Abstract
Background:
Scar formation is a major source of dissatisfaction among patients and surgeons. Individually, hyaluronan, or hyaluronic acid (HA), and zinc have been shown to reduce scarring. The authors evaluated the safety and efficacy of an HA sponge with zinc compared with placebo when applied to bilateral breast surgery scars; specifically, they evaluated whether the use of this product modulates inflammation and immediate scarring in treated patients after bilateral breast surgery.
Methods:
This double-blind, randomized, prospective study was approved by the local institutional review board. Bilateral breast surgery patients with right and left incision lines were randomly assigned to receive HA sponge with zinc or placebo within 2 to 4 days after their procedure. Participants were followed up at 6 weeks, 12 weeks, and 1 year and evaluated at 12 weeks. Three blinded evaluators reviewed photographs of the incision lines and assessed the scars using a visual analog scale, new scale, and a patient satisfaction survey.
Results:
Nineteen bilateral breast surgery patients were enrolled in the study. Statistical analysis was performed on 14 patients who completed the follow-up. The mean visual analog scale score was lower for the side receiving the HA sponge with zinc (2.6) than for the side receiving placebo (3.0), indicating a better outcome (t test; P = 0.08). The HA sponge with zinc was found to have significant positive findings on a patient satisfaction survey (P = 0.01).
Conclusions:
This is a preliminary study that shows zinc hyaluronan was associated with high patient satisfaction in achieving a better scar after bilateral breast surgery, irrespective of skin color. It seems to be safe and effective for early scars.
Scar formation after surgery and skin trauma is a major source of dissatisfaction among patients and surgeons.1 Some scars can be disfiguring and esthetically unpleasant and cause severe itching, tenderness, pain, and psychological disturbance. They can also diminish quality of life and result in posttraumatic stress reactions, loss of self-esteem, and stigmatization.2–4 Furthermore, discernible scarring is a major complication and a major cause of reoperation after breast surgery.5 Attempts to reduce scarring after plastic surgery, particularly breast surgery, have traditionally focused on choosing well-hidden incision sites and using silicon sheets, corticosteroid injections, and massage therapy postoperatively.6,7
Inflammation is the most important physiologic response to any form of wounding. The outcome of controlled inflammation in adults is a collagenous scar, which lacks the ordered structure of healthy skin.8 Fetal healing, on the other hand, resembles a regenerative process and does not result in a collagenous scar.9–11 Pathogenesis of scar deformity is still unclear.
When the skin is injured, the first glycosaminoglycan to be synthesized is hyaluronan, or hyaluronic acid (HA).12 Fetal healing is scarless and occurs in an environment rich in HA,9 suggesting that exogenous HA may be useful in wound repair in adults.13–18 Evidence suggests that using suitable HA under appropriate conditions leads to enhanced wound healing and a reduction in or elimination of scarring.19 More than 50% of the body’s HA is found in the skin, where it functions to stabilize and maintain the biomatrix between the cells.20,21 The intrinsic water-retaining capacity of HA suggests that it facilitates the transport of metabolites and preserves tissue hydration, especially in the dermal layer.22 In the early 1970s, Rydell13 observed that application of high-molecular weight, purified HA to surgical incisions in animals resulted in subcutaneous scars that were smoother with less connective tissue reaction than those of untreated incisions in the same animals. Although HA is not the sole factor in regulating wound repair, it is certainly a key factor. It modulates inflammation by affecting polymorphonuclear cells, macrophages, cells of the lymphomyeloid system, phagocytosis, and chemotaxis and is a scavenger of free radicals.23
Various HA formulations have been produced to help reduce scarring.24 One such formulation is the HA sponge (HylaSponge System, Matrix Biological Institute, Fort Lee, N.J.), which uses polymerization methods to produce a network of large HA molecular chains bound into an assembly of very large coils (Fig. 1). These coils form spheroidal particles of virtually infinite molecular weight. The spheroidal particles can absorb and release large volumes of water, or water-soluble molecules, much like a sponge. This system provides a “waterway” to the skin for large- and small-molecular weight HAs and biologically active molecules incorporated into them, thus providing longer lasting effects and more penetrating hydration.25
Fig. 1.
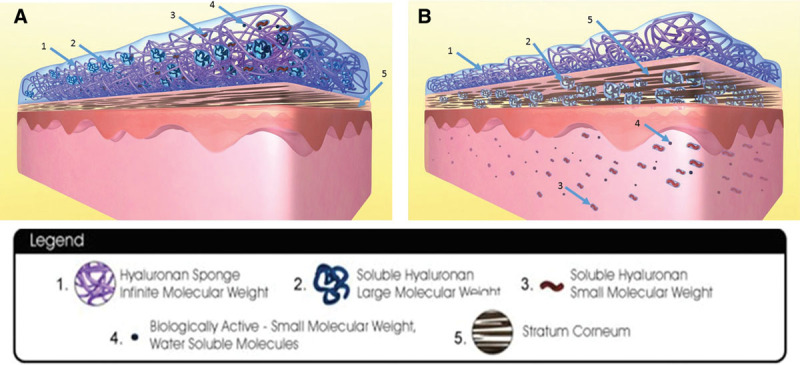
Hyaluronic acid sponge with zinc system. A, Hyaluronan sponge system with soluble hyaluronan of large molecular weight, soluble hyaluronan of small molecular weight, and biologically active molecules still within the sponge system. Stratum corneum is not hydrated and thinner. B, Hyaluronan sponge system with soluble hyaluronan of large molecular weight, soluble hyaluronan of small molecular weight, and biologically active molecules transferred into the layers of skin. Surface of skin and skin layers are kept hydrated by this mechanism. Stratum corneum is well hydrated and thicker. Reprinted with permission from Hylaco LLC.
The HA sponge system acts as a “second skin” and retains much of its water while delivering active components to the skin. The space in between the HA sponges is occupied by large-molecular weight, soluble HA (2) (Fig. 1A). Small-molecular weight, soluble HA (3) is inside and around the HA sponge. Optional additions of biologically active small molecules (4) such as vitamins, amino acids, and peptides are distributed in the water around and filling the HA sponges. The system releases water to the skin, enabling diffusion of the large (2) and small (3) HA molecules and the (4) biologically active molecules into the stratum corneum (5) of the skin, with the latter 2 molecules diffusing toward the deeper layers of the skin (Fig. 1B).
In this randomized prospective study, we evaluated the safety and efficacy of the HA sponge with zinc compared with placebo in the management of bilateral breast surgery scars. Specifically, we evaluated whether this product modulates inflammation and immediate scarring in treated patients after bilateral breast surgery. The moisture-intensive serum draws on the antibacterial and antiinflammatory properties of zinc to promote enhanced healing.
METHODS
This double-blind, randomized, prospective study was approved by the institutional review board of the University of Texas Southwestern Medical School. Participants included bilateral breast surgery patients who met the following inclusion and exclusion criteria:
-
Inclusion criteria
a. Elected to undergo bilateral breast surgery (augmentation or lift).
b. Female patients aged 21 to 65 years on date of signing the information and consent form.
c. Spanish-speaking patients.
-
Exclusion criteria
a. Not interested or unable to participate or complete the follow-up visits.
b. Known or suspected diagnosis of diabetes.
c. Known or suspected use of tobacco products within 14 days of signing informed consent through 12-week follow-up.
d. Women who were pregnant, nursing, or planning to become pregnant during the study or within 12 weeks after the procedure.
e. Women undergoing bilateral beast surgery for removal of cancerous tissue.
Randomization
Participants who had right and left incision lines were randomly assigned to receive the HA sponge with zinc or placebo (water without active ingredient) within 2 to 4 days of their surgical procedure. No occlusive dressings were applied to the patients’ scars after surgery.
Participants were asked to apply products twice daily to the specific side as designated by the research coordinator for 12 weeks. They were instructed to wash their hands between applications of products. The HA sponge with zinc and placebo were supplied by the study sponsor and packaged identically without any identifying marks. They were labeled product A and product B so that the investigator, the research team, and the participants were unable to distinguish which product was the HA sponge with zinc or which was the placebo.
Follow-Up
Participants were followed up at 6 and 12 weeks and again 1 year after their procedures. Each visit included evaluation by the investigator, patient satisfaction surveys, and photographs of the surgical sites. Participants applied the assigned product to each site for 12 weeks. Three blinded evaluators reviewed the photographs and assessed the scars with a Modified Manchester scale/visual analog scale (VAS) and a simpler New Scale at 12 weeks. The New Scale is a complementary scale to the validated VAS that we used exclusively in this article and in the sister article “A Randomized, Blinded Clinical Evaluation of Efficacy and Safety of Hyaluronan Sponge with Vitamin C (HylaSponge System) vs Placebo for Scar Reduction.” This is a 10-point New Scale created by adding the measures such as scar color, texture, relation to surrounding skin, and distortion. For all scales, lower scores indicated better outcomes. Photographs obtained at each interval were adjusted to the scar quality to reflect the best visualization by altering the lighting and shadowing to delineate the scars. Standard lighting was used when comparing the 2 sides during the same time interval (Fig. 2).
Fig. 2.
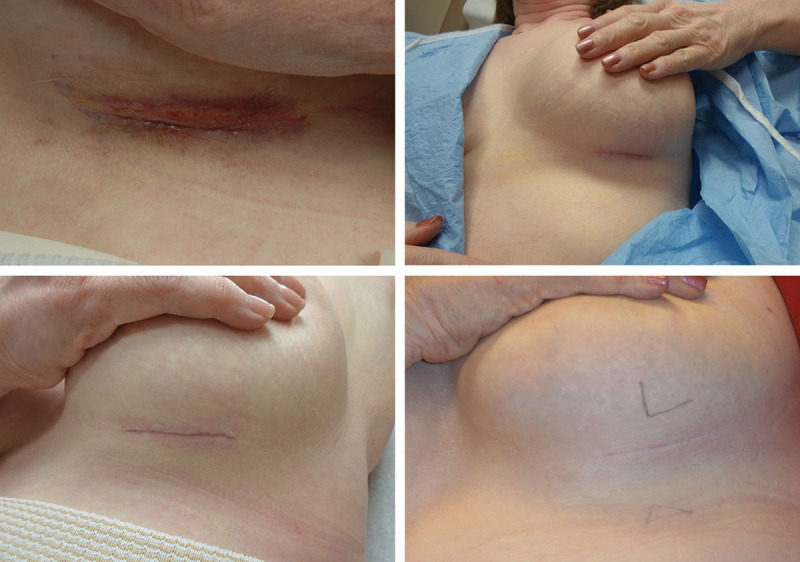
Example of maturing breast scar using variable lighting exposure to better delineate the scar.
For patients who consented to participate, their scar tissue was biopsied at the 12-week visit for wound healing and fibrosis analyses using hemotoxylin and eosin and trichrome stains, respectively. Biopsy specimens were examined by 1 blinded dermatopathologist and 1 blinded histopathologist.
At 12 weeks, participants were given the HA sponge with zinc to use on both incisional lines.
Statistical Analysis
All collected data sets that demonstrated nonparametric distributions used standard nonparametric tests. Findings were considered statistically significant if P < 0.05. Nonparametric data sets were analyzed using Wilcoxon or Kruskal–Wallis procedures. χ2 test was also used in statistical analysis.
RESULTS
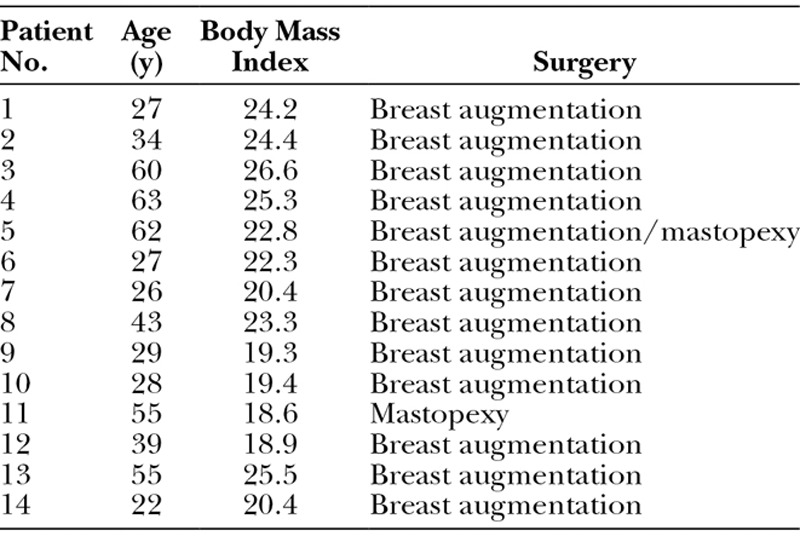
A total of 19 patients were enrolled in the study. Demographic characteristics of patients are presented in Table 1. Five patients withdrew or were removed from the study. Therefore, statistical analysis was performed on 14 patients who completed follow-up. Mean (range) age was 41 (22–63) years, and mean (range) body mass index was 22.2 (18.6–26.6). No adverse effects were reported in any patient.
Table 1.
Patient Demographics
At 12 weeks, the mean VAS score was lower for the side receiving the HA sponge with zinc (2.6) than for the side receiving placebo (3.0), indicating a better outcome, although this difference was not statistically significant (t test; P = 0.08). Fig. 3 shows distribution of these mean VAS scores by product. Figs. 4 and 5 show that no statistically significant difference was found between the 2 products for questions like “Which product do you prefer?” (P = 0.6) and “Satisfied with scar?” (P = 0.1). However, when “very satisfied” and “satisfied” answers were combined, a statistically significant difference was found (P = 0.01), as shown in Fig. 6. Fig. 7 shows that the majority of patients would buy the product.
Fig. 3.
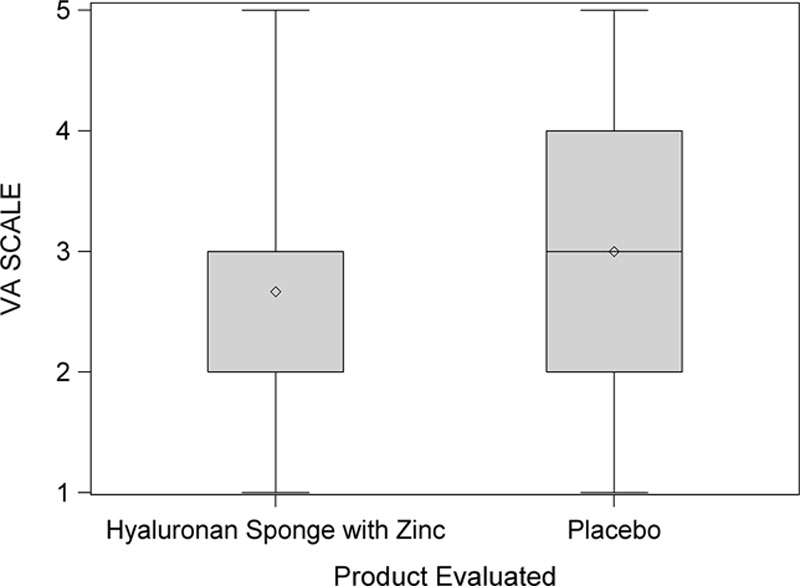
Distribution of visual analog (VA) scores by product. This box plot compares the mean (diamond) and the median (horizontal line in the box) scores.
Fig. 4.
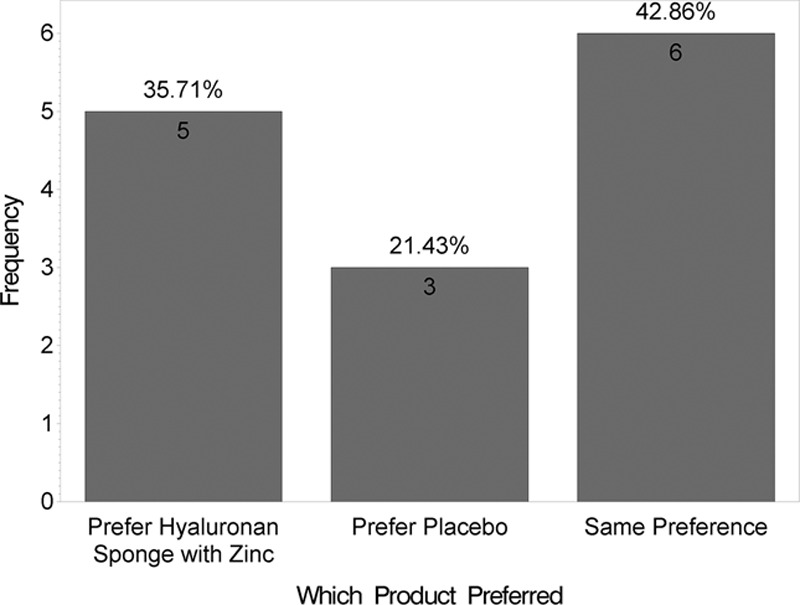
Satisfaction survey.
Fig. 5.
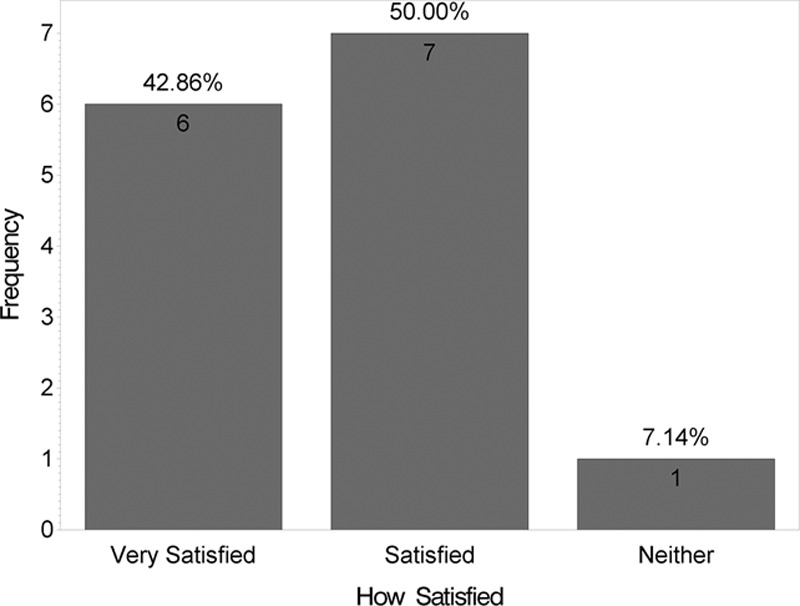
Satisfaction survey.
Fig. 6.
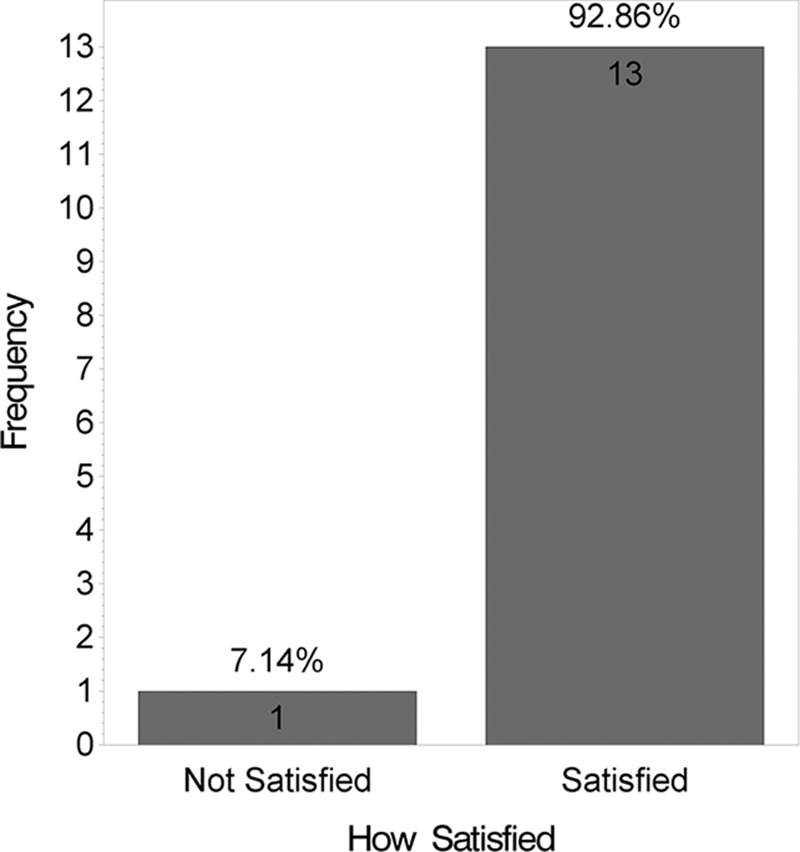
Satisfaction survey.
Fig. 7.
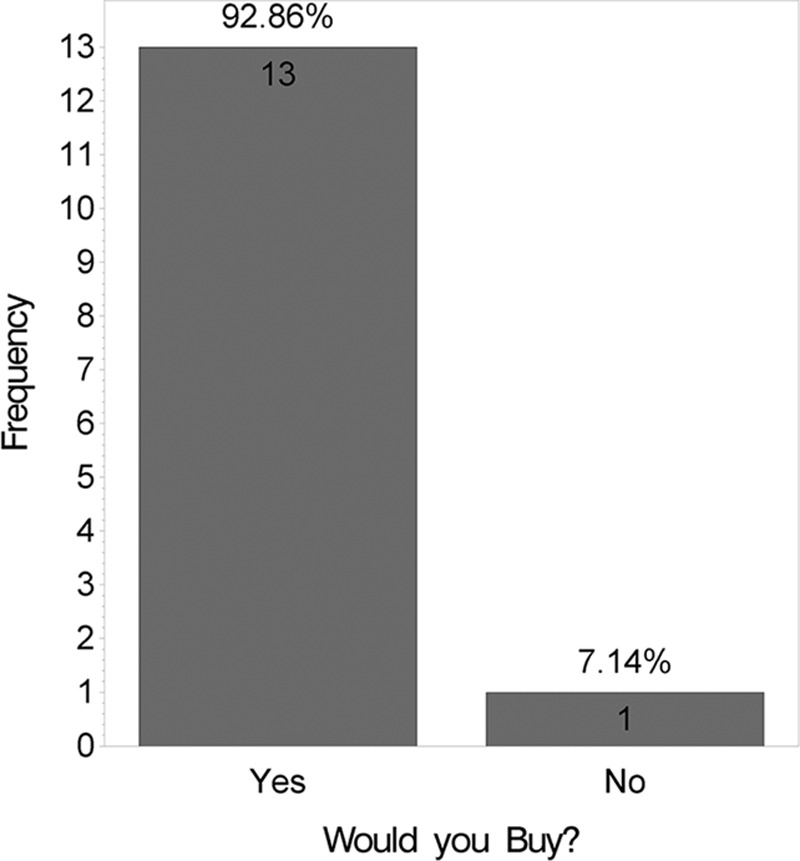
Satisfaction survey.
Fig. 8 shows a comparison of the products using the New Scale. It shows a box plot indicating a lower mean for the HA sponge with zinc, using a t test (P = 0.82).
Fig. 8.
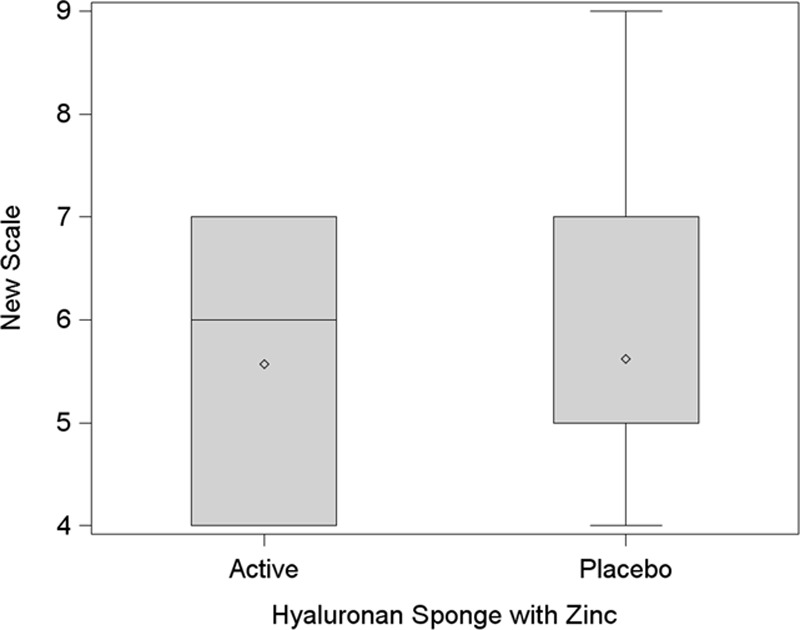
New Scale analysis. This box plot compares the mean (diamond) and the median (horizontal line in the box) scores.
Figs. 2, 9, 10, and 11 show changes in scar tissue over time. Scars improved over time, including the black patients. Fig. 12 shows histologic changes between the placebo product and HA sponge at 12 weeks. Skin treated with HA sponge with zinc showed increased density of collagen bundles with fewer aggregates of fibroblast.
Fig. 9.
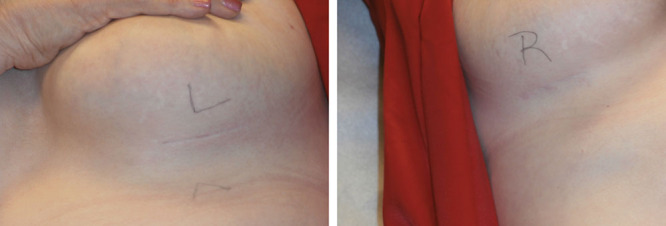
Scar depth at 1 y.
Fig. 10.
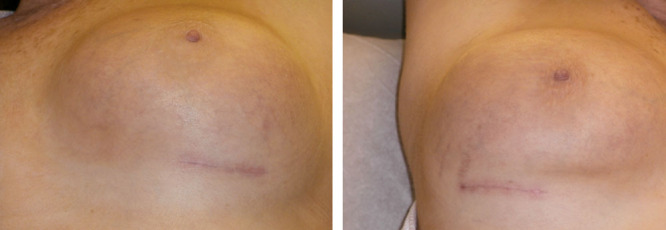
Scar color and width at 12 wk.
Fig. 11.
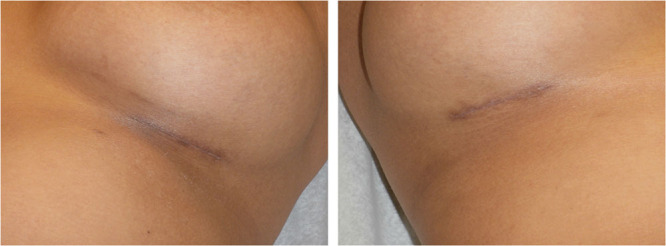
Black skin. Scar at 12 wk.
Fig. 12.
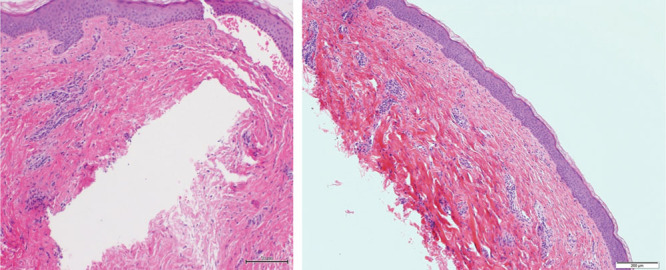
Scar biopsy specimen at 12 wk (hematoxylin and eosin stain; low magnification, 10×).
DISCUSSION
Patients’ dissatisfaction with displeasing scar formation can occur after any elective surgery and is particularly common after esthetic breast surgery.1,5 Traditionally, scar management has involved using tapes or butterfly closures to distribute tension equally or silicone strips and scar gels with various hypothetical mechanisms such as scar occlusion, negative ionic charge, and scar pressure.7,26,27 However, increased attention is now being directed at 2 other factors that play a critical role in achieving satisfactory healing: hydration and immunomodulation to control inflammation.7 Silicone sheeting, which increases hydration, is often used to achieve better scar quality. Massage therapy is also often advocated for scar management but lacks evidence of benefit.28,29 Without proper trials, benefits are difficult to quantify objectively because even a placebo benefit may be appreciated by patients.7
Hyaluronan likely plays a multifaceted role in mediation of cellular and matrix events during wound healing.24 Zinc is one of the trace elements considered to play an important role in wound healing and also has the additional benefits of antimicrobial and antiinflammatory properties.30–32 With this knowledge, it would be logical to combine zinc and HA in scar management to harness benefits of both to improve scar quality. One such formulation has been zinc HA, which is a proprietary biomolecule.
The HA sponge with zinc is based on research of the structure, function, and uses of various forms of HA: it hydrates the skin’s surface layer (stratum corneum) and maintains movement of water, biologically active ingredients, and small water-soluble molecules toward the skin’s deeper layers. Cosmetic applications of this product are used for hydration and to reduce the appearance of fine lines and wrinkles while smoothing the skin.25
Most studies of zinc and HA have been done in animal models, and none have been randomized, controlled, prospective trials.32,33 To our knowledge, our study is the first randomized, controlled, prospective trial to show significant patient satisfaction with this HA delivery system. Most of the patients in our study mentioned that they will buy the product. There was a histologic difference between the HA sponge with zinc and placebo in our biopsy samples, with changes in collagen fiber density and orientation noted. However, this difference may need further analysis with more samples and specific delineation of the location of the biopsy and the depth of the scar. Our understanding of the density and orientation of collagen fibers in a maturing scar is far better than our understanding of the changes that may appear in a superior esthetic scar.33–35 Multiple factors control the superior esthetic appearance of the scar, and these are hard to correlate with specific dermatopathologic findings.33–35 However, these changes further support the differential appearance of the placebo versus HA-sponge-treated skin. The timing of the application of this product is also important; the product should be applied soon after the time of wounding to modulate inflammation, at the time of initial scar matrix formation.12,14–16,21,23 For this study, we compared groups at 12 weeks, after which time all patients were allowed to apply the HA sponge with zinc on their scars. The longest follow-up was 1 year. We started using the product between 2 and 6 days. Additional research can determine how long this product needs to be applied for the best outcome, but based on our preliminary data it should be started within days of operation and continued at least for 3 months and up to 1 year after operation for maximal effect. Our findings support that the HA sponge with zinc is effective for early scars and that it can also function effectively for darker skin types.
One of the limitations of our study was the small number of patients. Another limitation of the study is the use of a VAS. Although this scale has been validated and is commonly used for skin care scar products, it is still subjective. Objective proof of benefit of HA sponge with zinc is demonstrated through histologic analysis, and although we observed histopathologic differences in the biopsy samples, there is no clear and large-scale study to demonstrate how a better esthetic scar should appear on histopathologic findings. Although we did see differences in scar depth, widening, and color, statistical significance was not reached, probably because of the small number of patients. Overall, results of the patient satisfaction surveys in our study were statistically significant with minimal side effects of the product to validate the use of this product on postoperative surgical scars.
CONCLUSIONS
This is a preliminary study that shows that the use of HA sponges with zinc was found to have high patient satisfaction in achieving a better scar after bilateral breast surgery, irrespective of skin color. Zinc HA seems to be safe and effective for early scars with minimal adverse effects and can be used in the management of surgical scars, with effective and easy application protocols.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported by Hylaco LLC/eraclea skincare. The Article Processing Charge was paid for by Hylaco LLC/eraclea skincare.
REFERENCES
- 1.Celebiler O, Sönmez A, Erdim M, et al. Patients’ and surgeons’ perspectives on the scar components after inferior pedicle breast reduction surgery. Plast Reconstr Surg. 2005;116:459–464. doi: 10.1097/01.prs.0000173060.02593.3a. discussion 465. [DOI] [PubMed] [Google Scholar]
- 2.Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert R, Meyer W, Bishop S, et al. Disfiguring burn scars and adolescent self-esteem. Burns. 1999;25:581–585. doi: 10.1016/s0305-4179(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 4.Dorfmüller M. [Psychological management and after-care of severely burned patients]. Unfallchirurg. 1995;98:213–217. [PubMed] [Google Scholar]
- 5.Manahan MA, Buretta KJ, Chang D, et al. An outcomes analysis of 2142 breast reduction procedures. Ann Plast Surg. 2015;74:289–292. doi: 10.1097/SAP.0b013e31829d2261. [DOI] [PubMed] [Google Scholar]
- 6.de Giorgi V, Sestini S, Mannone F, et al. The use of silicone gel in the treatment of fresh surgical scars: a randomized study. Clin Exp Dermatol. 2009;34:688–693. doi: 10.1111/j.1365-2230.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- 7.Widgerow AD, Chait LA, Stals PJ, et al. Multimodality scar management program. Aesthetic Plast Surg. 2009;33:533–543. doi: 10.1007/s00266-008-9276-x. [DOI] [PubMed] [Google Scholar]
- 8.Schilling JA. Wound healing. Surg Clin North Am. 1976;56:859–874. doi: 10.1016/s0039-6109(16)40983-7. [DOI] [PubMed] [Google Scholar]
- 9.Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: current biology. World J Surg. 2003;27:54–61. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- 10.Mackool RJ, Gittes GK, Longaker MT. Scarless healing. The fetal wound. Clin Plast Surg. 1998;25:357–365. [PubMed] [Google Scholar]
- 11.Namazi MR, Fallahzadeh MK, Schwartz RA. Strategies for prevention of scars: what can we learn from fetal skin? Int J Dermatol. 2011;50:85–93. doi: 10.1111/j.1365-4632.2010.04678.x. [DOI] [PubMed] [Google Scholar]
- 12.Aya KL, Stern R. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen. 2014;22:579–593. doi: 10.1111/wrr.12214. [DOI] [PubMed] [Google Scholar]
- 13.Rydell N. Decreased granulation tissue reaction after installment of hyaluronic acid. Acta Orthop Scand. 1970;41:307–311. doi: 10.3109/17453677008991516. [DOI] [PubMed] [Google Scholar]
- 14.Abatangelo G, Martelli M, Vecchia P. Healing of hyaluronic acid-enriched wounds: histological observations. J Surg Res. 1983;35:410–416. doi: 10.1016/0022-4804(83)90030-6. [DOI] [PubMed] [Google Scholar]
- 15.King SR, Hickerson WL, Proctor KG. Beneficial actions of exogenous hyaluronic acid on wound healing. Surgery. 1991;109:76–84. [PubMed] [Google Scholar]
- 16.Mast BA, Flood LC, Haynes JH, et al. Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix. 1991;11:63–68. doi: 10.1016/s0934-8832(11)80228-3. [DOI] [PubMed] [Google Scholar]
- 17.Balazs EA, Bland PA, Denlinger JL, et al. Matrix engineering. Blood Coagul Fibrinolysis. 1991;2:173–178. doi: 10.1097/00001721-199102000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Iocono JA, Ehrlich HP, Keefer KA, et al. Hyaluronan induces scarless repair in mouse limb organ culture. J Pediatr Surg. 1998;33:564–567. doi: 10.1016/s0022-3468(98)90317-7. [DOI] [PubMed] [Google Scholar]
- 19.Damodarasamy M, Johnson RS, Bentov I, et al. Hyaluronan enhances wound repair and increases collagen III in aged dermal wounds. Wound Repair Regen. 2014;22:521–526. doi: 10.1111/wrr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed RK, Lilja K, Laurent TC. Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand. 1988;134:405–411. doi: 10.1111/j.1748-1716.1988.tb08508.x. [DOI] [PubMed] [Google Scholar]
- 21.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 22.Ghersetich I, Lotti T, Campanile G, et al. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol. 1994;33:119–122. doi: 10.1111/j.1365-4362.1994.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 23.Neuman MG, Nanau RM, Oruña-Sanchez L, et al. Hyaluronic acid and wound healing. J Pharm Pharm Sci. 2015;18:53–60. doi: 10.18433/j3k89d. [DOI] [PubMed] [Google Scholar]
- 24.Weindl G, Schaller M, Schäfer-Korting M, et al. Hyaluronic acid in the treatment and prevention of skin diseases: molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol Physiol. 2004;17:207–213. doi: 10.1159/000080213. [DOI] [PubMed] [Google Scholar]
- 25. http://www.hylaco.com/benefits-advantages.html. Accessed May 22, 2016.
- 26.Hirshowitz B, Lindenbaum E, Har-Shai Y, et al. Static-electric field induction by a silicone cushion for the treatment of hypertrophic and keloid scars. Plast Reconstr Surg. 1998;101:1173–1183. doi: 10.1097/00006534-199804050-00001. [DOI] [PubMed] [Google Scholar]
- 27.Sharp PA, Pan B, Yakuboff KP, et al. Development of a best evidence statement for the use of pressure therapy for management of hypertrophic scarring. J Burn Care Res. 2015 May 28; doi: 10.1097/BCR.0000000000000253. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg. 2008;32:82–92. doi: 10.1007/s00266-007-9030-9. [DOI] [PubMed] [Google Scholar]
- 29.Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl. 2015;48:651–662. doi: 10.1016/j.msec.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juhász I, Zoltán P, Erdei I. Treatment of partial thickness burns with Zn-hyaluronan: lessons of a clinical pilot study. Ann Burns Fire Disasters. 2012;25:82–85. [PMC free article] [PubMed] [Google Scholar]
- 31.Kurmis R, Greenwood J, Aromataris E. Trace element supplementation following severe burn injury: a systematic review and meta-analysis. J Burn Care Res. 2016;37:143–159. doi: 10.1097/BCR.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 32.Du X, Hong G, Sun P, et al. Zn2+-SCMC versus HA for preventing intraperitoneal adhesions: a rat model study. Int J Med Sci. 2012;9:467–471. doi: 10.7150/ijms.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walmsley GG, Maan ZN, Wong VW, et al. Scarless wound healing: chasing the holy grail. Plast Reconstr Surg. 2015;135:907–917. doi: 10.1097/PRS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 34.Hardy MA. The biology of scar formation. Phys Ther. 1989;69:1014–1024. doi: 10.1093/ptj/69.12.1014. [DOI] [PubMed] [Google Scholar]
- 35.Carrico TJ, Mehrhof AI, Jr, Cohen IK. Biology of wound healing. Surg Clin North Am. 1984;64:721–733. doi: 10.1016/s0039-6109(16)43388-8. [DOI] [PubMed] [Google Scholar]


