By using two-photon (TP)[1] photoreleasable neurotransmitters like glutamate (Glu) or γ-aminobutyric acid (GABA), neuronal processes can be activated or inhibited with high temporal and spatial control (with around one micron three-dimensional precision) with reduced photodamage to cells or organs, and with deeper penetration of the light beam into living tissue compared to that of UV photoactivation.[2]
Whereas TP excitation of fluorophores can be achieved with high efficiency to allow accurate imaging in living systems,[3] TP uncaging is still in its early stages. This challenge requires the design of TP-sensitive caged neurotransmitters having a high TP uncaging action cross-section (expressed in Goeppert-Mayer GM, 1 GM = 1050 cm4s per photon), ideally with excitation wavelengths around λ = 800 nm.[2] To be useful, caged neurotransmitters must also be stable and biologically inert in the dark, and have good aqueous solubility (usually in the mM range in electrophysiological buffers). Additionally, the photolytic reaction should be fast (≤ 0.1 ms) and clean, thus leading to a stoichiometric release of the neurotransmitter.
Because of its primary role in neuronal excitation, the neurotransmitter Glu has been extensively targeted for one-photon and more recently for TP uncaging investigations.[4a–g] The most commonly used caged Glu, 4-methoxy-7-nitro-indolinyl-amino (MNI-Glu; see Scheme 1 for MNI structure),[4b–c] has enabled mapping of synaptic receptors,[4c] activation of individual spines[5] and neurons,[6] and has very recently been used in the cortex in vivo to look at the functional distribution of AMPA-type glutamate receptors (AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid)[7] and to induce continuous growth of a functional spine.[8] However, because none of the caged Glu systems described so far combine high TP uncaging action cross-sections with good quantum efficiency and sufficient buffer solubility, elevated concentrations of caged compounds and relatively high laser powers are needed, both of which can have deleterious effects. Accordingly, we have developed the (2,7-bis-{4-nitro-8-[3-(2-propyl)-styryl]}-9,9-bis-[1-(3,6-dioxa-heptyl)]fluorene (BNSF) photoremovable group[9] which displays to date the highest (5 GM) TP uncaging action cross-section at λ = 800 nm. Nevertheless, BNSF-Glu does not allow for a quantitative release of the neurotransmitter (60%) because of a competing photodegradation pathway, and it is sparingly soluble in aqueous media (0.1 mM).
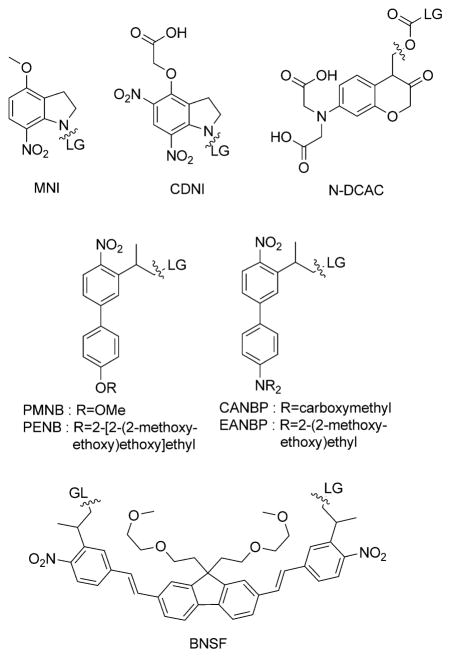
Scheme 1.
Two-photon-sensitive photoremovable groups. LG = leaving group.
Despite the primary role of GABA in neuronal inhibition, only a small number of caged GABA systems have been engineered so far, all with unfavorable photochemical (low TP uncaging action cross-section) and pharmacological properties.[10] CDNI is one cage used for TP uncaging of GABA in intact brain tissue, but it has a modest TP uncaging cross-section even at a low excitation wavelength (around 0.3 GM at λ = 720 nm[4d]). To our knowledge only N-DCAC-GABA (N-DCAC = 7-(dicarboxymethyl)-aminocoumarin) has been used for the TP release of GABA at λ = 800 nm on hippocampal neurons in brain slices,[11] but it suffers from slow GABA release with a long half time of about 4 ms and a moderate efficiency upon TP excitation (0.37 GM at λ = 800 nm[4a]). Therefore the development of efficient TP-sensitive caged GABA is one of the current challenges in the field of chemical neuroscience.[2e]
We have been developing new TP photoremovable groups in the 2-(o-nitrophenyl)-propyl series by applying the molecular engineering of one-dimensional donor–acceptor nonlinear optical chromophores for the optimization of the TP absorption cross-section. Such a strategy has already led to the PMNB cage[4f] as an efficient TP photolabile protecting group for glutamate, with a TP uncaging efficiency (δu=δa.ϕu) of 3.1 GM at λ = 740 nm.[4f] We report herein an important development related to this biphenyl donor–acceptor platform: replacing the alkoxy donor group with a functionalized di-alkylamino moiety leads to new photoremovable groups with improved aqueous solubility (up to 10 mM) and unprecedented (up to 11 GM) TP uncaging cross-sections at λ = 800 nm. We present here the synthesis and photophysical properties of two new photo-removable groups for carboxylic acids: 2-(4′-(bis(carboxymeth-yl)amino)-4-nitro-[1,1′-biphenyl]-3-yl)propan-1-ol (CANBP) and 2-(4′-(bis((2-methoxyethoxy)ethyl)amino)-4-nitro-[1,1′-biphenyl]-3-yl)pro-pan-1-ol (EANBP).TP excitation of CANBP-GABA (1) and EANBP-GABA (2) enabled rapid and spatially controlled GABA release in intact brain slices.
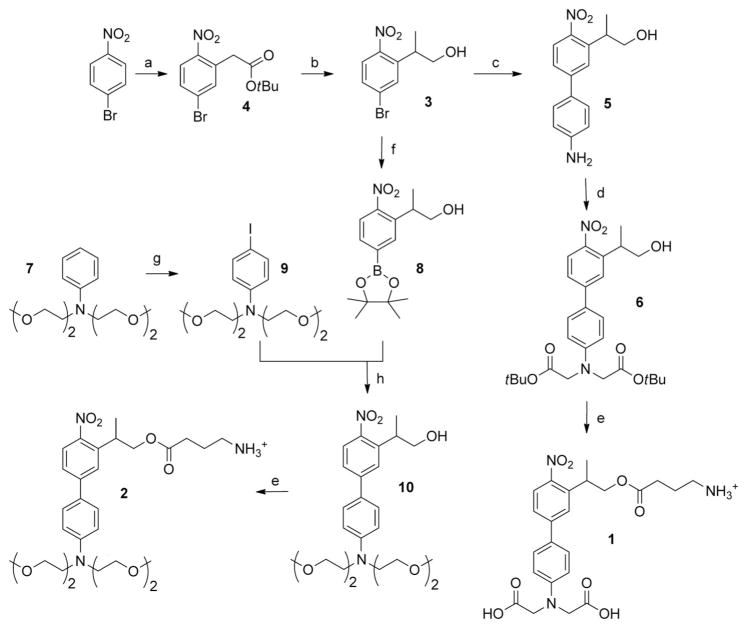
The syntheses of the probes 1 and 2 are summarized in Scheme 2. The key intermediate 2-(5-bromo-2-nitrophenyl)-propan-1-ol (3) was prepared in good yield by a modification of our previously described procedure,[4f] using a vicarious nucleophilic substitution[12] as the key reaction. Starting from 4-bromonitrobenzene, this latter reaction afforded the tert-butyl 2-(5-bromo-2-nitrophenyl)acetate intermediate quantitatively, which was then selectively methylated on the benzylic carbon atom and reduced with DIBAL-H, to afford 3 in three steps with an overall yield of 64%. The intermediate 3 was coupled to 4-aminophenylboronic acid hydrochloride by a Suzuki reaction using microwave activation,[13] thus leading to the 4-amino-4′-nitrobiphenyl 5, which could be conveniently bis(alkylated) with α-bromo tert-butylacetate to lead to the new protected cage 6.
Scheme 2.
Synthesis of 1 and 2. a) 1. tert-butylchloroacetate, tBuOK, DMF, 2 h, RT; 2. HCl, 96%. b) 1. NaH, CH3I, THF, 24 h RT, 83%; 2. DIBAL-H, THF, 3 h, 0 °C, 80%. c) 4-aminophenylboronic acid hydrochloride, K2CO3, Bu4NBr, Pd(OAc)2, EtOH/H2O (2:1), microwave 150 °C, 10 min, 81%. d) DIEA, tert-butyl bromoacetate, DMF, 90°C, 5 h, 40%. e) 1. N-Boc GABA, DCC, DMAP, CH2Cl2, 19 h, RT; 2. TFA/ CH2Cl2 (4:6), 5 h or 1 min, 94–96%. f) [PdCl2(dppf)], KOAc, bis(pinacolato)diboron, DMSO, 80 °C, 15 h, 49%. g) I2, 1,4-dioxane/pyridine (1:1), 0 °C–RT, 2 h, 84%. h) K2CO3, Bu4NBr, Pd(OAc)2, EtOH/H2O (2:1), microwave 150°C, 10 min, 70%. DCC = N,N′-dicyclohexylcarbodiimide, DIBAL-H = diisobutylaluminum hydride, DIEA = diisopropylethylamine, DMAP = 4-dimethylaminopyridine, DMF = N,N′-dimethylformamide, DMSO = dimethylsulfoxide, dppf = 1,1′-bis(diphenylphosphanyl)ferrocene, TFA = trifluoroacetic acid, THF = tetrahydrofuran.
A similar bis(alkylation) of the cage 10 occurred with very low yields (≤ 5%; data not shown). Accordingly, we elaborated an alternative synthesis starting from 3. The nitro-substituted pinacolato borate 8 was synthesized by borylation of 3 using pinacolatodiboron in the presence of a palladium catalyst at 80°C.[14] The alkylated 4-iodoaniline 9 was also synthesized by a selective para iodination[15] of the N,N-bis((2-methoxyethoxy)ethyl)aniline.[16] Then a Suzuki coupling reaction, using microwave activation,[13] between 9 and 8 led to the cage 10 with improved yields (30% overall).
Finally, N-Boc-protected GABA (N-α-tert-butoxycarbonyl GABA) was grafted onto the cages 6 and 10 to provide the new caged GABA analogues 1 and 2, respectively, after deprotection in acidic media.
The solubility of 1 and 2 at room temperature in phosphate buffer pH 7.4 (without any cosolvent) was 10 and 1.3 mM, respectively. The hydrolytic stability was explored by HPLC in phosphate buffer (pH 7.4) at room temperature; less than 1% hydrolysis was measured after 24 h for 1 and 2. The absorption maximum and molecular coefficient for both 1 and 2 are λ = 397 nm and 7500M−1cm−1, respectively.
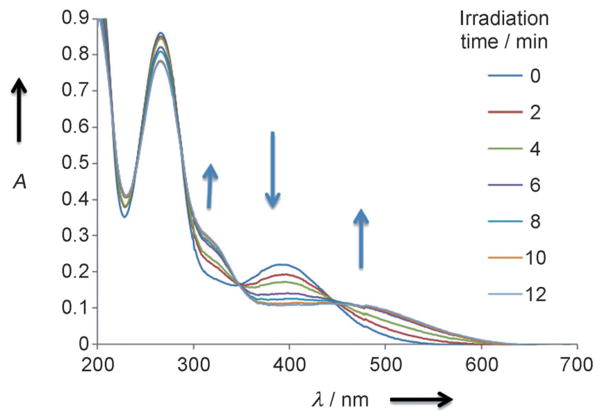
The one-photon photochemical properties of 1 and 2 were investigated by UV/visible spectroscopy. Photolysis was carried out by irradiation of samples (around 40 μM) at λ = 405 nm in phosphate buffer 50 mM pH 7.4. Both 1 and 2 showed a new and broad absorbance at λ = 450 nm, and the initial absorbance at λ = 397 nm gradually diminished and the isobestic points at λ = 350 and 450 nm appeared, thus indicating a homogenous photolysis reaction (Figure 1). The photolytic release of GABA was analyzed by UV and HPLC. To do so, 1 and 2 were coupled to a 4-methoxy benzoic acid chromophoric derivative by using an activated ester (see the Supporting Information). An almost quantitative (≥95%) formation of the 4-methoxybenzoic amide GABA derivatives was measured by HPLC after complete photoconversion of both 4-methoxybenzoic amide caged GABA derivatives. Altogether, this is in agreement with an almost quantitative photolytical reaction following β-elimination pathways with formation of biphenyl 2-(o-nitrophenyl)propene derivatives[17]. The quantum yield for GABA release, as determined by competition with the 2-(nitrophenyl)ethyl-ATP[18] reference molecule, was 15% for both 1 and 2, thus indicating a good photochemical efficiency (ϕ.ε≈1100M−1cm−1) at λ = 400 nm. Altogether, the good water solubility, the high photolytical efficiency, and quantitative release of GABA make the molecules remarkable caged GABA systems for visible light photolysis.
Figure 1.
Changes in the UV/visible spectrum of 2 during photolysis (λ = 405 nm) in phosphate buffer (50 mM pH 7.4) using a LUMOS 43 (Atlas Photonics Inc.).
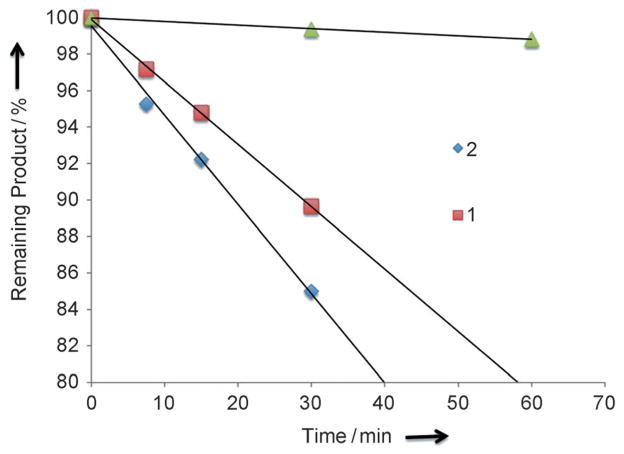
The TP uncaging action cross-section (δaΦu) of 1 and 2 were determined at λ = 800 nm by HPLC analysis of the photolysis products and compared to the 3-(2-propyl)-4′-tris-ethoxy(methoxy)-4-nitrobiphenyl caged 1,3-dichloro-9,9-dimethyl-9H-acridin-2(7)-one (PENB-DDAO) as a reference (0.45 GM).[19] The TP photolysis curves of 1, 2, and PENB-DDAO are presented in Figure 2, and show TP uncaging action cross-section values of 7.4 GM and 11 GM for 1 and 2, respectively.
Figure 2.
Two-photon photolysis of 1 and 2 at λ = 800 nm followed by HPLC analysis and compared to the reference molecule PENB-DDAO (green triangles).
The uncaging kinetics for ANBP photoremovable groups were estimated using a caged fluorescent derivative[19] ANBP-DDAO (see the Supporting Information). A time scale shorter than 5 μs was obtained for the release of the fluorescent DDAO, a value in accord with the reported kinetics for other 2-(o-nitrophenyl)propyl photoremovable groups,[4f,19] and in agreement with temporal control of neuronal processes.
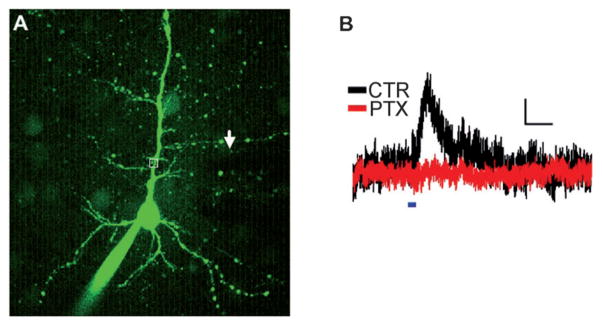
Finally, we used 1 and 2 for TP uncaging of GABA at λ = 800 nm in intact brain tissue. We performed whole-cell voltage clamp recordings from Layer 2/3 pyramidal cells in rat cortical brain slices (Figure 3A). Cells were held at 0 mV to record outward GABAergic currents. A 1 mM amount of 1 was applied focally to the proximal apical dendrite using a glass pipette. The λ = 800 nm laser light was scanned over an apical dendrite and resulted in an outward current (Figure 3B; mean current peak = (6.4 ± 0.3) pA, n = 3 cells). The GABAA receptor antagonist picrotoxin (100 μM) completely abolished the current, thus confirming that it was mediated entirely by GABAA receptors (Figure 3B; n = 3 cells). Experiments with 2 gave currents with similar amplitude (data not shown). Both caged GABA compounds were found to decrease the amplitude of miniature inhibitory post-synaptic currents before photolysis, thereby supporting the idea that like most other caged GABA systems described so far[9] these new cages still display antagonist properties at GABAA receptors (see the Supporting Information).
Figure 3.
Uncaging of 1 activates GABA receptors in brain slices. A) Alexa 488-filled L2/3 pyramidal cell. Uncaging location is within the white box. The puffer pipette containing 1 is visible as a shadow (arrow). B) Outward current evoked by λ = 800 nm uncaging flash (blue line) in the presence of 1 (black), which is blocked by picrotoxin (red). Scale bar = 2 pA/200 ms.
In conclusion, the replacement of the alkoxy donor group by a dialkyl amino group in the donor–acceptor biphenyl 2-(o-nitrophenyl)propyl series led to a 90 nm bathochromic shift (λmax = 397 nm), with no significant modification on the efficiency and kinetics of the photolytic reaction (95% release with a 15% quantum yield for one-photon photo-conversion, and 5 × 10−6 s time range kinetic for uncaging). Most importantly, the substitution of the methoxy group by a better electron-donating group (NR2) significantly increased the TP sensitivity of this photoremovable group, thus leading to an unprecedented TP uncaging action cross-section at λ = 800 nm (up to 11 GM). In addition, the presence of the dialkylamino group opens the possibility for improving significantly the aqueous solubility of these probes by grafting either oligoethyleneglycol chains or 2-carbonylmethyl groups on the amino functional group. The two new caged GABA compounds we have developed, CANBP-GABA (1) and EANBP-GABA (2), indeed have millimolar range buffer solubility, and were successfully used for TP GABA release in intact brain tissue at λ = 800 nm.
Altogether, the remarkable chemical and photophysical properties of the ANBP chromophore open the way for future developments using TP uncaging in neuroscience, cellular, and molecular biology. While the extension to the synthesis to caged glutamate or glycine is straight forward, the chemical strategy developed herein can be transposed to the modification of other functional groups such as phosphates (not shown), thereby leading to the synthesis of original caged nucleotides (ATP, cAMP) or inositolphosphates. Final efforts in the design of ANBP-GABA derivatives, fully devoid of antagonist properties, are currently under investigation for further study of the synaptic signaling both in slices and in vivo.
Supplementary Material
Footnotes
We thank Dr. M. Banghart for helpful discussions and critical comments on the manuscript. This work was supported by the Université de Strasbourg, the CNRS, the France-Berkeley Fund, and ANR (Contract No. 09-BLAN-0425-01 and PCV 07 1-0035).
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201106559.
Contributor Information
Loïc Donato, Laboratoire de Conception et Application de Molécules Bioactives UMR 7199, CNRS/UDS, Faculté de Pharmacie 74 Route du Rhin, 67400 Illkirch (France).
Dr. Alexandre Mourot, University of California Berkeley, Department of Molecular and Cell Biology, Berkeley, CA 94720-3200 (USA).
Dr. Christopher M. Davenport, University of California Berkeley, Department of Molecular and Cell Biology, Berkeley, CA 94720-3200 (USA).
Dr. Cyril Herbivo, Laboratoire de Conception et Application de Molécules Bioactives UMR 7199, CNRS/UDS, Faculté de Pharmacie 74 Route du Rhin, 67400 Illkirch (France)
David Warther, Laboratoire de Conception et Application de Molécules Bioactives UMR 7199, CNRS/UDS, Faculté de Pharmacie 74 Route du Rhin, 67400 Illkirch (France).
Dr. Jérémie Léonard, Institut de Physique et Chimie des Matériaux de Strasbourg UMR 7504, CNRS—Université de Strasbourg (France)
Dr. Frédéric Bolze, Laboratoire de Biophotonique et Pharmacologie, UMR 7213, CNRS/ UdS, Faculté de Pharmacie, 67400 Illkirch (France)
Prof. Dr. Jean-FranÅois Nicoud, Laboratoire de Biophotonique et Pharmacologie, UMR 7213, CNRS/ UdS, Faculté de Pharmacie, 67400 Illkirch (France)
Prof. Dr. Richard H. Kramer, University of California Berkeley, Department of Molecular and Cell Biology, Berkeley, CA 94720-3200 (USA)
Prof. Dr. Maurice Goeldner, Email: goeldner@bioorga.u-strasbg.fr, Laboratoire de Conception et Application de Molécules Bioactives UMR 7199, CNRS/UDS, Faculté de Pharmacie 74 Route du Rhin, 67400 Illkirch (France)
Dr. Alexandre Specht, Email: specht@bioorga.u-strasbg.fr, Laboratoire de Conception et Application de Molécules Bioactives UMR 7199, CNRS/UDS, Faculté de Pharmacie 74 Route du Rhin, 67400 Illkirch (France)
References
- 1.a) Denk W, Strikler JH, Webb WW. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]; b) Helmchen F, Denk W. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]; c) Cruz HG, Luescher C. Front Biosci. 2005;10:2263–2278. doi: 10.2741/1696. [DOI] [PubMed] [Google Scholar]; d) Molitoris BA, Sandoval RM. Am J Physiol. 2005;288:F1084– F1089. doi: 10.1152/ajprenal.00473.2004. [DOI] [PubMed] [Google Scholar]
- 2.a) Goeldner M, Givens R, editors. Dynamic Studies in Biology, Phototriggers, Photoswitches and Caged Biomolecules. Wiley-VCH; Weinheim: 2005. [Google Scholar]; b) Ellis-Davies GCR. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Specht A, Bolze F, Omran Z, Nicoud JF, Goeldner M. HFSP J. 2009;3:255–264. doi: 10.2976/1.3132954. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Warther D, Gug S, Specht A, Bolze F, Nicoud JF, Mourot A, Goeldner M. Bioorg Med Chem. 2010;18:7753–7758. doi: 10.1016/j.bmc.2010.04.084. [DOI] [PubMed] [Google Scholar]; e) Ellis-Davies GCR. ACS Chem Neurosci. 2011;2:185– 197. doi: 10.1021/cn100111a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Kim HM, Cho BR. Chem Commun. 2009:153–164. [Google Scholar]; b) Pawlicki M, Collins HA, Denning RG, Anderson HL. Angew Chem. 2009;121:3292–3316. doi: 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:3244–3266. doi: 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]; c) He GS, Tan LS, Zheng Q, Prasad PN. Chem Rev. 2008;108:1245–1330. doi: 10.1021/cr050054x. [DOI] [PubMed] [Google Scholar]; d) Terenziani F, Katan C, Badaeva E, Tretiak S, Blanchard-Desce M. Adv Mater. 2008;20:4641–4678. [Google Scholar]; e) Drobizhev MA, Makarov NS, Tillo SE, Hughes TE, Rebane A. Nat Methods. 2011;8:393– 399. doi: 10.1038/nmeth.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Furuta T, Wang SH, Dantzker JL, Dore TM, Bybee WJ, Callaway EM, Denk W, Tsien RY. Proc Natl Acad Sci USA. 1999;96:1193–1200. doi: 10.1073/pnas.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Papageorgiou G, Corrie JET. Tetrahedron. 2000;56:8197–8205. [Google Scholar]; c) Matsuzaki M, Ellis-Davies GCR. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ellis-Davies GCR, Matsuzaki M, Paukert M, Kasai H, Bergles DE. J Neurosci. 2007;27:6601–6604. doi: 10.1523/JNEUROSCI.1519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Specht A, Thomann JS, Alarcon K, Wittayanan W, Ogden D, Furuta T, Kurakawa Y, Goeldner M. ChemBioChem. 2006;7:1690–1695. doi: 10.1002/cbic.200600111. [DOI] [PubMed] [Google Scholar]; f) Gug S, Charon S, Specht A, Alarcon K, Ogden D, Zietz B, Leonard J, Haacke S, Bolze F, Nicoud JF, Goeldner M. ChemBioChem. 2008;9:1303–1307. doi: 10.1002/cbic.200700651. [DOI] [PubMed] [Google Scholar]; g) Fino E, Araya R, Peterka DS, Saliemo M, Etchenique R, Yuste R. Front Neural Circuits. 2009;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Araya R, Jiang J, Eisenthal KB, Yuste R. Proc Natl Acad Sci USA. 2006;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Carter AG, Sabatini BL. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]; c) Gasparini S, Magee J. J Neurosci. 2006;26:1412–1421. doi: 10.1523/JNEUROSCI.4428-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Sobczyk A, Scheuss V, Svoboda K. Neuron. 2003;38:277– 289. [Google Scholar]
- 6.Nikolenko V, Poskanzer KE, Yuste R. Nat Methods. 2007;4:943–950. doi: 10.1038/nmeth1105. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GCR, Kitamura K, Kano M, Matsuzaki M, Kasai H. J Physiol. 2011;589:2447–2457. doi: 10.1113/jphysiol.2011.207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon H-B, Sabatini BL. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gug S, Bolze F, Specht A, Bourgogne C, Goeldner M, Nicoud J-F. Angew Chem. 2008;120:9667–9671;. doi: 10.1002/anie.200803964. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:9525–9529. doi: 10.1002/anie.200803964. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki M, Hayama T, Kasai H, Ellis-Davies GCR. Nat Chem Biol. 2010;6:255–257. doi: 10.1038/nchembio.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantevari S, Matsuzaki M, Kanemoto Y, Kasai H, Ellis-Davies GCR. Nat Methods. 2010;7:123–125. doi: 10.1038/nmeth.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makosza M. Chem Soc Rev. 2010;39:2855–2868. doi: 10.1039/b822559c. [DOI] [PubMed] [Google Scholar]
- 13.a) Wallow TI, Novak BM. J Org Chem. 1994;59:5034–5037. [Google Scholar]; b) Allan GM, Vicker N, Lawrence HR, Tutill HJ, Day JM, Huchet M, Ferrandis E, Reed MJ, Purohit A, Potter BVL. Bioorg Med Chem. 2008;16:4438– 4456. doi: 10.1016/j.bmc.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Kaur G, Fang H, Gao X, Li H, Wang B. Tetrahedron. 2006;62:2583–2589. [Google Scholar]
- 15.Monnereau C, Blart E, Odobel F. Tetrahedron Lett. 2005;46:5421–5423. [Google Scholar]
- 16.Ros-Lis JV, García B, Jiménez D, Martínez-Máñez R, Sancenón F, Soto J, Gonzalvo F, Valldecabres MC. J Am Chem Soc. 2004;126:4064–4065. doi: 10.1021/ja031987i. [DOI] [PubMed] [Google Scholar]
- 17.Walbert S, Pfleiderer W, Steiner UE. Helv Chim Acta. 2001;84:1601–1611. [Google Scholar]
- 18.Walker JW, Reid G, McCray JA, Trentham DR. J Am Chem Soc. 1988;110:7170–7177. [Google Scholar]
- 19.Warther D, Bolze F, Léonard J, Gug S, Specht A, Puliti D, Sun X-H, Kessler P, Lutz Y, Vonesch J-L, Winsor B, Nicoud J-F, Goeldner M. J Am Chem Soc. 2010;132:2585–2590. doi: 10.1021/ja9074562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.