children.
Citation:
Lopez-Espinosa MJ, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. 2016. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6–9 years of age: a cross-sectional analysis within the C8 Health Project. Environ Health Perspect 124:1269–1275; http://dx.doi.org/10.1289/ehp.1509869
Introduction
Perfluorohexane sulfonate (PFHxS), perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), and perfluorononanoic acid (PFNA) are members of the class of human-made perfluoroalkyl substances (PFAS) that are widely used in household and commercial products (Renner 2001; WHO 2013). Studies around the world have shown measurable levels of these four PFAS in the serum of the studied populations (Fromme et al. 2009; Halldorsson et al. 2012; Kato et al. 2011; Mondal et al. 2012) and, given their long half-life in humans (Bartell et al. 2010), exposure to these compounds will persist for many years.
Even though humans are exposed to PFAS and they may disrupt the endocrine system (Jensen and Leffers 2008), little is known about their possible health effects. In some animal studies exposure to these contaminants has been linked with alterations in levels of sex hormones (Lau et al. 2007; Wan et al. 2011). However, few epidemiological data have been published on this topic despite the key roles played by sex hormones in developmental and reproductive functions during childhood and puberty (Norgil Damgaard et al. 2002; Schoeters et al. 2008). In a cross-sectional study of boys 8–18 years of age in the Mid-Ohio Valley, we reported a 6-month delay in the average age at which the boys entered puberty (using testosterone levels > 50 ng/dL as an indicator of having started puberty), comparing the highest and the lowest PFOS quartiles (Lopez-Espinosa et al. 2011). A study on Taiwanese boys 12–17 years of age (Tsai et al. 2015) reported an inverse association between PFOS and follicle-stimulating hormone (FSH), but found no significant associations between PFAS and estrogen, testosterone, luteinizing hormone (LH), or sex hormone–binding globulin (SHBG). Information on adult male populations is also scarce and inconclusive. PFOS and PFNA were associated inversely with testosterone and estradiol (Joensen et al. 2013) and PFOA positively with LH and FSH (Vested et al. 2013) in young Danish men with a mean age of around 20 years. In male American adults 30–66 years of age, positive associations between both PFOA and PFOS and LH were also reported (Raymer et al. 2012). Other studies, however, did not find such statistically significant associations (Olsen et al. 1998; Specht et al. 2012).
The relationship between PFAS and female reproductive hormones is not clear either. In girls 15 years of age from the ALSPAC (Avon Longitudinal Study of Parents and Children) cohort (UK), positive associations were found between prenatal PFHxS, PFOA, and PFOS exposure and testosterone (Maisonet et al. 2015). In contrast, inverse associations were found between PFOS and testosterone in Taiwanese girls 12–17 years of age (Tsai et al. 2015), but no significant associations were reported between prenatal PFOA or PFOS exposure and estradiol or testosterone in 20-year-old Danish women (Kristensen et al. 2013). In addition, an inverse association between PFOS and estradiol levels was reported among perimenopausal and menopausal women in the Mid-Ohio Valley (Knox et al. 2011). However, an explanation for the pattern found by Knox et al. (2011) may be reverse causality through menopause, leading to lower excretion rates (via menstrual blood loss) and thus higher PFAS concentrations in serum (Konkel 2014; Taylor et al. 2014). Other hormones and proteins involved in sexual maturation have also been studied in the previous studies. SHBG levels were inversely associated with PFOA in girls 12–17 years of age from the Taiwanese cohort (Tsai et al. 2015), but such significant association was not observed in 15-year-old girls from the UK cohort (Maisonet et al. 2015). Also, no statistically significant associations were found between PFAS and LH or FSH in adolescent girls (Kristensen et al. 2013; Tsai et al. 2015).
In addition to sex hormones, insulin-like growth factor-1 (IGF-1) also plays important roles in growth and sexual maturation. Dynamic interactions taking place among growth hormone (GH), IGF-1, and sex hormones increase muscle mass, affect the mineralization of the skeleton, and control growth and metabolic changes during the prepubertal and pubertal periods (Garnett et al. 2004; Le Roith and Butler 1999; Mauras 2001). IGF-1 stimulates testosterone synthesis and maintains normal fertility in males (Wan et al. 2011). It is also involved in the development and maturation of the pubertal mammary gland in females (Kleinberg and Ruan 2008), and has been shown to predict the age of menarche (Thankamony et al. 2012). In addition, IGF-1 levels in childhood and adolescence are predictive of IGF-1 levels in adulthood (Sandhu et al. 2006), and lower levels of IGF-1 in adults have been associated with diabetes, cardiovascular diseases, and an increased risk of mortality (Burgers et al. 2011; Ceda et al. 2005; Vaessen et al. 2001). Well-known factors influencing IGF-1 levels are diet and lifestyle (Baibas et al. 2003; Berg and Bang 2004; Savage 2013), but exposure to endocrine-disrupting chemicals may also alter the normal synthesis and/or secretion of IGF-1 (Zumbado et al. 2010). However, to our knowledge, no data on the association between IGF-1 and PFAS in humans have been published to date.
In this study we aimed to assess the association between serum concentrations of four PFAS (PFHxS, PFOA, PFOS, and PFNA) and total testosterone, estradiol, and IGF-1 in 6- to 9-year-old children from the Mid-Ohio Valley, USA.
Material and Methods
Study Participants
Data on 69,030 people were collected in the “C8 Health Project” between August 2005 and July 2006. The details of the study, participants, enrollment criteria, and consent procedures are described elsewhere (Frisbee et al. 2009). PFOA had been used in the manufacture of fluoropolymers at a chemical plant in Parkersburg, West Virginia, since 1951 and led to contamination of local drinking water with PFOA. Individuals were eligible to participate in the C8 Health Project if they had consumed water for at least 1 year between 1950 and 2004 from the six contaminated water districts or private wells in the proximity of the chemical plant.
For this study, we focused on the subset of children 6–9 years of age at enrollment. We set a minimum of 6 years because the proportion of children with hormone levels below the detection limit (LOD) rose sharply below age 6 years, and a maximum of 9 years was established to largely avoid including children who had started their pubertal development. Four boys had entered puberty using the criterion of testosterone levels > 50 ng/dL as a marker (Lopez-Espinosa et al. 2011), and 13 girls reported menarche by the time of the survey. These 17 children (0.7%) were excluded. Of the remaining children, 96% had measures of PFAS, hormones, and/or IGF-1, resulting in a final sample size of 2,292. Parents or guardians provided written informed consent on behalf of the children participating in the present study. The London School of Hygiene and Tropical Medicine Ethics Committee approved this study.
Determination of PFAS
Laboratory analyses of serum PFAS were conducted by a commercial laboratory (Exygen Research). Samples collected at survey were analyzed for 10 PFAS including PFHxS, PFOA, PFOS, and PFNA. The analytical methods and quality control procedures employed by the laboratory have been described elsewhere (Flaherty et al. 2005; Frisbee et al. 2009). Briefly, the technique used solid-phase extraction followed by reverse-phase high-performance liquid chromatography/mass spectrometry. Over a range of 0.5–40 ng/mL, the coefficient of variation (CV) for PFOA was generally < 10% for multiple samples measured in different batches. CVs for PFHxS, PFOS, and PFNA were similar to those for PFOA. The LOD was 0.5 ng/mL for all of them. A quality assurance program was carried out by analysis of duplicate samples at AXYS Analytical Service Ltd. Details on the interlaboratory reliability have been extensively reported by Frisbee et al. (2009).
Determination of Sex Hormones and IGF-1
Clinical laboratory tests were performed at an accredited clinical diagnostic laboratory (LabCorp). Estradiol and total testosterone levels were measured in serum samples using an electrochemiluminescence immunoassay (Roche Diagnostics) with LODs of 7 pg/mL and 10 ng/dL, respectively. Free testosterone was measured by a radioimmunoassay in serum (Coat-a-Count; Siemens Healthcare Diagnostics) with an LOD of 0.2 pg/mL. IGF-1 was measured in serum by an immunochemiluminometric assay (Siemens Healthcare Diagnostics) with an LOD of 25 ng/mL. Volumes used for analyses were: 35, 50, 50, and 20 μL for estradiol, total testosterone, free testosterone, and IGF-1, respectively. A total of 33.4% of the blood samples were drawn before 1200 hours, 43.6% between 1200 and 1600 hours, and 23.0% between 1700 and 1900 hours.
Covariates
Covariates selected a priori for the analyses included sex, race/ethnicity (non-Hispanic white and others), age (years), height (inches), weight (pounds), body mass index (BMI; kilograms per meter squared) transformed into a z-score based on the 2000 U.S. Centers for Disease Control and Prevention growth charts of height/weight/BMI-for-age (CDC 2010), household annual family income (classified in the original questionnaire as ≤ $20,000; $20,000–70,000; > $70,000; and not known), and the month and hour of sample collection.
Statistical Analyses
We present descriptive analyses for the three sex hormones and IGF-1, but free testosterone was excluded from the multivariate analyses due to the low proportion of samples above the LOD. Outcomes showed a non-normal distribution and were natural log transformed before inclusion in the models.
PFAS observations below the LOD (< 0.5% for all the PFAS) were replaced by the LOD divided by 2 for descriptive and statistical analyses. PFAS did not show a normal distribution either and were natural log transformed as well as categorized in quartiles for the statistical analyses. We used simple Pearson’s correlations to describe pairwise relationships between ln(PFAS).
We used interval regression for censored data to assess the relation between outcomes and ln(PFAS) or PFAS in quartiles. These models were constructed to provide maximum likelihood estimations of the coefficients in the presence of LODs in the dependent variable (Lubin et al. 2004). All models were adjusted for child’s age and sampling month (because of a trend in serum PFAS during the collection year as well as seasonal variations in levels of hormones and IGF-1). Total testosterone models were additionally adjusted for time of day of blood sampling due to diurnal variations in their levels. Race did not meet our operational definition of confounder because there was < 10% change in the PFAS coefficients when including or excluding it from the final regression models. Analyses were sex-stratified, but we also investigated differences by sex including the interaction of this variable with the contaminants in the joint models (i.e., models including both girls and boys).
For PFAS as continuous variables, we expressed results as percentage difference [with 95% confidence interval (CI)] in the outcomes associated with a sex-specific interquartile range (IQR) increment in PFAS concentrations, and the percentage was calculated as the complement of the exponentiated regression coefficient [100×(exp(β × IQR) – 1)]. For PFAS in quartiles, we expressed results as the percentage difference (95% CI) in outcome across quartiles of exposure (with the lowest as the reference). Tests for trend were conducted across quartiles using a variable with the median concentration of PFAS in each quartile added to models as a numerical regressor. Finally, we also fitted multi-pollutant models including all four ln(PFAS) in the same model.
We used the multivariate imputation procedure by chained equations (Horton and Kleinman 2007; van Buuren and Groothuis-Oudshoorn 2011) to impute missing values according to available information. Variables included in the procedure were eligible for inclusion based on prediction ability (correlation ≥ 0.05), their relation to the nonresponse (correlation ≥ 0.05), and the existence of at least 20% with known values within the subgroup of incomplete cases (van Buuren and Groothuis-Oudshoorn 2011), including the outcome and exposure variables, covariates, and other predictors not included in the main analyses, without interactions (see Table S1). We imputed 50 data sets (20 cycles for each) and assessed convergence by plotting parameters (mean and SD in each imputed data set) against iteration number. In addition, we checked imputations of missing values graphically, and compared them with observed data.
We performed sensitivity analyses with these imputed data. First, we considered the imputed variable household income as a possible confounder in our analyses although it did not meet our operational definition of confounder. Missing values for this variable (n = 344, 15%) were imputed according to available data for maternal education, current employment, and mother’s and child’s race (see Table S1). We also reran analyses after excluding individuals with missing household income data, and found no noteworthy changes in the results (data not shown). Second, the main analyses were not adjusted for height or BMI because these variables may be affected by outcomes. However, people who are larger at a given age might have higher IGF-1 levels, and there is some concern that growth (and increased body volume) resulting from higher IGF-1 levels might cause PFAS concentrations to decrease. The same process could occur with sex hormones, because they also influence growth. Therefore, to assess the possibility of reverse causality or confounding by growth, we also carried out sensitivity analyses by additionally adjusting for the imputed height or BMI variable (in quartiles according to sex). Missing values for BMI (n = 366) and height (n = 348) were imputed according to available data for child sex, frequency of exercise per week of the children, and anthropometric measures of the children and mothers (see Table S1). No remarkable changes in results were found when restricting analyses to individuals with available data for these two variables (data not shown).
In all the analyses, we excluded highly influential observations detected by examination of graphical representation of dfbetas (range, 0–3 observations excluded). We used the statistical software package STATA for all statistical analyses, and specifically the “intreg” command for interval (censored) regression (STATA Statistical Software, Release 12), and the statistical software R.3.1.1 (R Core Team 2014) for multiple imputation (mice package (van Buuren and Groothuis-Oudshoorn 2011). Where associations are referred to as statistically significant, this implies a p < 0.05.
Results
Table 1 shows the characteristics of the study participants (n = 2,292) and Table 2 the summary figures for sex steroid hormones, IGF-1, and PFAS. A total of 96% of the children were white, the annual household family income was < $20,000 in 34% of them, and 51% were boys (Table 1). Levels of sex hormones and IGF-1 were slightly higher in girls than in boys. The proportion of observations in boys and girls above the LODs were 73% and 80% for estradiol, 69% and 75% for total testosterone, 19% and 28% for free testosterone, and 99.9% and 100% for IGF-1 (Table 2). Median concentrations of PFHxS, PFOA, PFOS, and PFNA in boys were 8, 35, 22, 1.7 ng/mL, and in girls they were 7, 30, 21, 1.7 ng/mL, respectively (Table 2). In boys and girls, correlations between the four PFAS were low (range of r = –0.08 and 0.33), except for PFOS versus PFHxS (r = 0.56 and 0.61 in boys and girls) (see Table S2).
Table 1.
C8 Health Study Participants (n = 2,292), Mid-Ohio Valley (USA), 2005–2006.
| Variable | Boys (n = 1,169) | Girls (n = 1,123) |
|---|---|---|
| Race/ethnicity (white) | 1,120 (95.8) | 1,075 (95.7) |
| Household income (< $20,000) | 334 (33.9) | 336 (34.9) |
| Age (years) | 8.3 (7.3, 9.3) | 8.4 (7.3, 9.3) |
| Body mass index (kg/m2) | 17.9 (15.6, 20.8) | 17.4 (15.3, 20.5) |
| Height (inches) | 51.0 (48.0, 54.0) | 50.0 (48.0, 53.5) |
| Values are n (%) or median (interquartile range). The n (%) of missing values in boys and girls was as follows: household annual family income: 184 (16%) and 160 (14%); body mass index: 179 (15%), and 187 (17%); and height: 168 (14%) and 180 (16%). Missing values were not considered for descriptive analyses. | ||
Table 2.
Levels of sex hormones, IGF-1 and PFAS in children 6–9 years years of age (n = 2,292), Mid-Ohio Valley (USA), 2005–2006.
| Hormone, IGF-1, and PFAS | LOD | Boys (n = 1,169) | Girls (n = 1,123) | ||
|---|---|---|---|---|---|
| n > LOD (%) | Median (IQR) | n > LOD (%) | Median (IQR) | ||
| Estradiol (pg/mL) | 7 | 846 (72.6) | 10 (< LOD, 15) | 896 (80.0) | 12 (LOD, 17) |
| Total testosterone (ng/dL) | 10 | 809 (69.2) | 13 (< LOD, 18) | 840 (74.8) | 15 (< LOD, 21) |
| Free testosterone (pg/mL) | 0.2 | 216 (19.0) | < LOD (< LOD, 15) | 313 (28.0) | < LOD (< LOD, 17) |
| IGF-1 (ng/mL) | 25 | 1,159 (99.9) | 147 (116, 187) | 1,112 (100) | 185 (142, 234) |
| PFHxS (ng/mL) | 0.5 | 1,166 (99.7) | 8.1 (4.2, 17.1) | 1,120 (99.7) | 7.0 (3.8, 13.8) |
| PFOA (ng/mL) | 0.5 | 1,169 (100) | 34.8 (15.3, 82.2) | 1,123 (100) | 30.1 (13.5, 74.0) |
| PFOS (ng/mL) | 0.5 | 1,166 (99.7) | 22.4 (16.5, 32.0) | 1,123 (100) | 20.9 (15.3, 29.4) |
| PFNA (ng/mL) | 0.5 | 1,162 (99.4) | 1.7 (1.3, 2.3) | 1,119 (99.6) | 1.7 (1.3, 2.4) |
| Abbreviations: IGF-1, insulin-like growth factor-1; IQR, interquartile range; LOD, detection limit; PFAS, perfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate. The n (%) of missing values in boys and girls was as follows: estradiol: 3 (0.26%) and 3 (0.27%); total testosterone: 0 (0%) and 0 (0%); free testosterone: 5 (0.43%) and 5 (0.45%); and IGF-1: 9 (0.77%) and 11 (0.98%). Missing values were not considered for descriptive analyses. | |||||
Associations between PFAS and outcomes in children are shown in Table 3 and Figures 1–2 (see also Tables S3–S6). Any difference in effect by sex was assessed through interaction terms between sex and the contaminants in joint models (including boys and girls). The interaction with sex was not statistically significant except for PFOS and estradiol (p = 0.048) (Table 3). In the stratified models for boys, significant inverse associations were observed for PFOA and total testosterone (–4.9%; 95% CI: –8.7, –0.8%), PFOS and estradiol (–4.0%; 95% CI: –7.7, –0.1%), PFOS and total testosterone (–5.8%; 95% CI: –9.4, –2.0%), PFOS and IGF-1 (–5.9%; 95% CI: –8.3, –3.3%), and PFNA and IGF-1 (–3.5%; 95% CI: –6.0, –1.0%) (Table 3 and Figure 1). The magnitudes of the estimates were similar after adjustment for other PFAS or after including height or BMI in the models (see Table S3). Associations between each PFAS (in quartiles) and outcomes in boys are shown in Figure 1 (see also Table S5) where the lowest quartile is the reference exposure category. The decrease across PFAS quartiles was most clearly monotonic in the case of PFOS and both total testosterone (p-trend across quartiles = 0.002) and IGF-1 (p-trend < 0.001) and less clearly for PFNA and IGF-1 (p-trend = 0.031) (see Table S5).
Table 3.
Difference in levels of sex hormones and IGF-1 in relation to PFAS concentrations in children 6–9 years of age (n = 2,292), Mid-Ohio Valley (USA), 2005–2006.
| PFAS | Stratified, boys (n = 1,169) [% difference (95% CI)]a | Stratified, girls (n = 1,123) [% difference (95% CI)]a | Joint p-interb |
|---|---|---|---|
| Association with ln(estradiol)c | |||
| PFHxS | –1.3 (–5.5, 3.1) | 2.1 (–2.2, 6.5) | 0.165 |
| PFOA | 4.3 (–0.4, 9.1) | 4.2 (–0.7, 9.4) | 0.924 |
| PFOS | –4.0 (–7.7, –0.1) | –0.3 (–4.6, 4.2) | 0.048 |
| PFNA | –2.5 (–6.2, 1.4) | –2.4 (–6.3, 1.7) | 0.622 |
| Association with ln(total testosterone)d | |||
| PFHxS | –2.7 (–6.4, 1.2) | 0.2 (–3.5, 4.0) | 0.252 |
| PFOA | –4.9 (–8.7, –0.8) | –2.5 (–6.7, 1.8) | 0.425 |
| PFOS | –5.8 (–9.4, –2.0) | –6.6 (–10.1, –2.8) | 0.531 |
| PFNA | –2.1 (–5.5, 1.3) | –1.9 (–5.5, 1.9) | 0.700 |
| Association with ln(IGF-1)c | |||
| PFHxS | –2.5 (–5.2, 0.3) | –2.1 (–4.8, 0.7) | 0.829 |
| PFOA | –0.4 (–3.4, 2.7) | –3.6 (–6.6, –0.5) | 0.057 |
| PFOS | –5.9 (–8.3, –3.3) | –5.6 (–8.2, –2.9) | 0.761 |
| PFNA | –3.5 (–6.0, –1.0) | –3.8 (–6.4, –1.2) | 0.829 |
| Abbreviations: CI, confidence interval; IGF-1, insulin-like growth factor-1; PFAS, perfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate. aPercent difference in outcome in relation to 75th vs. 25th percentile of ln(PFAS), derived from multiple linear regression model of outcome on ln(PFAS). The interquartile ranges in boys were 1.40, 1.68, 0.66, and 0.57 for ln(PFHxS), ln(PFOA), ln(PFOS), and ln(PFNA), respectively. Corresponding data in girls were 1.29, 1.70, 0.65, and 0.61. bp-Value from the interaction term between PFAS and sex in joint models.cModels adjusted for age and month of sampling. dModel adjusted for age, month, and time of sampling. | |||
Figure 1.
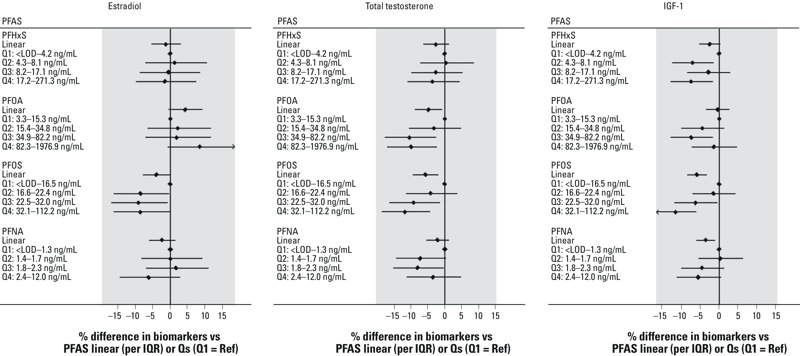
Difference in levels of sex hormones and IGF-1 in relation to PFAS (continuous or quartiles) in boys 6–9 years of age (n = 1,169), Mid-Ohio Valley (USA), 2005–2006. Abbreviations: IGF-1, insulin-like growth factor-1; PFAS, perfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; Q, quartile; Ref, reference. Models were adjusted for age and month of sampling. In addition, testosterone models were adjusted for time of sample collection. Estimates and 95% confidence intervals are presented. p-Trend was < 0.05 for PFOA or PFOS and total testosterone, and PFOS or PFNA and IGF-1.
Figure 2.
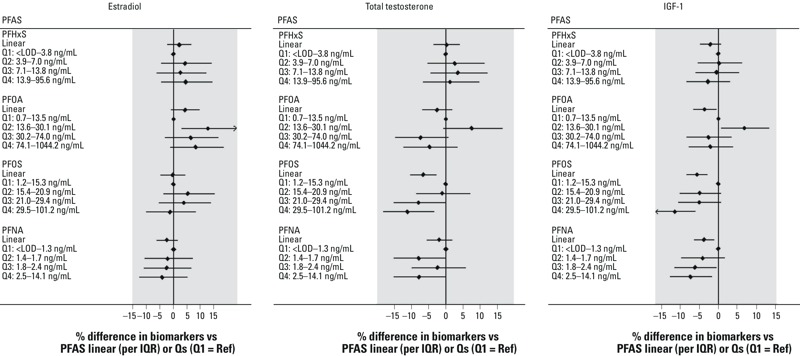
Difference in levels of sex hormones and IGF-1 in relation to PFAS (continuous or quartiles) in girls 6–9 years of age (n = 1,123), Mid-Ohio Valley (USA), 2005–2006. Abbreviations: IGF-1, insulin-like growth factor-1; PFAS, perfluoroalkyl substances; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; Q, quartile; Ref, reference. Models were adjusted for age and month of sampling. In addition, testosterone models were adjusted for time of sample collection. Estimates and 95% confidence intervals are presented. p-Trend was < 0.05 for PFOS and total testosterone or IGF-1, and PFNA and IGF-1.
In the stratified models for girls (Table 3 and Figure 2), significant inverse associations were found for PFOS and total testosterone (–6.6%; 95% CI: –10.1, –2.8%), PFOS and IGF-1 (–5.6%; 95% CI: –8.2, –2.9%), and PFNA and IGF-1 (–3.8%; 95% CI: –6.4, –1.2%). For PFOA, an association with IGF-1 was also found (–3.6%; 95% CI: –6.6, –0.5%) (Table 3), but this appeared to be an artifact of the raised mean IGF-1 in the second quartile (Figure 2; see also Table S6). Associations were similar after adjustment for other PFAS and results were also generally consistent when height or BMI was included (see Table S4). Across PFAS quartiles (Figure 2; see also Table S6), we found a more clearly monotonic decrease in total testosterone or IGF-1 in relation to concentrations of PFOS (p-trend across quartiles = 0.002 and < 0.001, respectively), and IGF-1 and PFNA (p-trend = 0.021).
Discussion
We found serum PFAS concentrations to be associated with lower levels of sex hormones and IGF-1 in children 6–9 years of age. Across sexes, PFOS exposure was found to be more clearly related to the outcomes in young children. Concerning outcomes, IGF-1 was the most consistently associated with PFAS exposures.
In boys 6–9 years of age, serum PFOS concentrations were associated with lower estradiol, total testosterone, and IGF-1 levels. PFOA was also inversely associated with total testosterone, and PFNA with IGF-1. A statistically significant delay in the age at which boys started pubertal development (defined by testosterone levels > 50 ng/dL) was found for PFOS but not PFOA in the 8- to 18-year group of the same population (Lopez-Espinosa et al. 2011), supporting the current findings. Although the age of hormone measurement is not the same, and comparisons ought to be made with caution, our results in young children are also consistent with inverse associations between PFOS (median, 7.79 ng/mL) and testosterone in Danish men with a mean age of 19.6 years (Joensen et al. 2013). In contrast, no significant associations between PFOS and these hormones were found in a study of Taiwanese adolescent boys 12–17 years of age (Tsai et al. 2015) or in adult men from different European countries (median range, 7.6–44.7 ng/mL) (Specht et al. 2012; Vested et al. 2013). Concerning PFOA, neither an epidemiological study in adolescent boys (Tsai et al. 2015) nor those conducted in adult men with background or with high occupational PFOA exposure (median range, 3.02 ng/mL–5.71 μg/mL) found statistically significant associations with estradiol or testosterone (Costa et al. 2009; Joensen et al. 2013; Raymer et al. 2012). PFHxS and PFNA have been more rarely studied (Joensen et al. 2013; Tsai et al. 2015), and only one study found an inverse association between PFNA (median, 1.07 ng/mL) and estradiol in young men (Joensen et al. 2013). PFOS and PFNA levels in young boys were also associated with IGF-1 in the present study, but, to our knowledge, there are no previous epidemiological studies with which to compare our results, and this observation should be investigated in other populations.
In girls 6–9 years of age, we found PFOS to be associated with lower total testosterone and IGF-1 levels. PFNA was also inversely associated with IGF-1. There was no significant association between any of the four PFAS that were studied and estradiol levels. In previous studies, testosterone was found to be inversely associated with PFOS among Taiwanese girls 12–17 years of age (Tsai et al. 2015). In contrast, prenatal concentrations of PFHxS, PFOA and PFOS (medians, 1.6, 3.6, and 19.2 ng/mL, respectively) were positively associated with testosterone in girls 15 years of age from the ALSPAC cohort (UK) (Maisonet et al. 2015). Neither estradiol nor testosterone measured in 20-year-old Danish women (Kristensen et al. 2013) was statistically significantly associated with in utero PFOA or PFOS concentrations (medians, 3.6 and 21.1 ng/mL, respectively). To our knowledge, the association between IGF-1 and PFOS or PFNA observed in the present study has not been reported previously in girls either and needs to be further investigated.
Levels of sex hormones and IGF-1 during the prepubertal period or around onset of puberty are associated with age at menarche (Apter and Vihko 1985; Thankamony et al. 2012). Therefore, our findings of a relatively modest decrease in levels of testosterone and IGF-1 in girls who had not reported their first period yet might be of concern, because later menarche attainment was associated with exposure to these contaminants in two previous investigations. A 5.3-month delay in the age of menarche between the lowest and highest tertile of prenatal PFOA exposure was reported in young Danish women (Kristensen et al. 2013). We also found a 4.3- and 4.6-month delay in the age of menarche when comparing the 1st and 4th quartiles of PFOA and PFOS concentrations in the 8- to 18-year-old girls of the C8 Health Project (Lopez-Espinosa et al. 2011). Our study was cross-sectional, so physiological changes, including menstrual blood loss, could have affected PFAS excretion and thus serum concentrations. In fact, a pharmacokinetic model (Wu et al. 2015) has recently indicated that growth dilution and excretion of the contaminants through menstruation may cause lower PFAS concentrations in serum. This might explain, in part, the association between higher PFAS concentrations and older age of menarche in our previous study (Lopez-Espinosa et al. 2011). Finally, a nested case–control study conducted in the ALSPAC cohort did not find a significant association between cord blood PFAS concentrations and the age of menarche (Christensen et al. 2011).
The possible biological mechanisms underlying the potential effects of PFAS on decreasing levels of sex hormones and IGF-1 are still not well understood, but several modes of action may be involved. Thus, some animal evidence exists on their possible effects on androgen synthesis. Concerning PFOA-exposed male rodents, a decrease in testosterone production was found to be due to the reduction in the conversion of 17α-hydroxyprogesterone to androstenedione (Cook et al. 1992) and to the inhibition of 3β- and 17β-hydroxysteroid dehydrogenase activities in Leydig cells (Zhao et al. 2010). For PFOS, the decrease in testosterone levels was attributed to a reduction in the expression levels of testicular receptors for FSH, LH, GH, IGF-1, and inhibin subunits as well as to the impairment of testicular steroidogenesis in PFOS-exposed male mice (Wan et al. 2011) and to the impairment of fetal Leydig cells in male fetus of prenatally PFOS exposed rats (Zhao et al. 2014). PFAS could also interfere with the androgen or estrogen pathway by disturbing the expression of genes associated with the metabolism of steroid hormones, as reported in liver tissue from PFOA-exposed mouse fetuses (Rosen et al. 2007) or decreasing the expression of genes related to the hypothalamic–pituitary–thyroid (HPT) axis, the growth hormone–insulin-like growth factor axis (GH–IGF), and the steroid hormone axis, as reported in salmon embryos and larvae exposed to PFOA and PFOS (Spachmo and Arukwe 2012).
The main strengths of the present study are the large sample size (over 2,000 participants) and the several individual potential confounders considered in our models. It is believed that these participants are representative, given the high participation rates in the C8 Health Project (77% in the 5–10 year age group) (Frisbee et al. 2009) among those children who drank contaminated water in the Mid-Ohio Valley, and this diminishes concern about potential selection biases. Notably, the strongest significant associations were found for PFOS, whose concentrations in the Mid-Ohio Valley participants were similar to the general U.S. population, assessed in the 2005–2006 National Health and Nutrition Examination Survey (NHANES) (Kato et al. 2011). Although NHANES information in children 6–9 years of age was not available, median concentrations of PFOS were 15 ng/mL in NHANES children 12–19 years of age versus 20 ng/mL in 2005–2006 C8 Health Project children 11–17 years of age (Lopez-Espinosa et al. 2012). However, patterns of human exposure to PFOS are changing worldwide as a consequence of changes in regulatory policies and PFOS exposure levels have decreased in the United States (Kato et al. 2011). Therefore, the levels reported in the present study may not be representative of current serum levels.
The 6–9 years age group was selected to investigate possible alterations in biomarkers involved in sexual maturation and growth due to PFAS exposure before or around the onset of puberty (i.e. entry to Tanner stage 2). We excluded boys who had started puberty (considered by the authors as testosterone levels > 50 ng/dL) and girls with menarche, but we were unable to differentiate children in different pubertal Tanner stages. We presume that some of our children might be in Tanner stage 2 or higher since according to a study (n = 2,070; collection period, 2005–2010), 26.1% (95% CI: 19.5, 33.6) and 8.1% (95% CI: 4.4, 13.4) of American non-Hispanic white boys had experienced the onset of genital and pubic hair development, respectively, at the age of 9 years (Herman-Giddens et al. 2012). In American non-Hispanic white girls (n = 420; collection period, 2004–2011), the median age of onset of breast development was 9.7 years (Biro et al. 2013). Our cross-sectional design has different limitations. First, we cannot rule out the possibility that growth (and associated IGF-1 increases) may actually cause a reduction in these PFAS by diluting them in a growing body or by affecting excretion rates. We consider this unlikely, because adding height or BMI (as markers of growth) to the models made no difference to the associations. Second, our findings suggest a possible alteration of sex hormones and IGF-1 due to PFAS exposure at 6–9 years of age, which is a period of high vulnerability to the possible effects of exposure to chemicals with endocrine properties, such as the PFAS. But apart from this period of life, there are also others, such as the fetal stage, in which the development of the hypthalamic–pituitary–gonadal (HPG), HPT, or GH-IGF axes, ovaries, and testicles could have been negatively affected at any level. Third, we had a single measure of the levels of these biomarkers, which are constantly changing in this period. In fact, their levels gradually increase from mid-childhood, rise sharply in puberty and decline steadily with age for IGF-1 (Pollak 2000), and continue to rise after puberty for sex hormones (Cole et al. 2015; Garnett et al. 2004). Therefore, further longitudinal studies need to be conducted to examine several periods of vulnerability. Another weakness of the present study is the lack of other markers of growth or pubertal development such as LH, FSH, or GH, which would have yielded more comprehensive information concerning the functioning of the HPG and GH-IGF axes. Finally, serum testosterone levels exhibit variable diurnal cyclicity (Ankarberg and Norjavaara 1999; Ankarberg-Lindgren and Norjavaara 2004), but the adjustment of models by the timing of sample collection enabled us to limit potential confounding from diurnal fluctuations.
Conclusions
Our findings show PFOS and PFNA concentrations to be associated with lower levels of IGF-1 in boys and girls 6–9 years of age. Our results also suggest an inverse association between PFAS and sex hormones with a decrease in testosterone in both boys and girls for PFOS and for PFOA in boys. PFOS was also inversely associated with estradiol only in boys. PFOS appears to be the most active of the four compounds studied in affecting sex hormones and IGF-1 in young children.
Supplemental Material
Acknowledgments
We thank the participants for their contributions to this study. We are also grateful for manuscript editing by A. Beierholm, and comments from B. Lasley and O. Costa.
Footnotes
This work (the “C8 Science Panel Community Study at LSHTM”) was supported by the C8 Class Action Settlement Agreement (Circuit Court of Wood County, WV) between DuPont and plaintiffs, which resulted from releases of perfluorooctanoate (PFOA, or C8) into drinking water. It is one of the C8 Science Panel Studies undertaken by the Court-approved C8 Science Panel established under the same Settlement Agreement. The task of the C8 Science Panel, of which T.F. is a member, is to undertake research in the Mid-Ohio Valley, and subsequently evaluate the results along with other available information to determine whether there are any probable links between PFOA and disease. Funds were administered by the Garden City Group (Melville, NY), which reports to the Court. M.-J. L.-E. holds grants from the Instituto de Salud Carlos III of Spain and the European Regional Development’s funds (FEDER) (Programa Miguel Servet-FEDER: MS11/0178 and FIS-FEDER: 14/00891).
The authors declare they have no actual or potential competing financial interests.
References
- Ankarberg C, Norjavaara E. Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: correlation with 17β-estradiol and dehydroepiandrosterone sulfate. J Clin Endocrinol Metab. 1999;84:975–984. doi: 10.1210/jcem.84.3.5524. [DOI] [PubMed] [Google Scholar]
- Ankarberg-Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 ml is a transition stage to puberty. Eur J Endocrinol. 2004;151:747–757. doi: 10.1530/eje.0.1510747. [DOI] [PubMed] [Google Scholar]
- Apter D, Vihko R. Premenarcheal endocrine changes in relation to age at menarche. Clin Endocrinol (Oxf) 1985;22:753–760. doi: 10.1111/j.1365-2265.1985.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Baibas N, Bamia C, Vassilopoulou E, Sdrolias J, Trichopoulou A, Trichopoulos D. Dietary and lifestyle factors in relation to plasma insulin-like growth factor I in a general population sample. Eur J Cancer Prev. 2003;12:229–234. doi: 10.1097/00008469-200306000-00010. [DOI] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. 2010. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect 118 222 228, doi: 10.1289/ehp.0901252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg U, Bang P. Exercise and circulating insulin-like growth factor I. Horm Res. 2004;62(suppl 1):50–58. doi: 10.1159/000080759. [DOI] [PubMed] [Google Scholar]
- Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132:1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers AM, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96:2912–2920. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Preventon) Epi Info™ 7. Version 3.5.2. 2010 Available: http://www.cdc.gov/epiinfo [accessed 12 March 2011]
- Ceda GP, Dall’Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, et al. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest. 2005;28(11 suppl proceedings):96–100. [PubMed] [Google Scholar]
- Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, et al. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ Int. 2011;37:129–135. doi: 10.1016/j.envint.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Ahmed ML, Preece MA, Hindmarsh P, Dunger DB. The relationship between Insulin-like Growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin Endocrinol (Oxf) 2015;82:862–869. doi: 10.1111/cen.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JC, Murray SM, Frame SR, Hurtt ME. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol. 1992;113:209–217. doi: 10.1016/0041-008x(92)90116-a. [DOI] [PubMed] [Google Scholar]
- Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med. 2009;51:364–372. doi: 10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- Flaherty JM, Connolly PD, Decker ER, Kennedy SM, Ellefson ME, Reagen WK, et al. Quantitative determination of perfluorooctanoic acid in serum and plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;819:329–338. doi: 10.1016/j.jchromb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks AP, Jr, Maher A, Flensborg P, Arnold S, Fletcher T, et al. 2009. The C8 Health Project: design, methods, and participants. Environ Health Perspect 117 1873 1882, doi: 10.1289/ehp.0800379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. Perfluorinated compounds—exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Garnett SP, Högler W, Blades B, Baur LA, Peat J, Lee J, et al. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr. 2004;80:966–972. doi: 10.1093/ajcn/80.4.966. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, et al. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 120 668 673, doi: 10.1289/ehp.1104034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130:e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- Horton NJ, Kleinman KP. Much ado about nothing: a comparison of missing data methods and software to fit incomplete data regression models. Am Stat. 2007;61:79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl. 2008;31:161–169. doi: 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, et al. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum Reprod. 2013;28:599–608. doi: 10.1093/humrep/des425. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population 1999–2008. Environ Sci Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Ruan W. IGF-I, GH, and sex steroid effects in normal mammary gland development. J Mammary Gland Biol Neoplasia. 2008;13:353–360. doi: 10.1007/s10911-008-9103-7. [DOI] [PubMed] [Google Scholar]
- Knox SS, Jackson T, Javins B, Frisbee SJ, Shankar A, Ducatman AM. Implications of early menopause in women exposed to perfluorocarbons. J Clin Endocrinol Metab. 2011;96:1747–1753. doi: 10.1210/jc.2010-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel L. 2014. PFCs and early menopause: association raises questions about causality. Environ Health Perspect 122 A59, doi: 10.1289/ehp.122-A59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, et al. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod. 2013;28:3337–3348. doi: 10.1093/humrep/det382. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Butler AA. Insulin-like growth factors in pediatric health and disease. J Clin Endocrinol Metab. 1999;84:4355–4361. doi: 10.1210/jcem.84.12.6208. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, et al. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol. 2011;45:8160–8166. doi: 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T. 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect 120 1036 1041, doi: 10.1289/ehp.1104370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112 1691 1696, doi: 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Calafat AM, Marcus M, Jaakkola JJ, Lashen H. 2015. Prenatal exposure to perfluoroalkyl acids and serum testosterone concentrations at 15 years of age in female ALSPAC study participants. Environ Health Perspect 123 1325 1330, doi: 10.1289/ehp.1408847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauras N. Growth hormone and sex steroids. Interactions in puberty. Endocrinol Metab Clin North Am. 2001;30:529–544. doi: 10.1016/s0889-8529(05)70200-0. [DOI] [PubMed] [Google Scholar]
- Mondal D, Lopez-Espinosa MJ, Armstrong B, Stein CR, Fletcher T. 2012. Relationships of perfluorooctanoate and perfluorooctane sulfonate serum concentrations between mother–child pairs in a population with perfluorooctanoate exposure from drinking water. Environ Health Perspect 120 752 757, doi: 10.1289/ehp.1104538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgil Damgaard I, Main KM, Toppari J, Skakkebæk NE. Impact of exposure to endocrine disrupters in utero and in childhood on adult reproduction. Best Pract Res Clin Endocrinol Metab. 2002;16:289–309. doi: 10.1053/beem.2002.0205. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med. 1998;40:614–622. doi: 10.1097/00043764-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36:1224–1228. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2014. R: A Language and Environment for Statistical Computing. Available: http://www.R-project.org/ [accessed 1 December 2014] [Google Scholar]
- Raymer JH, Michael LC, Studabaker WB, Olsen GW, Sloan CS, Wilcosky T, et al. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) and their associations with human semen quality measurements. Reprod Toxicol. 2012;33:419–427. doi: 10.1016/j.reprotox.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner R. Growing concern over perfluorinated chemicals. Environ Sci Technol. 2001;35(7):154A–160A. doi: 10.1021/es012317k. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Thibodeaux JR, Wood CR, Zehr RD, Schmid JE, Lau C. Gene expression profiling in the lung and liver of PFOA-exposed mouse fetuses. Toxicology. 2007;239:15–33. doi: 10.1016/j.tox.2007.06.095. [DOI] [PubMed] [Google Scholar]
- Sandhu J, Davey Smith G, Holly J, Cole TJ, Ben-Shlomo Y. Timing of puberty determines serum insulin-like growth factor-I in late adulthood. J Clin Endocrinol Metab. 2006;91:3150–3157. doi: 10.1210/jc.2005-2318. [DOI] [PubMed] [Google Scholar]
- Savage MO. Insulin-like growth factors, nutrition and growth. World Rev Nutr Diet. 2013;106:52–59. doi: 10.1159/000342577. [DOI] [PubMed] [Google Scholar]
- Schoeters G, Den Hond E, Dhooge W, van Larebeke N, Leijs M. Endocrine disruptors and abnormalities of pubertal development. Basic Clin Pharmacol Toxicol. 2008;102:168–175. doi: 10.1111/j.1742-7843.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Spachmo B, Arukwe A. Endocrine and developmental effects in Atlantic salmon (Salmo salar) exposed to perfluorooctane sulfonic or perfluorooctane carboxylic acids. Aquat Toxicol. 2012;108:112–124. doi: 10.1016/j.aquatox.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Specht IO, Hougaard KS, Spanò M, Bizzaro D, Manicardi GC, Lindh CH, et al. Sperm DNA integrity in relation to exposure to environmental perfluoroalkyl substances—a study of spouses of pregnant women in three geographical regions. Reprod Toxicol. 2012;33:577–583. doi: 10.1016/j.reprotox.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA, Daniels JL. 2014. Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES). Environ Health Perspect 122 145 150, doi: 10.1289/ehp.1306707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony A, Ong KK, Ahmed ML, Ness AR, Holly JM, Dunger DB. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J Clin Endocrinol Metab. 2012;97:E786–E790. doi: 10.1210/jc.2011-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MS, Lin CY, Lin CC, Chen MH, Hsu SH, Chien KL, et al. 2015. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int J Hyg Environ Health 218 437 443, doi: 10.1016/j.ijheh.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Vaessen N, Heutink P, Janssen JA, Witteman JC, Testers L, Hofman A, et al. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, et al. 2013. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect 121 453 458, doi: 10.1289/ehp.1205118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wong MH, Lee KF, Yeung WS, Giesy JP, et al. Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol Reprod. 2011;84:1016–1023. doi: 10.1095/biolreprod.110.089219. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) State of the Science of Endocrine Disrupting Chemicals – 2012. An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme and World Health Organization (Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, eds). 2013 Available: http://apps.who.int/iris/bitstream/10665/78101/1/9789241505031_eng.pdf?ua=1 [accessed 1 December 2014]
- Wu H, Yoon M, Verner MA, Xue J, Luo M, Andersen ME, et al. Can the observed association between serum perfluoroalkyl substances and delayed menarche be explained on the basis of puberty-related changes in physiology and pharmacokinetics? Environ Int. 2015;82:61–68. doi: 10.1016/j.envint.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Zhao B, Chu Y, Hardy DO, Li XK, Ge RS. Inhibition of 3β- and 17β-hydroxysteroid dehydrogenase activities in rat Leydig cells by perfluorooctane acid. J Steroid Biochem Mol Biol. 2010;118:13–17. doi: 10.1016/j.jsbmb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Liu J, Li H, Zhang C, Han P, et al. 2014. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS One 9 e78888, doi: 10.1371/journal.pone.0078888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbado M, Luzardo OP, Lara PC, Alvarez-León EE, Losada A, Apolinario R, et al. Insulin-like growth factor-I (IGF-I) serum concentrations in healthy children and adolescents: relationship to level of contamination by DDT-derivative pesticides. Growth Horm IGF Res. 2010;20:63–67. doi: 10.1016/j.ghir.2009.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


