Abstract
Background
Although combination antiretroviral therapy (cART) including tenofovir (TDF)+lamivudine (3TC) or emtricitabine (FTC) is recommended for treatment of HIV/HBV co-infected patients, TDF is unavailable in some resource-limited areas. Some data suggest that 3TC monotherapy-based cART may be effective in patients with low pre-treatment HBV DNA.
Methods
Prospective study of 151 Chinese HIV/HBV co-infected subjects of whom 60 received 3TC-based cART and 91 received TDF+3TC-based cART. Factors associated with HBV DNA suppression at 24 and 48 weeks, including anti-HBV drugs, baseline HBV DNA, and baseline CD4 cell count, were evaluated overall and stratified by baseline HBV DNA using Poisson regression with a robust error variance.
Results
Baseline HBV DNA≥20,000 IU/ml was present in 48.3% and 44.0% of subjects in the 3TC and TDF groups, respectively (P=0.60). After 48 weeks of treatment, HBV DNA suppression rates were similar between these two groups (96.8% vs. 98.0% for 3TC and TDF+3TC, P>0.999) in subjects with baseline HBV DNA<20,000 IU/ml; while in those with baseline HBV DNA ≥20,000 IU/ml, TDF+3TC was associated with higher suppression rates (34.5% vs. 72.5% in 3TC and TDF+3TC groups, respectively, P=0.002). In stratified multivariate regression, TDF use (RR 1.98, P=0.010) and baseline HBV DNA (per 1 log increase in IU/ml, RR 0.74, P<0.001) were associated with HBV DNA suppression only when baseline HBV DNA≥20,000IU/ml.
Conclusion
This study suggests that 3TC monotherapy-based cART is efficacious for HBV treatment through 48 weeks in HIV/HBV co-infection when baseline HBV DNA<20,000IU/ml. Studies with long-term follow-up are warranted to determine if this finding persists.
Keywords: HIV, HBV, lamivudine, tenofovir, Hepatitis B surface antigen
Introduction
Since human immunodeficiency virus (HIV) and hepatitis B virus (HBV) share the same transmission routes, co-infection is common, especially in areas where both diseases are prevalent. A recent retrospective multicenter study in China discovered that chronic HBV co-infection occurs in 12% of HIV-infected Chinese patients 1. Tenofovir (TDF)+lamivudine (3TC) or TDF+emtricitabine (FTC) based combination antiretroviral therapy (cART) is recommended for treatment of HIV/HBV co-infected patients 2 because they are both highly active against HIV and HBV viral replication. In addition, TDF is efficacious against 3TC-resistant HBV in HBV monoinfection 3. Despite this, TDF remains unavailable or expensive in some resource-limited areas; thus 3TC-based cART for HBV co-infection is given. Unfortunately, 3TC monotherapy has lower potency and genetic barrier to resistance, with around 20-25% mutation rate per year in HIV/HBV co-infected subjects 4; however, most of the subjects from whom resistance rate data come have high levels of HBV DNA prior to cART and resistance to 3TC does not usually develop when baseline HBV DNA levels are low. In a recent study from Côte d'Ivoire with treatment-naïve HIV/HBV co-infected subjects, those with high-level persistent HBV viremia and/or 3TC-associated HBV Pol mutations had high baseline HBV DNA levels (> 6 log IU/ml) 5. In most studies from developing countries 6, 7, including our previous report 8, a majority of HIV/HBV co-infected subjects had baseline HBV DNA <20,000IU/ml, which is the treatment threshold for HBV mono-infection 9. It is unknown whether 3TC-based cART has similar efficacy against HBV in comparison with TDF+3TC based cART in people with this low level of HBV DNA.
Before 2012, 3TC-based cART (zidovudine or stavudine plus 3TC as backbone) was the first-line treatment for all HIV-infected treatment-naïve patients in China 10, 11, including those with HIV/HBV co-infection. Since 2012, all newly treated HIV patients, including HIV/HBV co-infected patients, receive a TDF+3TC-based cART regimen. Thus, China is an ideal place to compare HBV outcomes between those who receive 3TC- or TDF+3TC-based cART to determine if 3TC-based cART is efficacious with low baseline HBV DNA.
Subjects and methods
Study subjects
The subjects in this study were previously-enrolled in one of the following four multicenter HIV cohorts in China: (1) 10th five-year (10-5) cohort (recruited between 2005 and 2007, reported in 12); (2) 11th five-year (11-5) cohort (recruited between 2008 and 2010, reported in 8); (3) 12th five-year cohort 4 (12-5-4, recruited between 2012 and 2014); (4) 12th five-year (12-5) cohort 6 (12-5-6, recruited between 2012 and 2014); (5) outpatient clinics (OP, recruited between 2012 and 2014) (Figure 1). The Institutional Review Board of Peking Union Medical College Hospital (PUMCH) approved the parent studies and each participant provided written informed consent. Inclusion criteria for the HIV cohorts were described previously 8, 12, including: (1) CD4 cell count lower than 350 cells/μl in 10th five-year cohort and 11th five-year cohort, CD4 cells lower than 500 cells/μl in 12th five-year cohort and outpatient clinics (CD4> 500 cells/μl can still be enrolled as long as adherence can be guaranteed in 12th five-year cohort 6 and outpatient clinic); (2) alanine transaminase (ALT) and aspartate aminotransferase (AST) lower than three times upper limit of normal (ULN, which is 40 IU/ml for both ALT and AST); (3) not pregnant. Enrollment criteria relevant to this study included: (1) HBV surface antigen positivity at baseline; (2) uninfected with hepatitis C virus (HCV) (anti-HCV negative or anti-HCV positive with negative HCV RNA); (3) no previous history of cART or anti-HBV treatment. All subjects from cohorts 10-5 and 11-5 received 3TC-based HBV-active cART (zidovudine or stavudine or didanosine plus 3TC plus nevirapine), while those from 12-5-4 received TDF+3TC-based HBV-active cART (TDF plus 3TC plus efavirenz or lopinavir/ritonavir). Subjects from cohorts 12-5-6 and OP received either 3TC-based or TDF+3TC-based HBV-active cART, which was determined by the standard of care at the time and locations they were enrolled. Thus, subjects from these cohorts contributed to both treatment arms.
Figure 1.
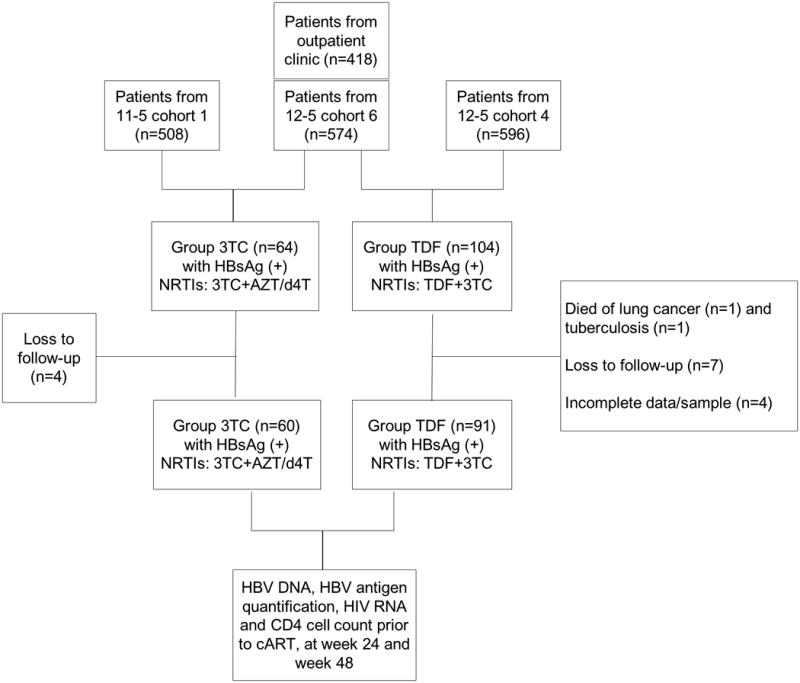
Subject recruitment.
Subjects visited local medical centers for clinical evaluation and blood collection prior to cART and at the following weeks after cART initiation: 4, 8, 12, and then every 12 weeks. In this study, we retrieved clinical data prior to cART (within two weeks of cART initiation), at week 24 and week 48.
Primary and secondary endpoints
The primary endpoint of this study was HBV DNA suppression (<20 IU/ml, the lower limit of detection) at week 48. Secondary endpoints included HBV DNA suppression at week 24, median HBV DNA at weeks 24 and 48, quantification of HBV surface antigen decline, HBV e antigen (HBeAg) loss and seroconversion, and HBsAg loss and seroconversion.
Clinical and laboratory data
At each visit, HIV RNA (COBAS Ampliprep/TaqMan48 real-time RT-PCR, Roche Diagnostics, Indianapolis, IN, USA), CD4 cell count (flow cytometry, Beckman-Coulter, Brea, California, USA), ALT and AST were measured (reported in our previous study 8).
HBV DNA measurement and HBsAg quantification
HBV DNA levels were measured using COBAS Ampliprep/TaqMan48 real-time PCR Test (Roche Diagnostics, Indianapolis, IN, USA) with a detection range of 20– 110,000,000 IU/ml. Quantification of HBV surface antigen (qHBsAg) may reflect HBV reservoir size13 and is associated with treatment response; 14 therefore we assessed qHBsAg using the Abbott Architect i2000 platform (Abbott Diagnostics, Abbott Park, IL, USA). This assay was performed according to the manufacturer's instructions, with a detection range of 0.05- 124,925 IU/ml. HBV DNA, qHBsAg and HBeAg were measured from frozen stored (-80°C) plasma samples.
Statistical analysis
Continuous variables were summarized with median and interquartile ranges (IQR) and analyzed by the Kruskal–Wallis test. Categorical variables were analyzed by Chi square test or Fisher's exact test. To determine whether TDF+3TC is superior to 3TC-based regimen at lower HBV DNA levels, we stratified statistical analyses by baseline HBV DNA (<20,000 vs. ≥20,000 IU/ml), a level chosen based on prior studies 15. Relative risks (RR) of factors associated with HBV DNA suppression (HBV DNA <20 IU/ml) were determined by Poisson regression with a robust error variance 16. Sex (male and female), age (categorical, 18-30 years, 31-40 years, 41-50 years, and 51-65 years), routes of transmission (men who have sex with men [MSM], heterosexual, blood and others/unknown), cART regimens (3TC-based or TDF+3TC-based), baseline HBV DNA (continuous) and baseline CD4 cell count (≤200 or >200 cells/μl) were forced into the multivariate models; other factors with P values<0.15 in univariate models were also adjusted for in the multivariate models. Mann-Whitney U test was used to determine whether difference of qHBsAg between the baseline and 48 week value was significant. Stata 13 (StataCorp, College Station, TX, USA) was used for all analyses. P values <0.05 were considered statistically significant.
Results
Baseline characteristics
This study included 151 HIV/HBV coinfected subjects, of whom 60 received 3TC-based regimens (3TC group) and 91 subjects received TDF+3TC based regimens (TDF group). Of these subjects, 48 were from Cohort 11-5, six were from Cohort 10-5, 45 were from 12-5 Cohort 4, 40 were from 12-5 Cohort 6 and 12 were from outpatient cohort. Most of patients were male, 30 to 40 years old and infected via sexual transmission (Table 1). Median CD4 cell count was 150 cells/μl in the 3TC group and 229 cells/μl in the TDF group (P<0.001), while HIV RNA, HBV DNA, and qHBsAg were comparable in these two groups (Table 1). Notably, 23 patients (15.2%) had undetectable HBV DNA prior to cART, while 69 patients (45.7%) had baseline HBV DNA≥20,000 IU/ml; these participants were equally distributed between the two treatment groups. Thirty-nine patients (25.8%) were HBeAg positive.
Table 1. Baseline Characteristics.
| Overall(n=151) | 3TC group (n=60) | TDF group (n=91) | P value (3TC vs. TDF) | |
|---|---|---|---|---|
| Male sex (n, %) | 125 (82.8) | 47 (78.3) | 78 (85.7) | 0.24 |
| Age (years, IQR) | 36 (30,45) | 34 (30, 41) | 37 (30, 47) | 0.16 |
| Route of transmission (n, %) | 0.29a | |||
| MSM | 44 (29.1) | 21 (35.0) | 23 (25.3) | |
| Heterosexual | 94 (62.3) | 33 (55.0) | 61 (67.0) | |
| Blood | 1 (0.7) | 1 (1.7) | 0 (0.0) | |
| Others/Unknown | 12 (7.9) | 5 (8.3) | 7 (7.7) | |
| CD4 cell count (cells/μl, IQR) | 200 (122, 307) | 150 (84, 241) | 229 (146, 332) | <0.001 |
| HIV RNA (log copies/ml, IQR) | 4.69 (4.22, 5.14) | 4.51 (4.08, 5.12) | 4.70 (4.37, 5.16) | 0.10 |
| HBV DNA (log IU/ml, IQR) | 3.59 (2.07, 7.52) | 3.69 (2.05, 8.04) | 3.49 (2.07, 6.53) | 0.39 |
| HBV DNA<20 IU/ml (n, %) | 23 (15.2) | 10 (16.7) | 13 (14.3) | 0.69 |
| HBV DNA>20000IU/ml (n, %) | 69 (45.7) | 29 (48.3) | 40 (44.0) | 0.60 |
| HBeAg positive (n, %) | 39 (25.8) | 18 (30.0) | 21 (23.1) | 0.34 |
| ALT (IU/l, IQR) | 29 (20, 43) | 28 (21, 44) | 30 (19, 43) | 0.98 |
| ALT>40IU/l (n, %) | 43 (28.5) | 17 (28.3) | 26 (28.6) | 0.98 |
| AST (IU/l, IQR)b | 28 (23, 39) | 28 (23, 40) | 29 (23, 34) | 0.68 |
| HBsAg quantification (log IU/ml, IQR) | 3.49 (2.76, 4.13) | 3.62 (2.77, 4.55) | 3.41 (2.70, 3.99) | 0.17 |
3TC, lamivudine; TDF tenofovir; MSM, men who have sex with men; IQR, interquartile ranges; HBeAg, HBV e antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IU, international unit; PEIU, Paul-Ehrlich-Institut unit.
using Fisher Exact test.
110 patients had AST data available.
Participants with HBV DNA ≥20,000 IU/ml had marginally lower median CD4 cell count (175 vs. 211 cells/μl, ≥ vs. < 20,000IU/ml, respectively P=0.067), higher median HIV RNA (4.76 vs. 4.58 log copies/ml, P=0.030), higher median qHBsAg levels (4.04 vs. 2.88 log IU/ml, P<0.001) and a higher proportion of HBeAg positivity (55.1% vs. 1.2%, P<0.001). Of the HBeAg positive participants, 97.4% had HBV DNA≥20,000 IU/ml compared to 27.7% of the HBeAg negative participants (P<0.001).
HBV DNA response
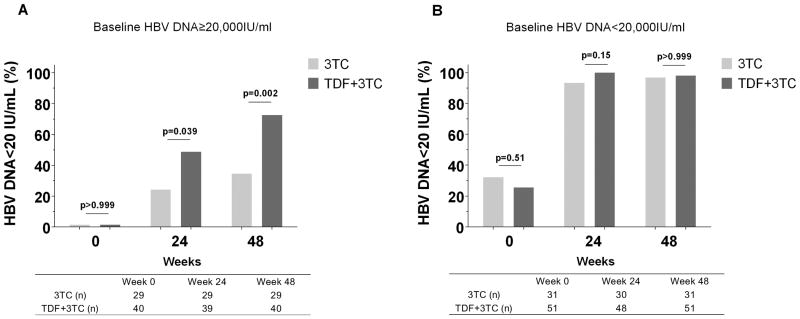
The primary endpoint, HBV DNA suppression to < 20 IU/ml after 48 weeks of cART, was achieved in 78.8% of subjects overall, but it was higher in the TDF than the 3TC group (86.8% vs. 66.7%, P=0.003). When baseline HBV DNA was <20,000 IU/ml, both the TDF and 3TC groups had similar HBV DNA suppression rates at week 48 (Figure 2B) even after excluding subjects with undetectable HBV DNA at baseline (95.2% and 97.4% in the 3TC and TDF groups, respectively, P=0.66). However, when baseline HBV DNA was ≥20,000 IU/ml, the TDF group was associated with higher viral suppression at week 48 (Figure 2A). HBV DNA suppression for the secondary endpoint of 24 weeks was similar (Figure 2A-B).
Figure 2.
HBV DNA suppression prior to, after 24 and 48 weeks of treatment. (A) Baseline HBV DNA≥20,000 IU/ml group; (B) baseline HBV DNA<20,000 IU/ml group. P values were calculated using Chi square test or Fisher exact test.
Similarly, analysis of median HBV DNA demonstrated that when baseline HBV DNA was <20,000 IU/ml, the median value was <20 IU/ml at weeks 24 and 48 in both the 3TC and TDF groups. However, when baseline HBV DNA was ≥20,000 IU/ml, median HBV DNA in TDF group was <20 IU/ml (IQR <20 IU/ml to 1.87 log IU/ml) at week 48, while it was 3.26 log IU/ml (IQR <20 IU/ml to 4.51 log IU/ml, P<0.001) in the 3TC group.
We also evaluated the association between HBeAg and HBV DNA suppression (Supplementary Figure S1). In HBeAg negative participants, the HBV DNA suppression rates were >90% at 48 weeks in both the 3TC and TDF groups (P=0.20). In contrast, in HBeAg positive participants, the 3TC group was less likely to achieve HBV DNA suppression than the TDF group (11.1% vs. 52.4%, respectively, P=0.006).
Factors associated with HBV DNA suppression at 48 weeks of HBV-active cART were determined with regression models stratified by baseline HBV DNA (Table 2). In univariate models, TDF use and lower baseline HBV DNA were the only variables significantly associated with HBV DNA suppression when baseline HBV DNA≥ 20,000 IU/ml (Table 2). In multivariate models, neither TDF use nor baseline HBV DNA levels were significantly associated with HBV DNA suppression in subjects with baseline HBV DNA< 20,000 IU/ml. However, in subjects with baseline HBV DNA≥ 20,000 IU/ml, TDF use was associated with HBV DNA suppression (adjusted RR [aRR] 1.98, 95% CI 1.18-3.34, P=0.010) as was lower baseline HBV DNA (aRR 0.74 per 1 log IU/ml increase, 95% CI 0.63-0.87, P<0.001). Interestingly, higher baseline CD4 cell count was associated with poorer HBV DNA suppression (aRR 0.66 for CD4 cell count>200 cells/μl compared with ≤200 cells/μl, 95% CI 0.46-0.96, P=0.028). When baseline HBeAg status or qHBsAg were included in the multivariable model in place of HBV DNA, both were associated with HBV DNA suppression only when baseline HBV DNA is ≥20,000 IU/ml (Supplemental Table S1). In particular, being HBeAg positive at baseline was associated with a 62% decreased likelihood for suppression (aRR 0.38, 95% CI 0.23-0.63, P<0.001). Higher baseline qHBsAg was also associated with decreased likelihood for suppression (aRR 0.49 per log IU/ml increase in qHBsAg, 95% CI 0.37-0.66, P<0.001).
Table 2. Factors associated with HBV DNA suppression after 48 weeks of treatment stratified by baseline HBV DNA.
| Baseline HBV DNA <20,000 IU/ml | |||||
|---|---|---|---|---|---|
| HBV DNA Suppression rate at week 48 [n/N (%)] | Crude RR (95%CI) | P | Adjusted RR(95%CI) | P | |
| cART | |||||
| 3TC-based | 30/31 (96.8) | Reference | Reference | ||
| TDF+3TC-based | 50/51 (98.0) | 1.01 (0.94-1.09) | 0.74 | 1.01 (0.93-1.11) | 0.78 |
| CD4 cell count | |||||
| ≤200 cells/μl | 35/37 (94.6) | Reference | Reference | ||
| >200 cells/μl | 45/45 (100.0) | 1.06 (0.98-1.14) | 0.16 | 1.03 ( 0.98-1.09) | 0.22 |
| Baseline HIV RNA (per 1 log copy/ml increase) | Not applicable | 0.97 (0.91-1.03) | 0.28 | ||
| Baseline HBV DNA (per 1 logIU/ml increase) | Not applicable | 1.00 (0.97-1.03) | 0.83 | 1.00 (0.97-1.03) | 0.99 |
| CD4 count at week 48 (cells/μL) | |||||
| ≤350 | 35/37 (94.6) | Reference | |||
| >350 | 45/45 (100.0) | 1.06 (0.98-1.14) | 0.16 | ||
| Baseline HBV DNA ≥20,000 IU/ml | |||||
| HBV DNA Suppression rate at week 48 [n/N (%)] | Crude RR (95%CI) | P | Adjusted RR(95%CI) | P | |
| cART | |||||
| 3TC-based | 10/29 (34.5) | Reference | Reference | ||
| TDF+3TC-based | 29/40 (72.5) | 2.10 (1.22-3.61) | 0.007 | 1.98 (1.18-3.34) | 0.010 |
| CD4 cell count | |||||
| ≤200 cells/μl | 23/39 (59.0) | Reference | |||
| >200 cells/μl | 16/30 (53.3) | 0.90 (0.59-1.39) | 0.65 | 0.66 (0.46-0.96) | 0.028 |
| Baseline HIV RNA (per 1 log copy/ml) increase | Not applicable | 1.12 (0.85-1.48) | 0.43 | ||
| Baseline HBV DNA (per 1 log IU/ml increase) | Not applicable | 0.74 (0.65-0.85) | <0.001 | 0.74 (0.63-0.87) | <0.001 |
| CD4 count at week 48 (cells/μL) | |||||
| ≤350 | 20/37 (54.1) | Reference | |||
| >350 | 19/32 (59.4) | 1.10 (0.72-1.66) | 0.66 | ||
RR, relative risk; cART, combination antiretroviral therapy; 3TC, lamivudine; TDF, tenofovir.
Since baseline CD4 cell count were not balanced in two treatment groups, we also adjusted for baseline CD4 cell count in multivariate models.
Sex, age and routes of transmission were also adjusted for in multivariate analyses. Of note, they were not significantly associated with HBV DNA suppression in univariate analysis. cART regimens (3TC-based or TDF+3TC-based), baseline HBV DNA and baseline CD4 cell count were forced into the multivariate models; other factors with P values<0.15 in univariate models were also included in the multivariate models.
We also performed a multivariable analysis stratified by HBeAg status (Supplementary Table S2), and found that TDF+3TC was associated with better HBV DNA suppression only in HBeAg positive group (aRR 10.07, 95% CI 2.30-44.22, P=0.002), but not in HBeAg negative group (aRR for TDF use 1.08, 95% CI 0.97-1.21, P=0.16).
Changes in qHBsAg
Median qHBsAg prior to therapy was 3.49 (IQR 2.76-4.13, n=151) and was 3.24 (IQR 2.24-3.74, n=137) after 48 weeks of cART (P=0.015). Overall, the median decrease of qHBsAg was 0.11 log IU/ml (IQR -0.029 to 0.40). The median decrease of qHBsAg was 0.086 log IU/ml (IQR -0.040 to 0.52 log IU/ml, n=47) in 3TC group and 0.12 log IU/ml (IQR -0.015 to 0.37 log IU/ml, n=90) in TDF group (P=0.92). When stratified by baseline HBV DNA levels, subjects in 3TC and TDF groups had comparable qHBsAg decline in both strata (data not shown).
HBeAg and HBsAg antigen loss/seroconversion
Thirty-five HBeAg-positive subjects had HBV serology data at week 48, of whom ten (28.6%) had HBeAg loss and seroconversion. In the 3TC group, 1/14 (7.1%) of subjects had HBeAg seroconversion; while 9/21 (42.9%) of subjects in TDF group had HBeAg seroconversion (P=0.028).
Of the 137 participants who had HBsAg obtained at week 48, five experienced HBsAg loss (two from 3TC group and three from TDF group) of whom four developed hepatitis B surface antibody. The two treatment groups had comparable rates of HBsAg loss and seroconversion.
HIV RNA suppression
After 48 weeks of cART, HIV RNA <400 copies/ml was achieved in 96.7% and HIV RNA <50 copies/ml was achieved in 86.1%. TDF and 3TC groups did not differ in HIV suppression rates (P=0.39 and P=0.52, at HIV RNA <400 copies/ml and <50 copies/ml, respectively).
Discussion
This is the largest multicenter cohort study to compare the efficacy of 3TC-based versus TDF+3TC-based cART against HBV infection in subjects with HIV/HBV co-infection stratified by baseline HBV DNA level. Since most HBV treatment guidelines use 20,000 IU/ml as a cut-off for treatment, there are limited data available on the response to 3TC monotherapy in those with low pre-treatment HBV DNA. We demonstrated that in HIV/HBV co-infected participants with baseline HBV DNA levels <20,000 IU/ml, 3TC and TDF+3TC based cART regimens had comparable efficacy for HBV treatment at 48 weeks, while in those with HBV DNA levels ≥20,000 IU/ml at baseline, TDF+3TC was more efficacious. These data are most applicable to resource-limited countries where TDF may not be universally available or affordable. They are also important for patients who cannot use TDF due to its nephrotoxicity.
At baseline, we found over 50% of HIV/HBV co-infected subjects had HBV DNA <20,000 IU/ml, the treatment threshold for HBV infection 9, 17. This finding is similar to other developing countries where 30 to 50% had baseline HBV DNA <20,000 IU/ml 6, 15, 18. It is in these people with HBV DNA < 20,000 IU/ml that our study demonstrates >95% HBV suppression rates for 48 weeks in both the 3TC and the TDF+3TC HIV/HBV co-infected groups. This is compared to other HIV/HBV co-infection studies, which did not stratify by baseline HBV DNA, that have shown approximately 30-60% achieving HBV DNA suppression with 3TC monotherapy at 48 weeks 19-21. A recent study from China with a small number of HIV/HBV co-infected subjects demonstrated that 3TC monotherapy was associated with higher rates of 3TC-resistant HBV compared to TDF+3TC 22, but in this study, the authors did not stratify their analyses with baseline HBV DNA levels. Our multivariable analysis demonstrates that TDF is associated with HBV DNA suppression when HBV DNA ≥ 20,000 IU/ml (Table 2) or when HBeAg is negative (Supplementary Table S2) at baseline. In a recent multicenter study, Thio et al. reported that in subjects with baseline HBV DNA <20,000IU/ml, monotherapy (3TC or FTC) and dual therapy (TDF+3TC or FTC) had similar efficacy in terms of HBV DNA suppression during 144 weeks of follow-up 15. Taken together, these studies suggest that 3TC-based monotherapy might be considered in patients with HBV DNA <20,000 IU/ml or HBeAg negative when TDF cannot be safely used or accessed. Since not all countries are able to determine HBV DNA levels, HBeAg status can also be used since we found that HBeAg-negative patients had a similarly high response to either TDF +3TC- or 3TC-based cART. However, HBeAg-positive subjects responded better to TDF+3TC.
In the multivariate analyses stratified by baseline HBV DNA, higher baseline HBV DNA was associated with lower HBV DNA suppression only when baseline HBV DNA was ≥20,000 IU/ml, which further supports that the precise level below 20,000 does not alter the response to treatment. HBeAg positive status was also a strong independent predictor for HBV DNA suppression. This finding together with the fact that 97% of the HBeAg positive participants and only 28% of the HBeAg negative participants had high HBV DNA supports the use of HBeAg testing to determine who is most likely to have a HBV DNA level that would require TDF +3TC based cART.
As has been shown in HBV mono-infected subjects, the HBsAg seroconversion rate and the decrease in qHBsAg are small regardless of treatment group. In HBV mono-infected subjects treated with ETV, the qHBsAg decline rate was 0.084 log IU/ml per year 23. While another study from France with HIV/HBV co-infected subjects reported that only 39% of subjects had constant decline in qHBsAg when they received TDF-based cART 24.
The strengths of our study are as follows. First, it is the first multicenter longitudinal study to compare the efficacy of 3TC-based and TDF+3TC based cART in HIV/HBV co-infected patients from a resource-limited setting designed to examine HBV treatment responses stratified by high and low baseline HBV DNA levels. Second, in addition to HBV DNA, we also stratified our findings by HBeAg status since HBV DNA requires more specialized equipment; thus, it cannot be obtained in all settings. Third, we determined whether decline in HBsAg levels differed by treatment group or by HBV DNA level.
One limitation to our study is that subjects from the two treatment groups were selected from cohorts established in different years; despite this, their baseline characteristics were well-balanced, except for CD4 cell count, which we adjusted for in the models. A second limitation is that the data to date are from one year; longer follow-up is warranted to evaluate HBV resistance and changes in qHBsAg in both treatment groups. Third, subjects with liver enzymes > 3xULN were excluded from the parent HIV cohorts, therefore it would be difficult to generalize this result to subjects who have high baseline ALT/AST.
In conclusion, HIV/HBV co-infected subjects with higher baseline HBV DNA (≥20,000 IU/ml) or HBeAg positivity, should receive TDF+3TC based cART since it is associated with higher HBV DNA suppression rates. However, in co-infected subjects with lower HBV DNA levels or HBeAg negative, 3TC-based cART could be considered if TDF is not readily available or affordable or is contraindicated. Further studies are warranted to determine if this stratification strategy can also be extrapolated to HBV mono-infected patients.
Supplementary Material
Supplementary Figure S1. HBV DNA suppression prior to, after 24 and 48 weeks of treatment. (A) Baseline HBV e antigen positive group; (B) baseline HBV e antigen negative group. P values were calculated using Chi square test or Fisher exact test.
Acknowledgments
We want to thank our HIV/HBV co-infected subjects for their participation. We also thank Doctor Shaoxia Xu from Department of Clinical Laboratory of Peking Union Medical College Hospital for his assistance with qHBsAg assays. The following clinical institutions or hospitals participated in this study. Northeastern centers included Peking Union Medical College Hospital, Beijing Youan Hospital, Beijing Ditan Hospital, 302 Hospital in Beijing, China Medical University and Zhengzhou Sixth Hospital. Northwestern center included Tangdu Hospital. Southeastern centers included Guangzhou Eighth People's Hospital, Shanghai Public Health Clinical Center, Shenzhen Third People's Hospital, Fuzhou Infectious Diseases Hospital. Southwestern centers included Chengdu Infectious Diseases Hospital, Changsha First Hospital, District CDC in Nanning, Longtan Hospital in Nanning, Nanning Forth People's Hospital, Yunnan AIDS Care Center, Kunming Third People's Hospital, and Honghe First People's Hospital.
This study is funded by the following grants: National Natural Science Foundation of China [grant 81071372]; National Institutes of Health [grant R01AI106586]; National Key Technologies R&D Program for the 12th Five-year Plan [grant 2012ZX10001003-001]; the 12th Five-Year Major New Drug Discovery Science and Technology [grant 2012ZX09303013]; J. X. was supported by the Johns Hopkins University Center for AIDS Research (JHU CFAR) NIH/NIAID fund [1P30AI094189-01A1].
This work was supported by National Natural Science Foundation of China [grant number 81071372]; National Institutes of Health [grant number R01AI106586]; National Key Technologies R&D Program for the 12th Five-year Plan [grant number 2012ZX10001003-001]; the 12th Five-Year Major New Drug Discovery Science and Technology [grant number 2012ZX09303013]. J. X. was supported by the Johns Hopkins University Center for AIDS Research (JHU CFAR) NIH/NIAID fund [grant number 1P30AI094189-01A1].
Footnotes
This study, in part, was presented at the 8th IAS Conference in Vancouver, Canada.
The funders had no role in study design, data collection, data analyses, preparation of the manuscript, or decision to publish.
Author's contributions: Chloe L Thio, Taisheng Li, and Huanling Wang designed this study. Yijia Li, Jing Xie, Chloe L Thio and Taisheng Li performed data analyses and prepared this manuscript. Yang Han, Ting Zhu, Yijia Li and Nidan Wang performed HIV RNA and HBV DNA assays. Jing Xie and Zhifeng Qiu performed flow-cytometry assays to determine CD4 cell count. Yijia Li performed qHBsAg assays. Yanling Li, Shanshan Du and Xiaojing Song were in charge of patient's follow-up visit and sample transportation. Huanling Wang, Wei Lv and Fuping Guo provided critical revision.
Conflict of interest: CLT has received grant support from Gilead Sciences. All other authors report no conflict of interest.
References
- 1.Zhang F, Zhu H, Wu Y, et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010-12: a retrospective observational cohort study. Lancet Infect Dis. 2014;14:1065–1072. doi: 10.1016/S1473-3099(14)70946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DHHS. [July 23, 2015];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 3.Fung S, Kwan P, Fabri M, et al. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2014;146:980–988. doi: 10.1053/j.gastro.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS. 2006;20:863–870. doi: 10.1097/01.aids.0000218550.85081.59. [DOI] [PubMed] [Google Scholar]
- 5.Boyd A, Moh R, Gabillard D, et al. Low risk of lamivudine-resistant HBV and hepatic flares in treated HIV-HBV co-infected patients from Cote d'Ivoire. Antivir Ther. 2015 doi: 10.3851/IMP2959. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Idoko J, Meloni S, Muazu M, et al. Impact of hepatitis B virus infection on human immunodeficiency virus response to antiretroviral therapy in Nigeria. Clin Infect Dis. 2009;49:1268–1273. doi: 10.1086/605675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann CJ, Charalambous S, Martin DJ, et al. Hepatitis B virus infection and response to antiretroviral therapy (ART) in a South African ART program. Clin Infect Dis. 2008;47:1479–1485. doi: 10.1086/593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Li Y, Zhang C, et al. Immunological and virological responses to cART in HIV/HBV co-infected patients from a multicenter cohort. AIDS. 2012;26:1755–1763. doi: 10.1097/QAD.0b013e328355ced2. [DOI] [PubMed] [Google Scholar]
- 9.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Guo F, Li Y, et al. An antiretroviral regimen containing 6 months of stavudine followed by long-term zidovudine for first-line HIV therapy is optimal in resource-limited settings: a prospective, multicenter study in China. Chin Med J (Engl) 2014;127:59–65. [PubMed] [Google Scholar]
- 11.Li T, Dai Y, Kuang J, et al. Three generic nevirapine-based antiretroviral treatments in Chinese HIV/AIDS patients: multicentric observation cohort. PLoS One. 2008;3:e3918. doi: 10.1371/journal.pone.0003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuping G, Wei L, Yang H, et al. Impact of hepatitis C virus coinfection on HAART in HIV-infected individuals: multicentric observation cohort. J Acquir Immune Defic Syndr. 2010;54:137–142. doi: 10.1097/QAI.0b013e3181cc5964. [DOI] [PubMed] [Google Scholar]
- 13.Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 14.Zoulim F, Carosi G, Greenbloom S, et al. Quantification of HBsAg in nucleos(t)ide-naive patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Thio CL, Smeaton L, Hollabaugh K, et al. Comparison of HBV-active HAART regimens in an HIV-HBV multinational cohort: outcomes through 144 weeks. AIDS. 2015;29:1173–1182. doi: 10.1097/QAD.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. [July 19, 2015];Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng.pdf?ua=1&ua=1. [PubMed]
- 18.Ive P, MacLeod W, Mkumla N, et al. Low prevalence of liver disease but regional differences in HBV treatment characteristics mark HIV/HBV co-infection in a South African HIV clinical trial. PLoS One. 2013;8:e74900. doi: 10.1371/journal.pone.0074900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews GV, Avihingsanon A, Lewin SR, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naive individuals in Thailand. Hepatology. 2008;48:1062–1069. doi: 10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 20.Matthews GV, Manzini P, Hu Z, et al. Impact of 3TC on HIV and HBV related outcomes in HIV/HBV individuals in a randomized clinical trial of antiretroviral therapy in South Africa. AIDS. 2011;29:29. doi: 10.1097/QAD.0b013e328349bbf3. [DOI] [PubMed] [Google Scholar]
- 21.Hamers RL, Zaaijer HL, Wallis CL, et al. HIV-HBV coinfection in Southern Africa and the effect of lamivudine- versus tenofovir-containing cART on HBV outcomes. J Acquir Immune Defic Syndr. 2013;64:174–182. doi: 10.1097/QAI.0b013e3182a60f7d. [DOI] [PubMed] [Google Scholar]
- 22.Gu L, Han Y, Li Y, et al. Emergence of Lamivudine-Resistant HBV during Antiretroviral Therapy Including Lamivudine for Patients Coinfected with HIV and HBV in China. PLoS One. 2015;10:e0134539. doi: 10.1371/journal.pone.0134539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevaliez S, Hezode C, Bahrami S, et al. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676–683. doi: 10.1016/j.jhep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Thibault V, Stitou H, Desire N, et al. Six-year follow-up of hepatitis B surface antigen concentrations in tenofovir disoproxil fumarate treated HIV-HBV-coinfected patients. Antivir Ther. 2011;16:199–205. doi: 10.3851/IMP1723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. HBV DNA suppression prior to, after 24 and 48 weeks of treatment. (A) Baseline HBV e antigen positive group; (B) baseline HBV e antigen negative group. P values were calculated using Chi square test or Fisher exact test.