Abstract
A proteomics screen of brown adipose tissue in 13-lined ground squirrels reveals protein changes underlying the extreme recruitment and activity cycles characteristic of this organ in small eutherian hibernators. Protein changes precede changes in physiology, indicating endogenous timing rather than ambient temperature controls the annual recruitment-atrophy cycle in this obligate hibernator.
Keywords: 14-3-3 proteins, adaptation, fatty acid binding proteins, hibernation, Ictidomys tridecemlineatus, non-shivering thermogenesis, torpor
Brown adipose tissue (BAT) is so elaborated in small eutherian hibernators that it was initially called “the hibernation gland.” BAT is a specialized organ that uses metabolic fuels to make heat rather than ATP, via the activity of uncoupling protein 1 (UCP1).1 The physiological dynamics of hibernation place extraordinary requirements on BAT form and function, making it an ideal experimental model system in which to decipher the molecular mechanisms controlling both recruitment and activation. In a recent paper, Hindle and Martin2 used a proteomics screening strategy coupled with an unusually comprehensive and carefully-collected set of BAT samples to define protein dynamics in hibernating 13-lined ground squirrels.
Ground squirrels segregate their year into active and hibernating phases, and during hibernation, oscillate between torpor and arousal (Fig. 1). In torpor, heart, respiratory and metabolic rates fall to a few percent of their euthermic values and core body temperature (Tb) plummets to near freezing. After about two weeks, the animals reverse all of these depressed physiological parameters and spontaneously rewarm. Non-shivering thermogenesis (NST) in BAT is wholly responsible for raising Tb early in arousal.1 Beginning with emergence from hibernation in the spring, 13-lined ground squirrels undergo a period of reproduction and growth that ends in obesity by late summer while maintaining Tb near 37 °C. Thus, throughout spring and summer, hibernators are typical mammalian homeotherms. In the fall, however, these animals begin to explore heterothermy, allowing Tb to fall to near ambient temperature (Ta) for short periods.3 This Tb plasticity quickly progresses to hibernation, with its characteristic cycles between prolonged torpor (~2 wk) and short arousals to euthermia (~12 h). The torpor-arousal cycle repeats scores of times throughout fall and winter.2
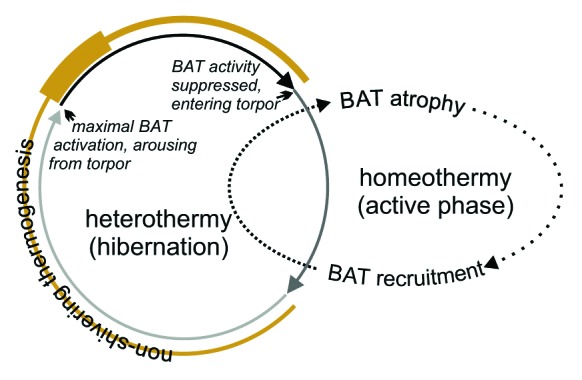
Figure 1. Schematic representation of BAT and hibernation cycles in 13-lined ground squirrels. The dotted and solid arrowed lines represent the annual and hibernation (torpor-arousal) cycles, respectively. Notice that BAT atrophies upon spring emergence and resumption of homeothermy, but undergoes intense recruitment as ground squirrels prepare for hibernation. BAT remains in its highly recruited form (closely spaced dashes) throughout the hibernation season but its activity must be suppressed to allow Tb to fall during entrance (medium gray). Slight BAT activity (thin gold line) maintains minimal Tb throughout torpor (light gray), but maximal activity occurs as animals initiate arousal from torpor (thickest gold line). The metabolically active periods of arousing from torpor and interbout arousal are indicated by the solid black line. The thickness of the gold line is directly proportional to the intensity of NST.
Brown adipose tissue likewise undergoes both a seasonal and a winter-specific (torpor-arousal) cycle (Fig. 1). BAT atrophies in spring and early summer, reaching minimal tissue and cell size. By late summer, cell and tissue size rebuilds. Full elaboration of the final, highly recruited state intensifies in the early fall and is maintained throughout hibernation2 while BAT repeatedly undergoes profound activity-quiescence cycles. Maximum activity occurs as the animal arouses from torpor, but maximum suppression of BAT activity necessarily occurs just 15–20 h later as Tb falls during entrance into the next bout of torpor.
To capture the protein differences underlying the transitions in both circannual atrophy-recruitment and activity-quiescence cycles, Hindle and Martin sampled axillary BAT from 13-lined ground squirrels in multiple, precisely-defined stages throughout the year. The study included eight “base” states. Three of the base states were from the homeothermic (active) period: spring cold and spring warm, Ta = 4 °C or 14–18 °C, respectively, and within 7–32days of their terminal arousal from hibernation, and summer active, representing mid-summer, Ta = 18–21 °C. The remaining five base states were during winter hibernation with Ta = 4 °C; all were collected at precise points in their spontaneous torpor-arousal cycles using remote telemetry to monitor Tb. These groups were interbout aroused, entrance, late in torpor, and early or late arousing. In terms of BAT function, these timepoints represent times of moderate, minimal, low, maximal and moderate activity, respectively. The study also included two groups spanning the transition between homeothermy and heterothermy: spring and fall transition. The precision of the sampling regimen synchronizes samples by their phase in the natural cycles of both homeothermy-heterothermy and torpor-arousal. This synchronization minimizes variability which, when taken together with the depth of sampling (6 animals/each of the eight base states) , maximizes discovery. The additional 45 spring and fall transition animals, although highly variable in their Tb rhythms, provided a rich resource for hypothesis testing. Protein differences were defined using a 2D-gel based method. Advantages of this approach include robust quantification of the abundant soluble fraction of the BAT proteome as well as detection of isoforms caused by alternative splicing and post-translational modification, however, the method precludes recovery of integral membrane and low abundance proteins. Nevertheless, nearly 3,000 BAT protein isoforms (e.g., 2D gel spots) were evaluated; just under 11% of these varied significantly among the eight base states, and 203 were unambiguously identified.
Few protein differences (0–3) distinguish the five winter groups despite their large differences in metabolic activity. This finding indicates that BAT is poised for maximal activity throughout winter and the few proteins controlling activity (e.g., transduction of the norepinephrine signal) must be too rare or too hydrophobic to be detected by these methods. Spring animals housed in the cold differ by just four proteins from those housed like summer animals, suggesting environmental temperature has little effect. The summer proteome is most distinct from that of the other groups, consistent with its distinct morphology; this feature in BAT is strikingly different from the response of the proteome in other organs that have been similarly examined.4-6 Interestingly, three animals in late torpor were collected late in the hibernation season and their proteomes were already shifting toward the spring profile, indicating that timing has a greater impact on the BAT proteome than physiological behavior since these animals were still cycling regularly between torpor and arousal. This apparent uncoupling of the proteome from hibernation behavior and Ta was tested using the fall and spring transition animals. In both cases, the transition groups were intermediate between the homeotherms and heterotherms but altered toward anticipation of the state that followed. Therefore, proteomic shifts in the ground squirrel’s BAT anticipate rather than respond to the physiological shifts related to hibernation.
The specific protein differences between homeotherms and heterotherms are consistent with a steady-state of maximally recruited BAT during hibernation, i.e., elevated mitochondrial membrane and fat burning capacity throughout winter. Fatty acid binding proteins also increase during winter. Interestingly, FABP4 abundance was positively correlated with the length of time in torpor across all of the base state animals. This correlation persisted in both spring and fall transition, plausibly linking torpor bout length to the availability of FABP4 for sequestration of intracellular fatty acids,2 because UCP1 activity (and hence NST and rewarming from torpor) depends on free fatty acid.1 One of the most striking and unanticipated findings to emerge from the proteomics screen was the several hundred-fold enrichment of 14-3-3 proteins among the proteins elevated in homeotherms compared with heterotherms.2 Because of their importance in regulating the activity of proteins involved in cell proliferation, survival and death,7 further investigation into the functional state and binding partners of these 14-3-3 proteins in samples from across the homeothermic season, when BAT is undergoing atrophy, regrowth and recruitment, may provide significant insight into mechanisms that could be exploited to enhance BAT in non-hibernators.
Glossary
Abbreviations:
- Ta
ambient temperature
- Tb
body temperature
- BAT
brown adipose tissue
- NST
non-shivering thermogenesis
- UCP1
uncoupling protein 1
Disclosure of Potential Conflicts of Interest
The Author states she has no conflict of interest
References
- 1.Cannon B, Nedergaard J. . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277 - 359; http://dx.doi.org/ 10.1152/physrev.00015.2003; PMID: 14715917 [DOI] [PubMed] [Google Scholar]
- 2.Hindle AG, Martin SL. . Intrinsic circannual regulation of brown adipose tissue form and function in tune with hibernation. Am J Physiol Endocrinol Metab 2014; 306:E284 - 99; http://dx.doi.org/ 10.1152/ajpendo.00431.2013; PMID: 24326419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell RL, O’Neill PH, Epperson LE, Martin SL. . Extensive use of torpor in 13-lined ground squirrels in the fall prior to cold exposure. J Comp Physiol B 2010; 180:1165 - 72; http://dx.doi.org/ 10.1007/s00360-010-0484-8; PMID: 20556614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hindle AG, Grabek KR, Epperson LE, Karimpour-Fard A, Martin SL. . Metabolic changes associated with the long winter fast dominate the liver proteome in 13-lined ground squirrels. Physiol Genomics 2014; 46:348 - 61; http://dx.doi.org/ 10.1152/physiolgenomics.00190.2013; PMID: 24642758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hindle AG, Karimpour-Fard A, Epperson LE, Hunter LE, Martin SL. . Skeletal muscle proteomics: carbohydrate metabolism oscillates with seasonal and torpor-arousal physiology of hibernation. Am J Physiol Regul Integr Comp Physiol 2011; 301:R1440 - 52; http://dx.doi.org/ 10.1152/ajpregu.00298.2011; PMID: 21865542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hindle AG, Martin SL. . Cytoskeletal regulation dominates temperature-sensitive proteomic changes of hibernation in forebrain of 13-lined ground squirrels. PLoS One 2013; 8:e71627; http://dx.doi.org/ 10.1371/journal.pone.0071627; PMID: 23951209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison DK. . The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 2009; 19:16 - 23; http://dx.doi.org/ 10.1016/j.tcb.2008.10.003; PMID: 19027299 [DOI] [PMC free article] [PubMed] [Google Scholar]


