Abstract
Pooled data from 16 radiology centers were retrospectively analyzed to seek patients with pathologically proven testicular lymphoma and grayscale and color Doppler images available for review. Forty-three cases were found: 36 (84%) primary and 7 (16%) secondary testicular lymphoma. With unilateral primary lymphoma, involvement was unifocal (n = 10), multifocal (n = 11), or diffuse (n = 11). Synchronous bilateral involvement occurred in 6 patients. Color Doppler sonography showed normal testicular vessels within the tumor in 31 of 43 lymphomas (72%). Testicular lymphoma infiltrates through the tubules, preserving the normal vascular architecture of the testis. Depiction of normal testicular vessels crossing the lesion is a useful adjunctive diagnostic criterion.
Keywords: color Doppler sonography, genitourinary ultrasound, non-Hodgkin testicular lymphoma, testicular lymphoma, testicular neoplasms, testicular sonography
Although primary testicular tumors usually present as enlarging masses that destroy and replace the normal parenchyma, the hallmark of lymphoma is an infiltrative growth pattern in which tumor cells surround and compress the seminiferous tubules and the normal testicular vessels.1,2 The grayscale and Doppler sonographic appearances of testicular lymphoma have been described previously, but only in single case reports or small patient series.1,3–7 Lymphoma is described as presenting on sonography as single or multiple focal regions with decreased echogenicity of variable size or as a diffuse enlargement and decreased echogenicity of the entire testis, maintaining the normal testicular shape. Occasionally, a striated appearance of the testis without apparent masses may be seen.8–10 Color Doppler interrogation is reported to show increased intralesional flow in focal lymphomatous infiltration and hypervascularization of the entire testis in diffuse infiltration.6,11 In clinical practice, based on imaging features alone, lymphoma is thought to be indistinguishable from other tumors or non-neoplastic conditions11–13 despite grayscale and color Doppler features reflecting possible infiltrative, nondestructive characteristics.
Previously, investigators have reported that in lymphoma, the distribution of vessels within the lesion may be relatively normal.8,11,13,14 Identification of normal testicular vessels with a regular course crossing the lesion could therefore be a specific feature distinguishing lymphomas and other infiltrative tumors from mass-forming lesions. Our clinical practice also reflects these anecdotal observations, but to the best of our knowledge, no systematic studies have investigated the prevalence of normal vessels with a straight course crossing the tumors in a large series of patients.
The aim of this series was to investigate the grayscale and Doppler sonographic features of a large group of patients with testicular lymphomas, with emphasis on identification of normal testicular vessels crossing the lesion.
Materials and Methods
This study was planned and conducted in accordance with the Declaration of Helsinki and good clinical practice rules. All patients gave informed verbal consent for the sonographic studies.
Through a call for scientific cooperation published on the website of the European Society of Urogenital Radiology, the databases of 16 European and American radiology centers were searched from 1993 to 2013 to find grayscale and color Doppler images from patients with pathologically proven testicular lymphoma.
The sonograms were evaluated retrospectively in random order by 2 independent radiologists (M.B. and L.E.D., with 20 and 38 years of experience in urologic imaging, respectively). Reviewers were asked to characterize the lesions as unifocal, multifocal, or diffuse, to assess lesion vascularization as hypervascular or not, and to check for normal testicular vessels with a straight course crossing the lesion.
In 4 cases in which there were differences of opinion regarding the grayscale classification of the lesion, discrepancies were resolved by discussion with final interpretation based on a consensus of the 2 readers. No differences were found with regard to assessment of overall vascularity. With regard to identification of normal testicular vessels crossing the lesion, these were considered present when identified by both readers. The age of the patient, laterality, and primary or secondary involvement were recorded.
Results
There were 43 testicular lymphomas (patient age range, 23–84 years). The final histologic report was available in all cases; 14 of 43 (33%) had paraffin blocks or images of histologic specimens available to the reviewers. A diagnosis of primary disease was made in 36 of 43 cases (84%); secondary involvement of the testis was observed in 7 of 43 cases (16%) with previous or concomitant extratesticular disease.
Primary testicular lymphoma occurred more often in patients aged 60 years or older (27 of 36 [75%]) but also occurred in younger men. There was no side prevalence for primary testicular lymphoma. The right testis was involved in 18 cases and the left in 14 cases. Involvement was bilateral in 3 patients. In the remaining case, a right primary testicular lymphoma was found, followed by contralateral recurrence 8 months later. There was involvement of the epididymis in 11 cases and of both the epididymis and spermatic cord in 2; the lymphoma was confined to testis in the remainder.
Most primary lymphomas (33 of 36 [92%]) were the diffuse large B-cell type belonging to the non–germinal center B-cell–like subgroup (Figure 1). They were more frequent in patients older than 60 years but were also found in younger patients (range, 23–84 years; median, 69 years). Additional histotypes included a primary centrofollicular lymphoma, lymphoblastic lymphoma (Figure 2), and natural killer-/T-cell lymphoma.
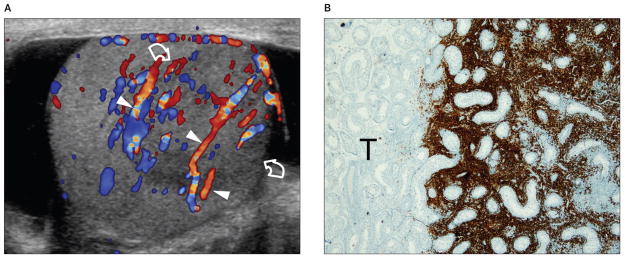
Figure 1.
Images from a 73-year-old patient with primary diffuse large B-cell lymphoma of the right testis presenting with a palpable testicular nodule. A, Color Doppler sonogram showing a unifocal hypoechoic lesion (curved arrows), which is hypervascular compared to the surrounding testis. Normal testicular vessels (arrowheads) cross the lesion with a rectilinear course, suggesting infiltrative disease. B, Immunohistochemical stain for CD20, a hallmark for B-cell lymphoma, showing the interface between the unstained uninvolved testis (T; left) and the portion of the testis involved by the tumor (right). CD20-positive tumor cells (brown) grow among the testicular vessels and seminiferous tubules without destroying them (original magnification ×20).
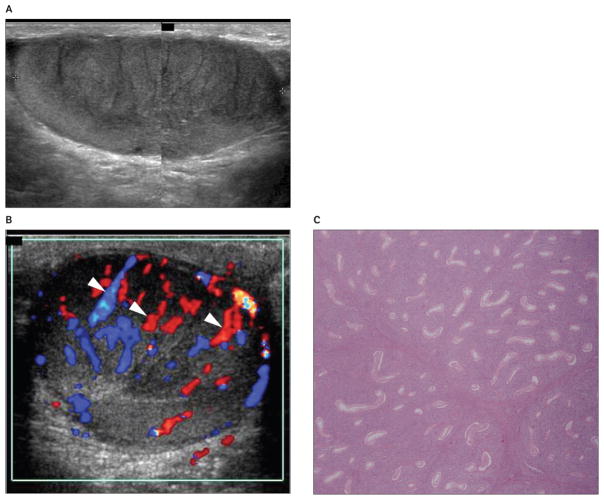
Figure 2.
Images from a 62-year-old patient with primary lymphoblastic lymphoma of the right testis presenting with a palpable right testicular nodule. A, Grayscale sonogram showing a striated appearance of a hypoechoic testicular mass involving most of the parenchyma. B, Color Doppler sonogram showing normal testicular vessels crossing the lesion (arrowheads). C, Photomicrograph of the histologic specimen showing diffuse peritubular and intratubular proliferation of lymphoma cells. Seminiferous tubules are infiltrated but not destroyed (hematoxylin-eosin, original magnification ×10).
The diffuse large B-cell type was also the prevailing histotype in secondary testicular lymphoma (Figure 3). The right testis was involved in 1 case and the left in 3 cases. Involvement was bilateral in the remaining 3 patients. Secondary testicular lymphoma was found more often in patients younger than 60 years (5 of 7 [71%]).
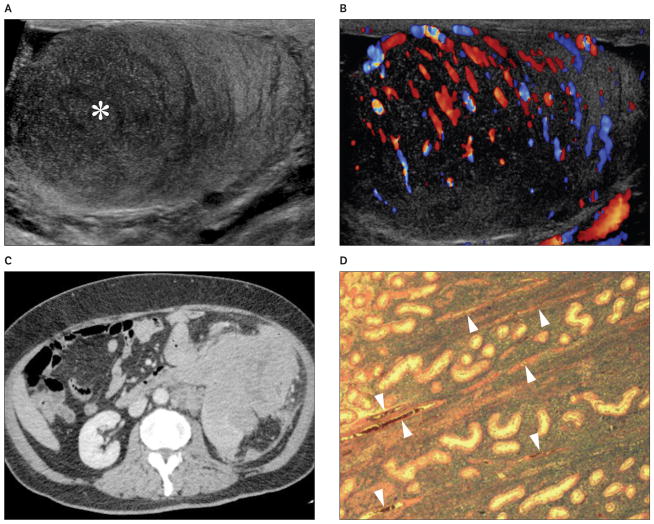
Figure 3.
Images from a 64-year-old patient with secondary lymphoma involving the left testis. The patient presented with a palpable left testicular nodule and abdominal mass. A and B, Grayscale (A) and color Doppler (B) sonograms showing a hypoechoic (asterisk) hypervascular lesion with poorly defined margins and normal intralesional testicular vessels. C, Contrast-enhanced computed tomogram showing extensive tumor involvement of the abdominal organs. D, Histologic specimen of the testis showing diffuse large B-cell lymphoma. Normal testicular vessels (arrowheads) and seminiferous tubules are surrounded by tumor cells (hematoxylin-eosin, original magnification ×20).
On grayscale sonography, lymphomatous unilateral involvement (n = 36), either primary (n = 32) or secondary (n = 4), appeared as unifocal (n = 11) or multifocal (n = 12) hypoechoic lesions. In the remaining 13 cases, diffuse enlargement and decreased echogenicity of the entire testis was found.
In 3 cases of synchronous bilateral lymphoma, lesion involvement was multifocal on both sides; in 1 case, it was diffuse on the left side and multifocal on the right side; and in the remaining 2 cases, it was diffuse on both sides. In the patient with metachronous bilateral involvement, the lesion was unifocal both in the right testis and in the left testicular recurrence (Figure 4).
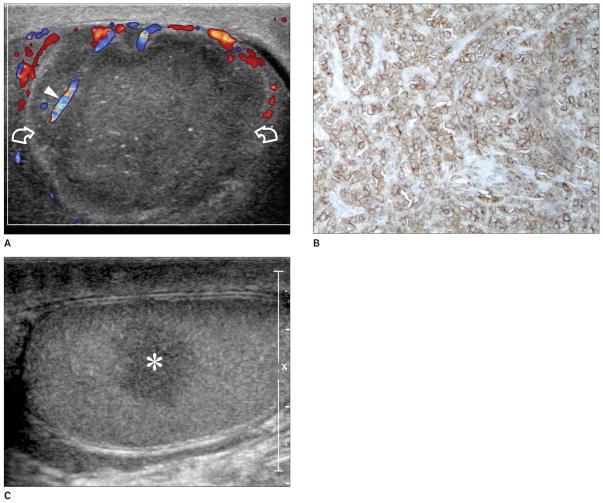
Figure 4.
Images from a 39-year-old patient with primary diffuse large B-cell lymphoma of the right testis and contralateral recurrence 8 months later. A, First examination: color Doppler sonogram of the right testis showing a unifocal hypoechoic lesion (curved arrows) presenting with an atypical hypovascular appearance compared to the surrounding testis. However, a normal vessel with a straight course (arrowhead) is visible within the lesion. The contralateral testis was normal at the time of the first investigation (not shown). B, Histologic specimen of the right testis showing CD20-positive tumor cells (original magnification ×40). C, Longitudinal sonogram of the left testis obtained 8 months after right orchidectomy showing contralateral recurrence (asterisk), which was confirmed histologically.
At color Doppler interrogation, 38 of 43 lymphomas (88%) were hypervascular. Normal testicular vessels with a straight course crossing the lesion were documented in 31 of 43 lesions (72%).
Discussion
Management of germ cell and stromal tumors has been standardized.15 On the contrary, treatment of testicular lymphoma is still debated.16 Biopsy to confirm diagnosis, followed by systemic chemotherapy using cyclophosphamide, doxorubicin, vincristine, and prednisolone, is currently the treatment of choice for stage IIIE and IVE disease. Irradiation in these patients is reserved for symptomatic and bulky localized deposits.16 In view of the high risk of central nervous system relapse, even in patients who achieve complete remission, central nervous system prophylaxis with intrathecal chemotherapy should be considered. For stages IE and IIE, there is universal agreement on orchidectomy as the initial treatment.3,16 The association between radiotherapy, chemotherapy, and central nervous system prophylaxis is debated, as testicular lymphoma may be very aggressive even when localized, with a high risk of relapse at various extranodal sites. Central nervous system relapses have been reported in 10% to 14% of cases.17
The practice of performing orchidectomy first in patients with testicular lymphoma, however, is not evidence based but is mainly due to the fact that preoperative diagnosis of lymphoma is rare, as in most cases, the features that suggest a specific diagnosis are not recognized preoperatively on color Doppler sonography. Indeed, several studies compared survival of patients who underwent orchidectomy alone or followed by chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone, radiation and chemotherapy, and irradiation of the contralateral testis, with or without central nervous system prophylaxis,3,16 but no study evaluated the impact of orchidectomy in these patients. Prospective multicenter trials incorporating a large number of patients would lead to a better understanding of the biology of testicular lymphoma and more targeted therapy, making a specific preoperative diagnosis possible in a substantial number of cases.
In our clinical practice, preoperative diagnosis of testicular lymphoma is also important. The waiting times for interventions and pathologic reports are reduced as much as possible in patients with suspected testicular lymphoma, and a multidisciplinary team of experts, including urologists, pathologists, and oncologists, is set to plan the best clinical workup.
In this series, we have described a group of 43 patients with pathologically proven testicular lymphoma investigated with grayscale and Doppler sonography. Results confirmed that testicular lymphomas present as hypoechoic lesions of the testis, either focal or diffuse, predominantly with a hypervascular appearance at color Doppler interrogation. In most cases (72%), we found normal testicular vessels traversing the lesion. This feature, however, is only indicative of the infiltrative nature of the pathologic process7 and not is specific for lymphoma, as it has been describedin other infiltrative neoplasms, such as plasmacytoma18,19 and leukemia infiltration.6,11 Importantly, non-neoplastic diseases, such as chronic granulomatous orchitis and other inflammatory conditions, may present as unifocal or multifocal hypoechoic hypervascular lesions, diffuse enlargement, decreased echogenicity, and hypervascularity of the entire testis or a striated pattern, identical to the grayscale and Doppler sonographic features of lymphoma.20 Accurate interpretation may therefore be difficult in the absence of clinical signs and symptoms of inflammation. Ultimately in these cases, biopsy is often necessary, and often orchidectomy is performed to establish the diagnosis.
This series had a number of limitations. Cases were pooled from the databases of many institutions and therefore were nonconsecutive. The prevalence of the features described and, in particular, that of normal testicular vessels crossing the lesion, cannot be extrapolated for all of the patients in the series. This finding may have been missed in some patients, as it was not specifically looked for or saved in the selected images that were available for retrospective evaluation. Normal intratesticular vessels tend to be oriented in vascular planes that intersect the mediastinum; ultrasound scanning along these planes allows recognition of the centripetal arteries and their origin from capsular arteries, whereas in views outside these planes, the vessels are foreshortened and are seen in cross section, appearing as color spots.13 Evaluation of interobserver and intraobserver variability was not attempted, as it was beyond the aim of this series. Last, the grayscale and color Doppler appearance of lymphomas has not been compared to that of germ cell and stromal tumors in an age-matched population.
Although the ability of color Doppler sonography to differentiate between lymphoma and mass-forming tumors cannot be substantiated from this series, the features described for lymphoma are not those of germ cell or stromal tumors, and we believe that an infiltrative disorder is the prime diagnostic consideration when a hypoechoic testicular lesion with rectilinear vessels running through it is encountered.
It is important to understand that testicular lymphoma is a neoplasm that is regarded as “rare,” and there is some difficulty in gathering experience with regard to any rare abnormality. In patients with rare tumors, the results of studies such as this series reflect the need for retrospective and multi-institutional review, even with their inherent bias. They may represent the highest level of evidence available for these rare conditions, for which knowledge of the imaging appearance is usually based solely on case reports or very small series.21
In conclusion, according to the literature, testicular lymphoma accounts for approximately 5% of all testicular tumors, is the most common malignant tumor of the testis in the elderly, and is the most common bilateral testicular tumor with a reported increasing incidence.22,23 Our data are in keeping with these previous results, as 75% of patients with primary testicular lymphoma were aged 60 years or older; 7 patients had secondary testicular involvement in previous or concomitant extratesticular disease; and synchronous bilateral involvement was found. Lymphoma must therefore be considered in patients presenting with a testicular mass, especially when older than 60 years or with a history of lymphoproliferative disease. Depiction of testicular vessels with a normal rectilinear course crossing the lesion is a useful adjunctive criterion to suggest the diagnosis. Differential diagnosis with other infiltrative tumors and with some inflammatory lesions may be problematic or even impossible based on imaging alone. The diagnostic accuracy of these grayscale and color Doppler criteria for differential diagnosis between lymphoma and other mass-forming testicular tumors needs to be substantiated with prospective studies.
Footnotes
This work was undertaken as a scientific activity of the European Society of Urogenital Radiology Scrotal Imaging Working Group.
References
- 1.Moorjani V, Mashankar A, Goel S, Khandelwal K, Patange V, Merchant N. Sonographic appearance of primary testicular lymphoma. AJR Am J Roentgenol. 1991;157:1225–1226. doi: 10.2214/ajr.157.6.1950870. [DOI] [PubMed] [Google Scholar]
- 2.Ferry JA, Harris NL, Young RH, Coen J, Zietman A, Scully RE. Malignant lymphoma of the testis, epididymis, and spermatic cord: a clinico-pathologic study of 69 cases with immunophenotypic analysis. Am J Surg Pathol. 1994;18:376–390. doi: 10.1097/00000478-199404000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Vitolo U, Ferreri AJ, Zucca E. Primary testicular lymphoma. Crit Rev Oncol Hematol. 2008;65:183–189. doi: 10.1016/j.critrevonc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Garcia AV, Alobeid B, Traina JM, Chen SS, Weiner MA, Middlesworth W. Isolated primary testicular B lymphoblastic lymphoma: an unusual presentation. J Pediatr Hematol Oncol. 2013;35:e88–e90. doi: 10.1097/MPH.0b013e318271c470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantz AG, Power N, Hutton B, Gupta R. Malignant lymphoma of the testis: a study of 12 cases. Can Urol Assoc J. 2009;3:393–398. doi: 10.5489/cuaj.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzu D, Jeffrey RB, Jr, Ralls PW. Lymphoma and leukemia involving the testicles: findings on gray-scale and color Doppler sonography. AJR Am J Roentgenol. 1995;164:645–647. doi: 10.2214/ajr.164.3.7863887. [DOI] [PubMed] [Google Scholar]
- 7.Zicherman JM, Weissman D, Gribbin C, Epstein R. Best cases from the AFIP: primary diffuse large B-cell lymphoma of the epididymis and testis. Radiographics. 2005;25:243–248. doi: 10.1148/rg.251045041. [DOI] [PubMed] [Google Scholar]
- 8.Emura A, Kudo S, Mihara M, Matsuo Y, Sato S, Ichigi Y. Testicular malignant lymphoma; imaging and diagnosis. Radiat Med. 1996;14:121–126. [PubMed] [Google Scholar]
- 9.Loberant N, Bhatt S, McLennan GT, Dogra VS. Striated appearance of the testes. Ultrasound Q. 2010;26:37–44. doi: 10.1097/RUQ.0b013e3181c6b284. [DOI] [PubMed] [Google Scholar]
- 10.Tweed CS, Peck RJ. A sonographic appearance of testicular lymphoma. Clin Radiol. 1991;43:341–342. doi: 10.1016/s0009-9260(05)80544-6. [DOI] [PubMed] [Google Scholar]
- 11.Ishigami K, Yousef-Zahra DM, Abu-Yousef MM. Enlargement and hypervascularity of both the epididymis and testis do not exclude involvement with lymphoma or leukemia. J Clin Ultrasound. 2004;32:365–369. doi: 10.1002/jcu.20046. [DOI] [PubMed] [Google Scholar]
- 12.Yang DM, Kim HC, Jin W, Lee HL, Kim GY. Lymphoma of the testis and epididymis mimics chronic inflammation upon sonography. J Clin Ultrasound. 2009;37:242–244. doi: 10.1002/jcu.20536. [DOI] [PubMed] [Google Scholar]
- 13.Horstman WG, Middleton WD, Melson GL, Siegel BA. Color Doppler US of the scrotum. Radiographics. 1991;11:941–957. doi: 10.1148/radiographics.11.6.1749858. [DOI] [PubMed] [Google Scholar]
- 14.Vara Castrodeza A, Torres Nieto A, Mendo González M, Rodriguez Toves A, Peñarrubia Ponce MJ, de la Fuente Bobillo MA. Diagnosis of a primary testicular lymphoma by echography and magnetic resonance imaging. Clin Transl Oncol. 2006;8:456–458. doi: 10.1007/s12094-006-0202-x. [DOI] [PubMed] [Google Scholar]
- 15.European Association of Urology. Guidelines on testicular cancer. European Association of Urology website; [Accessed September 10, 2014]. http://www.uroweb.org/guidelines/online-guidelines/ [Google Scholar]
- 16.Bhatia K, Vaid AK, Gupta S, Doval DC, Talwar V. Primary testicular non-Hodgkin’s lymphoma: a review article. Sao Paulo Med J. 2007;125:286–288. doi: 10.1590/S1516-31802007000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagrange JL, Ramaioli A, Theodore CH, et al. Non-Hodgkin’s lymphoma of the testis: a retrospective study of 84 patients treated in the French anti-cancer centres. Ann Oncol. 2001;12:1313–1319. doi: 10.1023/a:1012224123385. [DOI] [PubMed] [Google Scholar]
- 18.Walker FB, Bluth EI, Kenney A, Beckman EN. Plasmacytoma of the testis. J Ultrasound Med. 2005;24:1721–1725. doi: 10.7863/jum.2005.24.12.1721. [DOI] [PubMed] [Google Scholar]
- 19.Bude RO. Testicular plasmacytoma: appearance on gray-scale and power Doppler sonography. J Clin Ultrasound. 1999;27:345–346. doi: 10.1002/(sici)1097-0096(199907/08)27:6<345::aid-jcu6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology. 2003;227:18–36. doi: 10.1148/radiol.2271001744. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, Ozsahin M, Mirimanoff RO, Bhatia S, Chang K, Miller RC. The Rare Cancer Network: achievements from 1993 to 2012. Rare Tumors. 2012;4:e35. doi: 10.4081/rt.2012.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundrum JD, Mathiason MA, Moore DB, Go RS. Primary testicular diffuse large B-cell lymphoma: a population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol. 2009;27:5227–5232. doi: 10.1200/JCO.2009.22.5896. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad SS, Idris SF, Follows GA, Williams MV. Primary testicular lymphoma. Clin Oncol. 2012;24:358–365. doi: 10.1016/j.clon.2012.02.005. [DOI] [PubMed] [Google Scholar]