Abstract
RB +/− individuals develop retinoblastoma and, subsequently, many other tumors. The Rb relatives p107 and p130 protect the tumor-resistant Rb−/− mouse retina. Determining the mechanism underlying this tumor suppressor function may expose novel strategies to block Rb-pathway cancers. p107/p130 are best known as E2f inhibitors, but here we implicate E2f-independent Cdk2 inhibition as the critical p107 tumor suppressor function in vivo. Like p107 loss, deleting p27 or inactivating its Cdk inhibitor (CKI) function (p27CK−) cooperated with Rb loss to induce retinoblastoma. Genetically, p107 behaved like a CKI because inactivating Rb and one allele each of p27 and p107 was tumorigenic. While Rb loss induced canonical E2f targets, unexpectedly p107 loss did not further induce these genes but instead caused post-transcriptional Skp2-induction and Cdk2 activation. Strikingly, Cdk2 activity correlated with tumor penetrance across all the retinoblastoma models. Therefore, Rb restrains E2f, but p107 inhibits cross-talk to Cdk. While removing either E2f2 or E2f3 genes had little effect, removing only one E2f1 allele blocked tumorigenesis. More importantly, exposing retinoblastoma-prone fetuses to small molecule E2f or Cdk inhibitors for merely one week dramatically inhibited subsequent tumorigenesis in adult mice. Protection was achieved without disrupting normal proliferation. Thus, exquisite sensitivity of the cell-of-origin to E2f and Cdk activity can be exploited to prevent Rb pathway-induced cancer in vivo without perturbing normal cell division. These data suggest that E2f inhibitors, never before tested in vivo, or Cdk inhibitors, largely disappointing as therapeutics, may be effective preventive agents.
Keywords: Chemoprevention, Retinoblastoma, Cdk, E2f, p107
INTRODUCTION
The Rb-E2f and Cdk inhibitor (CKI)-Cdk2/1 interactions regulate cell cycle progression (Figure 1A). The Rb family (Rb, p107 and p130) bind and form repressor complexes with E2f family proteins. Activating E2fs, (E2f1/2/3) induce factors required for DNA replication, drive proliferation of quiescent cells (reviewed in (1, 2)) and, although not required for normal progenitor division (3), are essential for abnormal division of differentiating Rb null cells (4–8). Cip/Kip inhibitors (p21/p27/p57) on the other hand bind and inhibit the kinase activity of Cyclin A/E-Cdk2/1 complexes. Cdk2 fires replication origins, and feeds back to promote E2f activity by phosphorylating Rb family proteins (9).
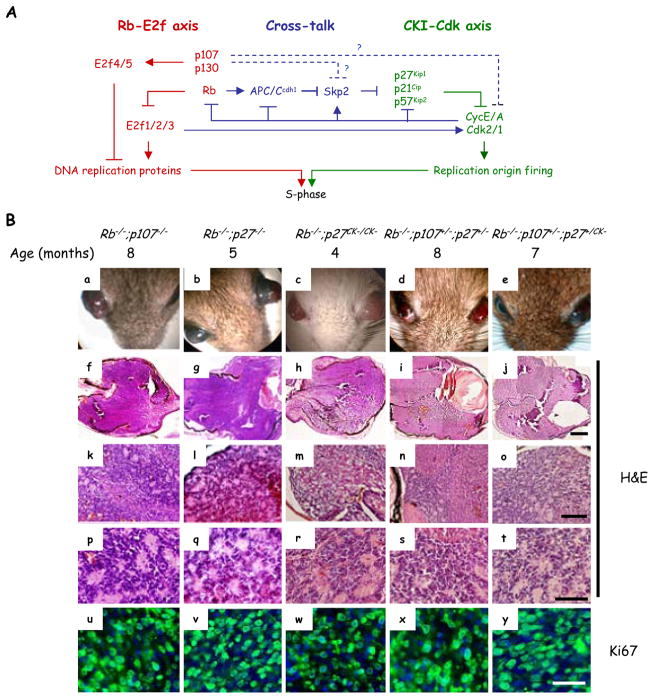
Figure 1. Both Rb-E2f and CKI-Cdk axes contribute to retinoblastoma initiation.
(A) The Rb-E2f (red) and Cip/Kip-Cdk2 (green) dual axes regulate G1/S progression. There are also links that affect cross-talk between the axes (blue). p107 is thought to inhibit cross-talk by regulating E2f targets, but in vitro data also suggest E2f-independent roles in controlling Skp2 stability and in binding Cdk2 (dotted blue lines). The relative contribution of these pathways to p107 function in vivo, especially to tumor suppression, is unclear. The figure does not include all regulators and links. (B) Loss of Rb together with inactivation of p107 and/or p27, either by deletion or inactivating the latter’s CKI activity, initiates the growth of protruding retinoblastoma (a–e) that fills the vitreous (f–J). H&E sections showed rosettes (k–t). Ki67 staining (u–y, green) reveals many dividing cells in tumors. For simplicity “Rb−/−” represents αCre;Rb f/f. Scale bars are 500 μm (f–j), 150 μm (k–o), 50 μm (p–t) and 25 μm (u–y). See also Figure S1.
To prevent uncontrolled G1-S progression, there are extensive controls limiting positive cross talk between E2f and Cdk2 (Figure 1A). For example, by preventing Rb family phosphorylation, CKIs inhibit Cdk2-mediated activation of E2f. Equally, by preventing Cyclin E/A gene induction the Rb family blocks E2f-mediated activation of Cdk2. Some work has emerged suggesting E2f-independent ways in which Rb proteins limit Cdk2 activity. For example, Rb promotes Skp2 degradation through APC and thus stabilizes CKIs (10, 11). Subsequent studies validated this link, as Skp2 is required in Rb-deficient pituitary tumors (12). p107/p130 do not bind APC, but p107 reduces Skp2 post-transcriptionally in vitro (13); whether this occurs in vivo is unclear. p107/p130 bind and inhibit Cdk2 in vitro (14), but a p107-Cdk2 complex in cells has only been detected in the absence of p21 and p27 (15). Thus, the extent to which p107 may utilize E2f-independent mechanisms to regulate Cdk2 in vivo and its relevance, if any, to tumorigenesis is unclear.
The ocular cancer retinoblastoma generated fundamental discoveries with broad relevance to cell cycle regulation and cancer, including the classic two-hit hypothesis and RB, the first recognized tumor suppressor. RB protein turned out to have universal relevance to cancer, and 50% of RB+/− survivors develop secondary tumors by the age of 50 (reviewed in (16)). The unique sensitivity of the human retina to RB loss implies that other human tissues and the retina in other species have extra protection. Indeed, p107 and p130 protect mouse retina, and current retinoblastoma models utilize loss of Rb and one relative (17–21). How p107/p130 protect the Rb−/− retina is unclear, but elucidating the mechanism could expose strategies to prevent tumors initiated by RB pathway defects in humans. One explanation for the quantum difference between the tumor-resistant Rb−/− and tumor-prone Rb/p107 null retina is that E2f targets become super-induced in the latter. This occurs in keratinocytes (22, 23), but in fibroblasts Rb and p107/p130 appear to regulate distinct targets (24, 25). Here, we show that the major function of p107 in the Rb null retina is not to regulate canonical E2f-regulated genes. Genetic, biochemical and pharmacological studies instead show that p107 prevents E2f-independent cross-talk to Cdk2 and that combined activation of E2f and Cdk2, through loss of Rb, and p107 or p27, respectively, underpins tumor susceptibility in the mouse retina. Strikingly exposure of the fetal retina to either E2f or Cdk small molecule inhibitors for merely one week blocked retinoblastoma without perturbing normal division. We suggest that the sensitivity of the human retina to RB loss not only reflects E2F activation, but poor buffering of feedback regulation of CDKs. Given the universal role of the RB pathway in cancer, further studies are needed to assess the potential clinical relevance of our findings to multiple cancers.
RESULTS
CKI Activity Suppresses Mouse Retinoblastoma
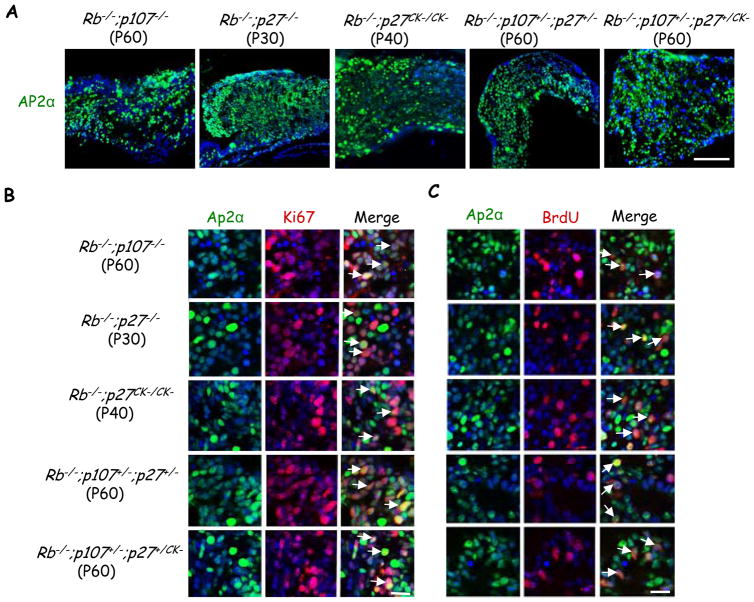
Current mouse knockout models of retinoblastoma require deletion of Rb plus either p107 or p130 (18, 17, 19–21). p107 or p130 could protect Rb−/− retina by repressing E2f targets, but we wondered if cross-talk to Cdk may also be important (Figure 1A). When we deleted floxed Rb (Rbf) in peripheral retina at embryonic day 10 (E10) using the αCre transgene as before (18, 5), we observed elevated p21 and p27 mRNAs, with p27 protein detected in virtually all cells at postnatal day 8 (P8), and sporadic p21 expression (Figure S1 and data not shown). To test whether CKI loss mimics p107 or p130 loss we therefore removed p27. Strikingly, αCre;Rbf/f;p27−/− mice developed retinoblastoma with 100% penetrance (Figure 1B and Table 1), double that of αCre;Rbf/f;p107−/− mice and similar to αCre;Rbf/f;p130−/− mice (18, 19). As in other double knockout (DKO) models of retinoblastoma (18, 17, 19–21), Rb/p27 DKO tumor cells expressed the amacrine cell marker Ap2α as well as markers in this and other cells such as Pax6, and Prox1, but lacked other cell type markers (Figure 2 and Figure S2). P30 tumor cells were positive for markers that label all cell cycle phases (Ki67), or S-phase (BrdU). In Rb−/−;p107−/−, Rb−/−;p27−/−, Rb−/−;p27CK−/CK−, Rb−/−;p107+/−;p27+/− and Rb−/−;p107+/−;p27+/CK− tumors, the fraction of Ki67+ cells that were also Ap2α+ was 92±4%, 91±1%, 92±5%, 92±2%, and 90±1% respectively, and the fraction of BrdU+ cells that were Ap2α+ was 89±11%, 91±10%, 85±4%, 84±5%, and 87±4%, respectively (Figure 2B–2C). The contaminating glutamine synthase (GS)-labelled Müller glia were quiescent (Figure S2). Thus, in the Rb−/− mouse retina, p27 is a potent tumor suppressor suggesting that low CKI activity in the human retina may contribute to the sensitivity of this tissue to RB loss.
Table 1.
Tumor penetrance in Rb null retinas with compromised p107 and/or CKI status
| p107 or p27 status of Rb null retina | Eyes with tumor/ Total eyes | Penetrance |
|---|---|---|
| p107−/− | 12/22 | 54% |
| p107+/− | 0/60 | 0% |
| p27−/− | 28/28 | 100% |
| p27+/− | 0/24 | 0% |
| p27CK−/CK− | 20/20 | 100% |
| p27+/CK− | 3/28 (3/3 LOH) | 10.7% (all LOH) |
| p107+/−;p27+/− | 10/28 (0/5 LOH) | 35.7% (no LOH) |
| p107+/−;p27+/CK− | 7/18 (1/7 LOH) | 38.9% (14% LOH) |
LOH: loss of heterozygosity
See also Figure S3.
Figure 2. Dividing Ap2α+ amacrine like cells in multiple mouse models of retinoblastoma.
(A) Tumors of the indicated genotypes and ages were stained for Ap2α (green) and DAPI (blue). Scale bar 100 μm. (B–C) Tumors of the indicated genotypes and ages were stained for the cell cycle markers Ki67 (B), and BrdU (C) (red), and the amacrine cell marker Ap2α (green). White arrows indicate double positive cells. Scale bar 20 μm. For simplicity “Rb−/−” represents αCre;Rbf/f. See also Figure S2.
p27 binds and regulates proteins other than Cyclin/Cdk2 (reviewed in (26)). To define the critical tumor suppressor activity we assessed the p27CK− allele in which four substitutions specifically disrupt CKI activity (27). αCre;Rbf/f;p27CK−/CK− mice developed retinoblastoma with 100% penetrance (Figure 1B and Table 1). p27CK−/CK− animals exhibit retinal dysplasia (27), which we confirmed, but they never developed retinoblastoma (data not shown). Furthermore, of 28 eyes from αCre;Rbf/f;p27+/CK− animals only three had tumors, and strikingly all showed loss of heterozygosity (LOH) (Table 1 and Figure S3). Thus, p27CK− is not a dominant oncoprotein either in normal or Rb−/− retina, contrasting lung where it causes tumors (27). Collectively, our results demonstrate that retinoblastoma requires loss of p27 CKI activity.
These data suggest a role for Cdk in retinoblastoma initiation. Conceivably p107 could, like p27, suppress tumorigenesis by limiting Cdk activity. To test this model, we first searched for genetic interaction between p107 and p27. In stark contrast to αCre;Rbf/f;p107+/− or αCre;Rbf/f;p27+/ − mice, which never developed retinoblastoma, αCre;Rbf/f;p107+/−;p27+/ − compound heterozygotes developed tumors (Figure 1B, Table 1). Importantly, none of five tumors analyzed showed LOH for p107 or p27 (Figure S3 and data not shown). We also analyzed compound heterozygotes harboring the p27CK− allele (αCre;Rbf/f;p107+/−;p27+/CK−) and similarly observed retinoblastoma with only 1/7 tumors displaying LOH (Figure 1B, Table 1, Figure S3C and data not shown). Previously, the Rb-E2f axis has been the focus in retinoblastoma, but these genetic data expose a new role for the CKI-Cdk axis.
Cdk2 Activity Correlates with Tumor Penetrance
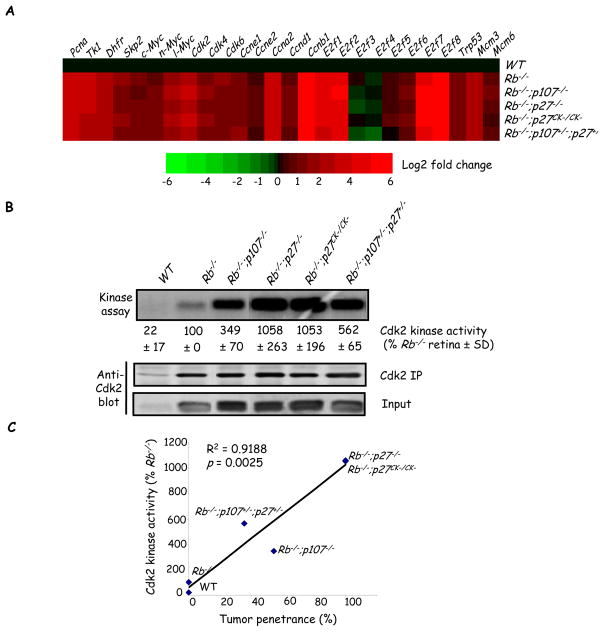
Quantifying tumor penetrance revealed various tumor frequencies across genetic models (Table 1). This variability might reflect differences in the activity of E2fs and/or Cdks. Normal retinal progenitor division, which is unaffected by Rb loss, ceases at post natal day 8 (P8) but ectopic division of differentiating Rb null neurons continues (18, 19). Thus E2f targets and Cdk activity were assessed at P8 to focus specifically on ectopically dividing differentiating cells. We assessed 24 E2f targets most of which were elevated in Rb−/− retinas (Figure 3A). Surprisingly, in four tumor-prone genetic models, expression of these targets was remarkably similar to Rb−/− levels (Figure 3A). We next analyzed Cdk pathway activity and found that Cdk2 protein was negligible in WT retina but induced similarly in Rb−/− and all tumor-prone models (Figure 3B). Strikingly, Cdk activity was strongly elevated in the tumor-prone state relative to Rb null tumor-resistant retinas, and quantification revealed an excellent correlation with tumor penetrance (Figure 3B–3C). However, we did not observe any correlation between Cdk2 kinase activity and cell cycle index (Figure S4), excluding the possibility that higher Cdk activity is a result of increased cell proliferation in tumor-prone genotypes. Thus, while E2f is deregulated similarly in the tumor-resistant Rb KO versus tumor-prone states, Cdk2 activity predicts susceptibility to sporadic transformation. This result, together with our new models of retinoblastoma, suggested that rather than constraining E2f target induction, p107 may protect the Rb−/− retina by blocking cross-talk to Cdk2.
Figure 3. Cdk activity correlates with tumor penetrance.
(A) RT-qPCR was used to measure the mRNA level of the indicated E2f target genes in P8 retinas of the indicated genotypes. Heat map shows the log2-fold changes of gene expression relative to WT. Red and green colors represent positive and negative expression changes, respectively. E2f4 & 5 are not known E2f targets but are included to show expression of the entire E2f family. (B) Cdk2 was immunoprecipitated from P8 retinas of the indicated genotypes. Kinase activity was determined using histone H1 as a substrate and the amount of Cdk2 in the IP was determined by Western blotting. (C) Cdk activity (percent of that in the Rb null retina) was plotted against tumor penetrance. p value was determined using a one-sample t-test for Pearson’s product-moment correlational coefficient, r. All assays were carried out 3–6 times and the mean ± SD is shown. For simplicity “Rb−/−” represents αCre;Rb f/f.
p107 Affects Skp2 and p27 Levels In Vivo
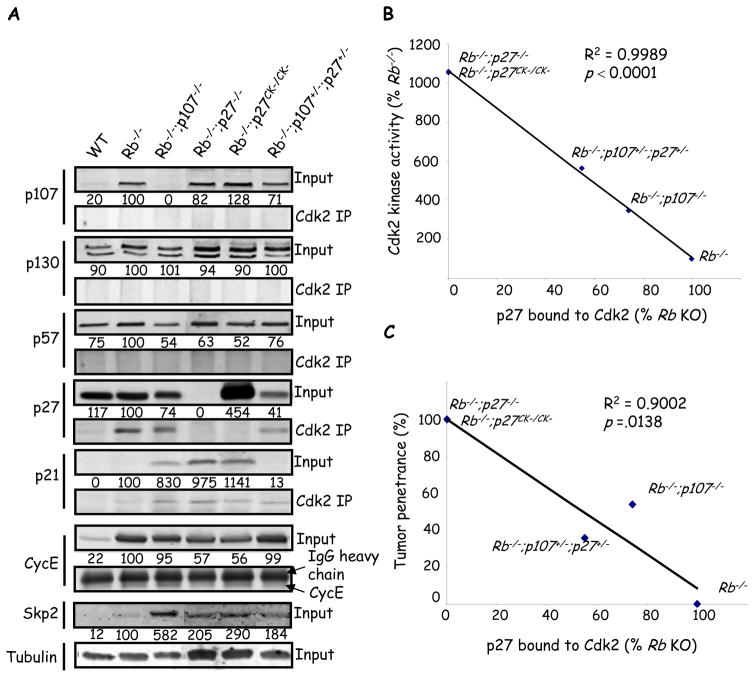
Typically, p107 is thought to influence Cdk activity through E2f-regulation of Cyclins. However, as noted above, Cyclin A/E mRNA induction was already maximal after Rb loss (Figure 3A). Cyclin E protein also showed similar or lower levels in tumor-prone retinas (Figure 4A). In vitro data link p107 to Cdk either directly by binding and inhibiting Cdk2 (14), or indirectly by decreasing Skp2 the substrate binding component of the SCFSkp2 E3 ubiquitin ligase which stimulates p27 degradation (Figure 1A) (13). Although, Cdk2 immunoprecipitates from all six of the genotypes described, showed interaction with p27 and p21 (see below), no interaction were observed between Cdk2 and p107 or p130, arguing against a direct CKI function in vivo (Figure 4A), These results are consistent with in vitro data showing that p107 only binds CyclinA-Cdk2 in MEFs lacking both p21 and p27 (15). p57 was also not detected in Cdk2 IPs, and as expected p27 and p27CK− were not associated with Cdk2 in the Rb−/−;p27−/− or Rb−/−;p27CK−/CK− retina, respectively (Figure 4A). However, p27 bound Cdk2 in the Rb−/− tumor-free retina and, consistent with a role in tumor suppression, the amount was reduced in tumor-prone Rb−/−;p107−/− and Rb−/−;p107+/−;p27+/− retinas (Figure 4A). In Rb−/−;p107−/− retina the total amount of p27 was reduced by 26±4%, and the amount bound to Cdk2 was reduced by 52±7%, suggesting negative effects of p107 loss on both the level and function of this CKI. The level of p27-Cdk2 complexes across multiple models correlated inversely with both Cdk2 activity and tumor penetrance (Figure 4B, C). In addition, Skp2 showed increased protein levels when p107 was reduced or absent, suggesting that the p107 --| Skp2 --| p27 pathway described in vitro may be relevant in vivo (Figure 1A) (13). Skp2 induction was post-transcriptional as Skp2 mRNA levels were similar in tumor-resistant Rb−/− retina versus tumor-prone retinas (Figure 3A). Total p21 levels were negligible in WT retina, low in Rb−/− and Rb−/−;p107+/−;p27+/− retinas and induced in Rb−/−;p107−/−, Rb−/−;p27−/− or Rb−/−;p27CK−/CK− retina (Figure 4A). p21 associated with Cdk2 in five genotypes (Figure 4A). Unlike p27, p21 levels rose with increasing Cdk2 activity, although this positive correlation was poor (Figure S5). Thus, p27 is the major CKI tumor suppressor in the Rb−/− retina, and when it is missing (p27−/−), unable to bind Cdk2 (p27CK−/CK−), or reduced (p107−/−), p21 is induced, but at insufficient levels to compensate for p27. Altogether, our data suggest that in Rb/p107 mutant retinas Rb loss enhances E2f activity, whereas p107 loss elevates Skp2, reduces p27 and elevates Cdk2 activity, yielding the tumor-prone state.
Figure 4. p27 bound to Cdk2 correlates inversely with kinase activity and tumor penetrance.
(A) Anti-Cdk2 immunoprecipitates or straight lysates (input) from P8 retina of the indicated genotypes were immunoblotted for the proteins indicated on the left. Values below input blots represent protein expression as a percentage of Rb KO retina in a representative blot. (B–C) Average amount of p27 bound to Cdk2 from 3 independent experiments was plotted against Cdk2 kinase activity (from Fig. 3) (B) or tumor penetrance (C). “Rb−/−” is used to indicate αCre;Rb f/f. p values were calculated using a one-sample t-test for Pearson’s product-moment correlational coefficient, r. All assays were run a minimum of 3 times. See also Fig S4 and S5.
Inhibiting Either of the Dual Axes Blocks Tumorigenesis
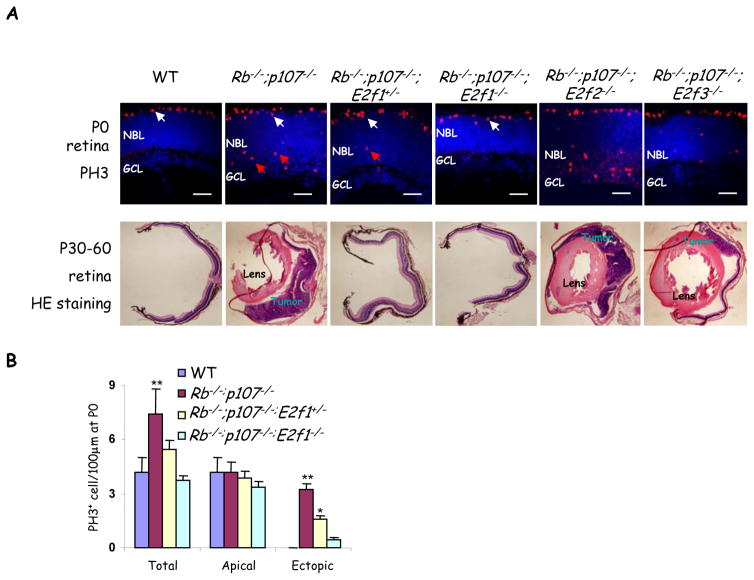
Our results suggest that E2f and Cdk2 form dual axes that can generate three states: WT, ectopically dividing (Rb null), and tumor-prone (Rb + p107 or p27-depleted), with only the latter being exquisitely dependent on elevated activity of both axes. These findings led us to hypothesize that lowering the activity of either axis could prevent sporadic retinoblastoma arising from cancer-prone cells. Removing E2f2 or E2f3 did not block retinoblastoma (Figure 5A, Table 2), concurring with the prior observation that E2f1, but not E2f2 or E2f3, drives ectopic division of differentiating neurons in the Rb−/− retina (5). p107 and E2f1 genes are in close proximity, hence to study E2f1 function in Rb/p107 DKO retina, we screened >150 pups to isolate a crossover event generating linked p107 and E2f1 null alleles. Although tumors occur in 54% or 100% of αCre;Rbf/f;p107−/− or αCre;Rbf/f;p27−/− retinas, respectively (Table 1), homozygous deletion of E2f1 completely blocked tumorigenesis in both models (Table 2). Notably, even reduction to heterozygosity completely blocked retinoblastoma in the αCre;Rbf/f;p107−/− retina, and reduced penetrance from 100% to 10% in the αCre;Rbf/f;p27−/− retina (Figure 5A, Table 2). Therefore, unlike normal cells which proliferate in the absence of E2f1–3 (3, 4), the tumor-prone state requires full E2f1 activity. Moreover, E2f1 heterozygosity did not affect progenitor division, but specifically reduced ectopic division in Rb/p107 deficient cells (Figure 5A and 5B). Thus, a therapeutic window of E2f activity exists that can be exploited to prevent abnormal pre-cancerous events without perturbing normal division.
Figure 5. Heterozygosity for E2f1 is sufficient to block retinoblastoma.
(A) P0 and adult (P30–P60) retinal sections of the indicated genotypes were stained for mitotic cells (PH3, red) and nuclei (DAPI, blue), or H&E (lower panels). Apical mitoses (white arrows) represent normal progenitors while ectopic mitoses (red arrows) represent abnormally dividing differentiating neurons that are abundant in the Rb/p107 null retina, and reduced or virtually absent when one or two E2f1 alleles are removed, respectively. Scale bar, 50μm. The lens or tumors in adult H&E sections are indicated. For quantification of tumor frequency see Table 2. (B) Quantification of mitoses in indicated genotypes. Data are mean ± SD and asterisks indicate significant difference from WT (* P<0.05, ** P<0.01. Students t-test For simplicity “Rb−/−” represents αCre;Rbf/f in (A & B). NBL: neuroblastic layer, GCL: ganglion cell layer.
Table 2.
Tumor frequency in retinas lacking E2f alleles
| Genotype | Age analyzed | No. of tumors/No. of eyes analyzed | Tumor penetrance |
|---|---|---|---|
| Rb−/−;p107−/− | 4 months | 26/48 | 54% |
| Rb−/−;p107−/−;E2f1+/− | 4 months | 0/24 | 0% |
| Rb−/−;p107−/−;E2f1−/− | 2 months | 0/28 | 0% |
| Rb−/−;p107−/−;E2f2−/− | 4 months | 22/48 | 46% |
| Rb−/−;p107−/−;E2f3−/− | 4 months | 4/8 | 50% |
| Rb−/−;p27−/− | 2 months | 24/24 | 100% |
| Rb−/−;p27−/−;E2f1+/− | 4 months | 2/20 | 10% |
| Rb−/−;p27−/−;E2f1−/− | 2 months | 0/6 | 0% |
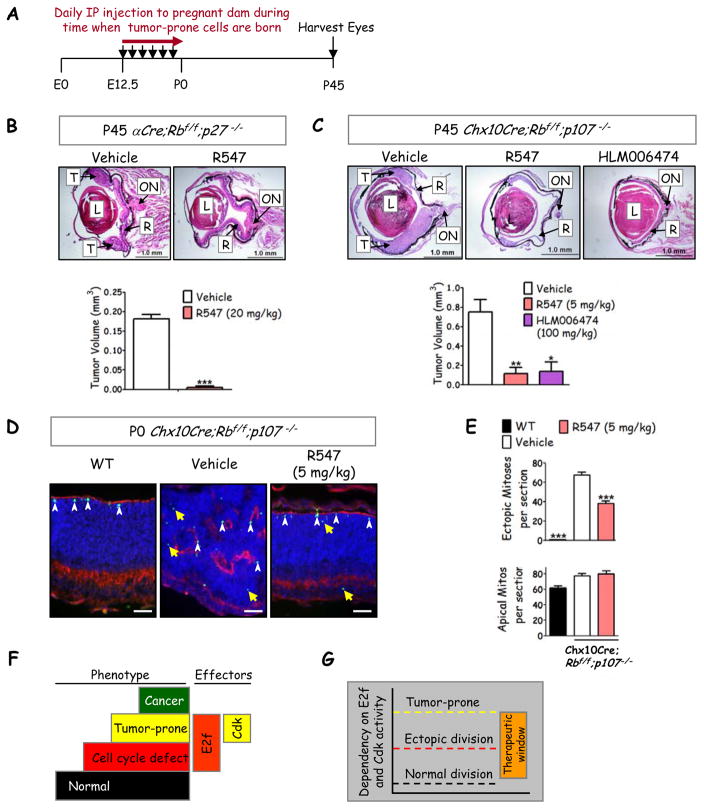
Next, we examined whether lowering Cdk activity might also inhibit retinoblastoma initiation. Cdk1 can functionally substitute for Cdk2 in vivo (28), thus we exploited a pharmaceutical approach to inhibit both and to test a novel chemoprevention strategy. Newborn neurons that survive Rb/p107 loss divide ectopically, but the vast majority (millions) of neurons escape tumorigenesis by eventually exiting the cell cycle (18). We hypothesized that - assuming drug crossed the placental barrier - mild and brief Cdk2-inhibition during this dangerous period of ectopic division would reduce sporadic transformation (Figure 6A). In contrast, if elevated Cdk activity is required only after transformation, this chemoprevention strategy would fail. For these assays we utilized R547, a potent Cdk inhibitor that passed preclinical evaluation (29) and is in phase I trials for solid tumors (30). It does not inhibit 113 other kinases, and requires >100 fold higher doses to inhibit Gsk3α/β versus Cdk1/2 (29). αCre;Rbf/f;p27−/− males were bred to αCre;Rbf/f;p27+/− females and pregnant dams received daily I.P. injections of vehicle or R547 (20 mg/kg) from embryonic day 12.5 (E12.5) to parturition and tumors were assessed at P45. Of 8 eyes examined in the treatment group, 6 eyes were tumor-free, and tumor volume in the affected eyes was considerably reduced, also consistent with reduced tumor frequency (Figure 6B). The failure to block all tumor formation may be due to some late stage amacrine cell birth that occurs in the far periphery up to ~P3, beyond the period of R547 exposure.
Figure 6. Chemoprevention of retinoblastoma through brief Cdk or E2f inhibition.
(A) Summary of chemoprevention strategy. (B) H&E stain of P45 retina in αCre;Rb f/f;p27−/− mice treated with either vehicle (n=8) or pan-Cdk inhibitor R547 (n=10) with quantification of tumor volume (bottom panel). (C) H&E stain of P45 retina in Chx10Cre;Rbf/f;p107−/− mice treated with vehicle (n=10), R547 (n=8), or E2f inhibitor HLM006474 (n=4) with tumor volume quantified (lower panel). (B,C) T: Tumor, R: retina, L: lens, ON: optic nerve (scale bar, 1 mm). (D) P0 retina of Chx10Cre;Rb f/f;p107−/− mice treated with vehicle or R547 were stained with PH3 (green) and the F-actin marker, phalloidin (red). Yellow arrows indicate ectopic PH3+ cells and white arrowheads represent apical mitotic progenitors (scale bar, 50 μm). (E) Quantification of ectopic (upper panel) or apical (lower panel) mitoses per section shows that the drug inhibits abnormal but not normal division. *= p<0.05; ** = p<0.01; *** = p<0.0001 compared to vehicle using an unpaired Student’s t-test (B) or One-way ANOVA followed by Bonferroni multiple comparisons posthoc test (C, F). Data represented as mean ± SEM (n represented per eye, n ≥ 4 for each condition). (F) Model summarizing critical molecular steps to the tumor-prone state. In the mouse retina Rb loss activates E2f1 and triggers ectopic division (red step), but additional genetic events are required to activate Cdk2 and thus create tumor susceptibility (yellow step). Sporadic mutations permit progression to cancer (green step). (G) The data suggest distinct dependence on E2f and Cdk activity for normal division, ectopic division and tumor susceptibility, and thus expose a therapeutic window of dual axes activity which can be exploited to block transformation.
We also examined models of retinoblastoma involving p107 instead of p27 loss, and to test a lower dose of R547. In αCre;Rb−/−;p107−/− mice, Rb knockout and tumorigenesis is limited to the periphery with 54% penetrance (Table 1) (18), whereas in Chx10Cre;Rbf/f;p107−/− mice, Cre is expressed across the entire retina, there is considerable dysplasia, and tumors emerge in multiple locations with 100% penetrance (21). This pattern was observed in P45 mice born to dams exposed to vehicle (Figure 6C). However, following brief exposure of fetuses to R547, 2/8 eyes in the resultant adult mice were tumor-free and the remainder showed much reduced tumor volume, most noticeably in the central retina, again consistent with the idea that Cdk inhibition in the embryonic retina blocks transformation of early-mid-born amacrine cells (Figure 6C, bottom panel). An appealing aspect of reduced E2f1 gene dosage was that it blocked tumorigenesis without perturbing normal division (Figure 5A–5B). We further examined Chx10Cre;Rbf/f;p107−/− retinas at P0, prior to tumor formation, for effects on division and the extensive dysplasia in this model. Strikingly, R547 reduced dysplasia (Figure 6D) and also modestly reduced ectopic mitoses but had no effect on progenitor mitoses that are distant or adjacent to phalloidin-marked apical membranes, respectively (Figure 6E), thus resembling the effect of lowered E2f activity (Table 2 and Figure 5). Thus only one week of pharmacological Cdk antagonism in fetuses is sufficient to inhibit the subsequent appearance of retinoblastoma in either Rb/p107 or Rb/p27 null cells, without perturbing normal progenitor division.
These results encouraged us to intervene pharmacologically with the E2f axis. Four inhibitors have been described of which three are peptides (31–33) and one is a small molecule inhibitor (HLM006474, abbreviated here to 6474) (34). To our knowledge, none have been tested in vivo. Although all four drugs inhibit division in vitro, only 6474 was tested on tissue, stalling tumor growth in a 3D skin model (34). Strikingly, short exposure of Chx10Cre;Rbf/f;p107−/− embryos to 6474 had a dramatic effect on tumorigenesis (Figure 6C). Thus, modest and temporary pharmaceutical E2f inhibition blocks retinoblastoma initiation.
Collectively, our results reveal greater dependency on E2f and Cdk activity for transformation than for normal progenitor proliferation (Figure 6F), creating a convenient therapeutic window in the cell-of-origin that, when targeted, dramatically impedes the subsequent emergence of cancer cells (Figure 6G).
DISCUSSION
Cross-talk to Cdk2 as the primary tumor suppressor function of p107
A simple explanation for p107 tumor suppressor function is that it replaces the E2f repressor role of Rb. However, we found that multiple E2f-regulated genes, including canonical targets, were expressed at similar levels in Rb and Rb/p107 null retina, contrasting the situation in keratinocytes (22, 23). We acknowledge that while deletion of p107 did not result in further induction of well known E2f target genes in Rb null retina, we can not rule out the possibility that the tumor-promoting effects of p107 loss may be mediated by E2f targets other than those tested in Fig 3A. However, akin to our in vivo findings, E2f-responsive reporter vectors show comparable activity in Rb or Rb/p107 null MEFs (35). Notably, Rb, but not p107/p130, inhibits E2f target expression during senescence (36), again mimicking our findings in terminally differentiating retinal neurons. Potentially, p107 cannot affect E2f targets in some Rb null contexts because it is not recruited to these genes (36), it is redundant with p130 (24), there is feedback inhibition of E2f by Cdk2-mediated phosphorylation (37, 38), and/or it is already sequestered in other complexes (39).
In stark contrast to E2f, we observed a marked increase in Cdk2 activity in the tumor-prone Rb/p107 null retina relative to the tumor-resistant Rb−/− tissue. E2f-induction of Cyclins did not explain elevated Cdk2 activity, but we observed post-transcriptional induction of Skp2, and reduced p27-Cdk2 binding that correlated with kinase activity and tumorigenesis. New retinoblastoma models developed here coupled with biochemical and pharmacological data strongly support the notion that this cross-talk to Cdk2 is central to tumorigenesis: i. Like the Rb/p107 null tissue the Rb/p27 deficient retina developed retinoblastoma, and p27 or p27CK− alleles behaved identically; ii. Compound heterozygosity for p107 and either p27 or p27CK− cooperated with Rb loss to drive retinoblastoma; iii. Cdk2 activity correlated with tumor penetrance across these multiple models; and iv. Short-term exposure to a Cdk inhibitor prevented tumorigenesis in Rb/p107 and Rb/p27 null retinas. The ability of Rb pathway and CKI defects to cooperate is well known (40–42), and we now extend this pattern to the retina where the focus had been primarily on Rb-E2f regulation. Our data are the first to prove unequivocally that it is the CKI function of p27 that cooperates with Rb to block tumorigenesis. Most importantly, they suggest that the primary tumor suppressive function of p107 in an Rb null tissue is to prevent activation of Cdk2 by E2f-independent and possibly Skp2-dependent means. Indeed, Skp2 is essential for retinoblastoma in the Rb/p107 null retina (M.S and R.B., unpublished data). These data justify further examination of the mechanism by which p107 might regulate Skp2 and cross-talk to Cdk2, and expose potent chemopreventive strategies for Rb pathway-initiated tumors. It is interesting to note that the kinetics and penetrance of tumorigenesis in the Rb/p27 null retina are similar to that observed in the Rb/p130 null retina (Macpherson et al., 2004). In theory, p27 loss could stimulate phosphorylation and inactivation of p130. However, we did not observe an increase in the slower migrating hyperphosphorylated form of p130 in the Rb/p27 null retina (Fig 4A). Further work would be required to comprehensively assess individual p130 phosphorylation sites. An alternate possibility is that p130 affects p27 levels, either through Skp2 or another mechanism. It would be interesting to assess Skp2 and p27 levels in the Rb/p130 null retina.
A Model to Explain Variable Sensitivity to RB Inactivation
The human retina is exquisitely sensitive to RB defects, but the underlying reason is unclear. Our data show that p107 protects mouse retina by preventing cross-activation of Cdk2 and thus cooperation with elevated E2f to create tumor susceptibility. We suggest, therefore, that low or negligible p107/p130/CKIs levels or activity in the human retinoblastoma cell-of-origin strengthens positive feedback regulation between E2Fs and CDKs. Rb loss alone in this case would be sufficient to raise Skp2 levels, reduce p27 further and thus efficiently activate Cdk2 (Figure 1A). Mouse pituitary shows the same sensitivity to Rb loss as the human retina (43), suggesting it also has unusually higher level of E2F and CDK activity. Finally, while inducing E2f or Cdk activity drives temporary ectopic proliferation in Drosophila, extended abnormal division requires both (44). Thus, a buffer that limits E2f to Cdk positive feedback regulation may be critical to avoid tumorigenesis in all animals.
Clinical Relevance
Chemoprevention is a growing field with important successes (45). Long-term exposure to anti-inflammatory drugs such as aspirin and non-steroidal anti-inflammatory drugs reduces cancer incidence, but failures, such as the lack of protection afforded by statins, highlight the need to define optimal targets (45). The notion of prevention as a viable goal has gained considerable ground in recent years, in particular for familial cancers of which >50 have been identified (46). In addition, effective chemopreventative strategies could also benefit cancer survivors, who are at higher risk of secondary tumors (47).
Here, we prevented cancer in an in vivo model of tumorigenesis using two distinct small molecule therapies, and with only one week of drug treatment. These striking observations have implicit clinical relevance, especially as they were obtained using genetic models that mimic human cancer, rather than cell-line derived xenografts in immune-deficient hosts. Our data raise the exciting notion that RB+/− patients, who often die of secondary tumors (48), may benefit from the preventive therapy we show is so potent in retina. Moreover, because the RB pathway is disrupted in many cancers, and elevated E2F and CDK activity is a universal feature of human tumors, our chemopreventive strategies may be broadly relevant. Although Cdk2 is dispensable for tumorigenesis in p27, p21 or p53 null mice (49–51) it acts redundantly with Cdk1 (28), thus the success of our chemotherapeutic strategy likely reflects inhibition of both kinases. Our data provide the first successful application of any E2f inhibitor in vivo and the remarkable efficacy indicates that like Cdk, E2f is an important chemopreventative target, although further work is required to confirm if this inhibitor lowers E2F target levels in vivo. Therapeutic trials with Cdk2 inhibitors in human cancer have been largely unsuccessful (9), but we suggest that their real benefit may lie in prevention. Recent work with Cdk inhibitors in genetic models of colon cancer support this notion (52). Future studies should examine whether the cell-of-origin in this and other tumor types also require elevated E2f activity. We found that E2f or Cdk inhibition could prevent retinoblastoma without perturbing normal retinal progenitor cell division. These data indicate a unique role for E2f and Cdk in supporting transformation versus normal cell cycle progression.
Note added in Proof
As we were preparing our manuscript for submission David Macpherson’s lab published work showing that microRNAs that down-regulate the CKI p21 are amplified or over-expressed in mouse and human retinoblastoma (53). These data support the notion that Rb and CKI inactivation cooperate to transform the human retina.
MATERIALS AND METHODS
Mouse strains and genotyping
Mice were treated according to institutional and national guidelines. αCre mice (P. Gruss), Rbf/f mice (A. Berns), p107−/− mice (M. Rudnicki), p27−/− mice (J. Roberts), and p27Ck−/Ck− mice (J. Roberts), were maintained on a mixed background. Different genotypes were compared within the same litter and across at least three litters. Genotyping was performed as before (18, 27).
Histology and immunofluorescence
BrdU-labelling, fixation and immunostaining were performed as before (5, 3). For p27, p21 and Ki67 antigen retrieval was performed by boiling sections in citric acid (H-3300, Vector Lab Inc.).
RNA-extraction, RT- PCR
RT-qPCR for E2f targets were run in duplicate on at least three separate biological samples as described (3).
Western blots
Mouse retinas were homogenized with a 30-gauge needle (BD) 5–10 times in lysis buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane. Blots were blocked and probed as described (5). Blots were scanned using ODYSSEY Infrared Imaging System (LI-COR).
Immunoprecipitation and kinase assays
Complete RIPA was prepared by combining PMSF, sodium orthovanadate, protease inhibitor cocktail (SantaCruz, CA, USA). Mouse retinas were lysed in RIPA and pre-cleared with 1.0 μg of rabbit IgG. Supernatant containing 250 μg total protein was incubated with 1μg primary antibody at 4°C for 2 hrs. 20 μl of resuspended Protein A/G Plus-Agarose was added and rocked at 4°C for 1hr to overnight. Kinase reactions were performed at 30°C for 15 min in kinase buffer containing 2.5 μg histone H1 (Upstate Biotechnology, NY, USA), 2 μCi of 32P-γ-ATP, and 20 μM ATP. Reactions were stopped with 2x Laemmli-buffer and boiled for 5 min before loading on 10% SDS-PAGE gels. Gels were dried and quantified using phosphoimager.
Chemoprevention
R547 and 6474 were synthesized by University Health Network Shanghai, and purity confirmed at >98% according to published methods (29, 34). Male and female mice were mixed in the early afternoon, checked the following morning and dams with vaginal plugs considered to be 0.5 days post-coitus (E0.5). After twelve days, pregnant dams were treated with either vehicle (2.5% v/v DMSO (5% for 20 mg/kg dose), 28% w/v 2-hydroxypropyl-β-cyclodextrin, 10% v/v PEG400 in distilled water), or R547 (5–20 mg/kg;) or 6474 (100 mg/kg) daily intraperitoneally until birth.
Tumor volume and stereology
Eyes were sectioned horizontally at 14 μm. Every 8th section was stained with H&E and scanned on Leica DMRB. Tumor volume was estimated using a Cavalieri Estimator in Stereo Investigator (MBF Bioscience, CA, USA).
Statistical analysis
Statistical analysis was performed using Prism software (Version 5.0a, GraphPad Software, LaJolla, CA, USA).
Supplementary Material
Acknowledgments
We thank Arnaud Besson and James Roberts for sharing p27CK−/CK− mice, and to Fred Dick, Gustavo Leone and Philippe Monnier for comments. This project was funded by grants to R.B. from the Canadian Institutes for Health Research (CIHR), Foundation Fighting Blindness Canada, Ontario Institute for Cancer Research through funding provided by the Government of Ontario, and the Terry Fox Research Institute. M.S., M.A., and S.R.M. were supported in part by fellowships from a CIHR training program.
Footnotes
Supplemental Information includes five figures.
References
- 1.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 2.Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. doi: 10.1038/sj.onc.1208612. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, Pacal M, Wenzel PL, Knoepfler PS, Leone G, Bremner R. Division and apoptosis of E2f-deficient retinal progenitors. Nature. 2009;462:925. doi: 10.1038/nature08544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, Hagan JP, et al. E2f1–3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D, Opavsky R, Pacal M, Tanimoto N, Wenzel P, Seeliger MW, et al. Rb-Mediated Neuronal Differentiation through Cell-Cycle-Independent Regulation of E2f3a. PLoS Biol. 2007;5:e179. doi: 10.1371/journal.pbio.0050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai KY, Hu Y, Macleod KF, Crowley D, Yamasaki L, Jacks T. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol Cell. 1998;2:293–304. doi: 10.1016/s1097-2765(00)80274-9. [DOI] [PubMed] [Google Scholar]
- 7.Ziebold U, Reza T, Caron A, Lees JA. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 2001;15:386–391. doi: 10.1101/gad.858801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClellan KA, Ruzhynsky VA, Douda DN, Vanderluit JL, Ferguson KL, Chen D, et al. Unique requirement for Rb/E2F3 in neuronal migration: evidence for cell cycle-independent functions. Mol Cell Biol. 2007;27:4825–4843. doi: 10.1128/MCB.02100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 10.Binne UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG, Jr, et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol. 2007;9:225–232. doi: 10.1038/ncb1532. [DOI] [PubMed] [Google Scholar]
- 11.Ji P, Jiang H, Rekhtman K, Bloom J, Ichetovkin M, Pagano M, et al. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol Cell. 2004;16:47–58. doi: 10.1016/j.molcel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J, et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nat Genet. 2010;42:83–88. doi: 10.1038/ng.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodier G, Makris C, Coulombe P, Scime A, Nakayama K, Nakayama KI, et al. p107 inhibits G1 to S phase progression by down-regulating expression of the F-box protein Skp2. J Cell Biol. 2005;168:55–66. doi: 10.1083/jcb.200404146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castano E, Kleyner Y, Dynlacht BD. Dual cyclin-binding domains are required for p107 to function as a kinase inhibitor. Mol Cell Biol. 1998;18:5380–5391. doi: 10.1128/mcb.18.9.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chibazakura T, McGrew SG, Cooper JA, Yoshikawa H, Roberts JM. Regulation of cyclin-dependent kinase activity during mitotic exit and maintenance of genome stability by p21, p27, and p107. Proc Natl Acad Sci U S A. 2004;101:4465–4470. doi: 10.1073/pnas.0400655101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balmer A, Zografos L, Munier F. Diagnosis and current management of retinoblastoma. Oncogene. 2006;25:5341–5349. doi: 10.1038/sj.onc.1209622. [DOI] [PubMed] [Google Scholar]
- 17.Robanus-Maandag E, Dekker M, van der Valk M, Carrozza ML, Jeanny JC, Dannenberg JH, et al. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 1998;12:1599–1609. doi: 10.1101/gad.12.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Livne-Bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5:539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 19.MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18:1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dannenberg JH, Schuijff L, Dekker M, van der Valk M, te Riele H. Tissue-specific tumor suppressor activity of retinoblastoma gene homologs p107 and p130. Genes Dev. 2004;18:2952–2962. doi: 10.1101/gad.322004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Schweers B, Dyer MA. The First Knockout Mouse Model of Retinoblastoma. Cell Cycle. 2004;3:952–959. [PubMed] [Google Scholar]
- 22.Lara MF, Garcia-Escudero R, Ruiz S, Santos M, Moral M, Martinez-Cruz AB, et al. Gene profiling approaches help to define the specific functions of retinoblastoma family in epidermis. Mol Carcinog. 2008;47:209–221. doi: 10.1002/mc.20376. [DOI] [PubMed] [Google Scholar]
- 23.Lara MF, Santos M, Ruiz S, Segrelles C, Moral M, Martinez-Cruz AB, et al. p107 acts as a tumor suppressor in pRb-deficient epidermis. Mol Carcinog. 2008;47:105–113. doi: 10.1002/mc.20367. [DOI] [PubMed] [Google Scholar]
- 24.Hurford RK, Jr, Cobrinik D, Lee MH, Dyson N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 25.Black EP, Huang E, Dressman H, Rempel R, Laakso N, Asa SL, et al. Distinct gene expression phenotypes of cells lacking Rb and Rb family members. Cancer Res. 2003;63:3716–3723. [PubMed] [Google Scholar]
- 26.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 27.Besson A, Hwang HC, Cicero S, Donovan SL, Gurian-West M, Johnson D, et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–1746. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, et al. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 29.DePinto W, Chu XJ, Yin X, Smith M, Packman K, Goelzer P, et al. In vitro and in vivo activity of R547: a potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol Cancer Ther. 2006;5:2644–2658. doi: 10.1158/1535-7163.MCT-06-0355. [DOI] [PubMed] [Google Scholar]
- 30.Malumbres M, Pevarello P, Barbacid M, Bischoff JR. CDK inhibitors in cancer therapy: what is next? Trends Pharmacol Sci. 2008;29:16–21. doi: 10.1016/j.tips.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Bandara LR, Girling R, La Thangue NB. Apoptosis induced in mammalian cells by small peptides that functionally antagonize the Rb-regulated E2F transcription factor. Nat Biotechnol. 1997;15:896–901. doi: 10.1038/nbt0997-896. [DOI] [PubMed] [Google Scholar]
- 32.Fabbrizio E, Le Cam L, Polanowska J, Kaczorek M, Lamb N, Brent R, et al. Inhibition of mammalian cell proliferation by genetically selected peptide aptamers that functionally antagonize E2F activity. Oncogene. 1999;18:4357–4363. doi: 10.1038/sj.onc.1202825. [DOI] [PubMed] [Google Scholar]
- 33.Montigiani S, Muller R, Kontermann RE. Inhibition of cell proliferation and induction of apoptosis by novel tetravalent peptides inhibiting DNA binding of E2F. Oncogene. 2003;22:4943–4952. doi: 10.1038/sj.onc.1206495. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Kurtyka CA, Boyapalle S, Sung SS, Lawrence H, Guida W, et al. A small-molecule E2F inhibitor blocks growth in a melanoma culture model. Cancer Res. 2008;68:6292–6299. doi: 10.1158/0008-5472.CAN-08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Classon M, Salama S, Gorka C, Mulloy R, Braun P, Harlow E. Combinatorial roles for pRB, p107, and p130 in E2F-mediated cell cycle control. Proc Natl Acad Sci U S A. 2000;97:10820–10825. doi: 10.1073/pnas.190343497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O, et al. Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence. Cancer Cell. 2010;17:376–387. doi: 10.1016/j.ccr.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu M, Sheppard KA, Peng CY, Yee AS, Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Molecular & Cellular Biology. 1994;14:8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dynlacht BD, Flores O, Lees JA, Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes & Development. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 39.Lee EY, Cam H, Ziebold U, Rayman JB, Lees JA, Dynlacht BD. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell. 2002;2:463–472. doi: 10.1016/s1535-6108(02)00207-6. [DOI] [PubMed] [Google Scholar]
- 40.Brugarolas J, Bronson RT, Jacks T. p21 is a critical CDK2 regulator essential for proliferation control in Rb-deficient cells. J Cell Biol. 1998;141:503–514. doi: 10.1083/jcb.141.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park MS, Rosai J, Nguyen HT, Capodieci P, Cordon-Cardo C, Koff A. p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice. Proc Natl Acad Sci U S A. 1999;96:6382–6387. doi: 10.1073/pnas.96.11.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, et al. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 44.Buttitta LA, Katzaroff AJ, Perez CL, de la Cruz A, Edgar BA. A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev Cell. 2007;12:631–643. doi: 10.1016/j.devcel.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Kelloff GJ, Lippman SM, Dannenberg AJ, Sigman CC, Pearce HL, Reid BJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–3697. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 46.Lindor NM, McMaster ML, Lindor CJ, Greene MH. J Natl Cancer Inst Monogr. 2. 2008. Concise handbook of familial cancer susceptibility syndromes; pp. 1–93. [DOI] [PubMed] [Google Scholar]
- 47.Ng AK, Kenney LB, Gilbert ES, Travis LB. Secondary malignancies across the age spectrum. Semin Radiat Oncol. 2010;20:67–78. doi: 10.1016/j.semradonc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu CL, Tucker MA, Abramson DH, Furukawa K, Seddon JM, Stovall M, et al. Cause-specific mortality in long-term survivors of retinoblastoma. J Natl Cancer Inst. 2009;101:581–591. doi: 10.1093/jnci/djp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padmakumar VC, Aleem E, Berthet C, Hilton MB, Kaldis P. Cdk2 and Cdk4 activities are dispensable for tumorigenesis caused by the loss of p53. Mol Cell Biol. 2009;29:2582–2593. doi: 10.1128/MCB.00952-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin A, Odajima J, Hunt SL, Dubus P, Ortega S, Malumbres M, et al. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1) Cancer Cell. 2005;7:591–598. doi: 10.1016/j.ccr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Tetsu O, McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–245. doi: 10.1016/s1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 52.Boquoi A, Chen T, Enders GH. Chemoprevention of mouse intestinal tumorigenesis by the cyclin-dependent kinase inhibitor SNS-032. Cancer Prev Res (Phila) 2009;2:800–806. doi: 10.1158/1940-6207.CAPR-09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conkrite K, Sundby M, Mukai S, Thomson JM, Mu D, Hammond SM, et al. miR-17~92 cooperates with RB pathway mutations to promote retinoblastoma. Genes Dev. 2011;25:1734–1745. doi: 10.1101/gad.17027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.