Abstract
Background
Despite the strong preclinical rationale, there are only very few data considering the utility of metformin as a potential pain therapeutic in humans. The aim of this study was to determine the association between metformin therapy and pressure pain threshold (PPT) in lean women with polycystic ovary syndrome (PCOS). We hypothesized that metformin therapy in lean PCOS women increases PPT.
Materials and methods
Twenty-seven lean PCOS women with free androgen index phenotype >5 and 18 lean healthy controls were enrolled in the study. Fifteen of the PCOS women were randomly assigned to be treated with metformin 1,500 mg daily for 6 months. PPT and plasma β-endorphin levels were measured in all women at the beginning of the study and after 6 months of observation.
Results
We observed an increase in PPT values measured on deltoid and trapezius muscle in the PCOS with metformin group after 6 months of metformin administration (4.81±0.88 kg/cm2, P<0.001 on deltoid muscle, and 5.71±1.16 kg/cm2 on trapezius muscle). We did not observe any significant changes in PPT values in the PCOS without treatment group and in controls. We did not observe any significant changes in serum β-endorphin levels in any studied groups during the 6-month observation.
Conclusion
We conclude that metformin therapy increases PPT in lean PCOS women, without affecting plasma β-endorphin concentration. Our results may suggest the potential role of metformin in pain therapy. We propose that larger, randomized studies on metformin impact on pain perception should be performed.
Keywords: metformin, pressure pain threshold, pressure algometry, polycystic ovary syndrome, pain therapy
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women. Not only does it impact the fertility of the patients, but it also increases the risk of metabolic disorders. Some evidence indicates that the evolution of PCOS is related to the activity of the endogenous opioid system, in which the main molecule β-endorphin is produced by the pituitary gland and may contribute to PCOS pathogenesis through central effects on gonadotropin secretion and peripheral effects on glucose metabolism.1–4 In our earlier study, we found higher plasma β-endorphin level in lean PCOS women compared to the healthy controls.4 Some data have reported that plasma opioid levels correlate with pain perception and pain threshold.5–7
Metformin, an antidiabetic drug of the biguanide class, is now one of the most widely used drugs in the treatment of PCOS.8–11 Metformin activates adenosine monophosphate-activated protein kinase (AMPK) and, through AMPK activation, inhibits the mammalian target of rapamycin (mTOR) pathway.12–15 AMPK may play a role in the pain mechanism pathway. Preclinical neuropathic and postsurgical pain studies implicate the AMPK as a potential pharmacological target for the treatment of chronic pain conditions. By inhibiting mTOR signaling in nociceptors, metformin has been shown to reduce mechanical allodynia and nociceptor excitability.12–15 Despite the strong preclinical rationale, there are only very few data, some of them case reports, considering the utility of metformin as a potential pain threshold modifier in humans.14,16,17
Given the effect of metformin therapy on pain perception, the objective of our study was to determine the association between metformin therapy and pressure pain threshold (PPT) in lean PCOS women. Earlier studies have demonstrated the role of β-endorphins and testosterone in this process. We hypothesized that metformin therapy in lean PCOS women would increase PPT.
Materials and methods
Forty-five lean women with body mass index (BMI) within the normal range (18.5–24.9 kg/m2) – 27 with PCOS and 18 without this disorder – were enrolled in the study and divided into three groups.
PCOS patients were divided into two groups. The first group consisted of 15 PCOS women who were treated with 1,500 mg metformin per day for 6 months. The second group consisted of twelve PCOS women who did not receive any medical therapy. Patients were assigned to groups randomly. The diagnosis of PCOS among all examined patients was based on the Androgen Excess Society criteria.1 We included patients with free androgen index (FAI) phenotype ≥5. Hyperprolactinemia, androgen-secreting tumors, Cushing’s disease, and congenital adrenal hyperplasia were excluded based on clinical and laboratory data.
The control group consisted of 18 healthy lean volunteers, who had regular menstrual cycles, without laboratory and clinical signs of hyperandrogenism. All patients had an FAI of <5 and did not receive any medical therapy over the entire period of the study.
All 45 subjects had normal renal and hepatic function and had not received any medication for at least 6 months before the study. Patients who showed evidence of thyroid dysfunction, diabetes, musculoskeletal disorders, or other internal pathologies were excluded from the study. Patients were also excluded if they exhibited any of following: symptoms in the neck, head, or upper extremities, previous history of motor organs injury, receiving any soft tissue therapy within the past 12 months, and regular use of analgesic or anti-inflammatory drugs.
There were no significant differences in BMI, age, prolactin, cortisol, estradiol, glucose in oral glucose tolerance test (OGTT), and thyroid hormones levels between the three studied groups at the beginning of the study. The clinical characteristics of all groups are listed in Table 1.
Table 1.
Clinical characteristics of lean women with polycystic ovary syndrome and controls at the beginning of the study
| Parameter | Total n=45 mean ± SD | PCOS with metformin group (1) n=15 mean ± SD | PCOS without treatment group (2) n=12 mean ± SD | Control group (3) n=18 mean ± SD | P-value |
|---|---|---|---|---|---|
| Age (years) | 23.3±2.81 | 23.27±3.24 | 23.83±2.89 | 22.89±2.47 | P=0.45 |
| Weight (kg) | 60±4.5 | 60.33±3.5 | 59.83±2.86 | 60.33±6.08 | P=0.38 |
| Height (cm) | 167.7±3.78 | 168.8±1.75 | 168.8±1.75 | 166±5.25 | P=0.39 |
| BMI (kg/m2) | 21.44±1.47 | 21.19±1.27 | 21.1±1.01 | 21.87±1.8 | P=0.63 |
| FT3 (pmol/L) | 4.54±0.49 | 4.41±0.49 | 4.52±0.42 | 4.67±0.62 | P=0.32 |
| TSH (IU/mL) | 1.8±0.59 | 1.99±0.62 | 1.51±0.33 | 1.83±0.64 | P=0.45 |
| FT4 (pmol/L) | 13.62±2.09 | 13.01±2.2 | 13.61±1.4 | 14.13±2.32 | P=0.29 |
| Prolactin (μIU/mL) | 248.4±87.14 | 243.9±87.18 | 271.5±81.2 | 236.7±92.7 | P=0.22 |
| Estradiol (pmol/L) | 276.2±113.7 | 270.8±113.7 | 289.8±96.7 | 271.6±169.1 | P=0.11 |
| LH (mIU/mL) | 8.85±3.36 | 11.46±2.74 | 10.51±1.98 | 5.57±1.03 | P1,2 =0.48, P1,3 <0.001, P2,3 <0.001 |
| FSH (mIU/mL) | 6.16±1.14 | 6.87±1.08 | 5.99±0.62 | 5.69±1.2 | P1,2 =0.09, P1,3 =0.008, P2,3 =0.72 |
| Testosteron (nmol/L) | 1.96±0.76 | 2.51±0.34 | 2.51±0.28 | 1.14±0.42 | P1,2 =0.1, P1,3 <0.001, P2,3 <0.001 |
| SHBG (nmol/L) | 41.57±8.07 | 37.59±6.33 | 39.78±4.43 | 46.06±9.23 | P1,2 =0.72, P1,3 =0.006, P2,3 =0.07 |
| FAI | 5.06±2.32 | 6.97±1.47 | 6.32±0.45 | 2.64±1.23 | P1,2 =0.33, P1,3 <0.001, P2,3 <0.001 |
| Cortisol-0800 hours (μm/dL) | 20.5±7.63 | 21.5±6.82 | 20.5±5.82 | 19.7±5.82 | P=0.25 |
| Cortisol-1700 hours (μm/dL) | 10.3±4.78 | 11.3±4.48 | 10.2±4.67 | 9.5±4.33 | P=0.19 |
| Glucose 0′ (mmol/L) | 5.1±0.95 | 4.8±0.6 | 5.0±0.86 | 5.4±1.15 | P=0.17 |
| Glucose 60′ (mmol/L) | 5.7±1.4 | 5.5±1.44 | 5.6±1.34 | 5.8±1.38 | P=0.18 |
| Glucose 120′ (mmol/L) | 5.41±1.14 | 5.1±0.92 | 5.2±0.94 | 5.0±1.34 | P=0.38 |
| Insulin 0′ (μU/mL) | 11.6±5.43 | 14.4±5.49 | 14.84±2.45 | 8.9±3.84 | P1,2 =0.54, P1,3 =0.003, P2,3 =0.003 |
| Insulin 60′ (μU/mL) | 62.5±34.83 | 83.8±32.26 | 85.6±20.21 | 42.6±24.12 | P1,2 =0.43, P1,3 <0.001, P2,3 <0.001 |
| Insulin 120′ (μU/mL) | 55.9±35.5 | 78.2±35.48 | 76.34±31.3 | 35.1±19.62 | P1,2 =0.32, P1,3 <0.001, P2,3 <0.001 |
| β-Endorphins (pg/mL) | 12.01±4.95 | 16.22±2.04 | 14.84±2.45 | 6.61±1.97 | P1,2 =0.22, P1,3 <0.001, P2,3 <0.001 |
| PPT deltoid muscle (kg/cm2) | 7.82±2.55 | 9.56±1 | 9.91±1.4 | 4.96±0.55 | P1,2 =0.63, P1,3 <0.001, P2,3 <0.001 |
| PPT trapezius muscle (kg/cm2) | 6.79±1.89 | 7.96±0.98 | 8.4±0.94 | 4.74±0.6 | P1,2 =0.38, P1,3 <0.001, P2,3 <0.001 |
Notes: Data are presented as mean ± SD. Analysis was carried out using ANOVA test. Significance level <0.05.
Abbreviations: BMI, body mass index; FT3, triiodothyronine; TSH, thyroid-stimulating hormone; FT4, thyroxin; SHBG, sex hormone-binding globulin; FAI, free androgen index; LH, luteinizing hormone; FSH, follicle-stimulating hormone; PPT, pressure pain threshold; PCOS, polycystic ovary syndrome.
Each patient underwent a full clinical evaluation. Fasting blood samples were collected at 0800 hours from a forearm vein between days 1 and 5 of the menstrual cycle. The following were the parameters measured: luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, testosterone, triiodothyronine (FT3), thyroid-stimulating hormone (TSH), thyroxin (FT4), sex hormone-binding globulin (SHBG), cortisol at 0800 hours and 1700 hours, prolactin, insulin, and glucose by OGTT, all according to the routine methods. Free testosterone was calculated by using the FAI ratio – the total testosterone level (nmol/L) was divided by the SHBG level (nmol/L) and then multiplied by 100. All parameters were measured two times – at the beginning of the study and after 6 months observation.
β-Endorphin measurement
Levels of endorphins were measured using the Endorphin beta (human) RIA Kit RK-022-14 (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) and calculated according to the Assay Protocol.4,18 In all patients, the β-endorphins concentrations were measured two times – at the beginning of the study and after 6 months observation.
PPT assessment
The PPT, defined as the pressure at which the sensation changed from pressure to pain, has been recognized as an effective and reliable way to quantify pain measures.19–22 In our study, PPT was measured using an electronic, wireless algometer (Commander Echo® Algometer, JTECH Medical, Midvale, UT, USA). The reliability of pressure algometry has been found to be high.23 The Algometer has a circular 1-cm2 tip applying a rate of pressure of 1 kg/s as standard. PPTs were measured in the midpoint of the anterior fibers of the deltoid muscle and on the upper fibers of the trapezius muscle (medial to the superior angle of the scapula), both in the nondominant side.24,25 Pressure was applied at a straight angle to the defined surface of the specified point. The examiner increased the pressure at the rate of 1 kg/s until the subject experienced pain. The participants were instructed to verbally express their first perception of pain. Three PPTs were measured at each designated point, so an average PPT at each point could be ascertained. Testing points were marked with a water-soluble pen to ensure reliable and rapid location during the experimental procedure. All patients were investigated by the same doctor, who underwent professional training according to JTECH Medical guidelines and completed 30 hours of practice before data collection. The investigator was not directly connected with the study to ensure objective results, and did not know to which study group the patient belonged. The study patients tested never viewed the recorded values. In all patients, the PPT values were measured two times – at the beginning of the study and after 6 months observation.
The study was performed in the Department of Gynecological Endocrinology, Jagiellonian University Hospital, Cracow, Poland. The study was approved by the Bioethics Committee at the Collegium Medicum, Jagiellonian University in Cracow, Poland (Resolution No KBET/249/B/2014), and informed consent was signed by each woman.
Statistical analysis
Clinical and laboratory parameters, expressed on a continuous scale, were presented as means with standard deviations. Data did not present any significant outliers. Kolmogorov–Smirnov test revealed a normal distribution of data. Comparisons in baseline parameters between groups were performed using analysis of variance (ANOVA). Changes in time after adjustment to the study groups were verified using ANOVA with repeated measures. In case of significant interaction between time and study group, paired t-test was also performed in each group. Finally, because of the simplicity of presentation, changes in all studied parameters in time were described in the metformin-treated PCOS group.
The mean of three PPT measurements executed at each designated point was applied in all calculations, their degree of consensus in terms of absolute agreement were verified using intraclass correlation coefficient (ICC), which is recommended to assess the degree of reproducibility of measurements. The 95% confidence interval (CI) and standard error of measurement (SEM) were also calculated to give an indication of the magnitude of disagreement between the PPT measurements.
Statistical analysis was made using Statistica 12 (Statsoft, Tulsa, OK, USA). Pearson correlations were used to assess the relation between pain threshold and β-endorphin levels.
Results
The main clinical characteristics of the study population at the beginning of the study are shown in Table 1. The PCOS women from both groups and controls were of a similar age and had a similar BMI. There were no significant differences in prolactin, estradiol, cortisol at 0800 hours and 1700 hours, glucose in OGTT, and thyroid hormones levels between the three studied groups. There were significant differences in LH, FSH, testosterone, SHBG, insulin in OGTT levels, and FAI ratios between PCOS women and controls. PCOS women have higher plasma β-endorphin levels and PPT values measured on deltoid and trapezius muscles than healthy controls.
ICC and SEM determined at specific points (Table 2), calculated separately for measurements performed at the beginning of the study and after 6 months observation, demonstrated strong reliability at both tested points (ICC =0.983–0.999).
Table 2.
ICC, 95% CI, and SEM of PPT values at each measurement point at the beginning of the study and after 6 months observation
| Parameter | ICC | 95% CI | SEM (kg/cm2) |
|---|---|---|---|
| PPT-deltoid muscle (beginning of the study) | 0.990 | 0.983–0.994 | 0.254 |
| PPT-trapezius muscle (beginning of the study) | 0.998 | 0.997–0.999 | 0.186 |
| PPT-deltoid muscle (after 6 months) | 0.990 | 0.983–0.994 | 0.188 |
| PPT-trapezius muscle (after 6 months) | 0.997 | 0.999–0.998 | 0.213 |
Abbreviations: ICC, intraclass correlation coefficient; CI, confidence interval; SEM, standard error of measurement; PPT, pressure pain threshold.
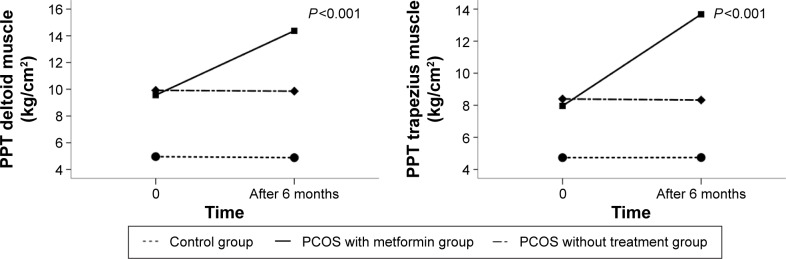
We observed significant interactions between time and study group in PPT levels (P<0.001). Figure 1 presents changes in PPT values measured on deltoid and trapezius muscles in all studied groups during the entire period of observation. We observed a significant increase in PPT values measured on deltoid and trapezius muscles only in the PCOS in the metformin group (4.81±0.88 kg/cm2 on deltoid muscle and 5.71±1.16 kg/cm2 on trapezius muscle, P<0.001). There were no significant changes in PPT values in time observed in the PCOS without treatment group and controls.
Figure 1.
Estimated marginal means of PPT values measured on deltoid and trapezius muscle at the beginning of the study and after 6 months of observation in all studied groups. Analysis was carried out using ANOVA test with repeated measures. Significance level <0.05.
Abbreviations: PPT, pressure pain threshold; PCOS, polycystic ovary syndrome.
We did not observe any significant changes in time in serum β-endorphin levels in any studied groups during 6 months observation (Figure 2).
Figure 2.
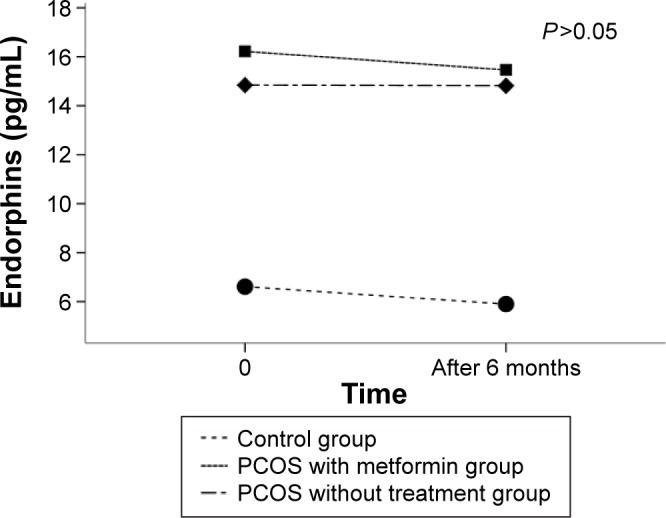
Estimated marginal means of plasma β-endorphins levels at the beginning of the study and after 6 months of observation in all studied groups.
Note: Analysis was carried out using ANOVA test with repeated measures. Significance level <0.05.
Abbreviation: PCOS, polycystic ovary syndrome.
Significant interactions between time and study group were observed also in insulin, testosterone, LH, FAI, and SHGB levels. Table 3 presents the comparison of all evaluated parameters in women from group 1 at the beginning of the study and after 6 months of metformin administration. We observed a decrease in testosterone level (−0.71±0.37 nmol/L, P<0.001) and an increase in SHBG level (9.13±6.51 nmol/L, P<0.001), and as a result, a significant decrease in the FAI ratio (−3.06±1.65, P<0.001) after 6 months of metformin therapy in lean PCOS women. We also observed a decrease in LH level (−5.91±1.95 mIU/mL, P<0.001) and insulin in OGTT levels (0′: −4.4±2.12 μU/mL, 60′: −33.6±14.89 μU/mL, 120′: −34.33±10.3 μU/mL, P<0.001) after 6 months metformin administration in group 1.
Table 3.
Clinical characteristics of lean women from the PCOS with metformin group (1) at the beginning of the study and after 6 months metformin therapy
| Parameter | PCOS with metformin group n=15 (beginning of the study) | PCOS with metformin group n=15 (after 6 months metformin therapy) | Mean difference in parameters between 6 months and baseline in PCOS with metformin group | P-value |
|---|---|---|---|---|
| Weight (kg) | 60.33±3.5 | 59.67±3.46 | −0.67±1.54 | 0.12 |
| BMI (kg/m2) | 21.19±1.27 | 20.95±1.23 | −0.24±0.54 | 0.11 |
| FT3 (pmol/L) | 4.41±0.49 | 4.51±0.76 | 0.1±0.52 | 0.46 |
| TSH (IU/mL) | 1.99±0.62 | 1.98±0.57 | −0.01±0.03 | 0.12 |
| FT4 (pmol/L) | 13.01±2.2 | 13.2±1.7 | 0.09±0.67 | 0.63 |
| Prolactin (μIU/mL) | 243.9±87.18 | 239.67±85.17 | −4.27±23.62 | 0.49 |
| Cortisol-0800 hours (μm/dL) | 21.5±6.82 | 19.8±5.08 | −1.7±2.65 | 0.45 |
| Cortisol-1700 hours (μm/dL) | 11.3±4.48 | 10.9±4.34 | −0.4±2.12 | 0.33 |
| Estradiol (pmol/L) | 270.8±113.7 | 270.33±98.42 | −0.47±31.3 | 0.96 |
| LH (mIU/mL) | 11.46±2.74 | 5.55±1.51 | −5.91±1.95 | <0.001 |
| FSH (mIU/mL) | 6.87±1.08 | 6.79±1.04 | −0.08±0.96 | 0.39 |
| Testosterone (nmol/L) | 2.51±0.34 | 1.8±0.29 | −0.71±0.37 | <0.001 |
| SHBG (nmol/L) | 37.59±6.33 | 46.7±4.75 | 9.13±6.51 | <0.001 |
| FAI | 6.97±1.47 | 3.91±0.73 | −3.06±1.65 | <0.001 |
| Glucose 0′ (mmol/L) | 4.8±0.6 | 4.67±0.34 | −0.13±0.28 | 0.47 |
| Glucose 60′ (mmol/L) | 5.5±1.44 | 5.2±0.1.23 | −0.3±0.63 | 0.54 |
| Glucose 120′ (mmol/L) | 5.1±0.92 | 5.0±0.87 | −0.1±0.43 | 0.67 |
| Insulin 0′ (μU/mL) | 14.4±5.49 | 10.2±3.45 | −4.4±2.12 | <0.001 |
| Insulin 60′ (μU/mL) | 83.8±32.26 | 50.2±28.97 | −33.6±14.89 | <0.001 |
| Insulin 120′ (μU/mL) | 78.2±35.48 | 43.87±21.23 | −34.33±10.3 | <0.001 |
| β-Endorphins (pg/mL) | 16.22±2.04 | 15.46±1.82 | −0.76±1.93 | 0.15 |
| PPT deltoid muscle (kg/cm2) | 9.56±1 | 14.37±0.94 | 4.81±0.88 | <0.001 |
| PPT trapezius muscle (kg/cm2) | 7.96±0.98 | 13.67±0.76 | 5.71±1.16 | <0.001 |
Notes: Data are presented as mean ± SD. Analysis was carried out using t-test for dependent samples. Significance level <0.05.
Abbreviations: PCOS, polycystic ovary syndrome; BMI, body mass index; FT3, triiodothyronine; TSH, thyroid-stimulating hormone; FT4, thyroxin; SHBG, sex hormone-binding globulin; FAI, free androgen index; LH, luteinizing hormone; FSH, follicle-stimulating hormone; PPT, pressure pain threshold.
We did not observe any significant changes in those hormonal parameters during the 6 months observation in PCOS without treatment group nor in healthy controls.
Discussion
To the best of our knowledge, this study is the first to address the question of whether there are changes in PPTs in lean women with PCOS after metformin therapy. We also tried to determine whether there were any changes in β-endorphins and testosterone levels in lean PCOS women after 6 months metformin administration.
The use of metformin in PCOS women is associated with a more regular menstrual cycle, improved ovulation, and a reduction in circulating androgen levels.10,11 In preclinical pain models, metformin, as an AMPK activator, has been shown to be remarkably effective in reducing mechanical allodynia and nociceptor excitability.12,26–29 Moreover, the activation of AMPK with metformin has led to decrease pain in neuropathic and postsurgical pain models, suggesting that these drugs and this mechanism of action might be effective in humans.30 Labuzek et al17 described a successful case of pain management using metformin in a patient with adiposis dolorosa. Taylor et al16 revealed that metformin use is associated with a decrease in pain in patients with lumbar radiculopathy. They proposed that metformin might have clinical utility for chronic pain conditions involving injury to the peripheral nervous system. On the other hand, Smith et al14 in their study concluded that the use of metformin was not associated with lower pain scores in diabetes patients. It is unclear what the dose and duration of metformin therapy is necessary to activate the AMPK pathway in humans to result in clinically significant pain reduction.
Because metformin is commonly prescribed for PCOS women, we could have tested the hypothesis that metformin would have an impact on pain perception in this group of patients. Because of studies that showed that β-endorphin levels are directly correlated to patients’ body weight, we first performed our study on lean patients only to eliminate the possible influence of overweight and obesity for β-endorphins and other hormonal parameters.1,4 Second, we decided to limit our group of PCOS patients only to the lean ones to avoid the effect of fat tissue on plasma insulin and testosterone levels during metformin treatment. As the lean PCOS patients also benefit from metformin treatment in regard to reduction of androgenicity, we decided to carry out the study on lean ones. The other reason we decided to choose lean patients was that the subcutaneous fat layer of overweight and obese PCOS subjects may interfere with the readings of the Algometer.
We tested this hypothesis through PPT measurements. PPT has been recognized as an effective and reliable way to quantify pain measures.19–22 In the beginning of the study, PPT values were higher in both PCOS groups than in healthy controls, which could be the cause for higher plasma β-endorphin levels in PCOS patients. Some studies, as well as our earlier findings showed a connection between the high level of endorphins and the pathogenesis of PCOS.3,4,31 Several studies have reported that plasma opioid peptide levels correlate with pain perception index measured from cutaneous tissues.5–7 It was found that a higher concentration of endorphins was associated with an increased peripheral PPT.6 Sheps et al7 showed an association between increased plasma β-endorphin levels and peripheral PPT. Moreover, some studies have shown that testosterone has an antianalgesic effect.32 Studies in humans have indicated that testosterone levels are positively correlated with activation in the middle frontal cortex during electrical and thermal pain stimulation, leading to a decrease in pain perception.33,34 Choi et al34 revealed that a decrease in the level of testosterone is a possible factor contributing to the increase in pain sensation during the stressful condition. Although studies were performed on males only, it could indicate the impact of testosterone on pain perception. In our opinion, it could be interesting in the light of latest reports that revealed a higher prevalence of self-harm in PCOS women than in healthy controls.35 To the best of our knowledge, there are no studies that evaluated the connection between testosterone levels and pain perception in lean PCOS women. All mentioned findings could explain differences that we observed between PCOS women and healthy controls in the beginning of the study. However, in our study, there were no differences in FAI ratios, plasma β-endorphin levels, and PPT values between the two PCOS groups, so data from the beginning of the study can be compared. In the PCOS with metformin group, after 6 months therapy, we observed a significant increase in PPTs in both measurement points (Figure 1). There were no differences in PPT values in the PCOS without treatment group and healthy controls. An increase in PPTs values was followed by a decrease in testosterone levels. Moreover, there were no differences in plasma β-endorphin levels after 6 months of metformin administration (Figure 2), so the increase in PPTs values could not be caused by an increase in testosterone levels or changes in plasma β-endorphin concentration, which can be explained by earlier findings.3–7,32–34 It may indicate the role of AMPK activation and concomitant decreases in mTOR activity by metformin in its effect on increased pain perception in PCOS women. Our findings are in agreement with studies that suggested metformin could be considered a potential pain therapeutic in humans.16,17 It has been argued whether metformin acts as an analgesic or only as an antihyperalgesic agent in chronic pain conditions. Some studies suggest that this drug has only antihyperalgesic effect,16,17 but our findings may give new insight into the problem.
Insulin resistance and hyperinsulinemia affect 65%–70% of women with PCOS.36 The benefits of metformin on insulin sensitivity have been demonstrated in nondiabetic women with PCOS.8–11 In our study, we observed a decrease in insulin levels in OGTT after 6 months of metformin administration, which is consistent with the results of earlier studies.8–11
It has been suggested that metformin reduces hyperandrogenism through its effect on both the ovary and adrenal gland, suppressing their androgen production, reducing pituitary LH, and increasing the production of SHBG by the liver.37 Our results are consistent with earlier findings.8,9,37 We observed a decrease in testosterone and an increase in SHBG levels as well as a decrease in LH levels. The changes in testosterone and SHBG levels resulted in a decrease in the FAI ratio in lean PCOS women after 6 months of metformin administration. In the same 6 months, we observed an increase in PPTs values, which may suggest the possible link between these two phenomena. This link may require further study evaluating the effect of other testosterone-reducing medications on PPT values.
Limitations
The main limitation of the present study is the small number of patients investigated. In our opinion, larger prospective trials are needed to test the hypothesis that metformin might impact on pain perception. The other possible limitation is collecting only single plasma samples to assess β-endorphin levels. We took samples on the same days of menstrual cycles, and PPT measurement and blood collection were performed at the same time. Another limitation could be only single-day PPT measurement and the length of the study. However, some studies showed no differences in PPT values over consecutive days of testing.38 Determinations of pain threshold are, by their nature, subjective and affected by habituation. It could be interesting to continue the study to 12 or 18 months so as to evaluate the influence of the long-term effect of metformin on PPT.
Conclusion
We conclude that metformin therapy increased PPT in lean PCOS women, without affecting plasma β-endorphin concentration. In addition, we found a decrease in testosterone, LH, and insulin and an increase in SHBG levels in the lean PCOS patients after 6 months metformin administration. Our results may suggest the potential role of metformin in pain therapy in humans and confirm the beneficial effect on hormonal parameters in PCOS women.
Footnotes
Disclosure
No financial contribution was received from any potentially interested party during preparation of this clinical study. The authors report no conflicts of interest in this work.
References
- 1.Eyvazzadeh AD, Pennington KP, Pop-Busui R, Sowers MF, Zubieta JK, Smith YR. The role of the endogenous opioid system in polycystic ovary syndrome. Fertil Steril. 2009;92:1–12. doi: 10.1016/j.fertnstert.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod. 2008;23:2564–2569. doi: 10.1093/humrep/den273. [DOI] [PubMed] [Google Scholar]
- 3.Guido M, Romualdi D, Lanzone A. Role of opioid antagonists in the treatment of women with glucoregulation abnormalities. Curr Pharm Des. 2006;12:1001–1012. doi: 10.2174/138161206776055895. [DOI] [PubMed] [Google Scholar]
- 4.Kiałka M, Milewicz T, Spałkowska M, et al. β-endorphins plasma level is higher in lean polycystic ovary syndrome group (PCOS) Exp Clin Endocrinol Diabetes. 2016;124:55–60. doi: 10.1055/s-0035-1564094. [DOI] [PubMed] [Google Scholar]
- 5.Falcone C, Specchia G, Rondanelli R, et al. Correlation between beta-endorphin plasma levels and anginal symptoms in patients with coronary artery disease. J Am Coll Cardiol. 1988;11:719–723. doi: 10.1016/0735-1097(88)90202-1. [DOI] [PubMed] [Google Scholar]
- 6.Jarmukli NF, Ahn J, Iranmanesh A, Russell DC. Effect of raised plasma beta endorphin concentrations on peripheral pain and angina thresholds in patients with stable angina. Heart. 1999;82:204–209. doi: 10.1136/hrt.82.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheps DS, Ballenger MN, De Gent G, et al. Psychophysical responses to a speech stressor: correlation of plasma beta-endorphin concentrations at rest and post psychological stress with thermally measured pain threshold in patients with coronary artery disease. J Am Coll Cardiol. 1995;25:1499–1503. doi: 10.1016/0735-1097(95)00045-6. [DOI] [PubMed] [Google Scholar]
- 8.Johnson NP. Metformin use in women with polycystic ovary syndrome. Ann Transl Med. 2014;2:56. doi: 10.3978/j.issn.2305-5839.2014.04.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Meab. 2010;1:117–128. doi: 10.1177/2042018810380215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 11.Marthur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Tillu DV, Melemedjian OK, Asiedu MN, et al. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain. 2012;8:5. doi: 10.1186/1744-8069-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melemedjian OK, Asiedu MN, Tillu DV, et al. Targeting adenosine monophosphateactivated protein kinase in preclinical models reveals the mechanism for the treatment of neuropathic pain. Mol Pain. 2011;7:70. doi: 10.1186/1744-8069-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith B, Ang D. Metformin: potential analgesic. Pain Med. 2015;16:2256–2260. doi: 10.1111/pme.12816. [DOI] [PubMed] [Google Scholar]
- 15.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor A, Westveld AH, Szkudlinska M, et al. The use of metformin is associated with decreased lumbar radiculopathy pain. J Pain Res. 2013;6:755–763. doi: 10.2147/JPR.S52205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Łabuzek K, Liber S, Suchy D, Okopień B. A successful case of pain management using metformin in a patient with adiposis dolorosa. Int J Clin Pharmacol Ther. 2013;51:517–524. doi: 10.5414/CP201878. [DOI] [PubMed] [Google Scholar]
- 18.Phoenix Pharmaceuticals Inc General protocol for RK-022-14: endorphin, beta (human) – RIA Kit (range: 10–1280 pg/ml) Available from: http://www.phoenixpeptide.com/catalog/repository/QCdata_RIK/RK-022-14.pdf.
- 19.Ohrbach R, Gale E. Pressure pain threshold in normal muscles: reliability, measurements effects, and topographic differences. Pain. 1989;37:257–263. doi: 10.1016/0304-3959(89)90189-9. [DOI] [PubMed] [Google Scholar]
- 20.Ylinen J, Nykanen M, Kautiainen H, Hakkinen A. Evaluation of repeatability of pressure algometry on the neck muscles for clinical use. Man Ther. 2007;12:192–197. doi: 10.1016/j.math.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Potter L, McCarthy C, Oldham J. Algometer reliability in measuring pain thresholds over spinal muscles quantification of anti-nociceptive treatment effects. Int J Osteopath Med. 2006;9:113–119. [Google Scholar]
- 22.Jones D, Kilgour R, Comtois A. Test-retest reliability of pressure pain threshold measurements of the upper limb and torso in young healthy women. J Pain. 2007;8:650–656. doi: 10.1016/j.jpain.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23:760–766. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg U, Dohns IE, Melin B, et al. Psychophysiological stress responses, muscle tension, and neck and shoulder pain among supermarket cashiers. J Occup Health Psychol. 1999;4:245–255. doi: 10.1037//1076-8998.4.3.245. [DOI] [PubMed] [Google Scholar]
- 25.Persson AL, Brogårdh C, Sjölund BH. Tender or not tender: test-retest repeatability of pressure pain thresholds in the trapezius and the deltoid muscles of healthy women. J Rehabil Med. 2004;36:17–27. doi: 10.1080/16501970310015218. [DOI] [PubMed] [Google Scholar]
- 26.Obara I, Géranton SM, Hunt SP. Axonal protein synthesis: a potential target for pain relief? Curr Opin Pharmacol. 2012;12:42–48. doi: 10.1016/j.coph.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Price TJ, Géranton SM. Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology. Eur J Neurosci. 2009;29:2253–2263. doi: 10.1111/j.1460-9568.2009.06786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melemedjian OK, Khoutorsky A, Sorge RE, et al. mTORC1 inhibition induces pain via IRS-1-dependent feedback activation of ERK. Pain. 2013;154:1080–1091. doi: 10.1016/j.pain.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melemedjian OK, Yassine HN, Shy A, Price TJ. Proteomic and functional annotation analysis of injured peripheral nerves reveals ApoE as a protein upregulated by injury that is modulated by metformin treatment. Mol Pain. 2013;9:14. doi: 10.1186/1744-8069-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Łabuzek K, Liber S, Marcol W, Okopień B. Controlling newly diagnosed type 2 diabetes mellitus with metformin managed pain symptoms in a patient affected with Dercum’s disease. Pain Med. 2012;13:1526–1527. doi: 10.1111/j.1526-4637.2012.01487.x. [DOI] [PubMed] [Google Scholar]
- 31.Wortsman J, Wehrenberg WB, Gavin JR, Allen JP. Elevated levels of plasma beta-endorphin and melanocyte stimulating hormone in the polycystic ovary syndrome. Obstet Gynecol. 1984;63:630–635. [PubMed] [Google Scholar]
- 32.Edinger KL, Frye CA. Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Choi JC, Yi DJ, Han BS, Lee PH, Kim JH, Kim BH. Placebo effects on analgesia related to testosterone and premotor activation. Neuroreport. 2011;22:419–423. doi: 10.1097/WNR.0b013e32834601c9. [DOI] [PubMed] [Google Scholar]
- 34.Choi JC, Chung MI, Lee YD. Modulation of pain sensation by stress-related testosterone and cortisol. Anaesthesia. 2012;67:1146–1151. doi: 10.1111/j.1365-2044.2012.07267.x. [DOI] [PubMed] [Google Scholar]
- 35.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term using data linkage. J Clin Endocrinol Metab. 2015;100:911–919. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 36.DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454–1460. doi: 10.1016/j.fertnstert.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 37.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 38.Nussbaum EL, Downes L. Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Phys Ther. 1998;78:160–161. doi: 10.1093/ptj/78.2.160. [DOI] [PubMed] [Google Scholar]