Abstract
Activation of central adenosine A1 receptors in the rat, a non-hibernating species, mimics the physiological characteristics of torpor and could thus represent a basis for the development of pharmacological approaches to induce therapeutic hypothermia in pathologies such as brain hemorrhage and ischemia, and to facilitate long-term space travel.
Keywords: Adenosine, brown adipose tissue, thermoregulation, hibernation, torpor
Our recent finding1 that the activation of central adenosine A1 receptors (A1AR) can induce a hypothermic, torpor-like state in rats, a non-hibernating species, reinforces the idea that it may be possible to induce in humans, a torpor-like state leading to a pharmacologically-based therapeutic hypothermia. The positive outcome that hypothermia contributes to neuroprotection following brain ischemia has stimulated clinical interest in the development of techniques to induce a hypothermic, hypometabolic state. Despite, the increased use of therapeutic hypothermia, the available induction techniques, employing cold‐exposure approaches rather than pharmacological methods, have limited utility in quickly reaching an appropriate hypothermic state, since they activate central thermoregulatory responses that counteract falls in core temperature by reducing heat loss (cutaneous vasoconstriction) and increasing thermogenic metabolism in brown adipose tissue and through shivering. Since the induction of a hypothermic, hypometabolic torpid state in hibernating animals requires a suspension of the thermogenic processes that would counteract falling core temperatures, many studies have tried to replicate this torpid state in non-hibernating animals, with the ultimate goal of reproducing this state in humans. Hypothermia can be induced pharmacologically,2-4 although not only can these approaches have unwanted side effects, but they may require intact central thermoregulatory circuits to produce hypothermia.
We recently demonstrated that the activation of central A1AR induces a hypothermic and hypometabolic state in rats that appears similar to the torpor in hibernating animals since it also includes the significant bradycardia, transient arrhythmic events, reduced electroencephalogram amplitude and increased cutaneous vasoconstriction observed in natural torpor. The inhibition of brown adipose tissue and shivering thermogenesis, through the activation of A1AR in the nucleus of the solitary tract (NTS) (Fig. 1), contributed to the deep hypothermia and reduced metabolism, from which the rats recovered with no apparent physiological or behavioral deficits.1 We hypothesize that natural torpor and the torpid state induced by central activation of A1AR both represent specific physiological states that are regulated by the central nervous system to allow survival of the animal at a low basal metabolic rate and at a low core body temperature and to ensure a spontaneous recovery to a normal physiological function upon return to a normothermic core temperature. Because the physiological state of natural torpor is maintained in an equilibrium that can last for many hours to days, it can be viewed as a “homeostatic” state - one in which physiological functions are regulated and balanced appropriately to maintain cellular, tissue and organismal viability, but at a basal metabolic rate and a core body temperature that are lower than normal. Although further research will be required, we speculate that, just as torpor is a regulated (i.e., defended) hypometabolic and hypothermic physiological state, a variety of pathological conditions may induce altered “homeostatic” states in patients in which the critical physiological systems are in an equilibrium that favors organismal survival under the specific conditions of the pathology. Thus, rather than immediately trying to return ‘normal’ physiological parameters in a clinical situation (e.g., oxygenating an ischemic patient or rapid warming of a hypothermic person), an understanding of the altered homeostatic state that is allowing survival at, for instance, a low core body temperature, could suggest different approaches in which the new equilibrium state might be maintained temporarily, or in which more appropriate physiological approaches might be pursued to return a patient to a normal homeostatic state.
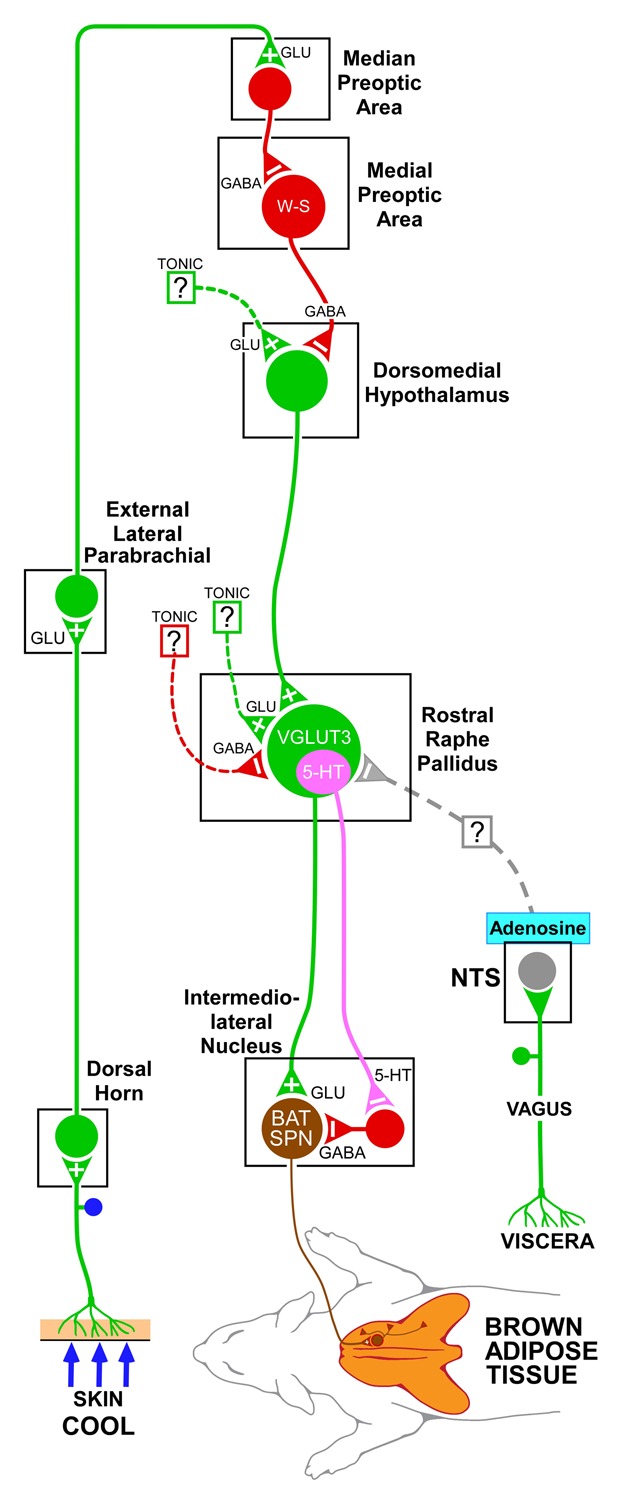
Figure 1. A model of the neural circuit through which adenosine receptor activation in the nucleus of the solitary tract (NTS) could contribute to the inhibition of brown adipose tissue thermogenesis activated by cutaneous cold stimulation. Cool cutaneous thermal sensory receptors transmit signals to dorsal root ganglia neurons which relay this thermal information to thermal sensory neurons in the dorsal horn, which activate neurons in the external lateral subnucleus of the lateral parabrachial nucleus. Thermosensory signals are then transmitted to the preoptic area to excite GABAergic interneurons in the median preoptic subnucleus which in turn inhibit a brown adipose tissue-regulating population of warm-sensitive (W-S) neurons in the medial preoptic area. Preoptic W-S neurons providing thermoregulatory control of brown adipose tissue thermogenesis inhibit BAT sympathoexcitatory neurons in the dorsomedial hypothalamus which, when disinhibited during skin cooling, excite brown adipose tissue sympathetic premotor neurons in the rostral ventromedial medulla, including the rostral raphe pallidus that project to brown adipose tissue sympathetic preganglionic neurons (SPN) in the spinal intermediolateral nucleus. Neurons in the NTS mediate the effects of afferents in the vagus nerve. The processing of metabolic, viscerosensory inputs by NTS neurons supports a potential role for an altered adenosinergic system in NTS in the modulation of the metabolic function of brown adipose tissue that could predispose to obesity. For simplicity, this figure describes only the thermoregulatory outcome, but not the other aspects of the more complex, torpor-like state induced by the central activation of the adenosine receptor. Dotted lines with question marks indicate that the pathway mediating the effect on brown adipose tissue activity is unknown. It is not yet clear if the adenosine-driven inhibition of the brown adipose tissue sympathetic premotor neurons in rostral raphe pallidus is mediated by a direct projection or a multisynaptic pathway from the NTS neurons to rostral raphe pallidus. 5-HT, 5-hydroxytryptamine; SPN, sympathetic preganglionic neuron; VGLUT3, vesicular glutamate transporter 3.
Although A1AR plays a role in the entrance to torpor in hibernating animals,5 exactly how the increase of adenosine gets triggered in natural torpor remains to be clarified. Speculatively, seasonal changes in nutrient availability could drive changes in viscerosensory inputs to the NTS that signal a declining energy status, and result in increasing local concentrations of adenosine, a byproduct of ATP metabolism, binding to A1AR in the NTS, with the consequent activation of central programs that initiate and sustain a hypometabolic, energy-conserving torpid state. What aspect of the torpor-inducing mechanism is absent in non-hibernating mammals, including humans, and thus prevents them from going into a natural torpor, remains unknown. However, our finding that central A1AR stimulation induces a torpor-like state in the non-hibernating rat is consistent with the idea that a neuronal program for the induction and support of a torpid state exists in non-hibernating mammals, including humans. For instance, prolonged, neonatal hypoxia and epilepsy, are associated with an increased central adenosine,6,7 and humans can occasionally survive severely hypoxic conditions when in a cold environment, both consistent with the possibility that humans possess a system (possibly adenosinergic) capable of triggering a torpor-like state contributing to survival. Parallels between such hypothermic, hypometabolic states and natural torpor remain to be investigated.
The importance of this study on the hypothermia induced by central administration of an A1AR agonist in a cool environment is the potential for induction of a safe, hypothermic and hypometabolic state could improve recovery and function in patients with myocardial infarction, brain hemorrhage, traumatic brain injuries, and brain ischemia. In these pathologies, a reduction in metabolism would reduce oxygen demand in ischemic tissues and improve outcomes. Futuristic applications could include extending the duration of space travel, by reducing the metabolism of astronauts. In a different vein, this study could contribute to our understanding of the pathophysiology of obesity. A chronically elevated release of adenosine in NTS, perhaps due to recurrent sleep apnea, to an alteration of the viscerosensory input to NTS, or to local inflammatory processes, could establish a hypometabolic state in which reduced energy expenditure predisposes or contributes to obesity. This study, together with those from scientists in the field of the hypothermia are leading to a new era of the physiology of hypothermia, in which a lot needs to be done to understand this fascinating homeostatic control that could revolutionize its clinical utility. In the field of hypothermia, we need to rethink the physiology and the pharmacology of the hypothermic state and the approaches used to treat hypothermic patients. For these reasons, the study of thermal biology and the central control of thermogenesis should be strongly supported.
Glossary
Abbreviation:
- 5-HT
5-hydroxytryptamine
- A1AR
adenosine A1 receptors
- ATP
adenosine triphosphate
- NTS
nucleus of the solitary tract
- SPN
sympathetic preganglionic neuron
- VGLUT3
vesicular glutamate transporter 3
- W-S
warm-sensitive
Disclosure of Potential Conflicts of Interest
The Author states he has no conflict of interest
References
- 1.Tupone D, Madden CJ, Morrison SF. . Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 2013; 33:14512 - 25; http://dx.doi.org/ 10.1523/JNEUROSCI.1980-13.2013; PMID: 24005302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackstone E, Morrison M, Roth MB. . H2S induces a suspended animation-like state in mice. Science 2005; 308:518; http://dx.doi.org/ 10.1126/science.1108581; PMID: 15845845 [DOI] [PubMed] [Google Scholar]
- 3.Muzzi M, Felici R, Cavone L, Gerace E, Minassi A, Appendino G, Moroni F, Chiarugi A. . Ischemic neuroprotection by TRPV1 receptor-induced hypothermia. J Cereb Blood Flow Metab 2012; 32:978 - 82; http://dx.doi.org/ 10.1038/jcbfm.2012.36; PMID: 22434066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, et al. . Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 2012; 32:2086 - 99; http://dx.doi.org/ 10.1523/JNEUROSCI.5606-11.2012; PMID: 22323721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinka TR, Tøien Ø, Drew KL. . Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J Neurosci 2011; 31:10752 - 8; http://dx.doi.org/ 10.1523/JNEUROSCI.1240-11.2011; PMID: 21795527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Gompel JJ, Bower MR, Worrell GA, Stead M, Chang SY, Goerss SJ, Kim I, Bennet KE, Meyer FB, Marsh WR, et al. . Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia 2014; 55:233 - 44; http://dx.doi.org/ 10.1111/epi.12511; PMID: 24483230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koos BJ, Kruger L, Murray TF. . Source of extracellular brain adenosine during hypoxia in fetal sheep. Brain Res 1997; 778:439 - 42; http://dx.doi.org/ 10.1016/S0006-8993(97)01207-9; PMID: 9459565 [DOI] [PubMed] [Google Scholar]


