Abstract
Staphylococcus aureus has developed many mechanisms to escape from human immune responses. To resist phagocytic clearance, S. aureus expresses a polysaccharide capsule, which effectively masks the bacterial surface and surface-associated proteins, such as opsonins, from recognition by phagocytic cells. Additionally, secretion of the extracellular fibrinogen binding protein (Efb) potently blocks phagocytic uptake of the pathogen. Efb creates a fibrinogen shield surrounding the bacteria by simultaneously binding complement C3b and fibrinogen at the bacterial surface. By means of neutrophil phagocytosis assays with fluorescently labelled encapsulated serotype 5 (CP5) and serotype 8 (CP8) strains we compare the immune-modulating function of these shielding mechanisms. The data indicate that, in highly encapsulated S. aureus strains, the polysaccharide capsule is able to prevent phagocytic uptake at plasma concentrations <10 %, but loses its protective ability at higher concentrations of plasma. Interestingly, Efb shows a strong inhibitory effect on both capsule-negative and encapsulated strains at all tested plasma concentrations. Furthermore, the results suggest that both shielding mechanisms can exist simultaneously and collaborate to provide optimal protection against phagocytosis at a broad range of plasma concentrations. As opsonizing antibodies will be shielded from recognition by either mechanism, incorporating both capsular polysaccharides and Efb in future vaccines could be of great importance.
Keywords: staphylococci, phagocytosis, capsule, fibrinogen
Introduction
Staphylococcus aureus is a major human pathogen responsible for many community- and hospital-acquired infections. Disease conditions may range from mild wound infections to more severe invasive illnesses such as endocarditis and bacteraemia (Lowy, 1998; Tong et al.‚ 2015).
The innate immune system is of high significance for the clearance of invading pathogens such as S. aureus (van Kessel et al.‚ 2014). Neutrophils, the predominant phagocytic cells of the innate immune system, rapidly engulf bacteria via phagocytosis and kill them intracellularly. Neutrophils recognize bacteria via specific receptors that are directed against bacterium-bound opsonins such as antibodies and complement components. The complement system is a complex proteolytic cascade of human plasma proteins that recognize surface-associated antibodies and specific bacterial surface structures (Gros et al.‚ 2008; Ricklin et al.‚ 2010). Activation of the cascade will result in deposition of several complement proteins at the bacterial surface. Complement component C3b is the major opsonin responsible for phagocytosis of bacteria by neutrophils and other phagocytic cells. Additionally, interaction of the Fc domain of bacterium-bound antibodies (IgG) with Fc receptors on the neutrophil contributes to effective phagocytosis.
To resist phagocytic clearance, S. aureus has evolved various immuno-modulatory mechanisms that frustrate the process of phagocytosis (Foster‚ 2005; Itoh et al.‚ 2010; Foster et al.‚ 2013; Stemerding et al.‚ 2013; Kang et al.‚ 2013). For instance, S. aureus produces several proteins that modulate binding of IgG to the bacterial surface (protein A and Sbi) or inhibit recognition of surface-bound IgG by Fc receptors (FLIPr). Also, S. aureus secretes multiple proteins that block activation of complement (e.g. SCIN, Ecb, Efb, Cna, SSL10). Furthermore, S. aureus has developed several ways to shield its surface from recognition by the host immune system. The first shielding mechanism is represented by the formation of a capsule, a polysaccharide structure surrounding the bacterial cell wall (O’Riordan & Lee‚ 2004). The two main serotypes produced by clinical S. aureus strains are the serotype consisting of capsular polysaccharide 5 (CP5) and capsular polysaccharide 8 (CP8), accounting for ~75 % of all clinical isolates, of which CP8 strains are the most prevalent (Sompolinsky et al.‚ 1985; Hochkeppel et al.‚ 1987; Albus et al.‚ 1988; Lee et al.‚ 1990). These capsules comprise trisaccharide repeating units of N-acetyl mannosaminuronic acid, N-acetyl l-fucosamine and N-acetyl d-fucosamine and are identical except for the glycosidic linkages between the sugars and the sites of O-acetylation (Jones‚ 2005). The CP5 and CP8 strains form non-mucoid colonies that are indistinguishable from colonies formed by unencapsulated strains. CP5 and CP8 are not only found among clinical isolates but are also expressed by commensal strains (Sompolinsky et al.‚ 1985; Albus et al.‚ 1988). The expression of CP5 or CP8 has been shown to enhance virulence and survival of S. aureus in vivo (Thakker et al.‚ 1998; Nilsson et al.‚ 1997; Watts et al.‚ 2005). Next to inhibition of phagocytic uptake, CP5 expression has been described to provide protection against intracellular killing of the bacterium (Nilsson et al.‚ 1997). However, S. aureus capsule expression (and therefore capsule size) is highly variable and depends on the presence or absence of certain environmental factors, such as CO2 (Herbert et al.‚ 2001). Therefore, capsule density and thus inhibition of phagocytosis are subject to the location of the bacterium in the body.
As a second shielding mechanism against phagocytosis, S. aureus secretes a protein that links specific plasma proteins to its surface. This extracellular fibrinogen binding protein (Efb) is a 16 kDa protein that binds to complement C3b on bacteria and simultaneously attracts fibrinogen to the surface. In doing so, Efb covers bacteria with a thick layer of fibrinogen that potently prevents recognition of surface-associated antibodies and C3b by phagocytic cells (Ko et al.‚ 2013).
Currently, it is not well understood why S. aureus evolved two separate mechanisms for shielding its surface from phagocytosis. In this study we further analyse the anti-phagocytic properties of both the capsule and Efb. Our findings indicate that these two shielding mechanisms can work in concert to enhance the resistance of S. aureus against phagocytosis.
Methods
Bacterial strains and fluorescence labelling.
In this study we used various wild-type S. aureus strains expressing different capsular polysaccharides: wild-type CP5-expressing strains include strain Reynolds (Jean Lee, Boston, MA, USA), COL (Andreas Peschel, Tübingen, Germany), USA100 (Jean Lee) and Newman (Jean Lee); wild-type CP8-expressing strains include Sanger 252 (Tim Foster, Dublin, Ireland), Becker and MN8 (Jean Lee). Capsule-negative strains included are USA300 (Frank Deleo, NIAID, Hamilton, MT, USA), 8325–4 (Tim Foster) and Wood 46 (ATCC-10832). ‘Isogenic capsule-negative mutants of strains Reynolds, Newman and MN 8 were created by deletion of the cap5 or cap8 as described (Watts et al.‚ 2005; Pohlmann-Dietze et al.‚ 2000). The CP8-negative mutant of strain Becker was created via transposon mutagenesis using Tn551.’ CP8-expressing strain Reynolds was generated by substitution of the cap5 region with the cap8 region (Watts et al.‚ 2005). Capsular serotypes were verified by flow cytometry analyses using specific CP5 and CP8 antisera (see below). Strains were fluorescently labelled by transformation with the pCM29 plasmid, constitutively expressing either GFP or mCherry under regulation of the sarA promoter as previously described (Pang et al.‚ 2010; Schenk & Laddaga‚ 1992). Alternatively, strains were fluorescently labelled with FITC (Sigma). To this end, bacteria were grown on Columbia agar (Oxoid) supplemented with 2 % (w/v) NaCl (CSA) for 24 h at 37 °C, suspended, washed and resuspended in PBS. FITC (0.5 mg ml−1 in DMSO) was added and incubated for 30 min on ice. Bacteria were washed twice and resuspended in RPMI containing 0.05 % human serum albumin (RPMI-HSA). All strains were grown on CSA for 24 h at 37 °C to guarantee optimal capsule expression (Thakker et al.‚ 1998; Pohlmann-Dietze et al.‚ 2000) and stored at −20 °C in RPMI-HSA before use.
Protein purification.
Recombinant Efb proteins were generated in E. coli as described previously (Ko et al.‚ 2011). Briefly, the efb gene from S. aureus strain Newman (Mal Horsburgh, Liverpool, UK) (without the signal peptide) was amplified by PCR and ligated into the pGEX-5x-1 vector (GE Healthcare) for N-terminal fusion with glutathione S-transferase (GST). Mutations of the fibrinogen and C3 binding domains were introduced in pGEX plasmids containing full-length GST-Efb as described previously (Ko et al.‚ 2011, 2013). The mutant EfbΔFg lacks both fibrinogen binding domains and was previously described as EfbΔFg1+2 (Ko et al.‚ 2013). EfbΔC3 has been altered in the C3d binding site and therefore lacks C3-binding ability. Recombinant proteins were expressed and purified according to the manufacturer’s manual (GE Healthcare).
Purification of human plasma and neutrophils.
To prepare plasma, blood was collected in 3 ml blood tubes (Roche) containing recombinant hirudin (15 µg ml−1) from four healthy volunteers. After centrifugation for 10 min at 2080 g plasma was collected, pooled and stored at −80 °C. For isolation of human neutrophils, blood from a healthy donor was collected in heparin vacutainers (BD) and cells were isolated using the Ficoll-Histopaque gradient method (Bestebroer et al.‚ 2007).
Capsule visualization with transmission electron microscopy.
GFP-labelled S. aureus strains Reynolds (CP5) and its isogenic CP-negative mutant (5×107 ml−1) were incubated with rabbit CP5 antiserum (Watts et al.‚ 2005, 1 : 100) in PBS-0.5 % BSA for 45 min at 4 °C and washed twice with PBS-0.5 % BSA. Subsequently, bacteria were adsorbed to 100 mesh hexagonal Formvar-carbon-coated copper grids (Stork-Veco). Samples were contrasted with 0.4 % uranyl acetate (pH 4.0) and 1.8 % methylcellulose (Slot & Geuze‚ 2007) and analysed in a Tecnai 12 transmission electron microscope (FEI) at 80 kV.
Capsule quantification by flow cytometry.
GFP- or FITC-labelled S. aureus strains (5×107 ml−1) were incubated with rabbit CP5 and CP8 antiserum (Watts et al.‚ 2005, 1 : 100) in PBS-0.5 % BSA for 45 min at 4 °C and washed twice with PBS-0.5 % BSA. Bacteria were incubated with Alexa647-conjugated Protein A (1 : 1000, Molecular Probes) and, after another washing step, fixed with formaldehyde (1 %) before flow cytometry measurement with a FACS Verse device (BD).
Phagocytosis assays.
All phagocytosis assays were performed in Falcon tubes (Corning). Freshly isolated human neutrophils (5×106 ml−1) were stained with Vybrant DiD cell-labelling solution (1 : 1000, Molecular Probes), and washed three times with and resuspended in RPMI-HSA before use. GFP- or FITC-labelled S. aureus (5×107 ml−1) were pre-incubated with human plasma in the presence or absence of Efb (0.5 µM) for 2 min at 37 °C. DiD-stained neutrophils (5×106 ml−1) were added and phagocytosis was allowed for 15 min at 37 °C, with shaking (600 r.p.m.). Cold formaldehyde (1 %) in RPMI-HSA was added to stop the reaction and samples were analysed by flow cytometry measurement of the fluorescence of the neutrophils.
Confocal microscopy.
S. aureus strains Reynolds (CP5 and CP−) (mCherry-labelled, 1×108 ml−1) were pre-incubated with human plasma (3 %) for 30 min at 37 °C in Veronal buffer containing 5 mM CaCl2 and 2.5 mM MgCl2 (VBS++) to deposit C3b on the bacterial surface. After a washing step with VBS++−0.5 % BSA, bacteria were incubated with Efb or Efb mutants (0.5 µM) for 1 h at 37 °C, with shaking (600 r.p.m.). Following another washing step, a 1 h incubation with Alexa-488-conjugated fibrinogen (60 µg ml−1, Invitrogen) at 37 °C shaking was performed, after which bacteria were fixed with formaldehyde (1 %). For visualization by confocal microscopy, samples were transferred onto poly-l-lysine-coated cover slips (0.45 µm; 12 mm diameter; Becton Dickinson) or, as a control, samples were analysed by flow cytometry. Confocal images were acquired using a Leica TCS SP5 inverted microscope equipped with HCX PL APO CS 63×/1.40–0.60 OIL objective (Leica Microsystems).
Results
Fluorescence labelling and capsule expression of S. aureus strains
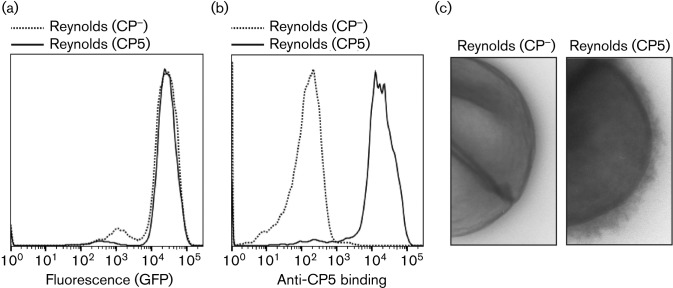
To compare the anti-phagocytic effects of the staphylococcal polysaccharide capsule with the Efb shield, we first performed in vitro neutrophil phagocytosis assays with different encapsulated S. aureus strains that show varying degrees of capsule expression (Fig. S1, available in the online Suplementary Material). Since the Reynolds strain expresses a thick CP5 capsule (Fig. S1; Thakker et al.‚ 1998; Watts et al.‚ 2005), we initially focused on this strain in our phagocytosis experiments and used its isogenic mutant (Reynolds CP−) as a capsule-negative strain. Both strains were fluorescently labelled by transformation with a pCM29-GFP plasmid that allows for intracellular production of GFP under a constitutive promoter (Pang et al.‚ 2010). Strains were grown on CSA for 24 h at 37 °C to guarantee that capsule expression was optimal (Thakker et al.‚ 1998). The fluorescence of the strains was measured by flow cytometry (Fig. 1a), which confirmed that GFP was properly expressed and that both strains were equally fluorescent. Furthermore, we confirmed expression of the polysaccharide capsule after fluorescence labelling by specific staining with a polyclonal antibody directed against CP5 (Fig. 1b). Finally, using transmission electron microscopy, we visualized the polysaccharide capsule of the GFP-labelled Reynolds (CP5) strain (Fig. 1c).
Fig. 1.
(a) Flow cytometry histogram showing the fluorescence of S. aureus strain Reynolds (CP5) and its isogenic capsule-negative mutant (CP−) after transformation with pCM29-GFP plasmid. (b) Flow cytometry histogram showing binding of rabbit anti-CP5 antibodies to GFP-labelled Reynolds (CP5) and isogenic mutant (CP−), detected with Alexa647-conjugated protein A. (c) Transmission electron micrographs showing the GFP-labelled Reynolds (CP5) and mutant (CP−) strain. Strains were pretreated with anti-CP5 antibodies to enhance stability and electron density of the capsule. Representative images are shown.
The polysaccharide capsule and Efb together protect against phagocytosis at a broad range of plasma concentrations
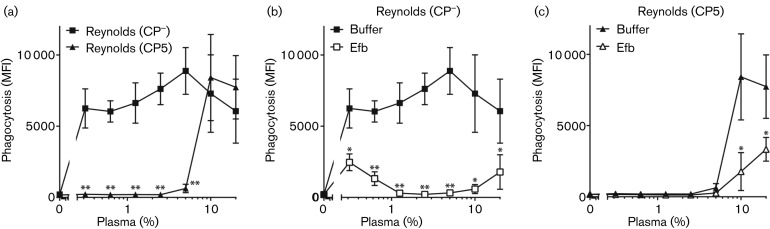
After confirming expression levels of both fluorescence and polysaccharide capsule, the strains were analysed in phagocytosis assays. We incubated the GFP-labelled S. aureus Reynolds (CP5) and mutant (CP−) strain with normal human plasma (as a source for antibodies, complement and fibrinogen) and freshly isolated human neutrophils. Phagocytosis of fluorescent bacteria by neutrophils was quantified using flow cytometry. As expected, we observed that the unencapsulated S. aureus strain was efficiently phagocytosed (Fig. 2a). As described previously (Cunnion et al.‚ 2003; Thakker et al.‚ 1998), the polysaccharide capsule of the Reynolds (CP5) strain potently blocked neutrophil phagocytosis at low plasma concentrations (Fig. 2a). However, at plasma concentrations ≥10 %, we observed little to no difference between the CP5-expressing Reynolds strain and its isogenic capsule-negative mutant. This suggests that CP5 does not protect against phagocytosis at higher plasma concentrations. As we previously observed that Efb prevents phagocytosis of S. aureus in plasma by shielding the bacterial surface with fibrinogen (Ko et al.‚ 2013), we wondered whether addition of Efb could also affect phagocytosis of encapsulated S. aureus strains. First, we found that purified GST-tagged Efb (0.5 µM) significantly blocked phagocytosis of the capsule-negative Reynolds (CP−) strain at all tested plasma concentrations (Fig. 2b). When the encapsulated strain Reynolds (CP5) was used, Efb also had an inhibitory effect on bacterial uptake at higher plasma concentrations, where the polysaccharide capsule itself is no longer protective (Fig. 2c). As a control, we showed that the GST-tag alone or GST-tagged Efb-N (the N-terminal domain of Efb) did not reduce phagocytic uptake (data not shown). These results suggest that the polysaccharide capsule and the Efb-dependent fibrinogen shield collaborate to fully protect S. aureus at an extensive range of plasma concentrations. Together, our findings indicate that these two anti-phagocytic mechanisms collaborate to fully protect S. aureus at an extensive range of plasma concentrations.
Fig. 2.
Phagocytosis of GFP-labelled Reynolds (CP− and CP5) by purified human neutrophils in the presence of human plasma alone (a) or plasma with 0.5 µM Efb (b, c), measured by fluorescence (geomean) of the neutrophils. (a) The polysaccharide capsule of S. aureus provides protection against phagocytosis at low plasma concentrations. (b) Addition of exogenous Efb inhibits phagocytic uptake of unencapsulated strain Reynolds (CP−) at all tested plasma concentrations. (c) Inhibition of phagocytosis of encapsulated strain Reynolds (CP5) is enhanced by addition of Efb. Graphs represent mean ± sd of three separate experiments. *P<0.05, **P<0.005 for Reynolds (CP5) versus Reynolds (CP−) or Efb versus buffer (two-tailed Student’s t-test). MFI, mean fluorescence intensity.
The Efb-dependent fibrinogen shield provides protection against phagocytosis on various encapsulated and capsule-negative S. aureus strains
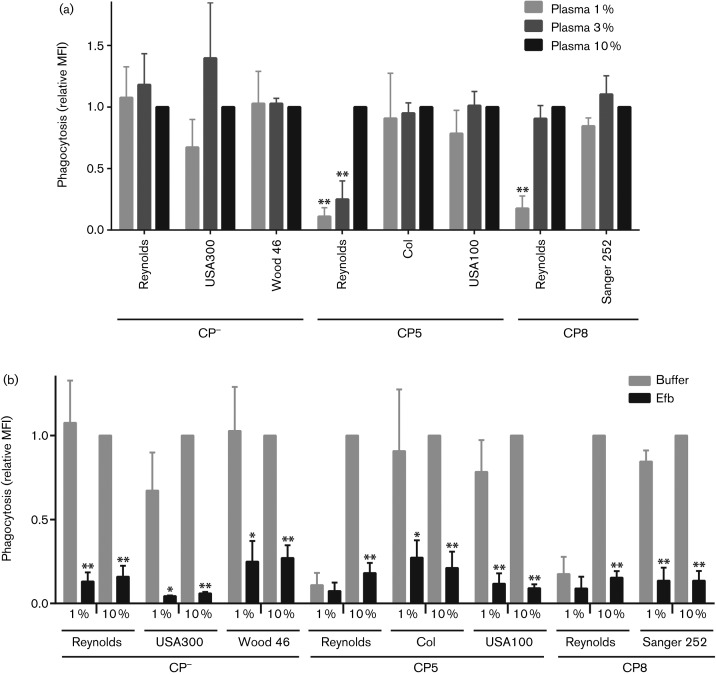
Since we used the highly encapsulated Reynolds strain in these experiments, we wondered whether the presence of different plasma concentrations also influences phagocytosis inhibition by other capsule-expressing S. aureus strains. We therefore performed phagocytosis assays in 1, 3 and 10 % plasma using either unencapsulated S. aureus strains (USA300 and Wood 46), CP5-expressing strains (Col and USA100) and CP8-expressing strains (Sanger 252 and Reynolds CP8, the latter an isogenic mutant of strain Reynolds in which the cap5 region was substituted with cap8). All strains were labelled with GFP and grown on CSA to ensure optimal capsule expression (Fig. S1). Since absolute fluorescence levels varied between strains, we expressed phagocytic uptake of each strain (Fig. 3a) as a relative value compared with the mean fluorescence intensity at 10 % plasma, at which phagocytosis had reached its maximum. Although this prohibits direct comparison between strains, this still allows us to analyse the effect of different plasma concentrations on phagocytosis efficiencies of each strain. Similar to the capsule-negative Reynolds strain, none of the other capsule-negative strains (USA300, 8325-4, Wood 46) showed a significant difference in phagocytic uptake at lower concentrations of plasma, compared with 10 % plasma (Fig. 3a). Notably, the other CP5- and CP8-expressing strains (COL, USA100, Sanger 252) did not show a substantial decrease in phagocytosis at the lower plasma concentrations. Only the isogenic Reynolds CP8 mutant showed a reduction in phagocytosis at 1 % plasma concentration. These experiments suggest that the anti-phagocytic effect of the S. aureus capsule depends both on the expression level of the capsule and on the plasma concentration. Next, we tested the anti-phagocytic effect of Efb on the other GFP-labelled CP5- and CP8-expressing strains. We observed that addition of Efb significantly reduced phagocytic uptake of all tested CP−, CP5 and CP8 strains at both 1 and 10 % plasma (Fig. 3b). Since phagocytosis of strain Reynolds (CP5 and CP8) was already considerably reduced at 1 % plasma, an additional significant decrease in phagocytic uptake in the presence of Efb was not measured. This shows that the Efb-dependent fibrinogen shield can be created and function properly on capsule-negative as well as capsule-expressing S. aureus strains.
Fig. 3.
(a) Phagocytosis of different GFP-labelled CP−-, CP5- and CP8-expressing S. aureus strains in the presence of 1, 3 or 10 % human plasma. Phagocytosis is displayed as the relative fluorescence compared with the 10 % plasma condition of each strain. Graph represents mean ± sd of three separate experiments. *P<0.05, **P<0.005 for 1 or 3 % plasma versus 10 % plasma of the same strain (two-tailed Student’s t-test). (b) Addition of Efb inhibits phagocytic uptake of different CP5 and CP8 encapsulated S. aureus strains at both 1 and 10 % human plasma. This was displayed by the relative fluorescence (geomean; compared with the buffer condition at 10 % plasma of the same strain) of the neutrophils. Graph represents mean ± sd of three separate experiments. *P<0.05, **P<0.005 for Efb versus buffer of the same strain (two-tailed Student’s t-test). MFI, mean fluorescence intensity.
Interplay between Efb and capsule on other isogenic mutants of CP5 and CP8
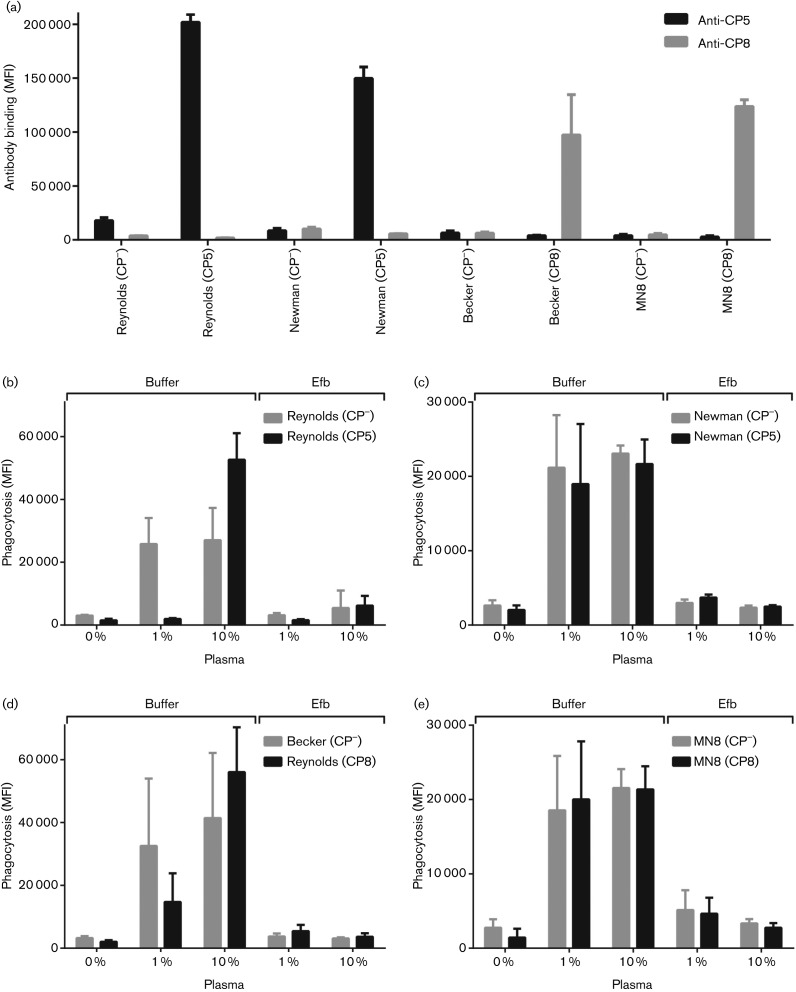
The phagocytosis experiment in Fig. 3(b) suggests that capsule-mediated inhibition in our assay system is only detectable for strain Reynolds, but not for other S. aureus strains. However, the exact contribution of the capsule in this experiment could not be studied due to the lack of isogenic capsule-negative mutants. Therefore, we decided to include three different capsule-expressing S. aureus strains [Newman (CP5), Becker (CP8) and MN8 (CP8)] in which the cap5 and cap8 loci are deleted (Watts et al.‚ 2005). These strains were fluorescently labelled and capsule expression was determined using specific CP5 and CP8 antibodies (Fig. 4a). When phagocytosis was analysed, results with FITC-labelled strain Reynolds (CP5 and CP−) were comparable with previous assays; at 1 % plasma the encapsulated strain showed a decrease in phagocytic uptake compared with the capsule-negative strain but at 10 % plasma this inhibitory effect was not present (Fig. 4b). Strikingly, the polysaccharide capsule of strain Newman, showing 74 % CP5 expression compared with Reynolds, was not able to block phagocytosis at 1 and 10 % plasma (Fig. 4c). Also, the capsules of strain Becker and MN8 showed no significant inhibition of phagocytosis (Fig. 4d, e). As anticipated, addition of Efb blocked the phagocytic uptake of all tested strains, regardless of their capsule expression (Fig. 4b–e). Again, this shows that the Efb-dependent fibrinogen shield can be created and function properly on capsule-negative as well as capsule-expressing S. aureus strains.
Fig. 4.
Interplay between Efb and capsule on other isogenic mutants of CP5 and CP8. (a) Binding of rabbit CP5 and CP8 antibodies to different FITC-labelled CP5- and CP8-expressing S. aureus strains. (b–e) Phagocytosis of different FITC-labelled CP5- and CP8-expressing S. aureus strains by purified neutrophils in the presence of 1 or 10 % human plasma and 0.5 µM Efb. (b) Phagocytosis of strain Reynolds (CP5) and Reynolds (CP−). (c) Phagocytosis of CP5-expressing strain Newman and its isogenic mutant Newman (CP−) (buffer vs Efb: P<0.05 at 1 and 10 % plasma). (d) Phagocytosis of CP8-expressing strain Becker and its isogenic mutant Becker (CP−) (buffer vs Efb: n.s. at 1 % plasma, P<0.05 at 10 % plasma). (e) Phagocytosis of CP8-expressing strain MN8 and its isogenic mutant MN8 (CP−) (buffer vs Efb: P<0.05 at 1 and 10 % plasma). Graphs represent mean ± sd of three separate experiments. At 10 % plasma, the inhibitory effect of Efb was statiscally significant for all strains, but at 1 % plasma was significant only for strains Newman and MN8. MFI, mean fluorescence intensity.
Efb attracts fibrinogen to the surface of both CP− and CP5 strains
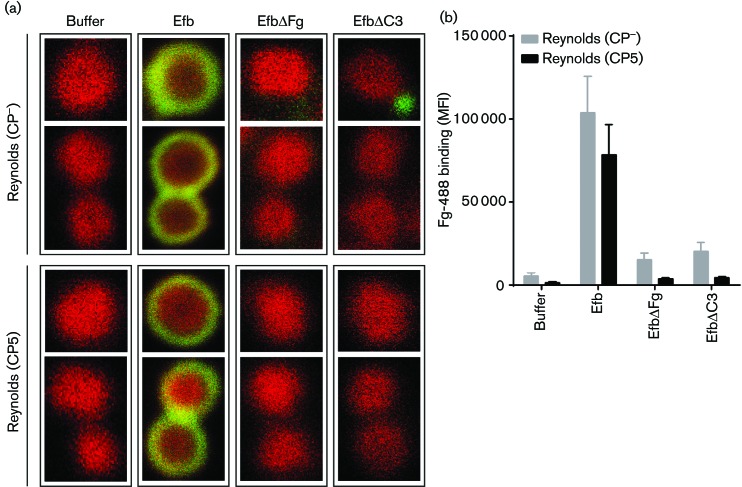
To confirm that Efb can indeed create a fibrinogen shield around the surface of encapsulated strains, we visualized this shield by confocal microscopy. We pre-opsonized mCherry-labelled Reynolds (CP− and CP5) with 3 % plasma and incubated them with Alexa488-labelled fibrinogen in the presence of Efb (0.5 µM). As a control, we included two Efb mutant proteins that cannot form this shield due to the lack of fibrinogen (EfbΔFg) or C3b (EfbΔC3) binding motifs. First, we observed that bacteria incubated without the addition of Efb did not show binding of fibrinogen to the bacterial surface, which was to be expected as washed bacteria were used and therefore no endogenously produced Efb was present (Fig. 5a). In the presence of Efb, both the CP− and the CP5 strain were completely surrounded by a layer of fluorescent fibrinogen. This fibrinogen layer was not present when bacteria were incubated with the Efb mutant proteins. These results were confirmed by flow cytometry analyses of the samples used for confocal microscopy, showing a considerable increase of fibrinogen binding in the presence of full-length Efb (Fig. 5b). Remarkably, no significant difference was observed between the CP− and CP5 strain incubated with full-length Efb with both confocal microscopy and flow cytometry. This suggests that formation of the Efb-dependent fibrinogen shield is equally efficient on encapsulated and capsule-negative strains.
Fig. 5.
(a) Confocal images of the binding of Alexa488-labelled fibrinogen to mCherry-labeled Reynolds (CP− and CP5) strains, pre-opsonized with human serum (3 %), in the presence of Efb variants (0.5 µM). Representative images are shown. (b) Flow cytometry analyses of samples shown in (a). Graph represents mean ± sd of three separate experiments. **P<0.005 for Efb versus buffer, EfbΔFg or EfbΔC3 (two-tailed Student’s t-test). MFI, mean fluorescence intensity.
Discussion
S. aureus has evolved many ways to evade and manipulate immune responses in order to survive inside the human host (Foster‚ 2005). As phagocytic uptake of S. aureus by neutrophils is crucial for clearance of the pathogen, suppressing this process will be of great importance to its persistence in the body. The polysaccharide capsule expressed by S. aureus has been shown to potently block killing by human neutrophils by covering C3b attached to the bacterial surface (Thakker et al.‚ 1998; Watts et al.‚ 2005). In this study, we observe that the capsule of strain Reynolds (CP5 and CP8) can efficiently block phagocytosis at low concentrations of plasma but that it loses its protective capacity at higher plasma concentrations. Furthermore, we have previously shown that Efb forms a shield of fibrinogen and thereby protects bacteria from phagocytosis (Ko et al.‚ 2013). Now, we demonstrate that the Efb-dependent fibrinogen shield can also effectively be formed on several encapsulated strains at a broad range of plasma concentrations. This shows that these two mechanisms of shielding can collaborate to ensure optimal protection against phagocytosis.
The results presented here suggest that the polysaccharide capsule of strain Reynolds has strong anti-phagocytic properties at lower plasma concentrations. Whereas the polysaccharide capsule has previously been shown to prevent recognition of staphylococcal surface-associated proteins by neutrophil receptors, it does not completely block the binding of specific antibodies nor the deposition of complement components at the bacterial surface (Cunnion et al.‚ 2001; Wilkinson & Sisson‚ 1979; Watts et al.‚ 2005). Complement activation was triggered by encapsulated S. aureus, which resulted in rapid deposition of C3b. However, purified capsular polysaccharides are not immunogenic and did not trigger complement activation (Watts et al.‚ 2005; Nemeth & Lee‚ 1995). Hence, deposition of C3b on an encapsulated strains does occur but was described to be located beneath the polysaccharide capsule on the bacterial cell wall and is thereby shielded from its surroundings (Watts et al.‚ 2005). Because C3b is able to deposit on top of other C3b molecules (Kinoshita et al.‚ 1988), it is possible that in high concentrations of plasma, C3b molecules can accumulate and eventually be displayed above the capsule, no longer shielded from complement receptors. This may explain the lack of phagocytic resistance by the polysaccharide capsule at high plasma concentrations in this study. An alternative explanation would be that antibodies against the polysaccharide capsule are present in normal human plasma, although concentrations have been shown to be too low to mediate phagocytic uptake (Thakker et al.‚ 1998; Fattom et al.‚ 1993). However, we previously showed that specific capsular antibodies can potently neutralize the anti-phagocytic effect of the polysaccharide capsule, as these antibodies enhance phagocytosis of encapsulated strains at low plasma concentrations (Ko et al.‚ 2013). This could indicate that at high plasma concentration, levels of antibodies directed against capsular polysaccharides are sufficient to efficiently activate complement, and thus C3b deposition, on top of the capsule leading to phagocytic uptake or to directly mediate phagocytosis through recognition by Fc receptors. Nevertheless, not all capsule-expressing strains tested in this study showed similar shielding capacities. Inhibition was most potent for the Reynolds (CP5) strain that is known for its thick capsule. CP8 and CP5 strains have been described to differ in their virulence, explained by the suggestion that CP5 strains commonly express more capsular polysaccharides than CP8 strains (Watts et al.‚ 2005). However, our in vitro data will not predict capsule expression inside the body and it is therefore also possible that these strains do produce a dense capsule in vivo, which is effective against phagocytic uptake. Interestingly, it was reported that the highly virulent USA300 isolates, prevalent in North America, lack the expression of capsular polysaccharide (Boyle-Vavra et al.‚ 2015). Also here, we observe that a USA300 isolate does not show impaired phagocytosis. Possibly, these strains use other mechanisms to circumvent phagocytic killing. For instance, expression of Efb and clumping factor A (ClfA) have been shown to be upregulated in USA300 strain LAC and therefore the fibrinogen binding capacity of this strain is high (Cheung et al.‚ 2011). Also, greater production of molecules that directly lyse neutrophils, such as Panton Valentine leukocidin (PVL) and phenol soluble modulin (PSM), could compensate for the lack of capsule expression by USA300 isolates (Otto‚ 2013; Cheung et al.‚ 2011).
Furthermore, our results indicate that Efb most potently prevents phagocytosis of the capsule-negative strain at plasma concentrations between 1 and 10 %. For Efb to completely cover S. aureus with a shield of fibrinogen and thus fully block phagocytosis, it not only requires simultaneous binding to both C3b and fibrinogen but also, very importantly, sufficient levels of these two plasma proteins. This explains the reduced efficiency of the Efb-dependent fibrinogen shield at very low plasma concentrations, as the layer generated at these levels of complement and fibrinogen will not be dense enough to completely mask the bacterial surface. We now show that Efb is also able to establish strong inhibition of phagocytosis on highly encapsulated strains. Therefore, binding of Efb seems not to be affected by the presence of capsular polysaccharides, even those of the highly encapsulated Reynolds strain (CP5). As the two shielding mechanisms provide protection at both low and high plasma concentrations, this could suggest that S. aureus has the ability to shield itself from phagocytic uptake at different locations inside the host, from tissue to bloodstream. Although we do not provide direct evidence that these shielding mechanisms occur during an infection in vivo, we believe that the concentrations of Efb used are relevant. Previously, we quantified the secretion of Efb in S. aureus (strain Newman) culture supernatants and found production levels of ~1 µM (Ko et al.‚ 2013). Although strain Newman has higher expression levels of Efb than most S. aureus strains due to a point mutation in the SaeR/S regulatory system (Voyich et al.‚ 2009), these levels are still more than 10 times higher than the calculated IC50 (0.08 µM) needed for inhibition of phagocytosis. Additionally, we showed that endogenously produced Efb mediates complex formation on the bacterial surface, as WT supernatants can attract fibrinogen to the bacterial surface whereas Efb-deficient supernatants do not introduce shield formation (Ko et al.‚ 2013). Furthermore, studies of the effect of Efb on the virulence of S. aureus in vivo show that the protein is expressed at levels high enough to be effective (Ko et al.‚ 2013; Palma et al.‚ 1996; Shannon et al.‚ 2005). Together, the data presented in this paper indicate that the balance between bacteria, plasma components and infiltrating immune cells can influence the anti-phagocytic properties of pathogenic S. aureus. Although it is generally believed that whole blood mimics a relevant physiological condition for S. aureus infections, we know that most S. aureus infections occur at localized sites of the body where bacteria encounter different concentrations of plasma and immune cells than in human whole blood. Furthermore, during an infection, the inflammatory response will alter the plasma-to-immune cell ratio because of rapid influx of immune cells. For this reason, the bacterium may have evolved additional mechanisms to subvert phagocytosis at different concentrations of plasma and neutrophils. This allows the bacterium to subvert immune clearance from different sites of the body and during different stages of an infection.
S. aureus is rapidly becoming more resistant to antibiotics (Rossolini et al.‚ 2014) and new therapeutic strategies are being explored (Vuong et al.‚ 2015). Despite interesting developments in preclinical studies (Lattar et al.‚ 2014; Wacker et al.‚ 2014; Park et al.‚ 2014), an effective vaccine against S. aureus is still not available. Although clinical studies in humans indicate that opsonic antibodies are successfully produced upon vaccination with different S. aureus antigens (including capsular polysaccharides) (Nissen et al.‚ 2015; Levy et al.‚ 2015), such antibodies fail to protect humans against S. aureus infections (Fattom et al.‚ 2015; Fowler et al.‚ 2013). Possibly, the shielding mechanisms described in this study complicate the effector mechanism of opsonic antibodies. Therefore, the inclusion of both capsular antigens and Efb could be important in the development of a protective S. aureus vaccine.
Acknowledgements
We thank the Cell Microscopy Center, Department of Cell Biology, UMC Utrecht, for assistance with electron microscopy. We thank Alexander Horswill (Iowa) for providing pCM29 and Eline van Yperen and Samantha van der Beek (UMC Utrecht) for their contribution to the development of the GFP- and mCherry-labelled S. aureus strains. This work was financially supported by: a European Research Council Starting Grant no. 639209 (to S.H.M.R.), the Netherlands Organization for Scientific Research Nederlandse Wetenschaps Organisatie (NWO-ZonMW) Vidi grant no. 91711379 (to S.H.M.R.), the Hamill Foundation (to Y.-P.K.) and NIH grant AI020624 (to M.H.).
Abbreviations:
- CP5
capsular polysaccharide 5
- CP8
capsular polysaccharide 8
- Efb
extracellular fibrinogen binding protein
References
- Albus A., Fournier J. M., Wolz C., Boutonnier A., Ranke M., Høiby N., Hochkeppel H., Döring G.(1998). Staphylococcus aureus capsular types and antibody response to lung infection in patients with cystic fibrosis. J Clin Microbiol 262505–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestebroer J., Poppelier M. J., Ulfman L. H., Lenting P. J., Denis C. V., van Kessel K. P., van Strijp J. A., de Haas C. J.(2007). Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 1092936–2943. 10.1182/blood-2006-06-015461 [DOI] [PubMed] [Google Scholar]
- Boyle-Vavra S., Li X., Alam M. T., Read T. D., Sieth J., Cywes-Bentley C., Dobbins G., David M. Z., Kumar N., et al. (2015). USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. MBio 61–10. 10.1128/mBio.02585-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G. Y., Wang R., Khan B. A., Sturdevant D. E., Otto M.(2011). Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 791927–1935. 10.1128/IAI.00046-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnion K. M., Lee J. C., Frank M. M.(2001). Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immun 696796–6803. 10.1128/IAI.69.11.6796-6803.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnion K. M., Zhang H. M., Frank M. M.(2003). Availability of complement bound to Staphylococcus aureus to interact with membrane complement receptors influences efficiency of phagocytosis. Infect Immun 71656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattom A., Schneerson R., Watson D. C., Karakawa W. W., Fitzgerald D., Pastan I., Li X., Shiloach J., Bryla D. A., et al. (1993). Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun 611023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattom A., Matalon A., Buerkert J., Taylor K., Damaso S., Boutriau D.(2015). Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother 11632–641. 10.4161/hv.34414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J.(2005). Immune evasion by staphylococci. Nat Rev Microbiol 3948–958. 10.1038/nrmicro1289 [DOI] [PubMed] [Google Scholar]
- Foster T. J., Geoghegan J. A., Ganesh V. K., Höök M.(2013). Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 1249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler V. G., Allen K. B., Moreira E. D., Moustafa M., Isgro F., Boucher H. W., Corey G. R., Carmeli Y., Betts R., et al. (2013). Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 3091368–1378. 10.1001/jama.2013.3010 [DOI] [PubMed] [Google Scholar]
- Gros P., Milder F. J., Janssen B. J.(2008). Complement driven by conformational changes. Nat Rev Immunol 848–58. 10.1038/nri2231 [DOI] [PubMed] [Google Scholar]
- Herbert S., Newell S. W., Lee C., Wieland K. P., Dassy B., Fournier J. M., Wolz C., Döring G.(2001). Regulation of Staphylococcus aureus type 5 and type 8 capsular polysaccharides by CO(2). J Bacteriol 1834609–4613. 10.1128/JB.183.15.4609-4613.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochkeppel H. K., Braun D. G., Vischer W., Imm A., Sutter S., Staeubli U., Guggenheim R., Kaplan E. L., Boutonnier A., et al. (1987). Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol 25526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S., Hamada E., Kamoshida G., Yokoyama R., Takii T., Onozaki K., Tsuji T.(2010). Staphylococcal superantigen-like protein 10 (SSL10) binds to human immunoglobulin G (IgG) and inhibits complement activation via the classical pathway. Mol Immunol 47932–938. 10.1016/j.molimm.2009.09.027 [DOI] [PubMed] [Google Scholar]
- Jones C.(2005). Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr Res 3401097–1106. 10.1016/j.carres.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Kang M., Ko Y. P., Liang X., Ross C. L., Liu Q., Murray B. E., Höök M.(2013). Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of Gram-positive bacteria inhibit complement activation via the classical pathway. J Biol Chem 28820520–20531. 10.1074/jbc.M113.454462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Takata Y., Kozono H., Takeda J., Hong K. S., Inoue K.(1988). C5 convertase of the alternative complement pathway: covalent linkage between two C3b molecules within the trimolecular complex enzyme. J Immunol 1413895–3901. [PubMed] [Google Scholar]
- Ko Y. P., Liang X., Smith C. W., Degen J. L., Höök M.(2011). Binding of Efb from Staphylococcus aureus to fibrinogen blocks neutrophil adherence. J Biol Chem 2869865–9874. 10.1074/jbc.M110.199687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y. P., Kuipers A., Freitag C. M., Jongerius I., Medina E., van Rooijen W. J., Spaan A. N., van Kessel K. P., Höök M., et al. (2013). Phagocytosis escape by a Staphylococcus aureus protein that connects complement and coagulation proteins at the bacterial surface. PLoS Pathog 9e1003816. 10.1371/journal.ppat.1003816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattar S. M., Noto Llana M., Denoël P., Germain S., Buzzola F. R., Lee J. C., Sordelli D. O.(2014). Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun 8283–91. 10.1128/IAI.01050-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Liu M. J., Parsonnet J., Arbeit R. D.(1990). Expression of type 8 capsular polysaccharide and production of toxic shock syndrome toxin 1 are associated among vaginal isolates of Staphylococcus aureus. J Clin Microbiol 282612–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Licini L., Haelterman E., Moris P., Lestrate P., Damaso S., Van Belle P., Boutriau D.(2015). Safety and immunogenicity of an investigational 4-component Staphylococcus aureus vaccine with or without AS03B adjuvant: Results of a randomized phase I trial. Hum Vaccin Immunother 11620–631. 10.1080/21645515.2015.1011021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy F. D.(1998). Staphylococcus aureus infections. N Engl J Med 339520–532. 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- Nemeth J., Lee J. C.(1995). Antibodies to capsular polysaccharides are not protective against experimental Staphylococcus aureus endocarditis. Infect Immun 63375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I. M., Lee J. C., Bremell T., Rydén C., Tarkowski A.(1997). The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun 654216–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen M., Marshall H., Richmond P., Shakib S., Jiang Q., Cooper D., Rill D., Baber J., Eiden J., et al. (2015). A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine 331846–1854. 10.1016/j.vaccine.2015.02.024 [DOI] [PubMed] [Google Scholar]
- O'Riordan K., Lee J. C.(2004). Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 17218–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M.(2013). Community-associated MRSA: what makes them special? IJMM 303324–330. 10.1016/j.ijmm.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma M., Nozohoor S., Schennings T., Heimdahl A., Flock J. I.(1996). Lack of the extracellular 19-kilodalton fibrinogen-binding protein from Staphylococcus aureus decreases virulence in experimental wound infection. Infect Immun 645284–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y. Y., Schwartz J., Thoendel M., Ackermann L. W., Horswill A. R., Nauseef W. M.(2010). agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun 2546–559. 10.1159/000319855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Gerber S., Lee J. C.(2014). Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates. Infect Immun 825049–5055. 10.1128/IAI.02373-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöhlmann-Dietze P., Ulrich M., Kiser K. B., Döring G., Lee J. C., Fournier J. M., Botzenhart K., Wolz C.(2000). Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect Immun 684865–4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J. D.(2010). Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11785–797. 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossolini G. M., Arena F., Pecile P., Pollini S.(2014). Update on the antibiotic resistance crisis. Curr Opin Pharmacol 1856–60. 10.1016/j.coph.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Schenk S., Laddaga R. A.(1992). Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett 73133–138. [DOI] [PubMed] [Google Scholar]
- Shannon O., Uekotter A., Flock J.(2005). Extracellular fibrinogen binding protein Efb from Staphylococcus aureus as an antiplatelet agent in vivo. Thromb Haemost, 1–5. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J.(2007). Cryosectioning and immunolabeling. Nat Protoc 22480–2491. 10.1038/nprot.2007.365 [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z., Karakawa W. W., Vann W. F., Schneerson R., Malik Z.(1985). Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol 22828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerding A. M., Köhl J., Pandey M. K., Kuipers A., Leusen J. H., Boross P., Nederend M., Vidarsson G., Weersink A. Y., et al. (2013). Staphylococcus aureus formyl peptide receptor-like 1 inhibitor (FLIPr) and its homologue FLIPr-like are potent FcγR antagonists that inhibit IgG-mediated effector functions. J Immunol 191353–362. 10.4049/jimmunol.1203243 [DOI] [PubMed] [Google Scholar]
- Thakker M., Park J. S., Carey V., Lee J. C.(1998). Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 665183–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S. Y., Davis J. S., Eichenberger E., Holland T. L., Fowler V. G.(2015). Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28603–661. 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel K. P., Bestebroer J., van Strijp J. A.(2014). Neutrophil-mediated phagocytosis of staphylococcus aureus. Front Immunol 5467. 10.3389/fimmu.2014.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyich J. M., Vuong C., DeWald M., Nygaard T. K., Kocianova S., Griffith S., Jones J., Iverson C., Sturdevant D. E., et al. (2009). The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 1991698–1706. 10.1086/598967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C., Yeh A. J., Cheung G. Y., Otto M.(2015). Investigational drugs to treat methicillin-resistant Staphylococcus aureus. Expert Opin Investig Drugs 378473–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker M., Wang L., Kowarik M., Dowd M., Lipowsky G., Faridmoayer A., Shields K., Park S., Alaimo C., et al. (2014). Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis 2091551–1561. 10.1093/infdis/jit800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A., Ke D., Wang Q., Pillay A., Nicholson-Weller A., Lee J. C.(2005). Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun 733502–3511. 10.1128/IAI.73.6.3502-3511.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K.(1979). Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun 261159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]