Abstract
Objectives
Major depressive disorder (MDD) is a common disorder, widely distributed in the population, and is often associated with severe symptoms and functional impairment. It has been estimated that 30% of MDD patients do not benefit adequately from therapeutic interventions, including pharmacotherapy and psychotherapy. Treatment-resistant depression (TRD) is generally defined as a failure to achieve remission, despite therapeutic interventions.
Aim
The most effective treatment alternatives for TRD are hospitalization, electroconvulsive therapy (ECT), and transcranial magnetic stimulation (TMS). Here we compared the clinical effectiveness of ECT and TMS, including success rates, patient responses, side-effect profiles, and financial worthiness.
Results
We found that ECT (P<0.0001) was more effective than TMS (P<0.012) (not statistically significant in group effect) in TRD patients. However, ECT patients reported a higher percentage of side effects (P<0.01) and the TMS treatment scored better in terms of patient preference. The cost benefit of ECT was higher than that of TMS (US$2075 vs US$814). Patient’s preferences for treatment could be more intense in the TMS, if the TMS is included in the Health Maintenance Organization’s service list.
Conclusion
We propose that both of these treatment options should be available in psychiatric wards, thus expanding the therapeutic toolkit for TRD.
Keywords: ECT, TMS, treatment-resistant depression, cost benefit, patient preference
Introduction
Depression is a common mental disorder, occurring in every decade of life and in all communities of the world.1–3 Previous Global Burden of Disease (GBD) studies conducted from 1990 to 2010 brought attention to depressive disorders as a leading cause of economic burden. In the 2010 GBD study, depressive disorders were the second most common cause of “years lived with disabilities” (YLDs). Major depressive disorder (MDD) accounted for 8.2% and dysthymia for 1.4% of global YLDs. Depressive disorders were also identified as a major cause of “disability-adjusted life years” (DALYs), with MDD and dysthymia accounting for 2.5% and 0.5% of global DALYs, respectively.2,3
Depression can be successfully treated with antidepressant medications and psychotherapy, either alone or in combination. A majority of, but not all, patients respond well to these treatments.1,4,5 Treatment-resistant depression (TRD) is defined as a failure to achieve remission, despite therapeutic interventions. About 30% of depressed patients do not achieve remission after two to four different antidepressant trials, with or without psychological treatment approaches. The three most common clinical treatment approaches in TRD are as follows:4,5 1) Patient hospitalization in a day-care unit or psychiatry ward. The average hospitalization time for TRD is 60 days. During this period, the patient receives more intense medications and various forms of psychotherapy. However, hospitalization affects the normal life of the patients, causes difficulties with their circle of life, and can become stigmatic for the patients. All Israeli citizens are mandatorily insured by one of the four insurance companies (Health Maintenance Organizations [HMOs]), according to the Israeli equal health to citizen’s plan (1995 law). Thus, the HMOs are responsible for meeting the cost of hospitalizations. The daily payment for hospitalization is set according to the Ministry of Health tariffs. For HMOs per patient, the average expenses arising from hospitalization in a psychiatric ward are greater than 50,000 shekels (US$12,988), excluding any indirect costs of hospitalization (eg, absence from work and full payment due to medical vacation); 2) Electroconvulsive treatment (ECT) has been an acceptable practice in psychiatry for more than 80 years.6 Today, ECT is considered acceptable, especially in cases of MDD, and its efficacy and safety are based on well-established clinical experience and research.6,7 ECT has historically been used to treat depressed patients and this remains the most effective treatment for TRD. However, ECT also benefits patients suffering from hostility, anxiety, and agitation.8 Each session of ECT costs the HMO up to 1,226 shekels ($318) and patients receive 8–12 sessions during treatment; 3) Repetitive transcranial magnetic stimulation (rTMS) has been proposed as an alternative treatment method.9 During rTMS, a rapidly changing electrical current creates a time-varying magnetic field, which passes unimpeded through the hair, scalp, and skull, entering the cortex, where it induces an electrical field that changes the neuronal activity at the site of stimulation and at related neuronal networks. A large number of 3–6-week long, randomized controlled studies have examined the antidepressant properties of rTMS applied over the left dorsolateral prefrontal cortex (LDLPFC).10,13 Although, the antidepressant properties of the prefrontal rTMS have been clearly demonstrated in patients who did not respond to antidepressant medication in depressive episodes, the response rates in these sham-controlled trials were from modest to moderate.10,13
Study design and theoretical background
Hospitalization, transcranial magnetic stimulation (TMS), and ECT are clinically accepted treatment approaches for resistant depression. We chose not to include the hospitalization arm in our study. This decision was made because of its high cost (direct and indirect costs), as well as the negative effect of hospitalization on routine life. Thus, we compared the effectiveness, patient’s preferences, and the associated side effects of ECT and TMS. We also compared the economics involved in these two procedures.
Thus, this study compared the clinical effectiveness of ECT and TMS in order to calculate and compare their success rates, patient responses, side-effect profiles, and financial worthiness, as well as patient’s preference.
Methods and materials
Subjects
TMS sample
Forty-one eligible subjects were included, consisting of depressed outpatients, aged between 18 and 68 years, with a DSM-IV diagnosis of MDD (recurrent episodes). The duration of the current episode was at least 3 months but not more than 5 years. Subjects were required to have a total score of at least 20 on the 21-item Hamilton Depression Rating Scale (HDRS-21) at the screening visit. Subjects were required to have failed at least two adequate antidepressant treatments or to have displayed intolerance to two different antidepressants during a previous depressive episode.
ECT sample
Forty ECT patients were referred by their clinician for ECT treatment, due to resistance to the current treatment regime (equal criteria for ECT patients). All patients underwent a physical workout, which included blood tests, neurological and physical examinations, electrocardiogram (ECG), and chest X-ray. Patients signed a consent form to receive ECT treatment. Physicians treating the ECT patients performed a physical examination and an ECG, and a specialist consultation was made every 6 months during the internal medicine-anesthesiologist renewed consultation.
Overview of study
This was a retrospective medical record review of 81 consecutive patients with treatment-resistant MDD who had received ECT or rTMS. The demographic characteristics of the patients, hospitalization history, the legal status of hospitalization, psychiatric diagnoses and medical co-morbidities, the reason for ECT–TMS, the treatment regimen, overall duration of ECT–TMS, previous pharmacological therapy, response to ECT or rTMS, and any side effects (including cognitive impairment) were collected from the ECT unit files. Information was also received from the medical staff involved in the treatment.
The severity of the patients’ psychopathology pre- and post-ECT–TMS was assessed using a questionnaire. The pre- and posttreatment depression levels of the patients were measured with the HDRS-2114 (completed by the physician/medical staff), Beck Depression Inventory (BDI-II)15 (completed by the patient), and Hamilton Anxiety Rating Scale (HARS)16 (completed by the physician/medical staff). The level of functioning pre- and posttreatment was evaluated using a Visual Analog Scale (VAS)17 (completed by the physician/medical staff).
Local, Beer Yaakov-Ness Zionah Mental Health Center, Institutional Review Board approval was obtained for the collection and analysis of the treatment data. ECT and rTMS treatments received Ministry of Health approval. We deleted any details from the data that may personally identify the patients.
Patients were recruited by referrals from a treating physician in outpatient clinics and private practice clinics from all over Israel. All subjects signed an informed consent document before undergoing the ECT and rTMS procedures.
rTMS
Magstim UK (Shefield, UK) rapid equipment was used for the rTMS treatment.10,13 The motor threshold (MT) over the left motor cortex (the area controlling the abductor pollicis brevis [APB]) was determined by the electromyography (EMG) method, which looks for the lowest machine power output that would provide an motor evoke potential (MEP) of at least 50 mV in five of ten stimulations. The MT was determined daily in all cases. Stimulations were given at 120% MT with continuous EMG monitoring. rTMS was administered over the LDLPFC.10,13
Placement of the electrode over the LDLPFC was determined following the method of Grunhaus et al:9,10 placing the coil 5 cm forward and at an angle of 45° from the vertex, which is the best spot for the APB control. We used a 10 cm wingspan figure-of-eight coil for the rTMS administration. We administered 30 pulses of rTMS at 10 Hz for 10 seconds (a total of 1,500 magnetic pulses per treatment day). rTMS was administered five times per week for 4 weeks (for a total of 20 treatment days).
ECT
After completing the work-up and signing the treatment consent form, patients were not allowed to eat or drink for 8 hours before the treatment. All patients received 1% intramuscular atropine 20–30 minutes before the treatment. ECT was performed according to the criteria taken from The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging, and a spECTrum 5000M™ (Mecta, Portland, OR, USA) machine was used to perform the ECT procedure.18
The patients received one of three different anesthetics during the ECT: propofol, etomidate, or thiopental. The anesthesiologist selected each patient’s anesthetic according to the patient’s medical background, additional medications, time taken to recover after the first treatment, and the patient’s response to the anesthetic in the past. Thiopental was given at 1.5 mg × body weight, propofol at 0.6 mg × body weight, and etomidate at 0.1 mg × body weight of the patient. Scoline (succinile choline) was added as a muscle relaxant and given as 0.6–0.8 mg × body weight of the patient. The effectiveness of the seizure was defined as 25 seconds minimum of the clinical seizure and 30 seconds minimum of the electroencephalogram-recorded seizure.19
Statistics
In general descriptive and demographics sections (Tables 1 and 2), we performed Student’s t-tests. For comparison between ECT and TMS, we performed ANOVA multivariate analysis (Figures 1–4).
Table 1.
General description
| N | Male | Female | |
|---|---|---|---|
| Number of patients and their sex | 81 | 39 | 42 |
| Number of patients in ECT | 40 | 21 | 19 |
| Number of patients in TMS | 41 | 18 | 23 |
| Mean | Standard deviation | ||
| Age (years) | 81 | 45.642 | 9.641 |
| Years of education | 81 | 13.148 | 2.214 |
| Current episode | 81 | 19.457 | 28.510 |
| Number of lifetime episodes | 81 | 2.395 | 1.069 |
| Past psychotherapy/cognitive therapy | 81 | 0.741 | 0.441 |
| HDRS-21 baseline | 81 | 26.802 | 3.172 |
| HDRS-21 after treatments | 81 | 14.802 | 13.688 |
| HARS baseline | 81 | 19.642 | 3.675 |
| HARS after treatments | 81 | 15.518 | 16.215 |
| BDI baseline | 81 | 25.197 | 4.247 |
| BDI after treatments | 81 | 11.457 | 4.093 |
| VAS baseline | 81 | 18.951 | 10.299 |
| VAS after treatments | 81 | 60.185 | 16.133 |
Abbreviations: HDRS, Hamilton Depression Rating Scale; HARS, Hamilton Anxiety Rating Scale; BDI, Beck Depression Inventory; VAS, Visual Analog Scale; ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation.
Table 2.
Student’s t-test, independent samples
| Mean | N | Standard deviation | t-test | df | Significance (2-tailed) | |
|---|---|---|---|---|---|---|
| Age, years (ECT) | 47.175 | 40 | 8.808 | 1.422 | 79 | 0.159 |
| Age, years (TMS) | 44.146 | 41 | 10.277 | |||
| Years of education (ECT) | 12.775 | 40 | 1.702 | −1.510 | 79 | 0.135 |
| Years of education (TMS) | 13.512 | 41 | 2.589 | |||
| Current episode (ECT) | 14.175 | 40 | 5.697 | −1.665 | 79 | 0.100 |
| Current episode (TMS) | 24.610 | 41 | 39.228 | |||
| Number of lifetime episodes (ECT) | 2.250 | 40 | 0.926 | −1.210 | 79 | 0.230 |
| Number of lifetime episodes (TMS) | 2.537 | 41 | 1.185 | |||
| Past psychotherapy/cognitive therapy (ECT) | 0.775 | 40 | 0.423 | 0.688 | 79 | 0.493 |
| Past psychotherapy/cognitive therapy (TMS) | 0.707 | 41 | 0.461 |
Abbreviations: ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation.
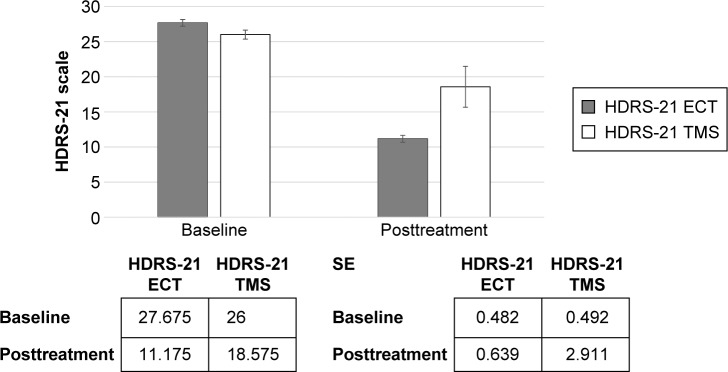
Figure 1.
21-item Hamilton Rating Scale for Depression (HDRS-21).
Abbreviations: ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation; SE, standard error.
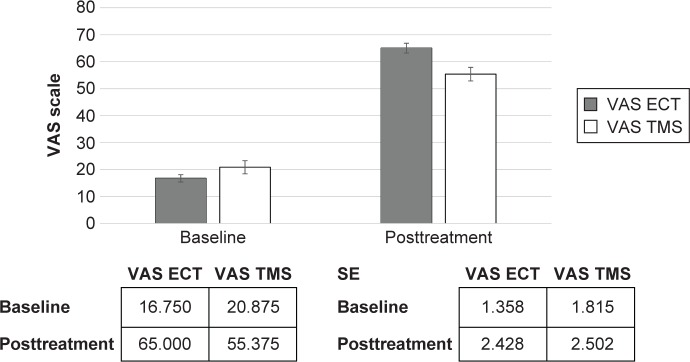
Figure 2.
Visual Analog Scale (VAS).
Abbreviations: ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation; SE, standard error.
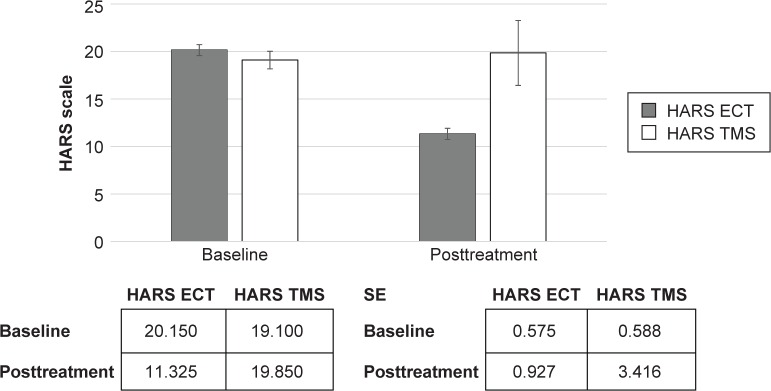
Figure 3.
Hamilton Anxiety Rating Scale (HARS).
Abbreviations: ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation; SE, standard error.
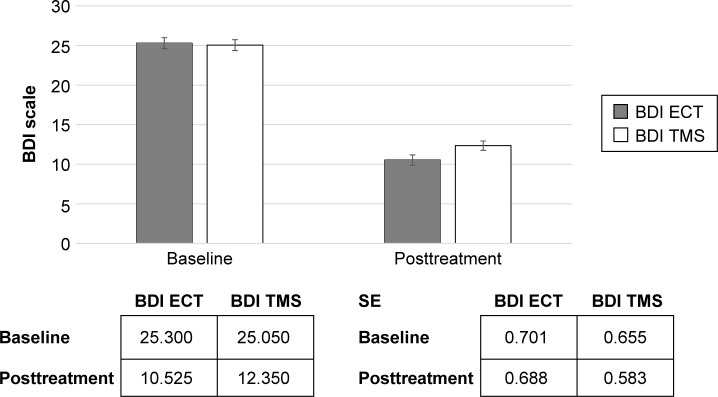
Figure 4.
Beck Depression Inventory (BDI).
Abbreviations: ECT, electroconvulsive therapy; TMS, transcranial magnetic stimulation; SE, standard error.
Results
Tables 1 and 2 summarize and compare the demographic findings of the ECT and TMS groups.
Effectiveness of treatments
We identified no differences between the two groups in terms of the demographic and baseline questionnaire results. Both groups were defined as TRD patients. Both ECT (df: 1; F: 32.72; P<0.0001) and rTMS (df: 1; F: 6.85; P<0.012) demonstrated their effectiveness in the HDRS-21, although the ECT was superior to rTMS (df: 1; F: 6.81; P<0.012). The ECT (df: 1; F: 333.5; P<0.0001) and rTMS (df: 1; F: 275.7; P<0.0001) both demonstrated their effectiveness in BDI-II. The ECT (df: 1; F: 423.4; P<0.0001) and rTMS (df: 1; F: 200.7; P<0.0001) were also demonstrated to be effective in the VAS improvement scale.
The ECT (df: 1; F: 70.1; P<0.0001), but not rTMS treatment (df: 1; F: 0.20; P<0.889, not significant), was effective in the treatment of anxiety symptoms, as determined by HARS.
Patients preferences, treatment effectiveness, and side effects
ECT treatment was superior (more effective) than rTMS in the treatment of TRD: anxiety symptoms did not improve with rTMS, but did improve significantly with ECT; the major disadvantage of ECT is the stigma that continues to surround the treatment, while high cost is the major drawback of TMS and patients who received ECT reported that they would have instead opted for rTMS if it had been financially available to them. They understood that the success rate of rTMS is lower than that of the ECT. This information is based on four basic questions and yes or no [y/n] answers: a) Do you believe that you responded well to ECT treatment? [y/n]; If the answer is yes: b) Would you consider receiving ECT in the future, if needed? [y/n]; c) Would you have considered receiving rTMS instead of ECT, if needed? [y/n]; d) If you know rTMS is less effective even than consider trying rTMS before ECT if needed? [y/n].
We summarized the income of two procedures for the hospital. Table 3 demonstrates that the hospital’s final income is higher with the ECT procedure.
Table 3.
Expenses and hospital income from the ECT-TMS treatments
| ECT
|
TMS
|
|||
|---|---|---|---|---|
| Shekels | US$ | Shekels | US$ | |
| Costs to HMO | 1,226 | 318 | – | – |
| Cost to patient (out of pocket) | – | – | 450 | 117 |
| Anesthesiologist expenses | 200 | 52 | – | – |
| Nursing staff expenses | 140 | 36 | – | – |
| Psychiatrist/resident in psychiatry expenses | 60 | 16 | 60 | 16 |
| Lease expenses of the TMS machine per daya | – | – | 125 | 32 |
| Daily department’s expensesb | 100 | 26 | 100 | 26 |
| Hospital earnings per treatment | 726 | 188 | 165 | 43 |
| Final incomec | 7,986 | 2,075 | 3,135 | 814 |
Notes:
Lease of the TMS machine costs 10,000 shekels per month. The TMS unit performs 80–120 treatments per month.
For ECT, daily department expense (including drugs, monitors, beds, sheets, and an ECT machine) is 15,000 shekels per month. The ECT unit performs 150–250 treatments per month; for TMS, department expense (including the rent and services) is 8,000 shekels per month. The TMS unit performs 80–120 treatments per month.
Patients complete 11.3±2.3 ECT treatments and 19.6±1.2 rTMS treatments, with total final income from ECT of 726×11=7,986 shekels ($2,074) and from TMS of 165×19=3,135 shekels ($814).
Abbreviations: ECT, electroconvulsive therap; TMS, transcranial magnetic stimulation; HMO, Health Maintenance Organization; rTMS, repetitive transcranial magnetic stimulation.
Clinician follow-up charts from patients’ medical records found that up to 60% of the ECT sample reported short- and intermediate-term side effects, especially memory loss. The TMS group suffered from relatively fewer (30% reported) and more minor side effects, such as headaches or pressure at the time of treatment, and these were resolved within a few hours after treatment.
Discussion
MDD is a common disorder that is increasing in prevalence and incidence. TRD is common, occurring in up to one-third of MDD patients,20 with TRD estimated to account for nearly $64 billion of the total cost of depression.21,22 TRD is responsible for a disproportionate amount of the disease burden, due to limited treatment possibilities and its chronic nature.21,22
Here we compared the “gold standard” ECT against rTMS during TRD treatment. We found that both the neurostimulation techniques are effective but that ECT is slightly more effective than rTMS. Our results are similar to recently published studies addressing ECT and/or rTMS.22–24
The cost-effectiveness of ECT vs rTMS has been explored in previous research, with conflicting results. Kozel et al25 published a decision model to evaluate the cost-effectiveness of ECT, rTMS, and rTMS followed by ECT in cases of rTMS failure. They found that rTMS was more cost-effective (in terms of health care and patient costs) than ECT but that rTMS followed by ECT was the most effective and least costly approach. Knapp et al26 conducted an economic evaluation comparing patients who were randomly treated with either ECT or rTMS in a 6-month follow-up study. Kozel et al25 found that rTMS had a very low probability of being cost-effective compared to ECT and that rTMS was not as effective as ECT. There were generally no differences between the direct health care costs, whereas the informal care costs were higher in the rTMS population than in the ECT population. Our findings are in line with those of Knapp et al26 and Vallejo-Torres et al.27 ECT seems to be more effective than rTMS in the treatment of TRD (but this is not statistically significant). The ECT procedure has more side effects than the rTMS. The ECT treatment is fully covered by the HMO’s health care system, whereas the rTMS treatment requires the patient to pay. The hospital earns 2.5 times more from the ECT than from the rTMS, due to expenses and patient fees.
Patients who received ECT reported that, if the rTMS had been financially available to them, they would have opted for this treatment instead, even if they understood that the therapeutic success rate of rTMS is lower than that of ECT.
Limitations
First, we included patients that were already willing to receive either ECT or TMS. Second, the rTMS population was required to pay for their treatment, which could have affected their willingness to get an effective treatment. Third, many of the ECT patients indicated that they would have preferred the rTMS treatment, if this had been financially viable. Fourth, the ECT treatment has an attached stigma in psychiatry, which makes it difficult to compare between any stigmatizing treatments and more patient-friendly procedures. Fifth, our therapist groups performed both treatments and were, therefore, not blinded to the treatment, although this also has the advantage of no bias between different groups of therapists.
Conclusion and future plan
The ECT procedure seems to be more effective than the rTMS in the treatment of TRD (but this does not reach statistical significance in this study). The ECT procedure has more side effects than the rTMS. The ECT is fully covered by the HMOs, whereas rTMS is self-funded by the patients. The hospital earns 2.5 times more from ECT than from rTMS.
The majority of patients who received ECT reported that, if rTMS had been financially available to them, they would have opted for rTMS instead of ECT, even if they were aware that the therapeutic success rate of rTMS was lower than that of ECT.
Patients receiving either TMS or ECT responded well to the treatments. However, due to the stigma and relatively high presence of side effects associated with ECT, rTMS was mostly preferred by patients. To make rTMS more widely available, we suggest that this treatment should be included within the HMOs systems and be made available to all TRD patients.
Footnotes
Disclosure
The authors report no conflict of interest in this study.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfeld RMA, Weissman MM. Risk factors for major depression and bipolar disorder. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 1017–1025. [Google Scholar]
- 3.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 6.Fekadu A, Wooderson SC, Markopoulo K, et al. What happens to patients with treatment-resistant depression? (A systematic review of medium to long term outcome studies) J Affect Disord. 2009;116(1–2):4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 7.UK ECT Review Group Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 8.Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):18–25. [PubMed] [Google Scholar]
- 9.Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;47:314–324. doi: 10.1016/s0006-3223(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 10.Grunhaus L, Schreiber S, Dolberg OT, Polack D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53(4):324–331. doi: 10.1016/s0006-3223(02)01499-3. [DOI] [PubMed] [Google Scholar]
- 11.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 12.Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Dannon PN, Dolberg OT, Schreiber S, Grunhaus L. Three and six-month outcome following courses of either ECT or rTMS in a population of severely depressed individuals – preliminary report. Biol Psychiatry. 2002;51(8):687–690. doi: 10.1016/s0006-3223(01)01274-4. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41(12 Pt 2):21–24. [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 17.Reips U-D. Web-based methods. In: Eid M, Diener E, editors. Handbook of Multi-Method Measurement in Psychology. Washington: American Psychological Association; 2006. pp. 73–85. [Google Scholar]
- 18.American Psychiatric Association . The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. 2nd ed. Washington: American Psychiatric Association; 2001. Committee on Electroconvulsive Therapy. Treatment procedures; p. 133. [Google Scholar]
- 19.Zahavi G, Dannon PN. Comparison of anesthetics in electroconvulsive therapy: an effective treatment with the use of propofol, etomidate, and thiopental. Neuropsychiatr DisTreat. 2014;10:383–389. doi: 10.2147/NDT.S58330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 21.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of tran scranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Nordenskjöld A. ECT is superior to pharmacotherapy for the short-term treatment of medication-resistant in patients with bipolar depression. Evid Based Ment Health. 2015;18(4):118. doi: 10.1136/eb-2015-102069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song GM, Tian X, Shuai T, et al. Treatment of adults with treatment-resistant depression: electroconvulsive therapy plus antidepressant or electroconvulsive therapy alone? Evidence from an indirect comparison meta-analysis. Medicine (Baltimore) 2015;94(26):e1052. doi: 10.1097/MD.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J, Chen J, Wei Q. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a meta-analysis of stimulus parameter effects. Neurol Res. 2013;35(10):1084–1091. doi: 10.1179/1743132813Y.0000000245. [DOI] [PubMed] [Google Scholar]
- 25.Kozel FA, George MS, Simpson KN. Decision analysis of the cost-effectiveness of repetitive transcranial magnetic stimulation versus electroconvulsive therapy for treatment of nonpsychotic severe depression. CNS Spectr. 2004;9(6):476–482. [PubMed] [Google Scholar]
- 26.Knapp M, Romeo R, Mogg A, et al. Cost-effectiveness of transcranial magnetic stimulation vs electroconvulsive therapy for severe depression: a multi-centre randomised controlled trial. J Affect Disord. 2008;109(3):273–285. doi: 10.1016/j.jad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Vallejo-Torres L, Castella I, Gonzales N, Hunter R, Serrano-Pérez P, Perestelo-Peres L. Cost-effectiveness of electroconvulsive therapy compared to repetitive transcranial magnetic stimulation for treatment-resistant severe depression: a decision model. Psychol Med. 2015;45(7):1459–1470. doi: 10.1017/S0033291714002554. [DOI] [PMC free article] [PubMed] [Google Scholar]