Abstract
Objective:
While compensatory saccades indicate vestibular loss in the conventional head impulse test paradigm (HIMP), in which the participant fixates an earth-fixed target, we investigated a complementary suppression head impulse paradigm (SHIMP), in which the participant is fixating a head-fixed target to elicit anticompensatory saccades as a sign of vestibular function.
Methods:
HIMP and SHIMP eye movement responses were measured with the horizontal video head impulse test in patients with unilateral vestibular loss, patients with bilateral vestibular loss, and in healthy controls.
Results:
Vestibulo-ocular reflex gains showed close correlation (R2 = 0.97) with slightly lower SHIMP than HIMP gains (mean gain difference 0.06 ± 0.05 SD, p < 0.001). However, the 2 paradigms produced complementary catch-up saccade patterns: HIMP elicited compensatory saccades in patients but rarely in controls, whereas SHIMP elicited large anticompensatory saccades in controls, but smaller or no saccades in bilateral vestibular loss. Unilateral vestibular loss produced covert saccades in HIMP, but later and smaller saccades in SHIMP toward the affected side. Cumulative HIMP and SHIMP saccade amplitude differentiated patients from controls with high sensitivity and specificity.
Conclusions:
While compensatory saccades indicate vestibular loss in conventional HIMP, anticompensatory saccades in SHIMP using a head-fixed target indicate vestibular function. SHIMP saccades usually appear later than HIMP saccades, therefore being more salient to the naked eye and facilitating vestibulo-ocular reflex gain measurements. The new paradigm is intuitive and easy to explain to patients, and the SHIMP results complement those from the standard video head impulse test.
Classification of evidence:
This case-control study provides Class III evidence that SHIMP accurately identifies patients with unilateral or bilateral vestibulopathies.
In the conventional head impulse paradigm (HIMP), the compensatory saccade is an indicator of semicircular canal loss.1–4 Here, the patient is instructed to maintain fixation on an earth-fixed target during head rotation toward their tested ear (video 1 on the Neurology® Web site at Neurology.org). In patients with vestibular loss, the vestibulo-ocular reflex (VOR) does not correct for the head movement, so that fixation is taken off the target, requiring a compensatory saccade to regain the target (videos 2 and 3). In contrast, healthy participants barely make compensatory saccades, as their VOR corrects for the head movement to maintain visual fixation on the earth-fixed target (videos 2 and 4).
Herein, we present a modified “suppression” head impulse paradigm (SHIMP) resulting in a complementary saccadic pattern: now the patient is instructed to follow a target from a head-mounted laser, which is moving with the head (video 1). Patients with vestibular loss complete this task without corrective saccades, because their eyes move with the head (videos 2 and 3). Instead, it is the healthy participants who make anticompensatory saccades to regain the target after the head turn, because their healthy VOR drives their eyes off the head-fixed target (videos 2 and 4).
Both paradigms provide 2 indicators of semicircular canal function: VOR gain and the presence of corrective saccades. While the VOR gain measures are predicted to be similar in both paradigms, the saccades are expected to be complementary: with HIMP, compensatory saccades indicate vestibular loss, whereas with SHIMP, anticompensatory saccades indicate vestibular function. These predictions were tested in healthy participants and in patients with unilateral (UVL) and bilateral vestibular loss (BVL).
METHODS
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all participants, and the protocol was approved by the Sydney South West Area Health Service Ethics Committee and the Cassino Ethics Committee in accordance with the Helsinki Declaration.
Participants.
Participants were tested in Sydney, Australia, and Cassino, Italy, between February and April 2011. Five patients (age range 37–73 years) with BVL (2 systemic gentamicin vestibulotoxicity, 3 idiopathic BVL) fulfilled the inclusion criterion of a total caloric response of <30°/s (table e-1).5 Five patients with UVL (operated unilateral vestibular Schwannoma with unilateral vestibular nerve transection, age range 40–70 years) were enrolled. Six participants (age range 28–68 years) without any history of vestibular disease served as healthy controls.
Study design.
The case-control study was a prospective comparison of HIMP and SHIMP using video head impulse test (vHIT) to test horizontal semicircular canal function in healthy controls and patients with prior, independently identified vestibular deficits. In every case both testing paradigms were undertaken in the one testing session. The results of the study are reported in accordance with the STROBE statement.6 The primary purpose of the study was to provide Class III evidence that the SHIMP accurately identifies patients with unilateral or bilateral vestibulopathies.
Experimental procedure.
HIMP.
Participants were instructed to fixate an earth-fixed dot on a wall about 90 cm away. Approximately 20 horizontal head impulses, with unpredictable timing and direction, were manually delivered by the experimenter to each side. Target peak head velocity of the impulses was about 150°/s to 250°/s. To preserve any corrective saccades, particular care was taken to minimize overshoot and return at the end of the head turn (“bounce”).
SHIMP.
Exactly the same procedure was used as for HIMP with the sole difference being that the participants were asked to fixate a target, which moved with the head. This target was a spot projected onto the wall in front of the participant by a miniature class 1 laser mounted onto the goggles.
Video-oculography.
The methods for video head impulse recording have been described in detail previously.2,3,7,8 A high-speed, lightweight, digital video camera (Firefly MV; Point Grey Research Inc., Vancouver, BC) mounted on a glasses frame viewed the right eye via an infrared reflecting mirror and recorded eye position at a frame rate of 250 Hz. The low weight of the system (approximately 60 g) minimized slippage of the glasses. Two infrared light-emitting diodes (TSUS502; Vishay Intertechnology, Malvern, PA) run at 20 mA illuminated the eye with infrared levels far below exposure risk levels.9 Head velocity was measured by triaxial orthogonal gyroscopes (IDG-300; InvenSense, Santa Clara, CA) mounted on the glasses frame. The in vivo calibration of eye position required participants to fixate on projected targets from small lasers mounted on the glasses. A laptop running online programs in LabVIEW (National Instruments, Austin, TX) detected the pupil center by a center-of-gravity algorithm, and a 2-point differentiator yielded eye velocity, which was then low-pass-filtered (0–30 Hz bandwidth) for further processing.10
Data analysis.
Offline data analysis used customized LabVIEW software. Analysis bias was avoided by fully automated data analysis without manual interference. Each head impulse was detected and aligned at peak head acceleration.4 If the eye velocity lay outside an envelope around the expected eye velocity response, it was classified as a blink or outlier and automatically excluded.3 An eye acceleration algorithm was used to detect saccades, which were removed for VOR gain analysis.2 The gain of the VOR for each impulse was calculated as the ratio of the area under the de-saccaded eye velocity to the area under the head velocity.2 The points defining the boundaries of the head impulse were defined from the moment when head velocity exceeded 5% of peak head velocity to the moment when head velocity crossed zero again.4 Cumulative HIMP and SHIMP saccade amplitude was calculated as the sum of the amplitudes of all saccades for each side divided by the number of trials. Weighted median HIMP and SHIMP saccade latency was calculated for each side as the median latency of all saccades weighted by their amplitudes.
Statistical analysis.
Receiver operating characteristic statistics were calculated with MedCalc software (Ostend, Belgium). To test whether VOR gains with standard HIMPs were significantly different from VOR gains with SHIMPs, we used paired sample t tests (significance level p = 0.05).11 The goodness of fit of the linear correlation between VOR gains from HIMP and SHIMP was estimated by the coefficient of determination (R2).
RESULTS
We measured horizontal vHIT in 6 healthy controls, 5 patients with UVL, and 5 patients with BVL. We analyzed saccade patterns as well as VOR gains to compare SHIMP with a head-fixed target to conventional HIMP with an earth-fixed target (table e-1).
Saccade analysis.
For comparison of the saccadic pattern during SHIMP to conventional HIMP, we juxtaposed examples of a healthy control (figure 1), a patient with BVL (figure 2), and a patient with UVL (figure 3, see also videos 2–4). In all participants, SHIMP and HIMP resulted in a reversed saccadic pattern: during HIMP, healthy controls elicited only few positive catch-up saccades, while during SHIMP, they elicited large negative saccades back to the head-fixed target after the end of the head impulse (figure 1). Patients with BVL showed the opposite pattern with mostly overt saccades back to the stationary target during HIMP, but only few downward saccades during SHIMP (figure 2). Patients with UVL often elicited covert saccades with impulses to the affected side during HIMP but large downward saccades with impulses to the healthy side during SHIMP (figure 3).
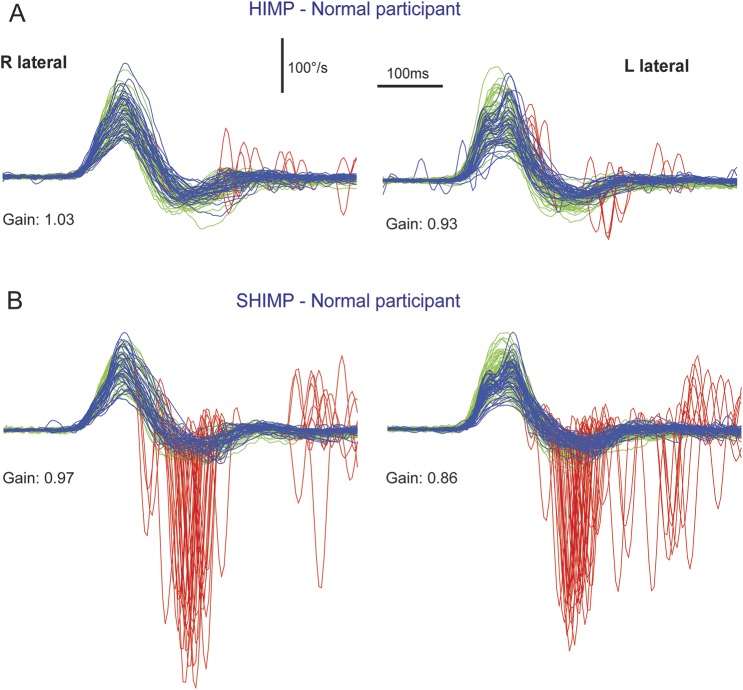
Figure 1. Video head impulse test of a healthy control with SHIMP compared to conventional HIMP.
During SHIMP (B), the participant's task is to fixate a target, which is moving with the head, whereas in conventional HIMP (A), the target remains stationary. The figure illustrates the typical HIMP and SHIMP saccade pattern in a healthy control. (A) During conventional HIMP, a healthy control elicits only few mostly positive catch-up saccades (red) after the end of the head impulse. (B) During SHIMP, the same healthy control shows large negative saccades after the end of the head impulse reflecting anticompensatory eye movements back to the head-fixed target. Both paradigms give similar but slightly lower vestibulo-ocular reflex gain values during SHIMP compared to HIMP, but a complementary saccade pattern. Head velocity = green traces; inverted slow phase eye velocity = blue traces; saccades = red traces; HIMP = conventional head impulse paradigm; SHIMP = suppression head impulse paradigm.
Figure 2. Video head impulse test of a patient with BVL using SHIMP compared to conventional HIMP.
Typical patient with complete BVL showing a reversed saccadic pattern during HIMP and SHIMP compared to a healthy control (figure 1). (A) During standard HIMP, the patient with BVL elicits mostly overt positive catch-up saccades after the head impulse. (B) During SHIMP, the same patient with BVL shows only very few downward saccades reflecting anticompensatory saccades after the end of the head impulse back to the head-fixed target. Both paradigms give similar but slightly lower vestibulo-ocular reflex gain values during SHIMP compared to HIMP, but a complementary saccade pattern, which is reversed compared to healthy controls. Head velocity = green traces; inverted slow phase eye velocity = blue traces; saccades = red traces. BVL = bilateral vestibular loss; HIMP = conventional head impulse paradigm; SHIMP = suppression head impulse paradigm.
Figure 3. Video head impulse test of a patient with UVL using SHIMP compared to conventional HIMP.
Typical patient with UVL showing reversed saccadic patterns during HIMP compared to SHIMP to the healthy and affected side. (A, affected) With standard HIMP, the patient elicits stereotyped covert saccades during head impulses to the affected side. (B, affected) With SHIMP, the patient elicits only small negative saccades after impulses to the affected side. Note that compared to HIMP (A, affected), SHIMP (B, affected) clears the eye velocity traces from covert saccades during head impulses to the affected side, thus facilitating gain calculation. Head impulses to the healthy side produce only small negative saccades during HIMP (A, healthy), but large negative saccades during SHIMP (B, healthy). Vestibulo-ocular reflex gain values to the healthy side are slightly lower during SHIMP compared to HIMP, but very similar to the affected right side. Head velocity = green traces; inverted slow phase eye velocity = blue traces; saccades = red traces; HIMP = conventional head impulse paradigm; SHIMP = suppression head impulse paradigm; UVL = unilateral vestibular loss.
For summarizing the saccadic patterns in the different patient groups, histograms with cumulative saccade amplitude comprising all participants were calculated (figure 4). For HIMP, positive saccades were cumulated as a function of latency after head impulse onset (upward histogram bars), while for SHIMP, negative saccades were cumulated (downward histogram bars).
Figure 4. Cumulative saccade amplitude as a function of latency after head impulse onset.
(A) In healthy controls, HIMP elicits only a few saccades (upward histogram bars), while SHIMP elicits a multitude of saccades (downward histogram bars) with a peak latency of about 176 milliseconds (ms). (C) Patients with BVL show a reversed saccadic pattern with large saccades in HIMP but only a few saccades in SHIMP. Patients with UVL often produce covert HIMP saccades with head impulses to the affected side (B) and overt SHIMP saccades to the healthy side (D). Note that in the same patients with UVL, overt SHIMP saccades to the healthy side (D) have a longer peak latency (176 ms) compared to the covert HIMP saccades to the affected side (104 ms, B). Histogram bars represent summated amplitudes of HIMP saccades (positive) and SHIMP saccades (negative) in 8-ms bins after head impulse onset. Saccade amplitude was normalized relative to the number of head impulses and participants and kept in proportion between participant groups (A, n = 6 controls × 2 sides), patients with UVL (B, affected side; D, healthy side, n = 5), and patients with BVL (C, n = 5 × 2). BVL = bilateral vestibular loss; HIMP = conventional head impulse paradigm; SHIMP = suppression head impulse paradigm; UVL = unilateral vestibular loss.
Healthy controls elicited only a few HIMP saccades but a multitude of SHIMP saccades with a mean weighted median latency of 185 milliseconds (ms) ± 20 SD, indicating normal vestibular function (figure 4A). In contrast, patients with BVL produced mainly HIMP saccades with a mean weighted median latency of 223 ms ± 35 SD (figure 4C). However, because BVL was incomplete in some patients with BVL, they also produced a few SHIMP saccades, indicating residual vestibular function. With 292 ms ± 69 SD, the mean weighted median latency of these SHIMP saccades was significantly longer than the corresponding HIMP saccades in the same patients with BVL (p = 0.0032). Cumulative HIMP saccade amplitude with a >0.78°/trial cutoff discriminated patients with BVL from healthy controls with 100% sensitivity (95% confidence interval 69%–100%) and 100% specificity (74%–100%) and an area under the receiver operating characteristic curve (AUC) of 1.0 (0.85–1.0, p < 0.0001; table e-2). Cumulative SHIMP amplitude with a more than −2.51°/trial cutoff discriminated patients with BVL from healthy controls with 90% sensitivity (56%–100%) and 100% specificity (74%–100%) and an AUC of 0.99 (0.83–1.0, p < 0.0001).
To their affected side, some patients with UVL elicited covert HIMP saccades with weighted median latencies of 120 to 140 ms, others only late overt saccades (mean weighted median latency 269 ms ± 128 SD) (figure 4B). However, patients with UVL produced only overt SHIMP saccades with a mean weighted median latency of 238 ms ± 46 SD to their affected side. To their healthy side, patients with UVL produced almost no HIMP saccades and mostly overt SHIMP saccades with a mean weighted median latency of 202 ms ± 41 SD (figure 4D). Both cumulative HIMP saccade amplitude (>0.78°/trial) and SHIMP saccade amplitude (more than −2.51°/trial) discriminated patients with UVL on their affected side from healthy controls with 100% sensitivity (48%–100%) and 100% specificity (74%–100%) and an AUC of 1.0 (0.81–1.0, p < 0.0001). While cumulative HIMP saccade amplitude could not discriminate between the healthy side of UVL and normal controls (AUC 0.51, p = 0.96), cumulative SHIMP amplitude (more than −5.18°/trial) distinguished the two with 80% sensitivity (28%–100%) and 83% specificity (52%–98%) and an AUC of 0.82 (0.56–0.96, p = 0.0049).
VOR gain.
Both HIMP gains (<0.76) and SHIMP gains (<0.66) discriminated patients with BVL from normal controls with 100% sensitivity (69%–100%) and 100% specificity (74%–100%) and an AUC of 1.0 (0.85–1.0, p < 0.0001; table e-2). Similarly, HIMP gains (<0.76) and SHIMP gains (<0.66) identified the affected side of UVL with 100% sensitivity (48–100) and 100% specificity (74–100) and an AUC of 1.0 (0.81–1.0, p < 0.0001). For separating the healthy side of UVL from healthy controls, both HIMP gains (<0.76) and SHIMP gains (<0.66) reached 60% sensitivity (15%–95%) but 100% specificity (74%–100%) with HIMP AUC of 0.85 (0.60–0.97, p = 0.0017) and SHIMP AUC of 0.84 (0.59–0.97, p = 0.0024).
The similarity of VOR gain measures for SHIMP and HIMP was compared across all patients and controls. SHIMP gains were slightly lower than HIMP gains (mean gain difference 0.06 ± 0.05 SD, p < 0.001). With the exception of the small gain values to the affected side in patients with UVL, this difference was significant in all subgroups. The coefficient of determination confirmed close correlation (R2 = 0.97) between the VOR gains of the 2 paradigms across all patients and controls (n = 16 participants × 2 sides).
vHIT model.
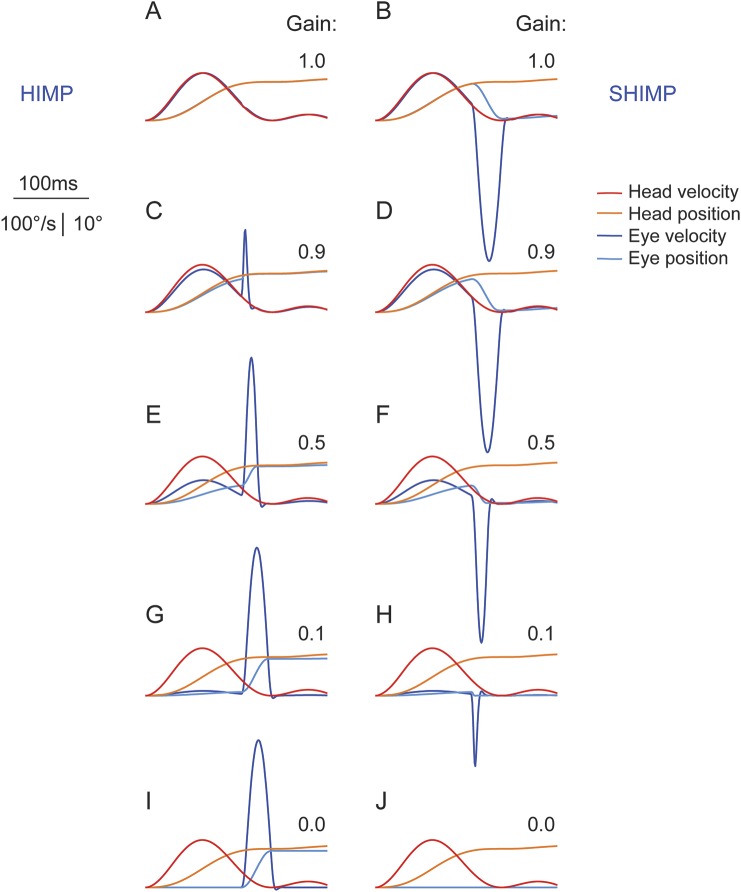
Figure 5 illustrates the salience of saccades of different peak velocity with respect to their amplitude and VOR deficit. Ideally, HIMP elicits no saccades in healthy controls with unity gain (figure 5A), while SHIMP elicits no saccades in patients with total BVL and zero gain (figure 5J), as no corrective eye movements should be necessary under these conditions. Little residual VOR in SHIMP (figure 5H), as well as small deficits in HIMP, are sufficient to trigger saccades (figure 5C). In the velocity domain, the size of these small saccades is overestimated by the naked eye, as the relationship between peak velocity and amplitude of saccades, often referred to as “main sequence,”12 is nonlinear. The salience of these small saccades makes them a sensitive indicator of residual VOR function in SHIMP and subtle deficits in HIMP, respectively.
Figure 5. Video head impulse test model for illustration of saccade size in relation to VOR gain deficit.
(A) In a healthy control, a head impulse with an earth-fixed target (HIMP) elicits no saccade. (B) In the same healthy control with a VOR gain of one, a head impulse with a head-fixed target (SHIMP) elicits an anticompensatory saccade of the size of the head rotation (16.5°). (J) Conversely, in a patient with total bilateral vestibular loss with a VOR gain of zero, SHIMP elicits no saccade while HIMP elicits a saccade the size of the head rotation (I). (C) Little VOR loss (gain 0.9) is sufficient to elicit a small compensatory saccade with HIMP. (H) In a patient with incomplete vestibular loss, little residual function (gain 0.1) is sufficient to elicit a small anticompensatory saccade with SHIMP. Note that on visual inspection in the velocity domain, the amplitude of smaller saccades (C, 1.5° amplitude) is overestimated compared to the amplitude of larger saccades (I, 15.8° amplitude). HIMP = conventional head impulse paradigm; SHIMP = suppression head impulse paradigm; VOR = vestibulo-ocular reflex.
DISCUSSION
In this study, we introduced a complementary head impulse paradigm (video 1). While for traditional HIMP, participants were instructed to fixate an earth-fixed target, in SHIMP we asked them to follow a target that moved with the head. We have shown that the VOR gain measures for the 2 paradigms correlate well with slightly lower gain values for SHIMP compared to traditional HIMP. However, the observed saccadic patterns during the 2 paradigms were complementary: While the compensatory saccades opposite to the head movement in HIMP indicate vestibular loss, the appearance of anticompensatory saccades with the head movement in SHIMP indicates vestibular function with high sensitivity and specificity (videos 2–4).
Catch-up saccades during traditional HIMP directly reflect the clinical sign of canal paresis as observed by the physician at the bedside.1 While overt saccades after the head movement are detectable by the naked eye, covert saccades during the head movement may be imperceptible to the clinical observer, as they cannot be distinguished from the residual VOR response.4 Nevertheless, cumulative amplitude of overt saccades after the head movement has been shown to be a useful marker for vestibular loss complementary to the VOR gain.13
Contrary to HIMP, SHIMP saccades in the direction of head rotation indicate vestibular function rather than loss, as they have to correct for any VOR in order to bring the eyes back to the head-fixed target. Our study has shown that the appearance of anticompensatory saccades in the direction of the head movement is a sensitive marker of residual vestibular function in SHIMP. Detecting residual vestibular function in patients with vestibular loss is of great clinical importance for vestibular rehabilitation, as it may help patients in compensating for their vestibular deficit by triggering early catch-up saccades.14,15
Traditionally, the main measurement parameter for head impulse testing was VOR gain. VOR gain as the ratio between head and eye movement has usually been measured during the first 80 to 100 ms before the appearance of the first catch-up saccades.16 Unfortunately, this time window is most susceptible to video recording artifacts caused by goggle slippage.2,17 Therefore, we recently proposed an improved algorithm, which calculates gain during the entire head impulse, but removes any catch-up saccades that can interfere with accurate VOR measures, before analysis.2 Because SHIMP saccades usually appear after the end of the head impulse, SHIMP eliminates most catch-up saccades in the sensitive time period for VOR gain calculation during the head impulse in patients with UVL (figure 3, affected side), thus facilitating more accurate gain measurements under these conditions. This may be of particular advantage in patients with acute vestibular neuritis, as SHIMP clears the head impulses to the affected side from contamination with spontaneous nystagmus.
Previous evidence has shown that healthy controls can, after a delay, suppress their slow phase eye velocity response elicited by semicircular canal stimulation. Crane and Demer18 found that the latency of VOR suppression with a visual target during high acceleration whole-body rotations was about 80 to 90 ms. Therefore, it may be expected that participants would be able to suppress their VOR to some extent during the head turn in SHIMP. Indeed, we found slightly but significantly lower VOR gains during SHIMP compared to HIMP. Correspondingly, the only subgroup that did not show such a difference was the one with the patients with UVL to the affected side where VOR gains were low a priori. Alternatively, the de-saccading algorithm,2 which is used to remove the catch-up saccades during the time window for VOR gain measurements, may be responsible for this systematic difference. This, in turn, would be an additional argument in favor of SHIMP, as it usually delays any saccades until after the end of the head impulse.
Proper vHIT examination technique is crucial to avoid measurement artifacts.17 For accurate VOR gain measurements, ballistic head impulses of sufficient speed (ideally approximately 200°/s) are important, while tight goggle fit must be ensured to avoid slippage. For the subsequent saccade pattern, the ending of the impulse is of paramount importance. Overshoot (“bounce”) of the head at the end of the impulse is destructive, as it diminishes the amplitude of both SHIMP saccades and HIMP saccades. The ideal head impulse is therefore a position step (“turn and stop”) rather than a bounce. Hence, a skilled operator and sufficient practice are necessary to ensure good examination quality.
We have found that SHIMP is equally simple to explain to patients as conventional HIMP, and patients reported that the task is easy to perform, comparing it to watching the ball during a tennis match. It is an easy intuitive task and the “game-like” test situation provides accurate, objective measures of vestibular function and saccadic compensation. The head-fixed target can be a cyclist's headlamp or a laser pointer on a bite bar, projecting a spot on the wall. SHIMP saccades can even be observed at the bedside: the clinician, standing to one side, can see SHIMP saccades easily since they are usually very large and later than HIMP saccades. In contrast to HIMP saccades, which are a sensitive indicator of vestibular loss, SHIMP saccades are a clinical sign of vestibular function. Therefore, the 2 complementary paradigms have their diagnostic strengths at opposite ends of the vestibular disease spectrum. Routine application will be necessary to acquire more experience about the clinical utility of SHIMP at the bedside, and further studies will be needed to determine its diagnostic accuracy in vHIT measurements.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all the participants of the study. The authors acknowledge Rosie Menzies for proofreading the manuscript and thank Muriel Dysli for help in recording the videos.
GLOSSARY
- AUC
area under the curve
- BVL
bilateral vestibular loss
- HIMP
conventional head impulse paradigm
- SHIMP
suppression head impulse paradigm
- UVL
unilateral vestibular loss
- vHIT
video head impulse test
- VOR
vestibulo-ocular reflex
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
G.M.H. discovered the SHIMP saccade. H.G.M. designed and built the vHIT system, programmed and analyzed the data, completed the statistical analysis, tested participants and patients, and developed the model. K.P.W. tested participants and patients, analyzed the data, and wrote the paper. L.A.M. tested participants and patients. S.J.R. developed the iPad VOR model for generating the supplementary videos. L.M. tested participants and patients and assisted in writing. A.M.B. assisted with analysis, figures, and writing. I.S.C. analyzed data, completed the statistical analysis, and wrote the paper.
STUDY FUNDING
This project was supported by the Garnett Passe and Rodney Williams Memorial Foundation, the National Health and Medical Research Council Australia (grants 632746 and 1046826), the Betty and David Koetser Foundation for Brain Research, and the Forschungskredit of the University of Zurich.
DISCLOSURE
H. MacDougall, L. McGarvie, G. Halmagyi, I. Curthoys, and K. Weber act as unpaid consultants and have received funding for travel and free equipment for beta testing from GN Otometrics. However, the study was conducted with a custom-built, noncommercial prototype and the authors have no commercial interest in video head impulse systems. S. Rogers, L. Manzari, and A. Burgess report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol 1988;45:737–739. [DOI] [PubMed] [Google Scholar]
- 2.MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. The video head impulse test (vHIT) detects vertical semicircular canal dysfunction. PLoS One 2013;8:e61488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology 2009;73:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology 2008;70:454–463. [DOI] [PubMed] [Google Scholar]
- 5.Barin K. Interpretation and usefulness of caloric testing. In: Jacobson GP, Shepard NT, editors. Balance Function Assessment and Management, 2nd ed. San Diego: Plural Publishing; 2016:319–345. [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 7.Weber KP, MacDougall HG, Halmagyi GM, Curthoys IS. Impulsive testing of semicircular-canal function using video-oculography. Ann NY Acad Sci 2009;1164:486–491. [DOI] [PubMed] [Google Scholar]
- 8.MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP. Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol Neurotol 2013;34:974–979. [DOI] [PubMed] [Google Scholar]
- 9.Delori FC, Webb RH, Sliney DH, American National Standards: I: maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J Opt Soc Am A Opt Image Sci Vis 2007;24:1250–1265. [DOI] [PubMed] [Google Scholar]
- 10.Moore ST, Curthoys IS, McCoy SG. VTM: an image-processing system for measuring ocular torsion. Comput Methods Programs Biomed 1991;35:219–230. [DOI] [PubMed] [Google Scholar]
- 11.Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design, 3rd ed. New York: McGraw-Hill; 1991. [Google Scholar]
- 12.Bahill A. The main sequence, a tool for studying human eye movements. Math Biosci 1975;24:191–204. [Google Scholar]
- 13.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology 2009;72:1417–1424. [DOI] [PubMed] [Google Scholar]
- 14.Lehnen N, Glasauer S, Jahn K, Weber KP. Head impulses in complete bilateral vestibular loss: catch-up saccades require visual input. Neurology 2013;81:688–690. [DOI] [PubMed] [Google Scholar]
- 15.Mantokoudis G, Schubert MC, Tehrani AS, Wong AL, Agrawal Y. Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol Neurotol 2014;35:148–154. [DOI] [PubMed] [Google Scholar]
- 16.Aw ST, Todd MJ, Halmagyi GM. Head impulse testing: angular vestibulo-ocular reflex (VOR). In: Scott DZE, David SZ, editors. Handbook of Clinical Neurophysiology. New York: Elsevier; 2010:150–164. [Google Scholar]
- 17.Mantokoudis G, Saber Tehrani AS, Kattah JC, et al. Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurootol 2014;20:39–50. [DOI] [PubMed] [Google Scholar]
- 18.Crane BT, Demer JL. Latency of voluntary cancellation of the human vestibulo-ocular reflex during transient yaw rotation. Exp Brain Res 1999;127:67–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.