Abstract
Recent research has suggested a potential for some of the physiological and cellular responses to heat acclimation to carry over to improved tolerance of the novel stresses of another environment. This cross-tolerance is evident in heat-acclimated animals that exhibit enhanced tolerance to either hypoxic or ischemic stress, and is primarily attributed to shared cellular stress response pathways. These pathways include Hypoxia-Inducible Factor-1 (HIF-1) and Heat Shock Proteins (HSP). Whether these shared cellular stress response pathways translate to systemic cross-tolerance (improved exercise tolerance, reduced risk of environment-associated illness) has not been clearly shown, particularly in humans. This review highlights the HIF-1 and HSP pathways and their relationship with systemic acclimation responses, and further examines the potential cellular and systemic adaptations that may result in cross-tolerance between hot and hypoxic environments.
Keywords: heat acclimation, high altitude acclimatization, heat shock protein, hypoxia-inducible factor
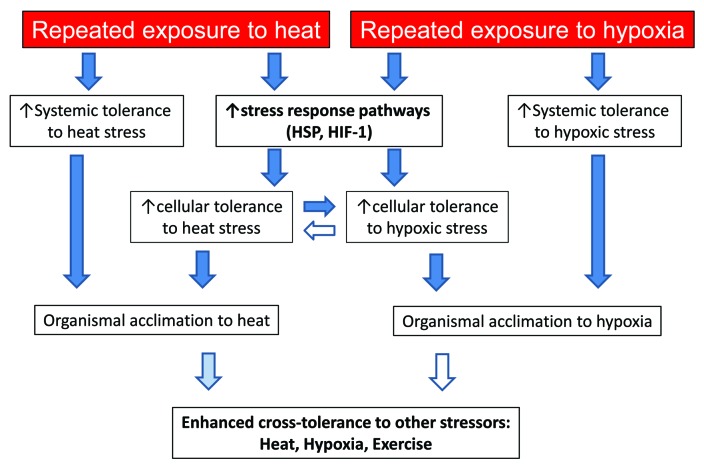
Chronic or repeated acute exposure of an organism to a hot and/or hypoxic environment results in a series of systemic physiological responses collectively referred to as acclimation. These include cardiovascular, thermoregulatory, ventilatory, and hematological adjustments in an attempt to maintain homeostasis in the face of environmental stress. On a cellular level, molecular responses during acclimation both contribute to the systemic response and protect individual cells from heat or hypoxic stress. While myriad cellular pathways are involved in the acclimation response, two of the key contributors in both heat and hypoxia are heat shock proteins (HSP) and Hypoxia-Inducible Factor-1 (HIF-1). These shared pathways have brought forth the potential for cellular cross-tolerance between hyperthermic and hypoxic stress1,2 (Fig. 1). This topic has been examined in plant models with promising results as well.3
Figure 1. The transition between exposure to heat or hypoxic stress, cellular stress response, systemic response, and acclimation/enhanced tolerance. Dark blue arrows indicate clear experimental evidence to support this relationship. Pale blue arrows indicate emerging evidence to support, while unfilled arrows indicate a possible relationship that has not yet been experimentally examined.
Hot and hypoxic cross-tolerance has been a subject of recent interest for military personnel and/or athletes who work or compete in both hot and high-altitude environments.4,5 However, the link between the observed cellular cross-tolerance and systemic acclimatory responses to hot or hypoxic environments has not been fully elucidated. Currently, cross-tolerance is primarily attributed to changes at the cellular level.2 There is some evidence lending support to the idea that heat acclimation may confer systemic benefits in both environments.6,7
The purpose of this review is to highlight cytoprotective pathways and systemic acclimatory responses in hot and hypoxic environments, and to examine the evidence for cellular and systemic cross-tolerance between environments. Links between cellular pathways and systemic responses supported by empirical evidence will be referenced, whereas areas of speculation will be identified as such. Throughout this review, the term “acclimation” will be used to describe the process of short-term (days to weeks) physiological adjustment to an environment, whether that is a simulated lab environment or a natural setting (commonly referred to acclimatization). Hypoxic (or hypoxia) will be used similarly to describe any environment with reduced partial pressure of oxygen, whether it is lab-simulated (normobaric hypoxia) or environmental (hypobaric hypoxia). Human systemic and cellular acclimation responses are the focus of this review and will be used whenever possible; however, animal data will be used when human data are unavailable.
Acclimation to Heat and Hypoxia
The process of heat acclimation in humans is generally thought to occur within one to two weeks of repeated exercise-heat stress. Based on the vast majority of published studies in the area, the classic systemic thermal, cardiovascular, sudomotor, and hematological acclimation responses are observed within 7–14 d of intermittent exercise/heat stress in humans.8,9 With human exercise-heat acclimation, cardiac output and regional blood flow are increased through plasma volume expansion to maintain perfusion of metabolically active tissues while simultaneously providing adequate blood flow to the skin for thermoregulation. Further heat acclimation responses include enhanced sensitivity of sweating, increased sweating rate of a more dilute sweat, and reduced thermoregulatory strain (lower core temperature at rest and during exercise) and cardiovascular strain (lower heart rate for a given workload) during exercise (For a review see refs. 10–11). These thermoregulatory and cardiovascular adjustments combine to reduce the risk of heat injury such as heat stroke in heat-acclimated individuals. Similar cardiovascular and thermoregulatory changes are observed using passive heat acclimation models in humans, but the addition of exercise appears to enhance the response.12 Interestingly, the longest known exercise-heat acclimation study in humans was six months in duration, however, measurements were limited by the technology available in this classic study13 and the majority of heat acclimation work in humans utilizes an exercise-heat stress protocol lasting 21 d or less. In contrast, rodent models often employ 30-d passive heat acclimation. Following this heat acclimation protocol, a reduction in resting heart rate,14 expansion of plasma volume,15 and enhancement of evaporative cooling mechanisms (salivary gland hypertrophy and enhanced salivation)16 are noted, comparable to the decreased heart rate response, increased plasma volume, and increased sweating response following human heat acclimation. While exercise-heat acclimation in animal models is less common, similar responses have been observed.17-20 The systemic adaptations and protection from heat illness are universally noted following heat acclimation in both humans and animals, but their relationships to consensus cytoprotective pathways such as HSP and HIF-1 are relatively unexplored.
With acclimation to hypoxia, the key systemic changes occur within 1–3 wk of high-altitude living (1500–3500 meters) or intermittent exposure to hypoxia. Changes associated with acclimation to hypoxia include carotid body-mediated increased minute ventilation to improve oxygen saturation,21 diuresis to effectively increase hemoglobin and hematocrit concentrations22 and stimulation of erythropoiesis to increase red blood cells and oxygen carrying capacity.23 There are also several metabolic adaptations aimed at augmenting both oxidative and glycolytic metabolism,24 and potentially enhanced capillarization to improve oxygen delivery to tissues.25 Due to these adaptations, organisms that are acclimated to hypoxia are provided protection from illnesses associated with rapid ascent to high altitude such as acute mountain sickness (AMS), high-altitude pulmonary edema (HAPE) and high-altitude cerebral edema (HACE).26 In contrast to heat acclimation, these systemic responses have been experimentally linked to several downstream effects of the HIF-1 and HSP pathway through induction of genes to produce the hormones, enzymes, and receptors associated with hypoxic acclimation.
Heat Shock Proteins
Heat shock proteins, first described by Ritossa,27 have been widely studied for their protective role in response to heat and other stressors such as oxidative damage and hypoxia. The most widely studied HSPs in response to heat or hypoxia are the HSP 70 and 90 families, so named for their molecular weight. Changes in HSP expression and the cytoprotective effects exhibited are collectively referred to as “acquired thermal tolerance.”28 HSPs play key regulatory roles in protein transport across cell membranes, re-folding of denatured proteins and preventing initiation of the apoptotic cascade observed in response to acute cellular stressors.
Heat shock proteins in heat acclimation
Previous studies in both humans and animals have noted increases in HSP-70 and HSP-90 following heat acclimation.29-32 These increases in HSP activation are associated with enhanced cytoprotection, such that cells survive longer when exposed to severe heat shock.30 Similar work in animals33 has suggested the timeline of HSP 70 and 90 expression correlates with the timeline of observed systemic heat acclimation responses and acquired thermal tolerance, indicating a possible role of HSPs in inducing systemic heat acclimatization.
The HSP 70 family (including HSP-70 and HSP-72) is highly inducible with heat stress, and these proteins serve as molecular chaperones, assist in refolding stress-denatured proteins, and are anti-apoptotic. Basal expression of HSP-70 in peripheral blood mononucleocytes (PBMCs) tracks small circadian variations in core temperature,34 and has been shown to increase following exercise-heat acclimation in humans.30,35 Similar increases were observed in basal expression of HSP-90 in PBMCs with ten days of exercise-heat acclimation.30 The increased basal expression of HSP-72 and-90 following heat acclimation resulted in a blunted HSP response to ex vivo heat shock (41–43 °C), suggesting that these cells were more tolerant to heat stress.30
Heat shock proteins in acclimation to hypoxia
Increased expression of HSPs have been observed during hypoxic acclimation. Taylor et al.36 subjected 8 healthy males to 10 d of intermittent hypoxic exposure (75 min per day at a simulated altitude of ~3000 meters), and observed increased basal levels of HSP-72 in PBMCs. HSP responses were also examined in animal models37 by exposing high elevation (yak) and low-elevation (rabbit) mammals to altitudes from 2300 meters to 6000 meters for a period of 3 wk, then analyzing brain and cardiac tissue for HSP-70 induction. While both high and low-altitude mammals increased gene transcription post-exposure, HSP-70 protein expression increased more in high elevation mammals, indicating that an enhanced HSP response to hypoxic stress may occur in altitude-acclimatized mammals.37
Heat shock proteins and systemic acclimatory responses
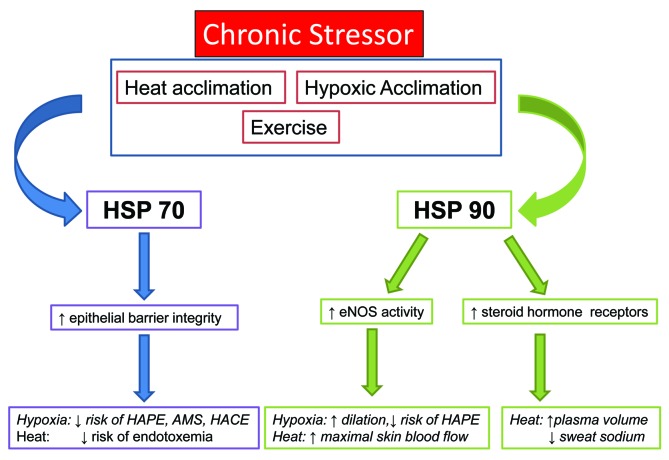
While changes in HSP expression and induction have been observed with heat and hypoxic acclimation, their relationship with systemic acclimation responses remains to be firmly established. Unexplored potential exists for HSP-90 to be involved in heat acclimation responses related to fluid balance (Fig. 2) and there is some experimental evidence for HSP-70 to aid in protection from illnesses related to heat (endotoxemia, heat stroke).38 HSP-70 may play a similar role in protection from illnesses associated with exposure to hypoxia (AMS, HAPE, and HACE; Figure 2), although this has not been experimentally elucidated. These relationships are briefly discussed in the following section.
Figure 2. HSP induction with various stressors and potential relationships between HSP expression and systemic acclimation responses to heat and hypoxia. Italics indicate a potential link between HSP and an acclimation response that has not yet been experimentally examined.
Plasma volume expansion39 and reduced sodium content of sweat40 have been noted following heat acclimation, and have been attributed in part to the sodium-reabsorbing actions of aldosterone in the kidney and sweat gland. HSP-90 regulates steroid hormone receptors,41 and could therefore act on mineralcorticoid receptors in the kidney and sweat glands to limit sodium losses through urine and sweat, thereby enhancing plasma volume expansion and creating more dilute sweat.
Heat acclimated humans display an enhanced ability to tolerate exertional heat stress without sequelae.42 Heat injury is thought to be related to cytokine production and endotoxemia, or release of toxins from the gastrointestinal tract through disruption of the epithelial border. HSP-70 plays a key role in maintaining epithelial barrier integrity, and may be related to the decreased risk of heat injury observed following acclimation.38 In addition, HSP-27 and 70 appear to be involved in suppressing inflammatory cytokine production, which may further protect a heat-acclimated organism on a systemic level.43
Another less-discussed aspect of interest is the effect of HSPs on systemic functions via interaction with other proteins involved with synaptic transmission in the brain.44 Li et al.45 demonstrated in rats that HSP-70 acts on the nucleus tractus solitarius and affects the baroreflex during heat stress while Kelty et al.46 showed HSP-70 effects on synaptic transmission in neuronal network linked to respiratory rhythms during thermal stress. To date, these effects have not yet been studied in acclimated phenotypes; however, similar roles can be hypothesized. Longer term heat acclimation protocols may be needed to fully realize changes in blood pressure regulation in humans.
Individuals who are acclimatized to hypoxia have a reduced risk of hypoxia-related illnesses26 including AMS, HACE, and HAPE, which are due in part to compromised endothelial barrier integrity in the cerebral (AMS and HACE) and pulmonary (HAPE) microcirculation. HSP-70 may play a similar role in maintaining barrier integrity and reducing the risk of cerebral and pulmonary edema in individuals acclimatized to hypoxia, however this has not been explored in human or animal models. The other factor involved in HAPE is dysregulation of pulmonary blood pressure due to exaggerated hypoxic pulmonary vasoconstriction. HSP-90 is a co-factor in production in endothelial nitric oxide synthase,47 and could therefore enhance vasodilation in acclimated individuals and further reduce the risk of developing HAPE or pulmonary hypertension.
In addition to the potential direct systemic effects of HSPs, HSP-70 and HSP-90 also interact with the HIF-1 pathway by increasing the molecular stability of HIF-1α, resulting in reduced degradation and enhanced HIF-1α formation as described in the following section. This influence of HSPs results in downstream acclimation effects attributed to the HIF-1 pathway, such as stimulation of erythropoiesis.36 This interaction between pathways is one of the foundations of heat/hypoxia cross-tolerance.
Hypoxia-Inducible Factor-1
Hypoxia inducible factors HIF-1α and HIF-1β are constitutively expressed in cells; however, HIF-1α is rapidly degraded by prolyl hydroyxylase under normal conditions while HIF-1β remains stable. With the addition of stressors including heat, hypoxia, cytokines, growth factors, reactive oxygen species, and nitric oxide, HIF-1α degradation is reduced and transcription is increased, allowing HIF-1α to accumulate and combine with HIF-1β to form the activated HIF-1 heterodimer.48 This HIF-1 dimer then signals multiple downstream pathways involved in stress responses and acclimation.49
HIF-1 in heat acclimation
HIF-1 response to chronic heat stress has primarily been examined in animal models. Its role in heat acclimation was first discovered using the Ceonorhabditis elegans genetic model. Treinin et al.50 demonstrated that C. elegans with loss of HIF-1 function cannot acclimate to heat. Consequently, HIF-1α transcription has been studied in rats and mice undergoing a 30-d passive heat acclimation protocol. Following heat acclimation, HIF-1α increased in basal conditions and augmented induction of HIF-1α transcription was observed in myocardial cells with acute heat stress in heat acclimated animals.51 This increase in the HIF-1 pathway was associated with many of the same downstream effects observed in response to high altitude acclimation, including increased expression of vascular endothelial growth factor (VEGF) in myocardial cells, as well as increased erythropoietin (EPO) and EPO receptors in renal tissue. Horowitz and Assadi2 added to this list of targets several mitochondria enzymes and Umshweif et al.52 emphasized the role of HIF-1 in aquaporin 4 and GLUT-1 transporters, which relate to enhanced metabolic (glucose uptake and utilization) and fluid regulatory (reduced cerebral edema due to water transport in aquaporin channels) mechanisms. The increased HIF-1α expression in heart tissue observed following heat acclimation lead to enhanced tissue tolerance to ischemia/anoxia, suggesting that heat acclimation may confer cross-adaptive advantages in response to ischemic or hypoxic stressors.51 Recently, Sugimoto et al.53 have substantiated that post-heat acclimation, a network of aquaporin channels controlled by HIF-1α are involved regulation of salivation, the major evaporative cooling system in rodents. The same group54 demonstrated that in long-term heat acclimation in mouse fibroblast cells both HSP and HIF-1α expression are upregulated, and that heat shock-induced and hypoxia-induced apoptoses are attenuated, suggesting that the hypoxia response pathway is an intrinsic part of the cytoprotective heat acclimation repertoire.
Increases in HIF-1α may be activated by increases in HSP associated with heat acclimation. Baird et al.55 demonstrated that Heat shock transcription factor 1 (HSF1) is upregulated during hypoxia due to direct binding of HIF-1α to the hypoxia response elements in a heat shock factor intron in Drosophila embryonic cells. Thus HIF-1 controls HSF1 transcriptional levels is a cross- regulatory mechanism for sensitizing heat shock pathway activity in order to maximize production of protective HSPs.
HIF-1 in acclimation to hypoxia
While cellular HIF-1α has not been measured over time during acclimation to hypoxia, HIF-1α transcription and accumulation are reliant on small decreases in oxygen tension56 and therefore reliably occurs in various cell types57 in any hypoxic environment sufficient to decrease arterial or cellular oxygen tension. HIF-1α accumulation begins within minutes of exposure to hypoxia and initiates a cascade of effects that, over time, constitute many of the systemic benefits associated with altitude acclimation.58,59
The HIF-1 pathway was first described as mediating the increase in serum EPO60 observed within 90 min of exposure to hypoxia.61 The downstream effects of HIF-1 in human acclimation to hypoxia have been observed in a variety of studies,62-64 but to date no human work has measured cellular HIF-1α over the course of acclimation to hypoxia.
HIF-1 and systemic acclimatory responses
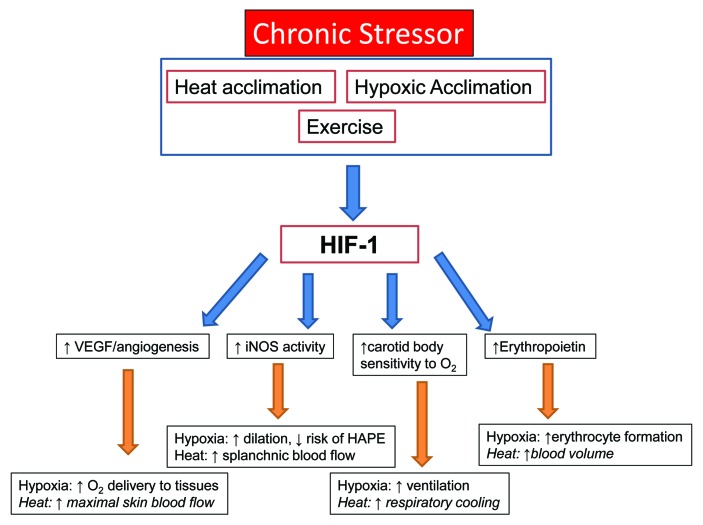
The acclimation responses to high altitude or hypoxic environments appear to be initiated by cellular events in various tissues through the HIF-1 pathway.65 These include an enhanced ventilatory response through changes in carotid body sensitivity,66 induction of erythropoietin (EPO) genes in renal tissue to increase erythrocyte production,60 induction of vascular endothelial growth factor (VEGF) to enhance angiogenesis in cardiac myocytes or other under-perfused tissues,67 increased glycolytic enzyme expression and activity to maintain anaerobic metabolism in the presence of reduced oxygen delivery to skeletal and cardiac myocytes,68 and enhanced vasodilatory mechanisms such as increased inducible nitric oxide synthase (iNOS) in endothelial cells to further aid in tissue perfusion and limit hypoxia-induced vasoconstriction69 (Fig. 3). While these responses were studied in animal models, similar systemic responses are observed in humans and are likely mediated by the same cellular pathways.
Figure 3. HIF-1α induction with various stressors and potential relationships between HIF-1and systemic acclimation responses to heat and hypoxia. Italics indicate a potential relationship between HIF-1 and acclimation that has not yet been experimentally examined.
HIF-1 is involved with changes in carotid body chemoreceptor sensitivity that results in sustained hyperventilation.66 The hypoxic ventilatory response (HVR) is reliant on the HIF-1 pathway, as HIF-1α knockout mice displayed a blunted HVR with 3-d hypoxic exposure.70 In addition, erythropoietic and angiogenic responses are dependent on a robust HIF-1α response, as HIF-1α deficient animals display delayed polycythemia and impaired vascular remodeling with hypoxic exposure.71
In heat acclimation, the relationship between the HIF-1 pathway and systemic responses has not been established in humans, but potential exists for HIF-1 mediated changes to play a role in systemic heat acclimation responses (Fig. 3). The increased maximal skin blood flow72 and sweating response post-heat acclimation could be influenced by HIF-1-regulated angiogenesis and enhanced vasodilation. Similarly, the improved exercise performance and lactate threshold noted following heat acclimation73 could potentially be related to increased capillarization, increased glycolytic activity and altered metabolic rate in skeletal muscle,74 and vasodilatory mechanisms regulated by HIF-1. In an animal model, splanchnic blood flow during heat stress is regulated by nitric oxide,75 also pointing to the HIF-1 pathway as protective from endotoxemia secondary to gastrointestinal ischemia.
Systemic Acclimatory Responses and Cross-Tolerance Between Hot and Hypoxic Environments
Among the best supported explanations for cross tolerance between heat and hypoxic acclimation are the observed cytoprotective interactions between HSPs and HIF-1. The increased HSP response to heat acclimation can independently stimulate the HIF-1 pathway and vice versa55 and results in many of the same responses observed with acclimation to hypoxia or high altitude. The cytoprotective interactions per se have been discussed in detail elsewhere.2
In a study designed to examine heat acclimation cross-tolerance, cardiac survival was modeled with an ischemia-reperfusion insult following heat acclimation in animals. Hearts were analyzed for total infarct area as a marker of cellular protection from ischemic/anoxic stress.51 The results indicate a reduced infarct area in heat-acclimated rats and mice and suggest that the increase in HIF-1α following heat acclimation contributes to cytoprotection with ischemic-reperfusion stress. Research from the same lab76 indicated that these protective effects are conferred with long-term (30 d) but not short-term heat acclimation (2 d). As most heat acclimation protocols in humans are 10–14 d, the potential for a long-term cross-tolerance response has not yet been studied. However, the potential of heat/hypoxia cross-tolerance in humans has spurred recent research efforts during a shorter heat acclimation protocol. Heled et al.6 examined systemic cross-tolerance to hypoxia (15.6% O2) following a 12-d heat acclimation protocol using both physical and cognitive performance parameters. Following heat acclimation, researchers observed a delayed onset of lactate threshold during graded exercise in both normoxia and hypoxia, and improved cognitive parameters in hypoxia. Additionally, a trend toward improved oxygen saturation during exercise in hypoxia was also noted, providing some of the first evidence of mutually beneficial systemic adaptations in humans.
Most human heat acclimation protocols involve moderate intensity endurance exercise, which can independently increase HSP expression in muscle.77,78 This HSP response is thought to be related to increased redox signaling rather than increased temperature in active muscle.79 Chronic exercise training confers similar changes in HSPs as heat acclimation, with enhanced basal expression80 and an attenuated stress response to acute exercise.81 HIF-1α transcription has also been observed to increase following acute exercise,82,83 although this response is more consistently observed with the addition of hypoxia.84 Interestingly, Lindholm et al.85 provided evidence that long-term exercise training negatively regulates HIF-1α levels and thus leads to attenuation of PDK-1 and contributes to skeletal muscle adaptation to exercise. In view of the HSP-HIF-1 cross-talk, heat acclimation-hypoxia-exercise interaction is an intriguing question. Given systemic responses we hypothesize, however, that an exercise-heat acclimation protocol may, further increase the potential for heat/hypoxia cross tolerance through both exercise- and heat-related stimulation of cellular pathways.
While evidence supporting enhanced cell survivability to severe hypoxic stress through heat acclimation is promising, the transition from cellular to systemic cross-tolerance in humans using long-term heat acclimation models has not been fully explored. Some systemic responses show promise in being beneficial in both heat and hypoxic stress. For example, the enhanced heat loss effector responses observed with heat acclimation results in reduced body core temperature at rest and a slowed rise during physical activity. The reduced temperature would cause a leftward shift in the oxyhemoglobin dissociation curve, indicating a potential for enhanced oxygen saturation of hemoglobin for a given partial pressure of oxygen as is observed following acclimation to hypoxia. The HIF-1-mediated increase in glycolytic enzymes and mitochondrial proliferation may also contribute to increased oxygen saturation during exercise via improved lactate threshold. This shift in lactate threshold was observed in humans following heat acclimation in both normoxic6,73 and hypoxic6 test environments, showing further promise for heat-hypoxia cross-tolerance and physical performance. Conversely, some systemic responses in heat and/or hypoxia are in opposition, such as plasma volume expansion during heat acclimation and diuresis or plasma volume contraction in acclimation to hypoxia. However, since plasma volume appears to return toward baseline in longer heat acclimation models86 and most animal work displaying cross-tolerance employed long-term heat acclimation (30 d), it is possible that greater cross-tolerance in humans would also be evident using a longer heat acclimation protocol that allowed plasma volume to return toward baseline.
In summary, both HSPs and HIF-1α increase following acclimation to heat or hypoxia, and both pathways may contribute to observed systemic acclimation responses. While cellular cross-tolerance has been observed with heat and hypoxic stress in animal models, little is known about systemic hypoxia and heat cross-tolerance in humans. Translational studies focused on exploring the links between cellular pathways and associated systemic responses in humans are warranted to further develop our understanding and application of environmental cross-tolerance.
Glossary
Abbreviations:
- AMS
Acute Mountain Sickness eNOS, Endothelial Nitric Oxide Synthase EPO, Erythropoeitin HACE, High-Altitude Cerebral Edema HAPE, High-Altitude Pulmonary Edema HIF-1, Hypoxia Inducible Factor 1 HSP, Heat Shock Protein HVR, Hypoxic Ventilatory Response iNOS, Inducible Nitric Oxide Synthase PBMC, Peripheral Blood Mononucleocytes VEGF, Vascular Endothelial Growth Factor
Citation: Ely BR, Lovering AT, Horowitz M, Minson CT. Heat acclimation and cross tolerance to hypoxia: bridging the gap between cellular and systemic responses. Temperature 2014; 1:0 - -1; 10.4161/temp.29800
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Horowitz M. . Heat acclimation and cross-tolerance against novel stressors: genomic-physiological linkage. Prog Brain Res 2007; 162:373 - 92; http://dx.doi.org/ 10.1016/S0079-6123(06)62018-9; PMID: 17645928 [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, Assadi H. . Heat acclimation-mediated cross-tolerance in cardioprotection: do HSP70 and HIF-1α play a role?. Ann N Y Acad Sci 2010; 1188:199 - 206; http://dx.doi.org/ 10.1111/j.1749-6632.2009.05101.x; PMID: 20201904 [DOI] [PubMed] [Google Scholar]
- 3.Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P. . Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant Cell Environ 2008; 31:1029 - 37; http://dx.doi.org/ 10.1111/j.1365-3040.2008.01816.x; PMID: 18410489 [DOI] [PubMed] [Google Scholar]
- 4.Salgado AM, White AC, Schneider SM, Mermier CM. . A Novel Mechanism for Cross-Adaptation between Heat and Altitude Acclimation: The Role of Heat Shock Protein 90. Physiol J 2014; 2014:1 - 12; http://dx.doi.org/ 10.1155/2014/121402 [DOI] [Google Scholar]
- 5.White AC, Salgado RM, Schneider S, Loeppky JA, Astorino TA, Mermier CM. . Does heat acclimation improve exercise capacity at altitude? A cross-tolerance model. Int J Sports Med 2014; Forthcoming PMID: 24816886 [DOI] [PubMed] [Google Scholar]
- 6.Heled Y, Peled A, Yanovich R, Shargal E, Pilz-Burstein R, Epstein Y, Moran DS. . Heat acclimation and performance in hypoxic conditions. Aviat Space Environ Med 2012; 83:649 - 53; http://dx.doi.org/ 10.3357/ASEM.3241.2012; PMID: 22779306 [DOI] [PubMed] [Google Scholar]
- 7.Yacobi A, Stern Bach Y, Horowitz M. . The protective effect of heat acclimation from hypoxic damage in the brain involves changes in the expression of glutamate receptors. Temperature 2014; Forthcoming http://dx.doi.org/ 10.4161/temp.29719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandolf KB. . Time course of heat acclimation and its decay. Int J Sports Med 1998; 19:Suppl 2 S157 - 60; http://dx.doi.org/ 10.1055/s-2007-971985; PMID: 9694426 [DOI] [PubMed] [Google Scholar]
- 9.Senay LC, Mitchell D, Wyndham CH. . Acclimatization in a hot, humid environment: body fluid adjustments. J Appl Physiol 1976; 40:786 - 96; PMID: 931907 [DOI] [PubMed] [Google Scholar]
- 10.Sawka MN, Wenger CB, Pandolf KB. Thermoregulatory responses to acute exercise-heat stress and heat acclimation. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am Physiol Soc, 1996, sect. 4, vol. II, chapt. 9, 157–185. [Google Scholar]
- 11.Sawka MN, Leon LR, Montain SJ, Sonna LA. . Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol 2011; 1:1883 - 928; PMID: 23733692 [DOI] [PubMed] [Google Scholar]
- 12.Taylor NA. . Human heat adaptation. Compr Physiol 2014; 4:325 - 65; http://dx.doi.org/ 10.1002/cphy.c130022; PMID: 24692142 [DOI] [PubMed] [Google Scholar]
- 13.Christensen WR. . Long term acclimatization to heat. Am J Physiol 1947; 148:86 - 90; PMID: 20283137 [DOI] [PubMed] [Google Scholar]
- 14.Horowitz M, Meiri U. . Central and peripheral contributions to control of heart rate during heat acclimation. Pflugers Arch 1993; 422:386 - 92; http://dx.doi.org/ 10.1007/BF00374295; PMID: 8437889 [DOI] [PubMed] [Google Scholar]
- 15.Horowitz M, Givol N. (1989) Heat acclimation and heat stress: cardiac output distribution, plasma volume expansion and the involvement of the adrenergic pathway. in: Thermoregulation: Research and Clinical Applications. Lomax and Schoenboum (eds.), Karger, Basel. pp.204-207. [Google Scholar]
- 16.Horowitz M. . Acclimatization of rats to moderate heat: body water distribution and adaptability of the submaxillary salivary gland. Pflugers Arch 1976; 366:173 - 6; http://dx.doi.org/ 10.1007/BF00585874; PMID: 1033519 [DOI] [PubMed] [Google Scholar]
- 17.Horowitz M, Kodesh E. . Molecular signals that shape the integrative responses of the heat-acclimated phenotype. Med Sci Sports Exerc 2010; 42:2164 - 72; http://dx.doi.org/ 10.1249/MSS.0b013e3181e303b0; PMID: 20404766 [DOI] [PubMed] [Google Scholar]
- 18.Kodesh E, Horowitz M. . Soleus adaptation to combined exercise and heat acclimation: physiogenomic aspects. Med Sci Sports Exerc 2010; 42:943 - 52; http://dx.doi.org/ 10.1249/MSS.0b013e3181c3ac3f; PMID: 19996992 [DOI] [PubMed] [Google Scholar]
- 19.Kodesh E, Nesher N, Simaan A, Hochner B, Beeri R, Gilon D, Stern MD, Gerstenblith G, Horowitz M. . Heat acclimation and exercise training interact when combined in an overriding and trade-off manner: physiologic-genomic linkage. Am J Physiol Regul Integr Comp Physiol 2011; 301:R1786 - 97; http://dx.doi.org/ 10.1152/ajpregu.00465.2011; PMID: 21957158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran D, Shapiro Y, Meiri U, Laor A, Epstein Y, Horowitz M. . Exercise in the heat: individual impacts of heat acclimation and exercise training on cardiovascular performance. J Therm Biol 1996; 21:171 - 81; http://dx.doi.org/ 10.1016/0306-4565(95)00042-9 [DOI] [Google Scholar]
- 21.Smith CA, Bisgard GE, Nielsen AM, Daristotle L, Kressin NA, Forster HV, Dempsey JA. . Carotid bodies are required for ventilatory acclimatization to chronic hypoxia. J Appl Physiol (1985) 1986; 60:1003 - 10; PMID: 3082845 [DOI] [PubMed] [Google Scholar]
- 22.Koller EA, Bührer A, Felder L, Schopen M, Vallotton MB. . Altitude diuresis: endocrine and renal responses to acute hypoxia of acclimatized and non-acclimatized subjects. Eur J Appl Physiol Occup Physiol 1991; 62:228 - 34; http://dx.doi.org/ 10.1007/BF00643747; PMID: 2044531 [DOI] [PubMed] [Google Scholar]
- 23.Reynafarje C, Ramos J, Faura J, Villavicencio D. . Humoral Control of Erythropoietic Activity in Man During and After Altitude Exposure. Proc Soc Exp Biol Med 1964; 116:649 - 50; http://dx.doi.org/ 10.3181/00379727-116-29331; PMID: 14194621 [DOI] [PubMed] [Google Scholar]
- 24.Green HJ, Sutton JR, Wolfel EE, Reeves JT, Butterfield GE, Brooks GA. . Altitude acclimatization and energy metabolic adaptations in skeletal muscle during exercise. J Appl Physiol (1985) 1992; 73:2701 - 8; PMID: 1490988 [DOI] [PubMed] [Google Scholar]
- 25.MacDougall JD, Green HJ, Sutton JR, Coates G, Cymerman A, Young P, Houston CS. . Operation Everest II: structural adaptations in skeletal muscle in response to extreme simulated altitude. Acta Physiol Scand 1991; 142:421 - 7; http://dx.doi.org/ 10.1111/j.1748-1716.1991.tb09176.x; PMID: 1927554 [DOI] [PubMed] [Google Scholar]
- 26.Gallagher SA, Hackett PH. . High-altitude illness. Emerg Med Clin North Am 2004; 22:329 - 55, viii; http://dx.doi.org/ 10.1016/j.emc.2004.02.001; PMID: 15163571 [DOI] [PubMed] [Google Scholar]
- 27.Ritossa F. . A new puffing induced by temperature shock and DNP in Drosophilia. Experientia 1962; 15:571 - 3; http://dx.doi.org/ 10.1007/BF02172188 [DOI] [Google Scholar]
- 28.Kregel KC. . Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol (1985) 2002; 92:2177 - 86; PMID: 11960972 [DOI] [PubMed] [Google Scholar]
- 29.Maloyan A, Palmon A, Horowitz M. . Heat acclimation increases the basal HSP72 level and alters its production dynamics during heat stress. Am J Physiol 1999; 276:R1506 - 15; PMID: 10233045 [DOI] [PubMed] [Google Scholar]
- 30.McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. . Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 2008; 294:R185 - 91; http://dx.doi.org/ 10.1152/ajpregu.00532.2007; PMID: 17977914 [DOI] [PubMed] [Google Scholar]
- 31.Sandström ME, Siegler JC, Lovell RJ, Madden LA, McNaughton L. . The effect of 15 consecutive days of heat-exercise acclimation on heat shock protein 70. Cell Stress Chaperones 2008; 13:169 - 75; http://dx.doi.org/ 10.1007/s12192-008-0022-8; PMID: 18759002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tetievsky A, Cohen O, Eli-Berchoer L, Gerstenblith G, Stern MD, Wapinski I, Friedman N, Horowitz M. . Physiological and molecular evidence of heat acclimation memory: a lesson from thermal responses and ischemic cross-tolerance in the heart. Physiol Genomics 2008; 34:78 - 87; http://dx.doi.org/ 10.1152/physiolgenomics.00215.2007; PMID: 18430807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horowitz M, Eli-Berchoer L, Wapinski I, Friedman N, Kodesh E. . Stress-related genomic responses during the course of heat acclimation and its association with ischemic-reperfusion cross-tolerance. J Appl Physiol (1985) 2004; 97:1496 - 507; http://dx.doi.org/ 10.1152/japplphysiol.00306.2004; PMID: 15155711 [DOI] [PubMed] [Google Scholar]
- 34.Sandström ME, Madden LA, Taylor L, Siegler JC, Lovell RJ, Midgley A, McNaughton L. . Variation in basal heat shock protein 70 is correlated to core temperature in human subjects. Amino Acids 2009; 37:279 - 84; http://dx.doi.org/ 10.1007/s00726-008-0144-4; PMID: 18665435 [DOI] [PubMed] [Google Scholar]
- 35.Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider SM. . Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol (1985) 2007; 103:1196 - 204; http://dx.doi.org/ 10.1152/japplphysiol.00242.2007; PMID: 17615280 [DOI] [PubMed] [Google Scholar]
- 36.Taylor L, Midgley AW, Chrismas B, Hilman AR, Madden LA, Vince RV, McNaughton LR. . Daily hypoxia increases basal monocyte HSP72 expression in healthy human subjects. Amino Acids 2011; 40:393 - 401; http://dx.doi.org/ 10.1007/s00726-010-0644-x; PMID: 20552383 [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Xu C, Wang X, Wang D, Wang Q, Zhang B. . Heat shock response and mammal adaptation to high elevation (hypoxia). Sci China C Life Sci 2006; 49:500 - 12; http://dx.doi.org/ 10.1007/s11427-006-2027-9; PMID: 17172058 [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. . Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol 1993; 265:R1447 - 57; PMID: 8285289 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen B, Hales JRS, Strange S, Christensen NJ, Warberg J, Saltin B. . Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 1993; 460:467 - 85; PMID: 8487204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirby CR, Convertino VA. . Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation. J Appl Physiol (1985) 1986; 61:967 - 70; PMID: 3759782 [DOI] [PubMed] [Google Scholar]
- 41.Bohen SP, Yamamoto KR. . Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci U S A 1993; 90:11424 - 8; http://dx.doi.org/ 10.1073/pnas.90.23.11424; PMID: 8248264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Federation of State High School Associations Sports Medicine Advisory Committee (NFSHA-SMAC). Heat Acclimatization and Heat Illness Prevention Position Statement, April 2012 [Google Scholar]
- 43.Moseley PL. . Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol (1985) 1997; 83:1413 - 7; PMID: 9375300 [DOI] [PubMed] [Google Scholar]
- 44.Jiang R, Gao B, Prasad K, Greene LE, Eisenberg E. . Hsc70 chaperones clathrin and primes it to interact with vesicle membranes. J Biol Chem 2000; 275:8439 - 47; http://dx.doi.org/ 10.1074/jbc.275.12.8439; PMID: 10722678 [DOI] [PubMed] [Google Scholar]
- 45.Li PL, Chao YM, Chan SH, Chan JY. . Potentiation of baroreceptor reflex response by heat shock protein 70 in nucleus tractus solitarii confers cardiovascular protection during heatstroke. Circulation 2001; 103:2114 - 9; http://dx.doi.org/ 10.1161/01.CIR.103.16.2114; PMID: 11319204 [DOI] [PubMed] [Google Scholar]
- 46.Kelty JD, Noseworthy PA, Feder ME, Robertson RM, Ramirez JM. . Thermal preconditioning and heat-shock protein 72 preserve synaptic transmission during thermal stress. J Neurosci 2002; 22:RC193; PMID: 11756523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brouet A, Sonveaux P, Dessy C, Balligand JL, Feron O. . HSP90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase (eNOS) in VEGF-exposed endothelial cells. J Biol Chem 2001; 276:32663 - 9; http://dx.doi.org/ 10.1074/jbc.M101371200; PMID: 11425855 [DOI] [PubMed] [Google Scholar]
- 48.Wang GL, Semenza GL. . Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 1995; 270:1230 - 7; http://dx.doi.org/ 10.1074/jbc.270.3.1230; PMID: 7836384 [DOI] [PubMed] [Google Scholar]
- 49.Semenza GL. . Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J 2007; 405:1 - 9; PMID: 17555402 [DOI] [PubMed] [Google Scholar]
- 50.Treinin M, Shliar J, Jiang H, Powell-Coffman JA, Bromberg Z, Horowitz M. . HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol Genomics 2003; 14:17 - 24; PMID: 12686697 [DOI] [PubMed] [Google Scholar]
- 51.Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. . HIF-1α-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics 2005; 23:79 - 88; http://dx.doi.org/ 10.1152/physiolgenomics.00279.2004; PMID: 16046617 [DOI] [PubMed] [Google Scholar]
- 52.Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. . Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab 2013; 33:524 - 31; http://dx.doi.org/ 10.1038/jcbfm.2012.193; PMID: 23281425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugimoto N, Matsuzaki K, Ishibashi H, Tanaka M, Sawaki T, Fujita Y, Kawanami T, Masaki Y, Okazaki T, Sekine J, et al. . Upregulation of aquaporin expression in the salivary glands of heat-acclimated rats. Sci Rep 2013; 3:1763; http://dx.doi.org/ 10.1038/srep01763; PMID: 23942196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugimoto N, Shido O, Matsuzaki K, Katakura M, Hitomi Y, Tanaka M, Sawaki T, Fujita Y, Kawanami T, Masaki Y, et al. . Long-term Heat Exposure Prevents Hypoxia-Induced Apoptosis in Mouse Fibroblast Cells. Cell Biochem Biophys 2014; Forthcoming http://dx.doi.org/ 10.1007/s12013-014-9912-9; PMID: 24648161 [DOI] [PubMed] [Google Scholar]
- 55.Baird NA, Turnbull DW, Johnson EA. . Induction of the heat shock pathway during hypoxia requires regulation of heat shock factor by hypoxia-inducible factor-1. J Biol Chem 2006; 281:38675 - 81; http://dx.doi.org/ 10.1074/jbc.M608013200; PMID: 17040902 [DOI] [PubMed] [Google Scholar]
- 56.Jiang BH, Semenza GL, Bauer C, Marti HH. . Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 1996; 271:C1172 - 80; PMID: 8897823 [DOI] [PubMed] [Google Scholar]
- 57.Maxwell PH, Pugh CW, Ratcliffe PJ. . Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A 1993; 90:2423 - 7; http://dx.doi.org/ 10.1073/pnas.90.6.2423; PMID: 8460154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semenza GL. . HIF-1 and human disease: one highly involved factor. Genes Dev 2000; 14:1983 - 91; PMID: 10950862 [PubMed] [Google Scholar]
- 59.Wenger RH. . Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 2002; 16:1151 - 62; http://dx.doi.org/ 10.1096/fj.01-0944rev; PMID: 12153983 [DOI] [PubMed] [Google Scholar]
- 60.Semenza GL, Wang GL. . A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992; 12:5447 - 54; PMID: 1448077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eckardt KU, Boutellier U, Kurtz A, Schopen M, Koller EA, Bauer C. . Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J Appl Physiol (1985) 1989; 66:1785 - 8; PMID: 2732171 [DOI] [PubMed] [Google Scholar]
- 62.Houston CS, Sutton JR, Cymerman A, Reeves JT. . Operation Everest II: man at extreme altitude. J Appl Physiol (1985) 1987; 63:877 - 82; PMID: 3654448 [DOI] [PubMed] [Google Scholar]
- 63.Moore LG. . Comparative human ventilatory adaptation to high altitude. Respir Physiol 2000; 121:257 - 76; http://dx.doi.org/ 10.1016/S0034-5687(00)00133-X; PMID: 10963780 [DOI] [PubMed] [Google Scholar]
- 64.Sutton JR, Reeves JT, Wagner PD, Groves BM, Cymerman A, Malconian MK, Rock PB, Young PM, Walter SD, Houston CS. . Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol (1985) 1988; 64:1309 - 21; PMID: 3132445 [DOI] [PubMed] [Google Scholar]
- 65.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. . Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci U S A 1991; 88:5680 - 4; http://dx.doi.org/ 10.1073/pnas.88.13.5680; PMID: 2062846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson DF, Roy A, Lahiri S. . Immediate and long-term responses of the carotid body to high altitude. High Alt Med Biol 2005; 6:97 - 111; http://dx.doi.org/ 10.1089/ham.2005.6.97; PMID: 16060845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pugh CW, Ratcliffe PJ. . Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 2003; 9:677 - 84; http://dx.doi.org/ 10.1038/nm0603-677; PMID: 12778166 [DOI] [PubMed] [Google Scholar]
- 68.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. . Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998; 12:149 - 62; http://dx.doi.org/ 10.1101/gad.12.2.149; PMID: 9436976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu DY, Liou HC, Tang CH, Fu WM. . Hypoxia-induced iNOS expression in microglia is regulated by the PI3-kinase/Akt/mTOR signaling pathway and activation of hypoxia inducible factor-1α. Biochem Pharmacol 2006; 72:992 - 1000; http://dx.doi.org/ 10.1016/j.bcp.2006.06.038; PMID: 16919605 [DOI] [PubMed] [Google Scholar]
- 70.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. . Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 α. Proc Natl Acad Sci U S A 2002; 99:821 - 6; http://dx.doi.org/ 10.1073/pnas.022634199; PMID: 11792862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. . Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999; 103:691 - 6; http://dx.doi.org/ 10.1172/JCI5912; PMID: 10074486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lorenzo S, Minson CT. . Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. J Appl Physiol (1985) 2010; 109:1736 - 43; http://dx.doi.org/ 10.1152/japplphysiol.00725.2010; PMID: 20864556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lorenzo S, Halliwill JR, Sawka MN, Minson CT. . Heat acclimation improves exercise performance. J Appl Physiol (1985) 2010; 109:1140 - 7; http://dx.doi.org/ 10.1152/japplphysiol.00495.2010; PMID: 20724560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young AJ, Sawka MN, Levine L, Cadarette BS, Pandolf KB. . Skeletal muscle metabolism during exercise is influenced by heat acclimation. J Appl Physiol (1985) 1985; 59:1929 - 35; PMID: 4077800 [DOI] [PubMed] [Google Scholar]
- 75.Haddad W, Horowitz M. . Heat acclimation alters nitric oxide response in the splanchnic circulation. J Therm Biol 1999; 24:403 - 8; http://dx.doi.org/ 10.1016/S0306-4565(99)00054-6 [DOI] [Google Scholar]
- 76.Assayag M, Gerstenblith G, Stern MD, Horowitz M. . Long- but not short-term heat acclimation produces an apoptosis-resistant cardiac phenotype: a lesson from heat stress and ischemic/reperfusion insults. Cell Stress Chaperones 2010; 15:651 - 64; http://dx.doi.org/ 10.1007/s12192-010-0178-x; PMID: 20221856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Locke M. . The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev 1997; 25:105 - 36; http://dx.doi.org/ 10.1249/00003677-199700250-00007; PMID: 9213090 [DOI] [PubMed] [Google Scholar]
- 78.Noble EG. Heat shock proteins and their induction with exercise. In: Locke M, Noble EG, editors. Exercise and stress response: the role of stress proteins. Boca Raton (FL): CRC Press LLC, 2002: 43-78. [Google Scholar]
- 79.Morton JP, Kayani AC, McArdle A, Drust B. . The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 2009; 39:643 - 62; http://dx.doi.org/ 10.2165/00007256-200939080-00003; PMID: 19769414 [DOI] [PubMed] [Google Scholar]
- 80.Morton JP, Maclaren DP, Cable NT, Campbell IT, Evans L, Kayani AC, McArdle A, Drust B. . Trained men display increased basal heat shock protein content of skeletal muscle. Med Sci Sports Exerc 2008; 40:1255 - 62; http://dx.doi.org/ 10.1249/MSS.0b013e31816a7171; PMID: 18580405 [DOI] [PubMed] [Google Scholar]
- 81.Smolka MB, Zoppi CC, Alves AA, Silveira LR, Marangoni S, Pereira-Da-Silva L, Novello JC, Macedo DV. . HSP72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. Am J Physiol Regul Integr Comp Physiol 2000; 279:R1539 - 45; PMID: 11049834 [DOI] [PubMed] [Google Scholar]
- 82.Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y. . Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J 2005; 19:1009 - 11; PMID: 15811877 [DOI] [PubMed] [Google Scholar]
- 83.Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. . Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc 2008; 40:691 - 8; http://dx.doi.org/ 10.1249/MSS.0b013e318160ff84; PMID: 18317375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vogt M, Puntschart A, Geiser J, Zuleger C, Billeter R, Hoppeler H. . Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol (1985) 2001; 91:173 - 82; PMID: 11408428 [DOI] [PubMed] [Google Scholar]
- 85.Lindholm ME, Fischer H, Poellinger L, Johnson RS, Gustafsson T, Sundberg CJ, Rundqvist H. . Negative regulation of HIF in skeletal muscle of elite endurance athletes - a tentative mechanism promoting oxidative metabolism. Am J Physiol Regul Integr Comp Physiol 2014; Forthcoming http://dx.doi.org/ 10.1152/ajpregu.00036.2013; PMID: 24898836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindinger MI, McCutcheon LJ, Ecker GL, Geor RJ. . Heat acclimation improves regulation of plasma volume and plasma Na(+) content during exercise in horses. J Appl Physiol (1985) 2000; 88:1006 - 13; PMID: 10710397 [DOI] [PubMed] [Google Scholar]