Abstract
Background:
As the use of acellular dermal matrices in breast reconstruction has become more commonplace and efforts are made to improve on postoperative outcomes, the method of acellular dermal matrix (ADM) processing (aseptic versus sterile) has become a subject of interest. This article provides an updated overview of the critical aspects of ADM processing in addition to application of ADMs in single- and two-stage breast reconstruction, a review of the morbidity associated with ADM use, and alternatives.
Methods:
A literature review was performed in PubMed identifying recent systematic reviews, meta-analyses, and head-to-head comparisons on aseptically processed ADM and sterile-processed ADM in implant-based breast reconstruction.
Results:
Recent meta-analyses have shown a 2- to 3-fold increase in infections and tissue expander/implant explantation rates and a 3- to 4-fold increase in seroma formation compared with non-ADM reconstruction techniques. Comparisons of aseptic and sterile ADMs in multiple studies have shown no significant difference in infection rates and equivocal findings for other specific complications such as seroma formation.
Conclusions:
Current evidence on the impact of processing techniques that improve ADM sterility on postoperative morbidity in implant breast reconstruction is unclear. Deficiencies of the available data highlight the need for well-designed, multicenter, randomized controlled studies that will aid in optimizing outcomes in implant-based breast reconstruction.
Over 232,000 new cases of invasive breast cancer were diagnosed in the United States in 2013.1 Although nearly 40% of women diagnosed with breast cancer ultimately undergo a total mastectomy, historically less than one quarter of these patients pursue immediate breast reconstruction.2–8 Among women who undergo breast reconstruction, over 70% of operations use a tissue expander/implant (TE/I)–based technique,9–12 and a majority of these cases incorporate the use of an acellular dermal matrix (ADM).13
Since the first reported application of ADM in breast reconstruction in 2005,14 the indications for its use in breast reconstruction have expanded dramatically. ADM is used routinely by plastic surgeons for aesthetic and revisionary breast surgery, nipple reconstruction, single-stage breast reconstructions, and primary TE/I reconstructions.15 Of the approximately 57,000 TE/I-based reconstructions performed annually in the United States, biologic mesh was used in nearly 56% of all cases.11,13,16
In trying to gain a better understanding of factors that influence the morbidity associated with use of ADMs in breast reconstruction, the method of ADM processing (aseptic versus sterile processing) has become a subject of interest with conflicting results from recent studies.17–21 Whether ADM sterility has an impact on postoperative outcomes is the question at hand, and this article attempts to summarize the current literature and to identify gaps in our knowledge. Furthermore, this article provides an updated overview of the critical aspects of ADM processing in addition to application of ADMs in single- and two-stage breast reconstruction. A review of the morbidity associated with ADM use and alternatives is also presented.
ACELLULAR DERMAL MATRICES IN BREAST RECONSTRUCTION
ADM offers several advantages when compared with the more traditional dual-plane and total submuscular reconstruction techniques for breast reconstruction.19,22 Advantages include decreased inferior pole rippling and contour abnormalities, greater TE/I control within the mastectomy pocket, improved inferolateral pole coverage, avoidance of serratus fascia/musculature elevation, greater intraoperative expander fill and potentially fewer in-office expansions, improved aesthetic outcomes, and decreased capsular contracture.15,19,22–30 Some of these advantages have made possible the addition of a single-stage reconstruction technique to the reconstructive armamentarium.
Single-stage breast reconstruction, although in many cases a misnomer, bypasses the previously unavoidable tissue expansion stage of implant breast reconstruction. The ADM allows for immediate placement of a full-size implant at the time of mastectomy—acting as an inferior and inferolateral extension of the pectoralis major muscle—obviating the need for expansion of the submuscular pocket. In appropriately selected patients with adequate skin preservation at the time of mastectomy, the use of ADM offers an attractive option and simplifies the overall reconstruction process (Fig. 1).15
Fig. 1.
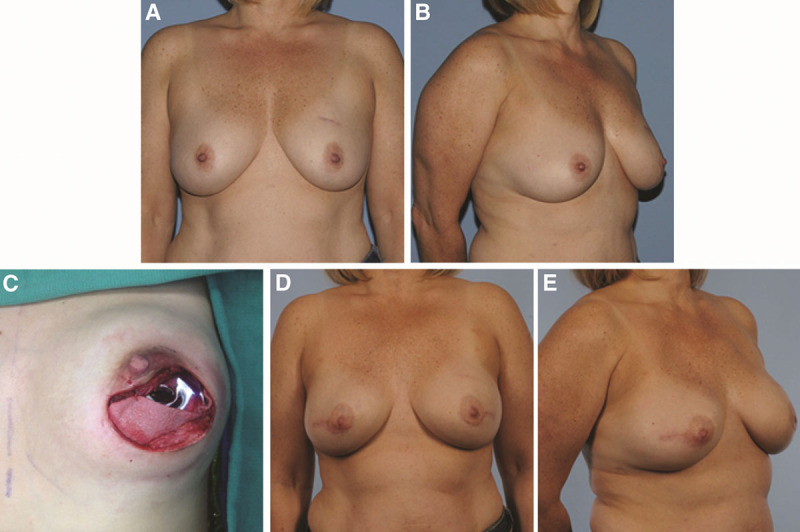
Patient with a high risk of breast cancer with grade I ptosis of the breasts (A and B), periareolar incision for nipple-sparing prophylactic mastectomies with placement of silicone implant underneath pectoralis major muscle and acellular dermal matrix (C); and 6 mo after single-stage implant breast reconstruction (D and E).
The well-established two-stage breast reconstruction technique continues to be the more common form of implant-based breast reconstruction. Here again, ADM is used to provide support and coverage of the inferolateral pole15,25,31 with benefits of greater control of the inframammary fold and reduced lateral migration of the prosthesis during expansion (Fig. 2).14,15,25,31 Compared with the total submuscular coverage technique, several studies have reported increased intraoperative expander fill volumes with the use of ADM resulting in fewer in-office fills and a potentially shortened time to definitive reconstruction with expander–implant exchange.15,25,28,32,33
Fig. 2.
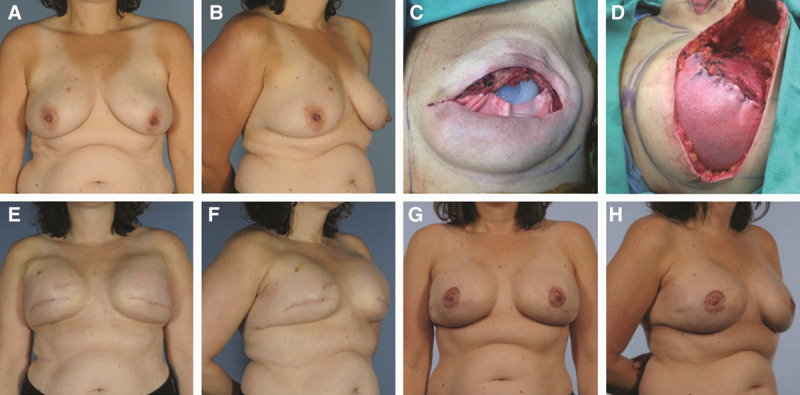
Patient with right breast ductal carcinoma in situ and grade II ptosis of the breasts (A and B); subpectoral placement of tissue expanders with acellular dermal matrix for inferior pole coverage after skin-sparing mastectomies with expander partially filled intraoperatively (C and D); results after completion of expansion process (E and F); and final postoperative results after exchange of expanders for silicone implants, nipple reconstruction, and tattooing (G and H).
Although clearly beneficial, the overwhelming evidence indicates that ADM use in breast reconstruction increases postoperative morbidity when compared to similar implant reconstructions without ADM.16,34,35 However, with the evolution and growth in the number and variety of available ADM products, questions exist on the effect of processing on postoperative morbidity.
ADM PROCESSING—ASEPTIC VERSUS STERILE
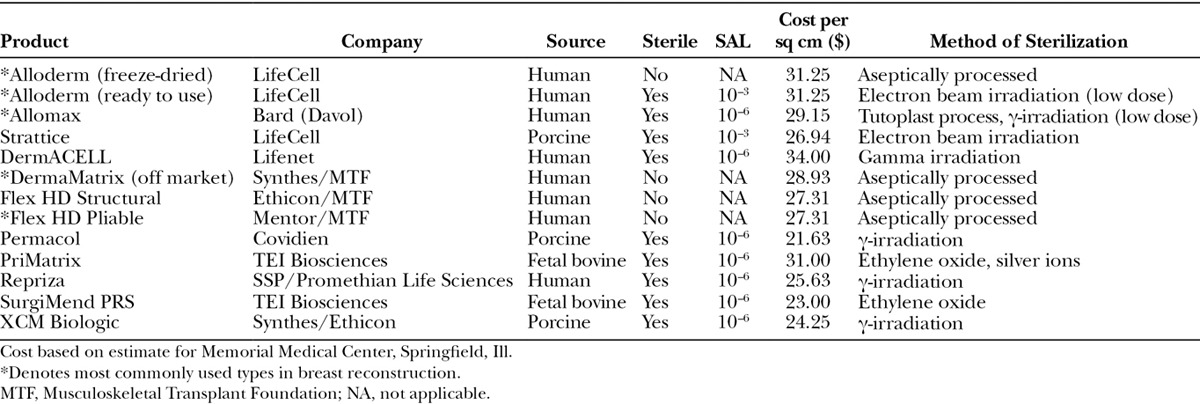
Aseptic processing refers to the technique of preventing, restricting, or minimizing contamination of a medical product with microorganisms from the environment, processing personnel, and/or equipment.17,36 For ADM, this often includes proprietary semicontained processes of washing the allograft with detergents, antibiotics, and mechanical means that ensure near complete decellularization and decontamination (Fig. 3).36 For example, AlloDerm is an aseptically processed ADM, which is treated with buffered salt solutions to remove the epidermis and is further subjected to mild, nondenaturing detergent agents to eliminate remaining epidermal and dermal cells while maintaining the integrity of the collagen matrix.37,38 Batches of ADM, processing solutions, and equipment are randomly sampled and extensively cultured to monitor for viable microorganisms using standards set by the US Pharmacopeia-71 sterility tests.39 The US Pharmacopeia-71 sterility testing procedures are not by themselves designed to ensure that a batch of product is sterile or has been sterilized, as this is ensured primarily by validation of the sterilization process or of the aseptic processing techniques.29 Some companies culture the ADM before and after processing and even purposely inoculate their product with known quantities of organisms before processing to calculate the log reduction in viable organisms to further validate their processes. Aseptic processing is common for pharmaceuticals and heat/chemical/radiation-sensitive medical devices and products. Aseptically processed items generally do not have an associated sterility assurance level (SAL) because they do not undergo a validated terminal sterilization process and are thus not labeled on the package as “sterile” (Table 1).
Fig. 3.
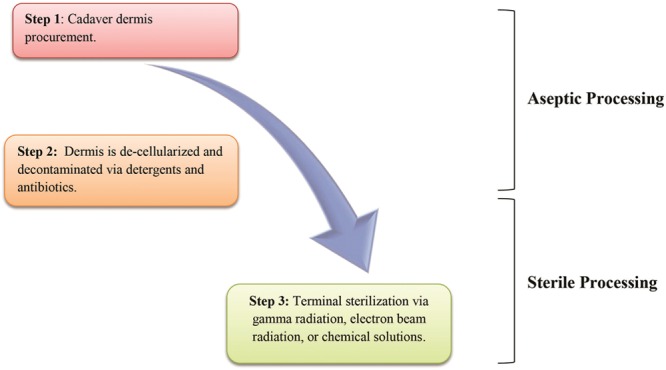
Schematic outline of processing for aseptic and sterile acellular dermal matrices.
Table 1.
Acellular Dermal Matrix Properties and Sterility Comparison
Sterile ADM, however, has undergone a validated terminal sterilization process after initial cleansing and decellularization (Fig. 3). For biologic tissues, the most common terminal sterilization techniques include treatment with gamma radiation, electron beam radiation, chemical sterilization solutions, or ethylene oxide. Each method has distinct advantages/disadvantages and all are known to alter protein/collagen structure that can impact tissue incorporation and mechanical properties.19,36,40 These alterations are usually dose-dependent, and newer sterilization processes can partially mitigate some of the detrimental effects. It has previously been shown that increasing gamma radiation doses in allograft material can cause increased scission of peptide bonds within collagen molecules, leading to reduced allograft tissue strength.40 This damage can be limited by using extremely low temperatures, free radical scavengers, or by freeze-drying the graft before the sterilization process.36,40 Theoretically, degradation of the allograft tissue's mechanical properties from high doses of gamma radiation could lead to TE/I malposition and worsened aesthetic outcomes. Complete sterility (i.e., the total absence of microorganisms) is not feasible nor practical when it comes to allografts or biologic tissues that are heat sensitive and cannot be autoclaved or radiated at high doses. Currently, a SAL of 10–6 is widely accepted as the definition of “sterile.”17,36,41 The SAL of 10–6 can be explained as the probability of 1 per 1,000,000 sterilized items having a viable microorganism after the sterilization process.17 Because of the dose-dependent relationship between terminal sterilization and allograft damage, some biologic tissues are terminally sterilized to a lower SAL such as 10–3, which theoretically offers the advantage of some level of sterility with less allograft compromise. However, this has not yet been experimentally proven in the published literature.
COMPLICATIONS WITH ASEPTIC VERSUS STERILE ADM BREAST RECONSTRUCTION
The current literature comparing sterile ADM to aseptically processed ADM in breast reconstruction is limited and has shown mixed results. Table 2 and Fig. 4 compare the outcomes of these studies and also include studies utilizing xenograft ADMs, which have always required terminal sterilization.42,43 In a prospective multicenter cohort study evaluating staged breast reconstruction with the sterile-processed ADM Allomax (Davol Inc, Warwick, R.I.), Venturi et al17 demonstrated a favorable complication profile. Over 12 months of follow-up of 65 breast reconstructions, they encountered one case of cellulitis (1.5%), two cases of partial flap necrosis (3.0%), and no cases of seromas or explantation.17 Unfortunately, the study did not include an aseptic ADM control group for direct comparison and attempted to compare their outcomes to previously published studies on aseptically processed ADM.9,26 Differences in patient demographics, comorbidities, and treatment variables limited the effectiveness of the attempted comparisons.
Table 2.
Studies Comparing Aseptically Processed to Sterile-processed ADM in Breast Reconstruction
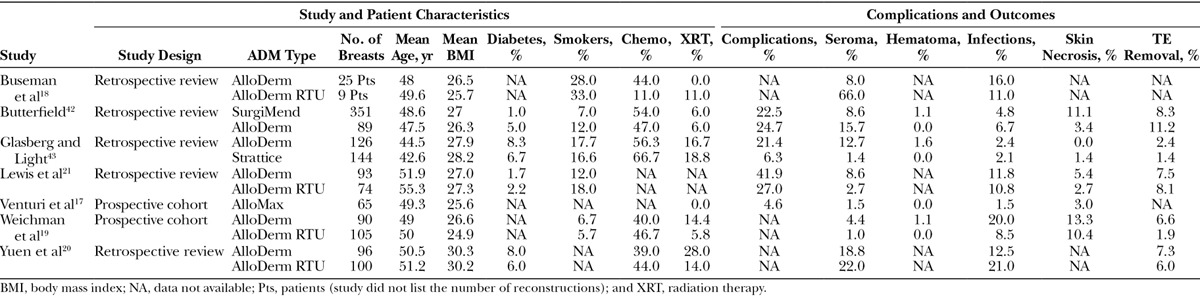
Fig. 4.
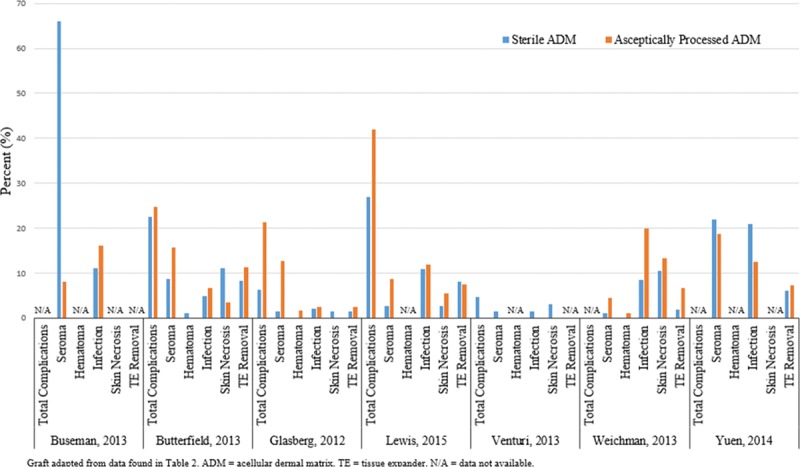
Study outcomes comparing aseptically processed to sterile-processed acellular dermal matrix in breast reconstruction.
A direct comparison of LifeCell's (Branchburg, N.J.)aseptic ADM (freeze-dried [FD] AlloDerm) to their sterile ADM (AlloDerm ready-to-use [RTU]) was performed retrospectively by Buseman et al.18 They found a statistically significant increase in seroma rates in the sterile Alloderm RTU group (P = 0.003), but no differences in infection rates. Similar findings were reported by Yuen et al,20 who retrospectively reviewed their outcomes after switching from aseptic FD AlloDerm (n = 51 patients) to sterile AlloDerm RTU (n = 52 patients). They also found higher seroma rates, in addition to higher cellulitis rates, in the AlloDerm RTU group compared with FD Alloderm (22.0% vs 18.8%; P = 0.599 and 21.0% vs 12.5%; P = 0.129, respectively). These findings, however, did not reach statistical significance.
In contrast, a prospective cohort study by Weichman et al19 comparing total submuscular coverage (351 breasts), aseptic AlloDerm (90 breasts), and sterile AlloDerm RTU (105 breasts) showed significantly fewer infections in the sterile group compared with the aseptic group (8.5% vs 20 %; P = 0.0088). Patients in the sterile group had a similar infection rate to the total submuscular coverage group (8.5% vs 5.7%; P = 0.36).19 Interestingly, they noted less incorporation of the sterile ADM during the implant exchange operations compared with aseptic ADM. Notable limitations with this study included a significantly higher body mass index in the patients who had reconstruction with aseptic Alloderm and the potential influence of surgeon learning curve, as the aseptic Alloderm was used earlier in the surgeon's experience. Lewis et al21 also recently reported on their comparison of aseptic AlloDerm with sterile Alloderm RTU and showed that although infection rates were similar (11.8% vs 10.8%, respectively; P = 1.000), overall complication rates were lower with sterile Alloderm RTU compared with aseptic Alloderm (27.0%–41.9%, respectively; P = 0.046).21
ALTERNATIVES TO ADM IN IMPLANT-BASED BREAST RECONSTRUCTION
Manufacturers have begun to offer an increasing variety of ADM alternatives for use in breast reconstruction. Patients who are not candidates for human ADM for medical, ideological, or religious reasons now have several options to choose from. However, given that most products have been on the market for a relatively short period of time, there are limited outcome data on their effectiveness and safety in breast reconstruction. One such product that has received interest is the SERI Surgical Scaffold (Allergan, Inc, Irvine, Calif.), approved for use in the United States in 2009. SERI is a knitted, bioresorbable scaffold composed of silk-derived fibroin, which is indicated for soft-tissue reinforcement and general soft-tissue reconstruction.44,45 Before packaging, SERI is washed and sterilized.44,45 In an ovine model evaluating the use of SERI surgical scaffold for two-stage breast reconstruction, Gross et al44 reported that SERI-incorporated tissue samples maintained at least 150% of native ovine fascial strength at all endpoints. Knitted polyglactin 910 (Vicryl; Ethicon, Inc., Somerville, N.J.) mesh has also been described as an alternative to ADM in direct-to-implant breast reconstruction after skin-sparing mastectomy because of its relatively inexpensive costs, ease of use, resistance to biofilm formation, and nonallergenic composition.12
Another alternative, the titanium-coated polypropylene mesh (TiLOOP Bra, pfm medical, Cologne, Germany) has been described for use in implant-based breast reconstruction.46 Despite approval in Europe since 2008, there remain limited data regarding safety and outcomes.46 As an autologous option, dermal sling-assisted breast reconstruction, also known as the Bostwick autoderm technique, has been reported as an alternative to ADM use.47,48 In this procedure, the inferior pole mastectomy skin is deepithelialized and adjoined to the inferior border of the pectoralis major muscle to create a tension-free vascularized pocket.47,48 The technique may be used in either one- or two-stage breast reconstruction patients who have excess lower pole skin after mastectomy.48
DISCUSSION
The use of ADM in single- and two-stage breast reconstruction remains popular because of its ease of use and improved TE/I control within the mastectomy pocket.15,19,22–24 There have been several studies that have reported low morbidity with the use of ADM in breast reconstruction,13,17,18,22 and there is a growing body of anecdotal evidence suggesting improved aesthetic outcomes in reconstruction patients.13,25–27,29,30,49,50 However, plastic surgeons continue to weigh the advantages of ADM against a growing body of the literature reporting on increased morbidity with its use. Alongside the clear benefits made possible by ADM in breast reconstruction, complications attributed to its use have led some authors to advocate for caution and selective use of the material.9,13 Numerous studies have reported on increased complication rates associated with ADM use in breast reconstruction,9,26,51,52 with a majority of the studies focused on the earliest available ADM, Alloderm (LifeCell, Branchburg, N.J.).9,14,24,26,49,52–55 Furthermore, several studies have attempted to isolate independent variables that may increase complication rates with ADM usage, such as elevated patient body mass index, higher patient age and axillary dissection,52 and potential variables such as surgeon experience and technique.15,26,56 Attempting to make sense of data from smaller, typically single-center studies, recent systematic reviews and meta-analyses have pooled complication data on ADM usage in implant-based breast reconstruction.
In a 2011 systematic review and meta-analysis, Hoppe et al34 compared complication rates of AlloDerm-assisted two-stage breast reconstruction with traditional non-ADM–assisted implant/expander techniques after mastectomy.34 Three of the included studies22,27,53 reported no difference in the complication rate and 4 studies9,26,51,52 reported an increased rate of complications between cohorts.34 When compared with the non-ADM cohort, the ADM-assisted group reported a 2-fold increase in infection rates (odds ratio [OR], 2.33), a 3-fold increase in seroma formation (OR, 3.00), and a 2-fold increase in the TE/I explantation rate (OR, 2.41).34 Similar complication rates were reported in a systematic review by Ho et al,35 which focused on single-stage and two-stage breast reconstructions using ADM. A systematic review by Sbitany et al57 in 2011 found higher seroma rates to be the only significant complication difference between ADM and non-ADM cohorts (8.4% vs 4.3%; p = 0.03). A meta-analysis by Kim et al16 in 2012 estimated complication rates in TE/I reconstructions using ADM. Nineteen studies reporting ADM use (4 of which reported on single-stage reconstruction with ADM) and 35 studies reporting submuscular techniques without ADM were used to estimate complication rates. Complication rates were higher with ADM use versus no ADM with specific complications including flap necrosis (6.9% vs 4.9%), infection (5.3% vs 4.7%), and seroma (4.8% vs 3.5%).16
One potential explanation for the reported increases in infection rates with ADM usage in breast reconstruction is that the ADM may serve as a nidus for bacteria. Some investigators have proposed a tiered sterility classification that is product specific with regard to product vulnerability to damage from sterilization and level of risk to the patient.41 For example, items that can withstand high heat (such as surgical instruments) would undergo high-level sterilization to an SAL of 10–6, whereas heat-sensitive products (such as biologics) would undergo low-level sterilization to a SAL of 10–3 and still be considered “sterile” for their intended use. Aseptically processed items generally do not have an associated SAL because they do not undergo a validated terminal sterilization process (Table 1).
Although the advent of terminally sterilized ADM has been marketed as a potentially “cleaner” alternative to the aseptically processed biologics, the current data comparing the two have been largely inconclusive because of study design flaws including unmatched cohorts, underpowered populations, and confounding variables ultimately resulting in studies with a low level-of-evidence. One of the highest quality studies to date that evaluated use of ADMs in implant breast reconstruction, the BREASTrial, recently reported on outcomes from the time of mastectomy and tissue expander placement to completion of the reconstruction process.58 This was a large prospective randomized trial comparing outcomes of immediate-staged tissue expander breast reconstruction using either AlloDerm or DermaMatrix.58 One hundred twenty-eight patients were randomly assigned to either of the ADM groups, and the impact of obesity, chemotherapy, and radiation was assessed in regard to complications and biointegration of the ADMs. Similar overall complication rates were observed between the AlloDerm and DermaMatrix groups (33.6% vs 38.8%, respectively). No differences in complications were noted on regression. In both groups, obesity contributed to poor matrix biointegration, longer drain times, and increased complication rates. As evidence of the rapidly evolving nature of ADMs, the study was initiated at a time that preceded the introduction of sterile-processed Alloderm and as such both ADMs in the study are aseptically processed. Similar well-designed prospective studies are needed to help tackle questions on the effect of ADM processing on outcomes.
CONCLUSIONS
Acellular dermal matrices have made significant contributions to the evolution of single- and two-stage implant breast reconstruction after mastectomy. Careful patient selection continues to be critical in efforts made to optimize outcomes of breast reconstruction with acellular dermal matrices. The impact of ADM processing techniques on postoperative morbidity in implant-based breast reconstruction is unclear at this point, highlighting the need for well-designed, multicenter, randomized controlled studies on this subject.
Footnotes
Disclosure: Dr. Neumeister is on the board of directors of the Musculoskeletal Transplant Foundation. Neither of the other authors has any financial disclosures. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle TM, Rueth NM, Abbott A, et al. Trends in the local treatment of breast cancer: should we be worried? J Surg Oncol. 2011;103:313–316. doi: 10.1002/jso.21699. [DOI] [PubMed] [Google Scholar]
- 3.Habermann EB, Abbott A, Parsons HM, et al. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28:3437–3441. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 4.Alderman AK, McMahon L, Jr, Wilkins EG. The national utilization of immediate and early delayed breast reconstruction and the effect of sociodemographic factors. Plast Reconstr Surg. 2003;111:695–703. doi: 10.1097/01.PRS.0000041438.50018.02. discussion 704. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins EG, Alderman AK. Breast reconstruction practices in North America: current trends and future priorities. Semin Plast Surg. 2004;18:149–155. doi: 10.1055/s-2004-829049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polednak AP. Postmastectomy breast reconstruction in Connecticut: trends and predictors. Plast Reconstr Surg. 1999;104:669–673. doi: 10.1097/00006534-199909030-00008. [DOI] [PubMed] [Google Scholar]
- 7.Reuben BC, Manwaring J, Neumayer LA. Recent trends and predictors in immediate breast reconstruction after mastectomy in the United States. Am J Surg. 2009;198:237–243. doi: 10.1016/j.amjsurg.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 9.Liu AS, Kao HK, Reish RG, et al. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg. 2011;127:1755–1762. doi: 10.1097/PRS.0b013e31820cf233. [DOI] [PubMed] [Google Scholar]
- 10.Warren AG, Morris DJ, Houlihan MJ, et al. Breast reconstruction in a changing breast cancer treatment paradigm. Plast Reconstr Surg. 2008;121:1116–1126. doi: 10.1097/01.prs.0000305516.93441.fd. [DOI] [PubMed] [Google Scholar]
- 11.Report of the 2009 Statistics: 2009 Reconstructive Surgery Trends. 2010. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2009-statistics/2009-reconstructive-trends-statistics.pdf. Accessed January 1, 2016.
- 12.Tessler O, Mattos D, Vorstenbosch J, et al. A methodological analysis of the plastic surgery cost-utility literature using established guidelines. Plast Reconstr Surg. 2014;133:584e–592e. doi: 10.1097/PRS.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 13.JoAnna Nguyen T, Carey JN, Wong AK. Use of human acellular dermal matrix in implant- based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg. 2011;64:1553–1561. doi: 10.1016/j.bjps.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232–239. doi: 10.1097/01.sap.0000168527.52472.3c. [DOI] [PubMed] [Google Scholar]
- 15.Israeli R. Complications of acellular dermal matrices in breast surgery. Plast Reconstr Surg. 2012;130(5 Suppl 2):159S–172S. doi: 10.1097/PRS.0b013e3182634e62. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 17.Venturi ML, Mesbahi AN, Boehmler JH, 4th, et al. Evaluating sterile human acellular dermal matrix in immediate expander-based breast reconstruction: a multicenter, prospective, cohort study. Plast Reconstr Surg. 2013;131:9e–18e. doi: 10.1097/PRS.0b013e3182729d4f. [DOI] [PubMed] [Google Scholar]
- 18.Buseman J, Wong L, Kemper P, et al. Comparison of sterile versus nonsterile acellular dermal matrices for breast reconstruction. Ann Plast Surg. 2013;70:497–499. doi: 10.1097/SAP.0b013e31827f52c8. [DOI] [PubMed] [Google Scholar]
- 19.Weichman KE, Wilson SC, Saadeh PB, et al. Sterile “ready-to-use” AlloDerm decreases postoperative infectious complications in patients undergoing immediate implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;132:725–736. doi: 10.1097/PRS.0b013e31829fe35b. [DOI] [PubMed] [Google Scholar]
- 20.Yuen JC, Yue CJ, Erickson SW, et al. Comparison between freeze-dried and ready-to-use AlloDerm in alloplastic breast reconstruction. Plast Reconstr Surg Glob Open. 2014;2:e119. doi: 10.1097/GOX.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis P, Jewell J, Mattison G, et al. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg. 2015;74(Suppl 1):S30–S32. doi: 10.1097/SAP.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 22.Preminger BA, McCarthy CM, Hu QY, et al. The influence of AlloDerm on expander dynamics and complications in the setting of immediate tissue expander/implant reconstruction: a matched-cohort study. Ann Plast Surg. 2008;60:510–513. doi: 10.1097/SAP.0b013e31816f2836. [DOI] [PubMed] [Google Scholar]
- 23.Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:1049–1058. doi: 10.1097/PRS.0b013e31824a2acb. [DOI] [PubMed] [Google Scholar]
- 24.Nahabedian MY, Mesbahi AN. Breast reconstruction with tissue expanders and implants. In: Nahabedian MY, editor. In: Cosmetic and Reconstructive Breast Surgery. London: Elsevier; 2009. pp. 1–20. [Google Scholar]
- 25.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. doi: 10.1007/s00266-008-9128-8. [DOI] [PubMed] [Google Scholar]
- 26.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 27.Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg. 2009;124:1735–1740. doi: 10.1097/PRS.0b013e3181bf803d. [DOI] [PubMed] [Google Scholar]
- 28.LifeCell Corporation (2011): AlloDerm Instructions for Use. Available at: http://www.lifecell.com/fileadmin/media/files/downloads/Alloderm_IFU_D.pdf. Accessed January 1, 2016. [Google Scholar]
- 29.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–381. doi: 10.1097/01.prs.0000267340.31742.1. [DOI] [PubMed] [Google Scholar]
- 30.Breuing KH, Colwell AS. Immediate breast tissue expander-implant reconstruction with inferolateral AlloDerm hammock and postoperative radiation: a preliminary report. Eplasty. 2009;9:e16. [PMC free article] [PubMed] [Google Scholar]
- 31.Breuing KH, Colwell AS. Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250–255. doi: 10.1097/SAP.0b013e31802f8426. [DOI] [PubMed] [Google Scholar]
- 32.Hanna KR, DeGeorge BR, Jr, Mericli AF, et al. Comparison study of two types of expander-based breast reconstruction: acellular dermal matrix-assisted versus total submuscular placement. Ann Plast Surg. 2013;70:10–15. doi: 10.1097/SAP.0b013e31822f6765. [DOI] [PubMed] [Google Scholar]
- 33.Collis GN, TerKonda SP, Waldorf JC, et al. Acellular dermal matrix slings in tissue expander breast reconstruction: are there substantial benefits? Ann Plast Surg. 2012;68:425–428. doi: 10.1097/SAP.0b013e318225833f. [DOI] [PubMed] [Google Scholar]
- 34.Hoppe IC, Yueh JH, Wei CH, et al. Complications following expander/implant breast reconstruction utilizing acellular dermal matrix: a systematic review and meta-analysis. Eplasty. 2011;11:e40. [PMC free article] [PubMed] [Google Scholar]
- 35.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356. doi: 10.1097/SAP.0b013e31823f3cd9. [DOI] [PubMed] [Google Scholar]
- 36.The Centers for Disease Control and Prevention. Processing of Orthopedic, Cardiovascular and Skin Allografts Workshop (October 2007) http://www.fda.gov/downloads/biologicsbloodvaccines/newsevents/workshopsmeetingsconferences/transcriptsminutes/ucm054425.pdf. Accessed May 3, 2015. [Google Scholar]
- 37.Bullocks JM. DermACELL: a novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur J Plast Surg. 2014;37:529–538. doi: 10.1007/s00238-014-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Pharmacopeial-71 Sterility Testing. Available at: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c71.html. [Google Scholar]
- 39.United States Pharmacopeial Convention (1211): In: United States Pharmacopeia and National Formulary (USP25-NF20) Rockville, MD: United States Pharmacopeial Convention; 2002. Sterility testing. [Google Scholar]
- 40.Conrad BP, Rappé M, Horodyski M, et al. The effect of sterilization on mechanical properties of soft tissue allografts. Cell Tissue Bank. 2013;14:359–366. doi: 10.1007/s10561-012-9340-2. [DOI] [PubMed] [Google Scholar]
- 41.von Woedtke T, Kramer A. The limits of sterility assurance. GMS Krankenhhyg Interdiszip. 2008;3:Doc19. [PMC free article] [PubMed] [Google Scholar]
- 42.Butterfield JL. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg. 2013;131:940–951. doi: 10.1097/PRS.0b013e3182865ab3. [DOI] [PubMed] [Google Scholar]
- 43.Glasberg SB, Light D. AlloDerm and Strattice in breast reconstruction: a comparison and techniques for optimizing outcomes. Plast Reconstr Surg. 2012;129:1223–1233. doi: 10.1097/PRS.0b013e31824ec429. [DOI] [PubMed] [Google Scholar]
- 44.Gross JE, Horan RL, Gaylord M, et al. An evaluation of SERI surgical scaffold for soft-tissue support and repair in an ovine model of two-stage breast reconstruction. Plast Reconstr Surg. 2014;134:700e–704e. doi: 10.1097/PRS.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 45.Fine NA, Lehfeldt M, Gross JE, et al. SERI surgical scaffold, prospective clinical trial of a silk-derived biological scaffold in two-stage breast reconstruction: 1-year data. Plast Reconstr Surg. 2015;135:339–351. doi: 10.1097/PRS.0000000000000987. [DOI] [PubMed] [Google Scholar]
- 46.Dieterich M, Paepke S, Zwiefel K, et al. Implant-based breast reconstruction using a titanium-coated polypropylene mesh (TiLOOP Bra): a multicenter study of 231 cases. Plast Reconstr Surg. 2013;132:8e–19e. doi: 10.1097/PRS.0b013e318290f8a0. [DOI] [PubMed] [Google Scholar]
- 47.Goyal A, Wu JM, Chandran VP, et al. Outcome after autologous dermal sling-assisted immediate breast reconstruction. Br J Surg. 2011;98:1267–1272. doi: 10.1002/bjs.7531. [DOI] [PubMed] [Google Scholar]
- 48.Ladizinsky DA, Sandholm PH, Jewett ST, et al. Breast reconstruction with the Bostwick autoderm technique. Plast Reconstr Surg. 2013;132:261–270. doi: 10.1097/PRS.0b013e3182958774. [DOI] [PubMed] [Google Scholar]
- 49.Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg. 2009;124:387–394. doi: 10.1097/PRS.0b013e3181aee95b. [DOI] [PubMed] [Google Scholar]
- 50.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64(5):674–78. doi: 10.1097/SAP.0b013e3181dba892. [DOI] [PubMed] [Google Scholar]
- 52.Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg. 2010;125:1606–1614. doi: 10.1097/PRS.0b013e3181d4fb2a. [DOI] [PubMed] [Google Scholar]
- 53.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 54.Spear SL, Pelletiere CV, Lockwood M. Immediate breast reconstruction with tissue expanders and AlloDerm. In: Spear SL, Wiley SC, Robb GL, et al., editors. In: Surgery of the Breast: Principles and Art. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 484–488. [Google Scholar]
- 55.Spear SL, Howard MA, Boehmler JH, et al. The infected or exposed breast implant: management and treatment strategies. Plast Reconstr Surg. 2004;113:1634–1644. doi: 10.1097/01.prs.0000117194.21748.02. [DOI] [PubMed] [Google Scholar]
- 56.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 57.Sbitany H, Serletti JM. Acellular dermis-assisted prosthetic breast reconstruction: a systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg. 2011;128:1162–1169. doi: 10.1097/PRS.0b013e318230c29e. [DOI] [PubMed] [Google Scholar]
- 58.Mendenhall SD, Anderson LA, Ying J, et al. The BREASTrial: stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plast Reconstr Surg. 2015;135:29e–42e. doi: 10.1097/PRS.0000000000000758. [DOI] [PubMed] [Google Scholar]


