Abstract
Scientists have long been fascinated by animals undergoing aestivation, a state of torpor at high temperature, due to its great potential in fields ranging from medicine to space travel. The brain of the African lungfish is able to coordinate a whole-body response to induce aestivation and to arouse from aestivation.
Keywords: growth hormone, metabolic rate reduction, osmoregulation, oxidative defence, prolactin, somatostatin
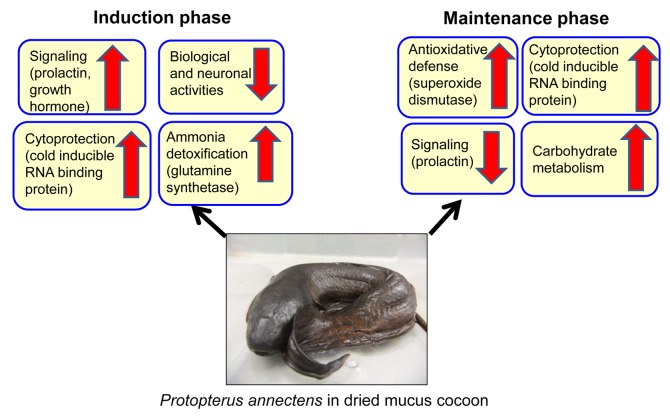
The present discovery article serves to highlight a recent study conducted by the authors1 that demonstrates the differential gene expression occurring in the brain of the African lungfish, Protopterus annectens, during the induction (6 d) or maintenance (6 mo) phases of aestivation (Fig. 1) as compared with the freshwater control using suppression subtractive hybridization polymerase chain reaction. Lungfishes are an archaic group of lobe-finned fishes. They possess lungs and hold an important position in the evolution of vertebrates with regard to the water-land transition and their close phylogenetic relationships with tetrapods. There are six species of extant lungfishes, of which four (P. annectens, P. aethiopicus, P. amphibius, and P. dolloi) are found in Africa. In nature, African lungfishes can undergo aestivation during desiccation, entering into a state of corporal torpor in subterranean mud cocoon without food and water for up to 5 y.2 In the laboratory, the authors have been able to induce African lungfishes to aestivate in pure dried mucus cocoons inside plastic boxes.3
Figure 1. Changes occurring in the brain of Protopterus annectens aestivated in dried mucus cocoon during the induction (6 d) and maintenance (6 mo) phases of aestivation.
Aestivation comprises three phases: induction, maintenance, and arousal. During the induction phase, the aestivating lungfish detects environmental cues and turns them into internal signals that will instil the necessary behavioral, structural, physiological, and biochemical changes in preparation of aestivation. It hyperventilates and secretes mucus which turns into a dried mucus cocoon within 6–8 d. The maintenance phase of aestivation begins when the lungfish is completely encased in a cocoon, and there is a complete cessation of feeding and locomotor activities. During the maintenance phase, the lungfish has to prevent cell death, preserve the biological structures, and sustain a slow rate of waste production to avoid pollution of the internal environment. The lungfish can be aroused from aestivation upon the return of water. After arousal, it has to excrete the accumulated waste products and feed for repair and growth. Logically, the brain of an aestivating lungfish would need to process the internal cues and coordinate a whole-body response to induce aestivation during desiccation and to arouse from aestivation when water becomes available. However, there is a dearth of knowledge in this regard.
The authors1 reported that more genes were upregulated than downregulated during both phases of aestivation, indicating that the brain was transcriptionally-, and hence to a certain extent metabolically-, active during aestivation. Traditionally, metabolic depression has been regarded as an important facet of the aestivation process, but there must be differences in metabolic responses between organs/tissues during aestivation.4 The muscle and kidney of an aestivating lungfish could probably undergo profound decrease in metabolic rate due to torpidity and anuria, respectively, but the same cannot be applied to the brain. In fact, an aestivating lungfish can respond to sensory disturbances,5 indicating that its brain would still maintain certain levels of metabolic and physiological activities.
The authors1 discovered that the mRNA expression levels of growth hormone and prolactin were upregulated in the brain of P. annectens during the induction phase of aestivation. In general, several endogenous hormonal substances related to sleep, e.g., growth hormone, somatostatin, and arginine vasotocin, have been proposed to be involved in initiating and maintaining aestivation in vertebrates. In humans, growth hormone secretion is associated with sleep and the maximal level occurs within minutes of the onset of slow wave sleep.6 Somatostatin is a cyclic tetradecapeptide, synthesized from a large precursor molecule and acts as a negative regulator for the secretion of growth hormone. The novel findings on the downregulation of preprosomatostatin 2, and the simultaneous upregulation of growth hormone in the brain of P. annectens indicate their possible involvement in the induction process.1 In fishes, prolactin is involved mainly in osmoregulation and salt absorption in fresh water and in terrestrial adaptation.7 As an aestivating lungfish is confronted with dehydration and uremic stresses, prolactin could be involved in coordinating a whole-body osmoregulatory response in P. annectens during the induction phase of aestivation. An upregulation in the expression of tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein γ polypeptide and a downregulation of phosphatidylethanolamine binding protein were also observed in P. annectens, suggesting that there could be a reduction in biological and neuronal activities in the brain.1 The mRNA expression levels of cold inducible RNA-binding protein and glucose regulated protein 58were also upregulated in the brain, probably to enhance their cytoprotective effects. Furthermore, the induction phase was characterized by reduction in glycolytic capacity and metabolic activity, suppression of protein synthesis and degradation, and an increase in defense against ammonia toxicity in the brain of P. annectens. During the maintenance phase, the mRNA expression of prolactin in the brain of P. annectens decreased drastically, corroborating the proposition that prolactin plays an important role in coordinating aestivation in P. annectens. In addition, the expression level of superoxide dismutase 1 was upregulated to enhance anti-oxidative defense.1 Thus, there could also be increases in capacities of transcription, glycolysis, transcription and translation during the maintenance phase, possibly in preparation for arousal.
Overall, the results obtained by the authors,1 signify the important role of the brain to coordinate a whole-body response in P. annectens during the three phases of aestivation. They provide new insights into the possible involvement of prolactin and growth hormone in the aestivation process, which deserve more in-depth studies in the future. They also point to the importance of understanding mechanisms involved in reconstruction of protein structures, regulation of energy expenditure, suppression of protein degradation, conservation of metabolic fuel stores, avoidance of oxidative damages, and prevention of apoptosis in the brain of aestivating P. annectens. More importantly, efforts should be made in the future to identify the adaptive responses particular to the arousal phase of aestivation in the brain of P. annectens, which is currently lacking in the literature.
Citation: Chew SF, Hiong KC. Aestivation and brain of the African lungfish Protopterus annectens. Temperature 2014; 1:0 - -1; 10.4161/temp.29650
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hiong KC, Ip YK, Wong WP, Chew SF. . Differential gene expression in the brain of the African lungfish, Protopterus annectens, after six days or six months of aestivation in air. PLoS One 2013; 8:e71205; http://dx.doi.org/ 10.1371/journal.pone.0071205; PMID: 23976998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith HW. . Metabolism of the lungfish, Protopterus aethiopicus. J Biol Chem 1930; 88:97 - 130 [Google Scholar]
- 3.Chew SF, Chan NK, Loong AM, Hiong KC, Tam WL, Ip YK. . Nitrogen metabolism in the African lungfish (Protopterus dolloi) aestivating in a mucus cocoon on land. J Exp Biol 2004; 207:777 - 86; http://dx.doi.org/ 10.1242/jeb.00813; PMID: 14747410 [DOI] [PubMed] [Google Scholar]
- 4.Ip YK, Chew SF. . Nitrogen metabolism and excretion during aestivation. Prog Mol Subcell Biol 2010; 49:63 - 94; http://dx.doi.org/ 10.1007/978-3-642-02421-4_4; PMID: 20069405 [DOI] [PubMed] [Google Scholar]
- 5.Delaney RG, Lahiri S, Fishman AP. . Aestivation of the African lungfish Protopterus aethiopicus: cardiovascular and respiratory functions. J Exp Biol 1974; 61:111 - 28; PMID: 4411892 [DOI] [PubMed] [Google Scholar]
- 6.Holl RW, Hartman ML, Veldhuis JD, Taylor WM, Thorner MO. . Thirty-second sampling of plasma growth hormone in man: correlation with sleep stages. J Clin Endocrinol Metab 1991; 72:854 - 61; http://dx.doi.org/ 10.1210/jcem-72-4-854; PMID: 2005213 [DOI] [PubMed] [Google Scholar]
- 7.Manzon LA. . The role of prolactin in fish osmoregulation: a review. Gen Comp Endocrinol 2002; 125:291 - 310; http://dx.doi.org/ 10.1006/gcen.2001.7746; PMID: 11884075 [DOI] [PubMed] [Google Scholar]