Supplemental Digital Content is available in the text.
Abstract
Background:
The practice of breast reconstruction continues to evolve with the introduction of new technologies. The authors describe a unique approach allowing immediate direct-to-implant reconstruction that can be performed on an outpatient basis.
Methods:
After a nipple-sparing mastectomy, acellular dermal matrix (ADM)-covered implants are placed in a prepectoral position in an immediate reconstruction. Assessment of results was performed via a retrospective review of demographic and procedural data.
Results:
Forty-five patients (79 breasts), mean age 46.8 years, were treated with direct-to-implant reconstruction using ADM-wrapped implants placed above the muscle with mean follow-up of 23.1 months (median 22 mo). Mean body mass index was 24.3, and 15 patients (33.3%) were current or former smokers. Twenty-seven patients (60%) had prior breast surgery with 22 (49%) exposed to chemotherapy and 34 (76%) radiation. Procedure time averaged 155 minutes and hospital length of stay averaged 0.6 days. Complications included flap necrosis in 22 cases (28%), seroma in 12 (15%), infection in 8 (10%), rippling in 28 (35%), and contracture in 8 (10%). In 14 breasts (18%), postoperative wound complications (flap necrosis or infection) led to implant loss.
Conclusions:
The availability of ADM and cohesive gel implants has allowed us to perform above-the-muscle implant breast reconstruction in reduced time and often on an outpatient basis. Complication rates were comparable to expected results of standard expander-to-implant, staged breast reconstruction. This technique is a viable option delivering clinically and aesthetically acceptable results in select patients.
Breast reconstruction after mastectomy continues to evolve thanks in part to the development of unique technologies and enabling devices. The earliest reconstructions were described in the late 1800s,1 and the practice was revolutionized with introduction of breast implants in the 1960s and tissue expanders in the 1980s. More recently, the availability of acellular dermal matrix (ADM) grafts introduced in early 2000s and the modern cohesive gel breast implants (FDA approved in 2006–2013) have permitted plastic surgeons to further enhance implant-based reconstruction and develop new protocols allowing for a shorter operative course and improved outcomes.2 Today, the majority of patients and their surgeons choose an implant-based approach for breast reconstruction.3 The immediate direct-to-implant reconstruction has been shown to reduce operating room time, cost, and potential added morbidity associated with a 2-step expander/implant reconstruction.4–6 We have taken a logical next step and have developed a technique that combines ADM coverage and above-the-muscle implant placement in a single immediate-to-implant reconstruction, which we can now perform in an outpatient setting.
METHODS
This is a retrospective review of 45 patients, 79 breasts treated with immediate above-the-muscle implant reconstruction performed by a single plastic surgeon between December 2011 and February 2015. Data describing baseline patient demographics, comorbidities, operative characteristics, and follow-up were collected from electronic health record reviews and patient interviews. Follow-up was obtained at least 7 months after the reconstruction or last-reported complication. We perform this procedure as standard of care for appropriate patients; thus, all patients provided standard surgical consent. Patient information was deidentified, complying with the Health Insurance Portability and Accountability Act.
SURGICAL TECHNIQUE
Preoperative patient counseling includes review of the direct-to-implant reconstruction and other surgical options including, but not limited to, staged expander/implant and autologous tissue reconstructions and no reconstruction. Expected outcomes, risks, and potential for additional revision procedures are discussed and patients consented for the direct-to-implant procedure and their preferred alternative options, should they become necessary.
Before surgery, the breast boundaries are marked to assist the mastectomy surgeon and aid in implant size selection (Fig. 1). A general surgeon performs a nipple-sparing mastectomy using an inframammary or lateral approach with or without a supra- or infraareolar extension. The approach is carefully discussed with the surgeon to review experience and preference, oncologic requirements, and aesthetic needs of the patient. If necessary, a small mastopexy is performed through the supra-areolar extension for minor ptosis correction.
Fig. 1.
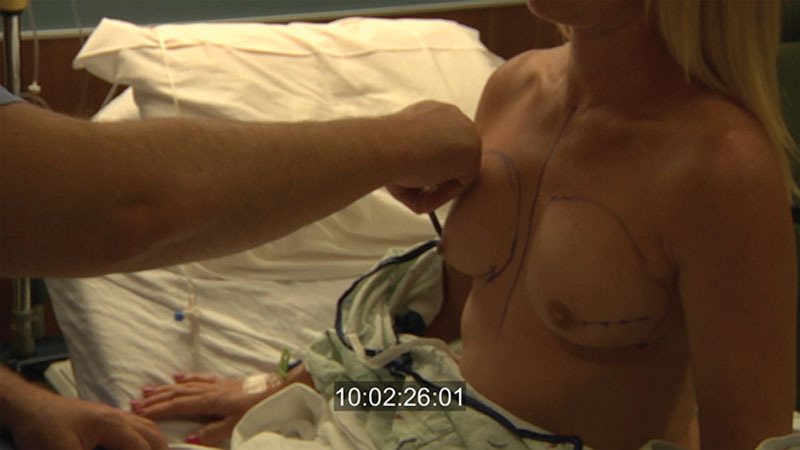
The breast boundaries are outlined with the patient sitting upright. For this technique, typically an inframammary or lateral breast incision is used.
At completion of the mastectomy, the surgical team evaluates the mastectomy flaps for viability using their best clinical judgment and available techniques. In our facility, we have a SPY Elite System (Novadaq, Bonita Springs, Fla.) available to measure intraoperative tissue perfusion. During this evaluation, a 100% cursor is applied to an obviously viable flap area and typically a relative reading of 30 or above is considered to predict an adequate measure of tissue viability. If there is a predicted area of tissue necrosis, it is debrided at this point. If this area is sizeable, we convert to an alternate approach.
Upon proceeding with the direct-to-implant route, the tissue is again evaluated with a sizer in place (Fig. 2). Appropriate pocket alterations are made to assist with a “hand in glove” implant fit. If necessary, a 2-0 PDS suture (Ethicon, Somerville, N.J.) is utilized to reinforce the inframammary fold or close the lateral pocket and axilla.
Fig. 2.
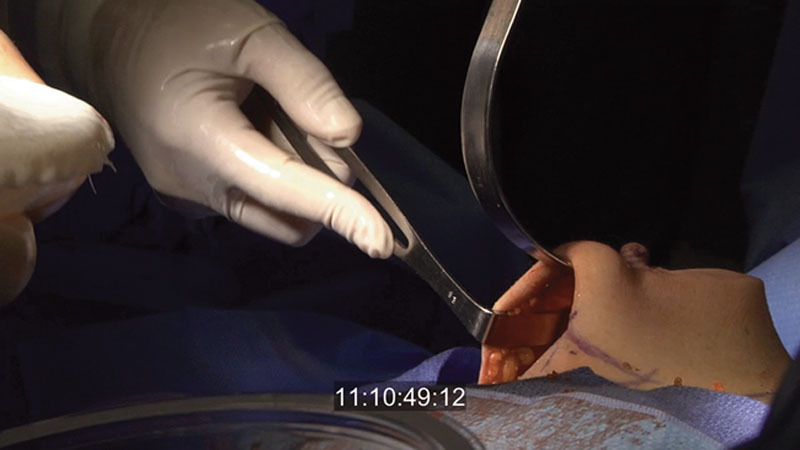
After the mastectomy, the pocket is sized and the skin flap viability is assessed. At this point, the decision to proceed with reconstruction using this technique or converting to an alternative approach is made.
The approximate implant size is determined preoperatively, sometimes with the aid of 3D imaging. A MemoryShape implant (Mentor, Santa Barbara, Ca.) is wrapped anteriorly and posteriorly with 16- × 20-cm-sized ADM [AlloDerm (LifeCell, Branchburg, N.J.) or FlexHD (MTF, Edison, N.J.)] (Fig. 3). In some cases, 2 pieces are used, forming a complete 360-degree implant wrap. The margins of the resultant unit are used to suture the implant to the chest wall and inframammary fold, avoiding migration or rotation of the implant. Alternatively, an anterior wrap using a single ADM piece can be used. This anterior wrap is secured with posterior spanning 2-0 PDS sutures, allowing the posterior texturing of the implant to assist in implant location and adhesiveness to avoid malposition. The implant/ADM is positioned in the pocket above the pectoralis and secured to the chest wall at 2 or more locations inferiorly. The posterior aspect of the nipple is positioned and sutured to the anterior portion of the ADM with 2-0 PDS suture to stabilize nipple location and avoid slippage. A 15Fr drain is placed in the mastectomy pocket, positioned around the periphery of the implant and fastened at the skin level with 3-0 silk (Ethicon, Somerville, N.J.). The incision is closed with 3-0 monocryl (Ethicon, Somerville, N.J.) in the subcutaneous and subcuticular levels and the wound is sealed with tissue adhesive. The inframammary fold and lateral pocket are reinforced with Mastisol and 1-in foam tape left in place for 24 hours. The patient is placed in a postoperative bra, reversed from anesthesia and sent to recovery. We use Exparel (Pacira, San Diego, Ca.) to further reduce postoperative discomfort, and typically narcotics are not required for pain. Patients may be kept overnight or sent home the same day. (See video, Supplemental Digital Content 1, which displays a short video clip of the operative technique. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A239.)
Fig. 3.
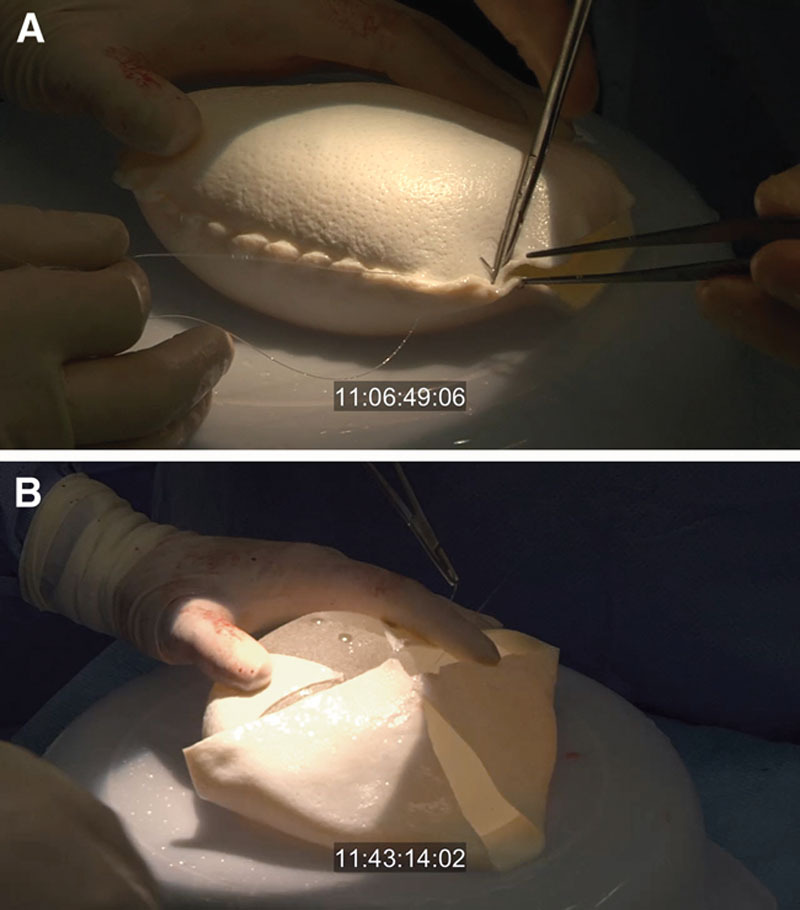
Implant preparation: (a) Full 360-degree coverage—The ADM is wrapped around the implant circumference and sutured with running 2-0 PDS. Edges are trimmed. B, Anterior coverage with posterior spanning sutures: this approach lowers the cost of the procedure and exposes the posterior texture of the implant to assist with implant positioning.
Video Graphic 1.
See video, Supplemental Digital Content 1, which displays a short video clip of the operative technique. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A239.)
Patients are seen within the first 3 postoperative days for wound and drain assessment. Drain removal usually occurs 10 days postoperatively or when drainage is below 30 cc/d per drain for 2 consecutive days. Fat grafting or other revision procedures are sometimes desired to enhance aesthetic outcomes and can be performed subsequently. Figures 4 and 5 depict representative pre- and postoperative outcomes.
Fig. 4.
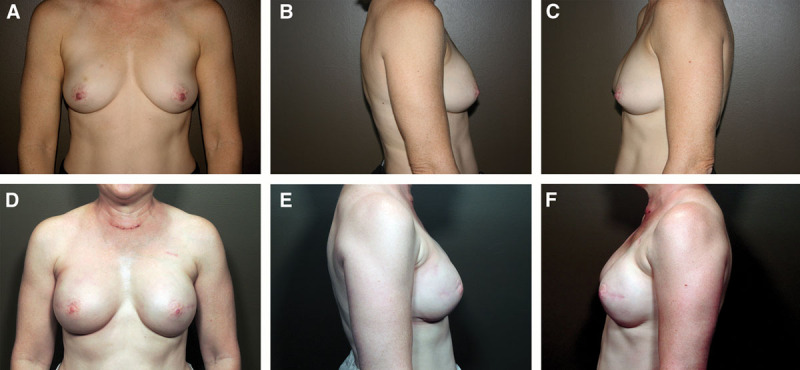
A 52-year-old patient with previous chemotherapy and radiation to right breast. Photographs (A, B, C) were taken before reconstruction. Photographs (D, E, F) at 6 months after bilateral nipple-sparing direct-to-implant reconstruction with cohesive gel implants in the prepectoral position.
Fig. 5.
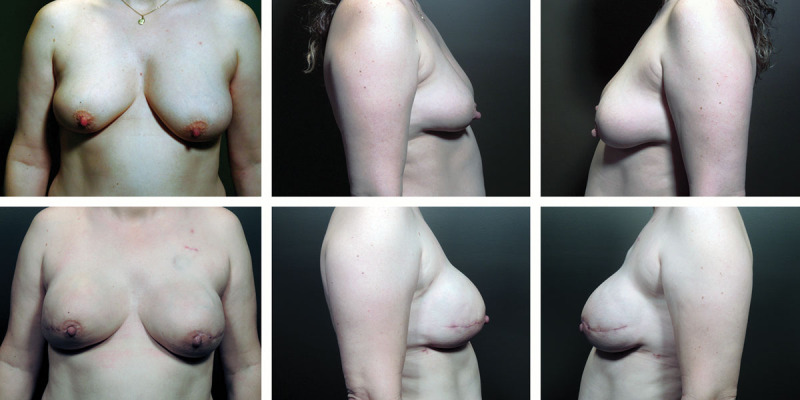
A 41-year-old patient who presented with recurrent cancer in right breast previously treated with lumpectomy. Left column images (a, b, c) were taken before bilateral nipple-sparing mastectomy and direct-to-implant reconstruction. Right column (d, e, f) is after reconstruction with cohesive gel implants in the prepectoral position.
STATISTICAL ANALYSIS
Descriptive statistics were generated for all demographic and surgical procedure-related and postoperative outcome variables. For outcomes other than length of stay, the number of breast reconstructions was used for analysis. Both univariate and multivariable mixed-effects logistic regression models, adjusting for nesting effect of multiple breast reconstructions within the same patient, were performed to identify risk factors associated with each complication outcome (rippling, flap necrosis, infection, implant loss, and capsular contracture) separately. Because of the small sample size, only variables with a significant P < 0.05 in the univariate analysis were entered in the multivariable regression models for further analysis. A 2-tailed P-value of less than 0.05 was considered significant. All statistical analyses were performed using SAS 9.4 statistical software (SAS Inc., Cary, N.C.).
RESULTS
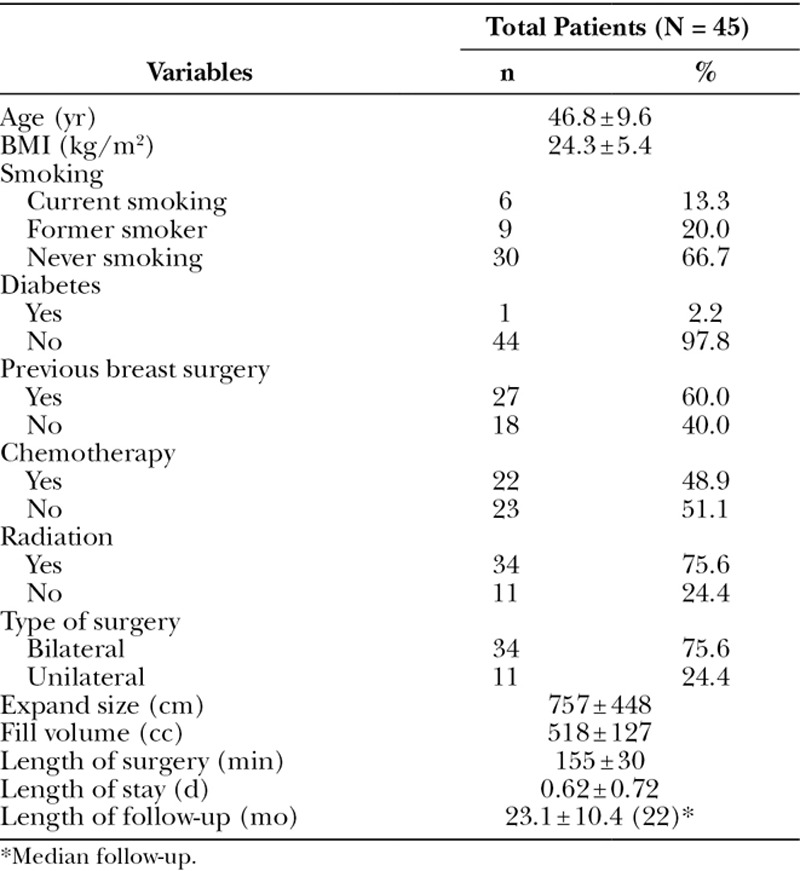
A total of 45 women had 79 direct-to-implant reconstructions, which are included in this analysis. Thirty-four patients (75.6%) had bilateral reconstructions and the remaining 11 (24.4%) had unilateral procedures. Patient characteristics are summarized in Table 1. Patients’ mean age was 46.8 ± 9.6 years and mean body mass index (BMI) was 24.3 ± 5.4 kg/m2. Approximately 33% were either former or current smokers and 2% had diabetes mellitus. Sixty percent had previous breast surgery with 49% and 76% having prior chemotherapy and radiation exposure, respectively.
Table 1.
Patient Characteristics
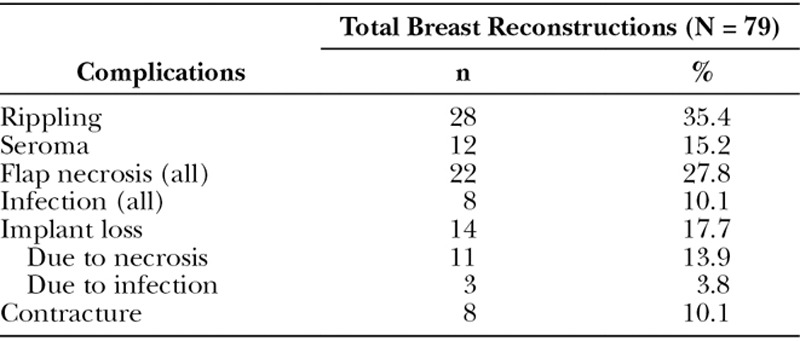
Operative time averaged 155 minutes and patients spent less than a day on average in the hospital. Median follow-up was 22 months after procedure. Postoperative complications, presented in Table 2, were most commonly rippling (35%) and flap necrosis (28%), seroma (15%), contracture (10%), and infection (10%). Infection was defined by the presence of cellulitis, purulent drainage, and/or elevated white blood cell count. Flap necrosis encompassed any area requiring debridement. Complications were managed conservatively in most cases; however, in 14 breast reconstructions (17.7%), postoperative implant loss was unavoidable: 11 due to tissue necrosis (13.9%) and 3 (3.8%) due to infection.
Table 2.
Postoperative Complications
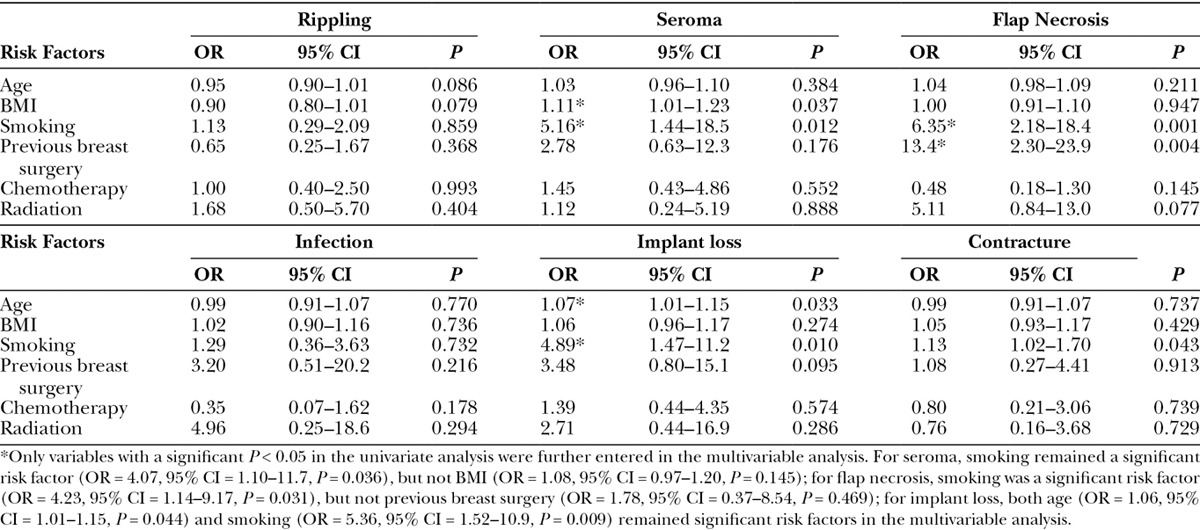
In the univariate logistic analysis adjusting for multiple breast constructions nesting within the same patient, shown in Table 3, higher BMI (OR = 1.11, P = 0.037) and smoking (OR = 5.16, P = 0.012) were associated with higher risk of postoperative seroma. Patients who were smokers (OR = 6.35, P = 0.001) and had previous breast surgery (OR = 13.4, P = 0.004) and radiation (OR = 5.11, P = 0.077) were also more likely to have postoperative flap necrosis. Older patients (OR = 1.07, P = 0.033) and smokers (OR = 4.89, P = 0.010) were at greater risk of implant loss. Finally, smoking (OR = 1.13, P = 0.043) was also associated with higher risk of postoperative capsular contracture. In the multivariable analysis, smoking remained an independent risk factor for seroma (OR = 4.07, P = 0.036), flap necrosis (OR = 4.23, P = 0.031), and implant loss (OR = 5.36, 0.009), whereas older age was also associated with higher risk of postoperative implant loss (OR = 1.06, P = 0.044).
Table 3.
Univariate Logistic Regression on Postoperative Complications by Breast Reconstruction (N = 79)
DISCUSSION
In 2014, the ASPS reported over 100,000 breast reconstructions in the United States with 83,000 incorporating an implant.3 Generally speaking, most plastic surgeons have concerns with placing the implant above the muscle because of thin mastectomy flaps and insufficient subcutaneous tissue remaining after a mastectomy. The potential risk of postoperative rippling, wound complications, implant erosion and extrusion, infection, and capsular contracture is felt to be higher than occurs with submuscular placement. Intuitively, the risk of complications increases with extended time in the OR; thus, minimizing the surgical time for breast reconstruction is desirable. Using our technique, we eliminated a second surgery and reduced our complete mastectomy/reconstruction OR time to 155 minutes, with the average patient hospital stay being 0.6 days. Now, the majority of patients are released the same day without significant narcotics.
Any quality breast reconstruction begins with a quality mastectomy. A close working relationship with the treatment team and a specific understanding by the mastectomy surgeon of the reconstructive plan and nuances are essential. Our patients are typically treated by 1 of 2 general surgeons who have become most familiar with this protocol, although over the course of this series 6 general surgeons performed the mastectomies. Variability among mastectomy surgeons may have played a role in our complications.
As adequate skin flaps with intact subcutaneous fat are critical for the success of the above-the-muscle technique, assessment of the mastectomy before proceeding with reconstruction is imperative. We believe real-time intraoperative tissue perfusion analysis is an extremely valuable tool. Our “Go/No Go” decision point is directly related to the amount of predicted nonviable tissue: if less than 10% of the flap has limited perfusion, it can likely be debrided, closed, and the direct-to-implant route maintained. Mastectomy flaps with 10% to 30% viability are more likely to need a tissue expander as opposed to an implant. Initially, we placed these expanders in the usual subpectoral plane with the ADM defining the inferior pole. Currently, we position the expanders in the subcutaneous pocket without disruption of the pectoralis musculature. If greater than 30% of the mastectomy flap is predicted to have limited to no perfusion, the direct-to-implant reconstruction should be abandoned in favor of alternative methods such as staged expander/implant or autologous tissue reconstructions, or in some cases depending on clinical judgment and patient preference, no reconstruction. In the absence of a SPY system, we recommend plastic surgeons rely on their clinical judgment when appraising tissue flap viability. Capillary refill, thickness of flaps, presence of subcutaneous tissue, and temperature to touch are several variables that may be evaluated when considering whether to proceed with direct-to-implant reconstruction.
Complications in this series were managed conservatively in most cases with implant loss occurring in 14 breasts. Unquestionably, comorbid conditions factor heavily into the complications and success of any procedure, particularly those depending on flap perfusion and viability. Smoking, diabetes mellitus, advanced age, and high BMI have all been shown to affect flap viability.7–9 In this series, we found a similar range of major complications that one would expect after a standard ADM/expander/implant submuscular placement.10–15
Twenty-two breasts had some degree of flap necrosis, of which 11 (13.9%) can be categorized as major, resulting in implant loss. Certainly, some may have been related to our learning curve, particularly as early in the series we did not objectively measure tissue perfusion. Although we were not alarmed with our rate of implant loss, a review of the literature was performed to assess skin-sparing mastectomy (SSM) flap complications and implant loss rates, confirming our outcomes were similar to those seen by others.7,16–19 Garwood et al16 reported an overall 16.8% rate of implant loss in a series of 170 SSMs, with loss ranging from 31% in an early cohort to 10% in a later group. Similarly, Hultman described flap loss in 24.3% of patients/17% of reconstructions after SSM with immediate reconstruction.7 Additionally, Chun et al17 reported an incidence of 11.8% major necrosis complications in nontumescent mastectomy patients. Comparable to our rate of 3.9% implant loss due to infection, Reish reported an overall explant rate of 3.2% in 1952 immediate implant-based reconstructions, specifically assessing implant salvage in the presence of postoperative infection.18
Predictably, multivariate analysis demonstrated that smokers were more likely to have postoperative flap necrosis. Smoking and age remained significant risk factors in the multivariable analysis. This underscores the importance of patient selection and intraoperative assessment of tissue viability. Even with previous radiation therapy, however, this technique can be performed with acceptable outcomes.
Seroma occurred in 12 breasts (15%); however, only 5 (6%) would be deemed clinically significant using the definition of requiring hospital stay, IV antibiotics, or device explant.14 One patient received IV antibiotics in the office, and the other 4 occurrences were associated with infection and flap necrosis, which combined necessitated explant. Other seromas were managed conservatively. As published reports suggest a potential increased seroma rate when ADMs are utilized, we typically leave drains in a bit longer, which may mitigate risk. When adjusting for multiple breast constructions within the same patient, higher BMI (OR = 1.11, P = 0.037) and smoking (OR = 5.56, P = 0.011) were associated with higher risk of postoperative seroma.
In our series with mean follow-up of close to 2 years, capsular contracture occurred in 8 breasts (5 patients) (10%), with the majority categorized as Baker II. This is well within the range anticipated with any breast reconstruction. Smoking was the only variable associated with a significantly higher risk of postoperative capsular contracture (OR = 1.12, P = 0.048).
We must continuously analyze and refine our surgical technique. Traditionally, an implant or expander is placed in a complete submuscular or partial submuscular plane and the muscle used to improve upper pole cosmesis and decrease implant visibility. Ultimately, the muscle becomes thin and attenuated over time, limiting its advantage. Additionally, disruption of the chest wall musculature not only significantly increases procedural discomfort but contributes to “hyperanimation” of the upper pole of the reconstruction, which can be unsightly.20
Initially, we used a “complete wrap” with two pieces of ADM placed around the anterior and posterior surfaces of the implant. With experience, we found that an anterior wrap using a single ADM piece was preferable. In addition to reducing cost, a free margin for positioning the implant is maintained and the textured posterior of the device is exposed to the chest wall to further secure its position preserving the benefits associated with the ADM. About 50% of these cases were done with the “complete wrap” and the remaining utilized an anterior wrap.
In our Midwest-based practice, we select the implant best suited to each patient from a wide range of available shapes and sizes. Initially, cohesive gel implants were chosen; however, we quickly progressed to using an ultracohesive implant with enhanced texturing. These implants maintain their shape and in our experience are associated with decreased incidence of capsular contracture.
Patient-reported outcomes scores are not measured routinely, so quantitative analysis of patient satisfaction was not performed. Subjectively, we find patients to be quite satisfied with their aesthetic outcomes. As with other reconstructive methods, fat grafting is a helpful adjunct for perceived small aesthetic defects. In this series, as in all our reconstructions, fat grafting was used in 60% to 70% of patients to address any resultant rippling or upper pole implant visibility, particularly in very thin patients. This was usually done in the office under a local anesthetic and has become quite routine.
Broadly speaking, we do not believe that the major complications in this series are directly related to implant type or size or use of ADM, but more a function of overall patient health and comorbid conditions. That said, to maximize success, we recommend surgeons wishing to incorporate this technique into their practice begin their experience in nonsmokers with small–moderate sized breasts. As a guide, but not a strict criterion, comorbidities are not hard contraindications to this approach, and although a higher BMI may in some cases increase perioperative risk, it also allows for a thicker subcutaneous layer and better vascularized mastectomy flaps, which are critical for a successful outcome. Based on our learning curve and experience to date, perhaps the most ideal patients are those with Grade 1 or 2 ptosis and BMIs of 25 to 35 as they tend to have adequate subcutaneous fat.
This technique requires use of an ADM to provide supplemental support to the remaining skin and subcutaneous tissue and minimize rippling and the risk of capsular contracture. There are a number of ADMs available, with the most clinical experience reported with human grafts. Recently, porcine ADM was used in a small series of direct-to-implant nipple-sparing mastectomies using a prepectoral approach.21 In our series, two human ADMs were used to wrap the implants: AlloDerm (69%) and FlexHD Pliable (31%). Both ADMs performed well; however anecdotally, we observed differences in cosmetic results which we hypothesize could be attributed to graft variability. We currently use a single type of ADM (FlexHD Pliable); however, we did not critically analyze FlexHD versus Alloderm, recognizing that a prospective comparative study to evaluate cosmetic outcomes and soft tissue complications between different ADM materials is needed to confirm our observations.
CONCLUSIONS
The availability of an ADM combined with ultracohesive-shaped breast implants allows us to perform an outpatient above-the-muscle immediate breast reconstruction with an acceptable rate of major complications, excellent cosmetic results, and no hyperanimation deformity or pain associated with pectoral muscle disruption. This approach is a faster, less invasive option that should be considered in select cases. It offers patients a complete outpatient mastectomy and reconstruction without compromising clinical outcomes or further postoperative oncologic therapy. Additionally, the costs and complications associated with tissue expanders and a second surgery are eliminated.
ACKNOWLEDGMENTS
The authors recognize and thank Edward Wang, PhD, who reviewed our data and performed all statistical analyses. Dr. Wang’s insights and assistance in the preparation of this article are greatly appreciated.
Ethical Research Statement: This research conformed to the Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects.
Supplementary Material
Footnotes
The Musculoskeletal Transplant Foundation (MTF), Edison, N.J. provided funding for this research. Funding was provided as an educational grant for the cost associated with data collection and analysis only. MTF was not involved with data analysis nor did they write the manuscript. Authors consulted with MTF before manuscript submission to assure accuracy of ADM description.
Disclosure: Dr. Ronald K. Downs works as a consultant and lectures on behalf of Mentor and MTF. Kellee Hedges has no financial interest to declare in relation to the content of this article. This study was funded, in part, by a research grant from MTF (Edison, N.J.). The Article Processing Charge was paid for by the author.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Uroskie TW, Colen LB. History of breast reconstruction. Semin Plast Surg. 2004;18:65–69. doi: 10.1055/s-2004-829040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. doi: 10.1097/01.sap.0000168527.52472.3c. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Plastic Surgeons. 2014 Statistics. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2014-statistics/plastic-surgery-statsitics-full-report.pdf. Accessed October 1, 2015.
- 4.Cassileth L, Kohanzadeh S, Amersi F. One-stage immediate breast reconstruction with implants: a new option for immediate reconstruction. Ann Plast Surg. 2012;69:134–138. doi: 10.1097/SAP.0b013e3182250c60. [DOI] [PubMed] [Google Scholar]
- 5.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 6.Colwell AS. Direct-to-implant breast reconstruction. Gland Surg. 2012;1:139–141. doi: 10.3978/j.issn.2227-684X.2012.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hultman CS, Daiza S. Skin-sparing mastectomy flap complications after breast reconstruction: review of incidence, management, and outcome. Ann Plast Surg. 2003;50:249–255. doi: 10.1097/01.sap.0000046784.70583.e1. discussion 255. [DOI] [PubMed] [Google Scholar]
- 8.Pannucci CJ, Antony AK, Wilkins EG. The impact of acellular dermal matrix on tissue expander/implant loss in breast reconstruction: an analysis of the tracking outcomes and operations in plastic surgery database. Plast Reconstr Surg. 2013;132:1–10. doi: 10.1097/PRS.0b013e318290f917. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal JP, Mendenhall SD, Anderson LA, et al. The breast reconstruction evaluation of acellular dermal matrix as a sling trial (BREASTrial): design and methods of a prospective randomized trial. Plast Reconstr Surg. 2015;135:20e–28e. doi: 10.1097/PRS.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 10.Hoppe IC, Yueh JH, Wei CH, et al. Complications following expander/implant breast reconstruction utilizing acellular dermal matrix: a systematic review and meta-analysis. Eplasty. 2011;11:e40. [PMC free article] [PubMed] [Google Scholar]
- 11.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356. doi: 10.1097/SAP.0b013e31823f3cd9. [DOI] [PubMed] [Google Scholar]
- 12.Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg. 2009;123:1–6. doi: 10.1097/PRS.0b013e3181904bff. discussion 107. [DOI] [PubMed] [Google Scholar]
- 13.Seth AK, Persing S, Connor CM, et al. A comparative analysis of cryopreserved versus prehydrated human acellular dermal matrices in tissue expander breast reconstruction. Ann Plast Surg. 2013;70:632–635. doi: 10.1097/SAP.0b013e318250f0b4. [DOI] [PubMed] [Google Scholar]
- 14.Brooke S, Mesa J, Uluer M, et al. Complications in tissue expander breast reconstruction: a comparison of AlloDerm, DermaMatrix, and FlexHD acellular inferior pole dermal slings. Ann Plast Surg. 2012;69:347–349. doi: 10.1097/SAP.0b013e31824b3d97. [DOI] [PubMed] [Google Scholar]
- 15.Liu DZ, Mathes DW, Neligan PC, et al. Comparison of outcomes using AlloDerm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg. 2014;72:503–507. doi: 10.1097/SAP.0b013e318268a87c. [DOI] [PubMed] [Google Scholar]
- 16.Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249:26–32. doi: 10.1097/SLA.0b013e31818e41a7. [DOI] [PubMed] [Google Scholar]
- 17.Chun YS, Verma K, Rosen H, et al. Use of tumescent mastectomy technique as a risk factor for native breast skin flap necrosis following immediate breast reconstruction. Am J Surg. 2011;201:160–165. doi: 10.1016/j.amjsurg.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. doi: 10.1097/PRS.0b013e31828bd377. [DOI] [PubMed] [Google Scholar]
- 19.Davies K, Allan L, Roblin P, et al. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast. 2011;20:21–25. doi: 10.1016/j.breast.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Sbitany H. Management of the post-breast reconstruction “hyperanimation deformity”. Plast Reconstr Surg. 2014;133:897e–898e. doi: 10.1097/PRS.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 21.Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68(2):162–167. doi: 10.1016/j.bjps.2014.10.012. [DOI] [PubMed] [Google Scholar]


