Abstract
Purpose
In the era of increasingly complex surgical techniques for peripheral nerve repair, there is a need for high spatial resolution imaging of the neural plexuses in the body. We describe our experience with chemical shift encoded MRI and its implications for patient management.
Materials and methods
IDEAL water-fat separation is a chemical shift based method of homogeneously suppressing signal from fat, while maintaining adequate signal. This technique was used in clinical practice and the patient images reviewed.
Results
IDEAL water-fat separation was shown to improve visualization of the brachial and lumbosacral plexuses with good fat suppression and high signal to noise ratio.
Conclusion
IDEAL water − fat separation is an excellent technique to use in the imaging of the brachial and lumbosacral plexuses as it balances the need for homogeneous fat suppression with maintenance of excellent signal to noise ratio.
Keywords: Brachial plexus, Lumbosacral plexus, Neurography, IDEAL
1. Introduction
High quality imaging of the brachial and lumbosacral plexus is critical for accurate diagnosis of a variety of conditions that affect the peripheral nervous system, including trauma, neoplasm and inflammation. The anatomic complexity and the small size of the peripheral nerves necessitate high-resolution imaging, which is made more challenging by the need for homogeneous fat suppression, despite non-uniform body regions. Fat signal is bright on most pulse sequences and can therefore obscure pathology.
There are several options for fat suppression with magnetic resonance imaging (MRI). Traditional fat suppression methods using spectrally selective fat saturation pulses are widely available and relatively fast; they are performed as an adjunct and can be used both before and after contrast administration. Fat suppression sequences add minimal additional scan time but often suffer from inhomogeneous fat suppression, particularly in challenging locations such as the brachial plexus [1]. Susceptibility differences at tissue-air interfaces can result in areas of incomplete fat suppression and even water suppression. Short-tau inversion recovery (STIR) exploits difference in T1 recovery of fat from other tissues to suppress the fat signal. Using a non-selective inversion pulse, STIR can achieve very homogenous fat suppression, but does not maintain the same level of signal to noise ratio and is incompatible with post contrast imaging given the heavy T2 weighting and potential inadvertent suppression of enhancing tissue [2,3]. Water excitation pulses (spatial-spectral) have been explored but can be limited by areas of failure of fat suppression [4]. Chemical shift encoded MRI (CSE-MRI) methods take advantage of the known precessional difference between water and fat and, by measuring the local magnetic field inhomogeneity, can create very homogeneous separation of water and fat into individual images. Iterative Decomposition of water and fat using Echo Asymmetry and Least squares estimation (IDEAL) is a CSE-MRI method that has been developed to optimize signal while obtaining homogeneous fat suppression.
IDEAL has been used in many applications including musculoskeletal and body MRI [5–7]. We have successfully used IDEAL water-fat imaging as part of our routine brachial and lumbosacral plexus protocols to optimize anatomic visualization and detection of pathology.
2. Methods
2.1. IDEAL technique
IDEAL water-fat separation is an advanced chemical shift encoded MRI (CSE-MRI) method, related to 3-point Dixon water-fat suppression methods, but with optimized echo spacing and reconstruction algorithms [8–10]. CSE-MRI water-fat separation is based on the known 217 Hz (434 Hz) difference between the precession of hydrogen in water and fat at 1.5 T (3.0 T) (the chemical shift scales linearly with magnetic field strength). By measuring local magnetic field inhomogeneities, CSE-MRI thereby compensates for their effects on water-fat signal separation. Further, CSE-MRI methods are inherently insensitive to B1 inhomogeneity. With IDEAL, echoes are acquired asymmetrically with respect to the spin-echo and water and fat signals are separated using the IDEAL reconstruction algorithm to maximize noise performance [11]. IDEAL uses an iterative algorithm to estimate, and thereby remove the magnetic field inhomogeneity. This allows for demodulation of the effects of the field map to be divided out leaving a linear equation:
water (W) and fat (F) signals are subsequently separated through simple linear least squares estimation from the measured signals (s(t)) acquired at echo times (tn) relative to the spin-echo. Δf is the known frequency shift between water and fat signals (eg. 217 Hz at 1.5 T).
IDEAL has the advantage of allowing arbitrary echo times, which allows for optimized echo times that maximizes noise performance [12]. It facilitates a maximum effective number of signal averages of 3.0, although this requires acquisition of images at three different echo times. This would lead to a three-fold increase in scan time; however, there are several options to address this problem. Performing an IDEAL acquisition simultaneous acquires non-fat suppressed sequences (water + fat) as the water and fat images do not need to be separated and can be used as a traditional T1 FSE image. Multi-echo IDEAL can also be performed where all echoes are acquired in one TR; however, this can remove the advantage of allowing optimization of echo spacing. Further, IDEAL is compatible with homodyne reconstruction, which further reduces scan time through partial ky acquisitions. We routinely accelerate IDEAL with parallel imaging methods such as Autocalibrating Reconstruction for Cartesian imaging (ARC) [13]; IDEAL is an optimal choice to use with parallel imaging as the signal to noise ratio penalty of parallel MRI is offset by the high noise performance of IDEAL.
2.2. Plexus protocols
Our plexus studies are performed on a 3 T scanner (GE Healthcare, Discovery MR750). The brachial plexus protocol consists of coronal T1 FSE, sagittal T1 FLAIR, coronal T2 IDEAL, axial T1 FSE, axial T2 IDEAL and post contrast three plane T1 IDEAL sequences. Coil selection is based on patient body habitus and may include 8-channel neurovascular phased array coil, head neck coil or 8 channel cardiac coil. The fields of view are defined as follows:
Coronal—inferior to axilla from C3, covering from posterior spinous process through clavicle.
Sagittal—humeral head to contralateral neural foramina, covering from C2 to inferior axilla.
Axial—C5–T5 covering mid humeral head through spinal cord.
The lumbosacral plexus protocol consists of coronal SSFSE, sagittal SSFSE with fat suppression, sagittal T1 FLAIR, oblique coronal T1, axial T1, sagittal PD 3D-FSE (CUBE), oblique coronal T2 IDEAL and postcontrast three plane T1 IDEAL sequences. Coil selection is either 8 channel cardiac coil or 32 channel torso coil. The fields of view are as follows:
Coronal—L3 through greater trochanter; oblique coronal scans aligned parallel to the nerves.
Sagittal—L3 through pubic symphysis, including the bilateral acetabula; CUBE sequences are angled to individual side and parallel to nerves.
Axial—L4 through greater trochanters.
Contrast (gadobenate dimeglumine) is administered at a typical dose of 0.1 mmol/kg (maximum dose 20 mL) when needed. All plexus examinations are interpreted by fellowship trained neuroradiologists.
Fig. 1 demonstrates a normal lumbosacral plexus with excellent visualization of the sciatic nerve, as well as delineation of the internal architecture. With one acquisition, a routine T1 weighted fat suppressed image is obtained along with a water suppressed (fat) image and in and out of phase images.
Fig. 1.
41 year old male with normal anatomy. Coronal T1 IDEAL post contrast images of the lumbosacral plexus, specifically the sciatic nerves (arrows): A—fat only image clearly delineates the course of the sciatic nerves, as well as the internal architecture; B—water only image demonstrates no abnormal enhancement in the sciatic nerves; C (in-phase) and D (out-of-phase) images can be useful in evaluation of marrow abnormalities.
2.3. Patient demographics
A retrospective review of the picture archiving and communication system (PACS) was performed to identify patients that underwent either brachial or lumbosacral plexus imaging using the IDEAL technique. Patients that were included in this paper include those above 18 years of age with technically successful water-fat separation images. Patient symptomatology, age, gender and imaging findings were not used as inclusion or exclusion criteria, but are listed in Table 1. Of note, only one normal patient was included (Fig. 1) in order to demonstrate the appearance of the different acquired sequences.
Table 1.
Demographic information for patients included in this study. Pathology is reported, when available, with presumed diagnosis or imaging findings supplied where appropriate.
| Patient Age (years) | Gender | Clinical Indication | Imaging Findings/Pathology |
|---|---|---|---|
| 23 | Male | Flail arm after trauma | Pseudomeningocele formation with multilevel nerve root avulsions and hematoma compressing trunks |
| 44 | Male | Dropped head syndrome | Paraspinal muscle edema following radiofrequency rhizotomy |
| 35 | Male | Leg pain and weakness | Lymphadenopathy proven to be anaplastic lymphoma |
| 51 | Female | Palpable neck mass | Neurofibroma |
| 18 | Female | Right quadriceps atrophy | Perineuroma (presumed) |
| 37 | Female | Right arm radicular symptoms | Schwannoma |
| 56 | Male | Right groin and testicular pain | Malignant peripheral nerve sheath tumor |
| 72 | Male | Upper back and left scapular pain after trauma | High grade spindle cell malignancy, favor sarcomatoid carcinoma |
| 77 | Male | Low back pain extending down left leg with associated leg weakness | Initially, nonspecific enhancement felt to represent either radiation changes or perineural spread. At 2 year follow up, enhancement had progressed and androgen deprivation therapy was started with improvement of symptoms (presumed metastatic prostate cancer). |
| 41 | Male | Chronic low back pain, left buttock and leg pain | No imaging findings to explain the patient’s symptoms. Presumptively treated for piriformis syndrome. |
3. Results/patient cases
3.1. Trauma
Acute trauma to the brachial plexus can result in devastating and incapacitating injuries, particularly when the dominant arm is affected. Young male patients predominate [14] and are often involved in high velocity accidents. In the acute setting, differentiating continuity vs avulsion injury can be important, both prognostically and in terms of directing treatment. Injuries in continuity manifest as T2 hyperintensity and occasional enlargement of the nerve roots. These injuries are managed non-operatively initially and a good proportion of these patients recover function. In avulsion injuries, the radiologist can aid management by localizing the site of avulsion and measuring the distance between avulsed edges. Chances of spontaneous recovery of function following avulsion injuries approach zero and surgery is indicated in these cases.
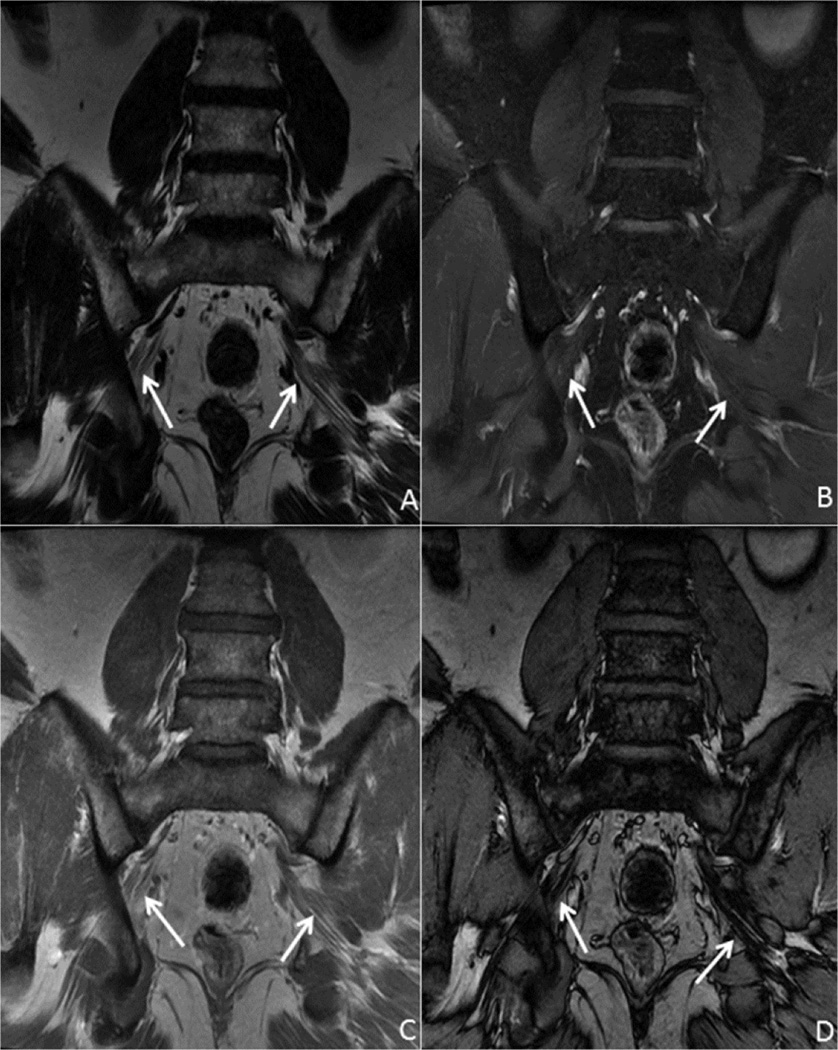
The imaging finding of pseudomeningocele formation implies the presence of nerve avulsion and 80% of patients with avulsions will develop pseudomeningoceles [15]. Fig. 2 demonstrates a young male patient who presented after sustaining an injury to the right arm and supraclavicular region from a falling tree branch. The patient had a flail arm and a right Horner’s syndrome and was found to have absent motor action potentials on nerve conduction studies, suggesting C5 through T1 nerve root avulsions. Imaging confirmed pseudomeningocele formation at C7, C8 and T1 with separation and compression of the trunks secondary to hematoma formation.
Fig. 2.
24 year old male with C6-T1 nerve root avulsions. Coronal T2 IDEAL images of the right brachial plexus from posterior (A) and anterior (B) demonstrate complex hematoma (arrow) associated with the trunks and divisions of the brachial plexus with pseudomeningocele formation at C8 and T1 (arrowheads). The nerve roots are retracted and present within the complex hematoma. Incidentally noted is a T2 hyperintense cystic lesion in the right paraspinal soft tissues adjacent the T2 and T3 vertebral bodies, most consistent with a congenital duplication cyst (asterisk).
The lumbosacral plexus is less susceptible to injury; however, can be involved, particularly in the setting of complex pelvic and sacral fractures.
3.2. Inflammatory
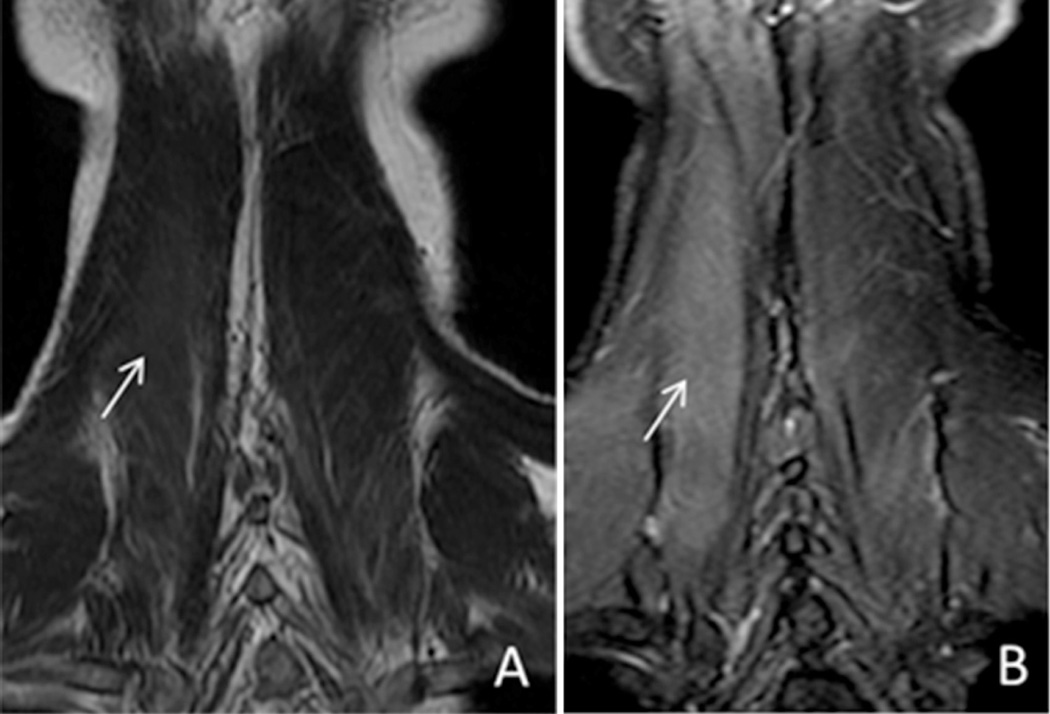
The brachial and lumbosacral plexuses can be involved in the setting of infectious or inflammatory conditions. In these cases, the homogeneous fat suppression is indispensable to accurately diagnose subtle enhancement of the nerves and associated musculature. Fig. 3 clearly delineates the importance of fat suppression in diagnosing paraspinal muscle edema in an older patient who presented with severe anterocollis provoked by a previous C3–C6 radiofrequency rhizotomy (performed for chronic cervicalgia). Without homogeneous fat suppression, the subtle T2 hyperintensity (edema) and enhancement within the right paraspinal musculature may easily have been overlooked.
Fig. 3.
44 year old male with dropped head syndrome. Coronal T2 FSE (A) and T2 IDEAL (B) images demonstrate improved visualization of edema within the right paraspinal musculature with the use of a fat suppression technique (arrows).
3.3. Compression
The brachial and lumbosacral plexuses are susceptible to compression from adjacent structures and pathology. In the setting of trauma, hematoma can lead to compression (as in Fig. 2). Given the close proximity of the plexus to adjacent vascular structures (subclavian artery for the brachial plexus and iliac arteries for the lumbosacral plexus), aneurysm should be considered, particularly if consideration is given to tissue sampling. Soft tissue tumors, including lipoma and soft tissue sarcomas, may cause compression; osseous compression, usually secondary to degenerative changes, is an important cause to exclude.
Fig. 4 demonstrates images from a patient with a history of anaplastic lymphoma presenting with progressive right leg weakness and pain radiating from the low back into the groin. Multiple enlarged lymph nodes were present along the course of the lumbosacral plexus with inflammatory changes present in the iliopsoas fossa, presumed secondary to lymphomatous involvement given the absence of infectious symptoms.
Fig. 4.
36 year old male with anaplastic lymphoma presenting with back and testicular pain. Coronal oblique IDEAL post-contrast water (A) and fat (B) images demonstrate multiple rounded soft tissue masses along the iliac vessels, consistent with lymphadenopathy (asterisks). Note the proximity of the sciatic nerve (arrow).
3.4. Neoplastic
Benign tumors are common causes for focal or diffuse enlargement of the nerve roots. Differentiation of subtypes of nerve tumors is difficult on imaging, but high quality imaging is imperative in surgical planning.
Fig. 5 contains images from a female patient that presented with a palpable deep neck mass, pathologically proven to be a neurofibroma. The classic imaging appearance of a neurofibroma is described as a “target sign” with peripheral T2 hyperintensity and central low to intermediate T2 signal. Neurofibromas can assume localized or diffuse morphologies and can be seen as part of Neurofibromatosis type 1, classically in a plexiform distribution. Neurofibromas arise from the nerve proper and are rarely encapsulated.
Fig. 5.
51 year old female with neurofibroma. Sagittal T2 IDEAL (A) and coronal (B) T1 IDEAL post-contrast administration images demonstrate a well circumscribed T2 hyperintense lesion with avid contrast enhancement, consistent with pathologically proven neurofibroma (arrows). The high signal external to the patient in the left supraclavicular region represents a saline bag intended to help to improve the fat suppression (labelled).
Perineuriomas may occur in both the intraneural and extraneural compartments. Intraneural perineuriomas tend to occur in young patients and reflects a benign neoplastic process (World Health Organization Grade I). Classic imaging findings consist of fusiform enlargement of the nerve with tapered ends and avid enhancement [16]. Fig. 6 contains images from a young woman that presented with weakness and atrophy of the right quadriceps. Electromyography findings included active denervation with marked decrease in right quadriceps recruitment and slightly decreased right iliopsoas recruitment without evidence of active denervation.
Fig. 6.
17 year old female with perineurioma. Coronal oblique T2 IDEAL (A) and 3D reconstructed (B) images demonstrate diffuse enlargement of the right femoral nerve, consistent with perineurioma (arrows).
Schwannomas have a true capsule composed of epineurium and are easily separated from the associated nerve at surgery. Schwannomas often demonstrate a higher rate of cystic degeneration and/or hemorrhage than other nerve sheath tumors. Fig. 7 demonstrates images obtained in a patient with persistent right radicular symptoms. Dedicated imaging of the brachial plexus demonstrated a mass associated with the C5 nerve root, which was pathologically proven to be schwannoma.
Fig. 7.
36 year old female with schwannoma. Coronal T2 IDEAL (A) and coronal T1 IDEAL following contrast administration (B) demonstrate a T2 heterogeneous, homogeneously enhancing well circumscribed mass associated with the C5 nerve root, consistent with schwannoma (arrows).
Malignant tumors can be found within the plexuses. Although rare, malignant peripheral nerve sheath tumors should be considered, particularly in patients with a known history of neurofibromatosis 1 [17,18], or a history of radiation. The risk of malignant transformation in neurofibromas ranges between 2.4–29% [19]. Clinical findings concerning for malignant degeneration include rapid enlargement, pain or new motor deficits. Correlative imaging findings include increased metabolic activity on PET imaging [20,21]. Fig. 8 delineates the findings in a patient who presented with right groin and testicular pain. Of note, the patient did have a history of prior right testicular seminoma treated with orchiectomy and radiation therapy. Imaging was performed, followed by an inconclusive biopsy, necessitating surgical resection. The final pathology was shown to be a malignant peripheral nerve sheath tumor of the posterior cutaneous nerve of the thigh in the region of the sciatic notch.
Fig. 8.
56 year old male with malignant peripheral nerve sheath tumor. Coronal T1 IDEAL post contrast water (A) and fat (B) images demonstrate heterogeneously enhancing mass (asterisk), shown to be a malignant peripheral nerve sheath tumor. Note that the mass abuts the sciatic nerve with preservation of normal internal architecture (arrows).
Metastatic disease can spread to the plexus via multiple routes: direct/contiguous spread, hematogenous spread or perineural spread. It is important to have a high index of suspicion to exclude metastatic involvement. The classic example of direct spread to the brachial plexus is via a superior sulcus (Pancoast) tumor. Fig. 9 contains images from a patient presenting with upper back pain radiating to below the left scapula following a history of trauma. Radiographs were obtained demonstrating a left apical lung mass with associated osseous destruction of the ribs. The patient went onto have both CT and MRI evaluation with tissue diagnosis of a high grade spindle cell malignancy, favoring sarcomatoid carcinoma.
Fig. 9.
72 year old male with left apical mass (Pancoast tumor). Coronal T1 IDEAL water (A) and fat (B) images demonstrate a large left apical mass with central necrosis (asterisk). There is effacement of the interscalene fat pad (arrow). The proximal nerve roots and trunks of the brachial plexus are preserved with normal architecture (arrowhead) on image B.
Radiation treatment can lead to immediate or delayed injury to the brachial plexus. Acute radiation injury is defined as within the 6 months immediately following radiation therapy, particularly with doses over 6000 cGray, and occurs secondary to vascular ischemia. This type of injury is often not reversible; however, delayed radiation injury (greater than 6 months following radiation therapy) can be a transient and reversible phenomenon [15]. Differentiating radiation changes from progressive or recurrent metastatic disease is predicated on the pattern of enhancement, but is often difficult. Radiation tends to present with diffuse fusiform enlargement of the nerve roots with T2 hyperintensity and enhancement, while metastatic involvement usually has nodular areas of enhancement.
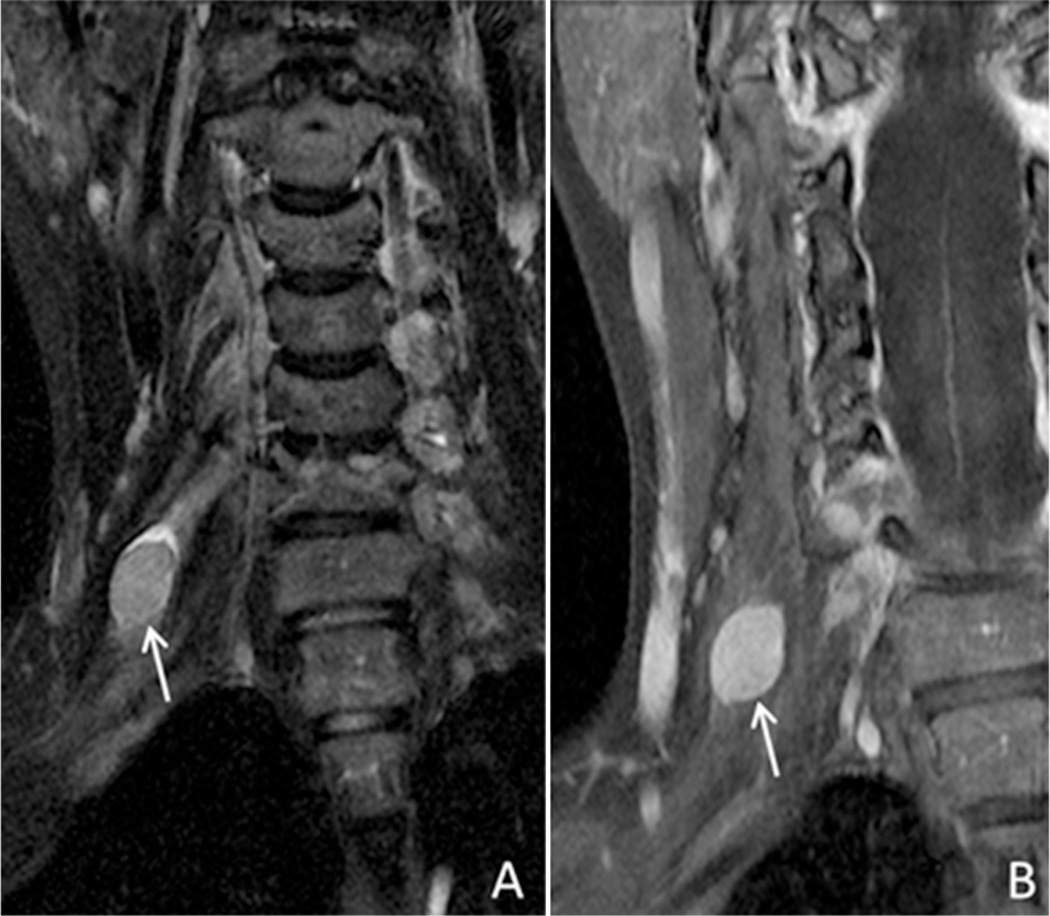
Differentiating radiation change from metastatic involvement is an unfortunate dilemma. Fig. 10 contains images from a patient with a history of prostate cancer who presented with worsening lumbosacral pain with extension along the posterior left leg and associated leg weakness. Imaging was performed and documented abnormal enhancement and configuration of the left S1 and S2 nerves with extension to the proximal left sciatic nerve and left gluteal nerves. There was associated atrophy of the left gluteal muscles. This was felt to represent either perineural spread of prostate cancer or delayed radiation plexitis. The patient’s prostate specific antigen (PSA) level was normal at this time and conservative management was undertaken. Two years later, the patient returned with persistent leg pain, but now the PSA level was 1.48 ng/mL (previously 0.1 ng/mL). Repeat imaging was performed with increased mass like enhancement of the S2 nerve root. Androgen deprivation therapy was initiated and there was an improvement in his PSA and clinical symptoms of leg pain and weakness.
Fig. 10.
79 year old male with prostate cancer. Coronal T1 IDEAL post contrast images water (A and B) and fat (C). Image A was obtained in 2011, when the patient was in biochemical remission. Images B and C were obtained in 2013, when the patient had a biochemical recurrence. Images demonstrate abnormal enhancement of the left S2 nerve root and proximal sciatic nerve (arrows) as well as marked architectural distortion of the left sciatic nerve (arrows image C), consistent with perineural spread of tumor.
4. Discussion
Imaging of the brachial and lumbosacral plexuses has traditionally been limited secondary to technical constraints, particularly the need for a large field of view and the difficulty of obtaining homogeneous fat suppression. The potential for both focal and diffuse pathologies necessitates a large field of view, which necessarily decreases SNR performance. However, given the complicated anatomy of the plexus, high anatomic resolution is necessary for diagnosis. Recent research has focused on methods to increase the water-fat separation by using anatomical mapping to improve the B0 field map estimation [22]. Creating more robust water fat separation would be advantageous in suppressing the high signal from fat, thus allowing the identification of subtle edema or enhancement. Additionally, there is growing interest in using the fat-only image to examine the microstructure of the larger nerves, which would also be aided by increased signal to noise.
Limitations of the IDEAL sequence are mostly secondary to the longer acquisition time; however, there is also a possibility of water-fat swapping [23] which are most common in areas of field inhomogeneity, such as those seen in the supraclavicular region. This can be compensated for by attempting to maintain the area of interest in the iosocenter of the magnet.
The advantage of using an IDEAL based protocol in conjunction with accelerated imaging techniques is the ability to obtain high quality images within clinically acceptable time parameters. Although an IDEAL sequence does take longer (approximately 5 min total scan time), it eliminates the need for T1 without and with fat saturation FSE sequences (which are approximately 2 and 3 min each respectively). There is also a balance of the longer time required for IDEAL with the decrease in time obtained by eliminating the magnetization preparation or selective excitation scans [23].
5. Conclusions
High quality imaging of the brachial and lumbosacral plexus is critical for identification of anatomy and pathology. Use of IDEAL sequences in combination with parallel imaging allows for robust fat suppression and adequate field of view with maintenance of high signal to noise ratio in clinically acceptable time parameters. Further directions include increased use of acceleration factors to shorten examination time as well as correlation with further advanced imaging techniques, including 3D volumetric acquisition and diffusion tensor imaging.
Acknowledgments
The authors want to thank GE Healthcare who provided research support to the University of Wisconsin.
Footnotes
Conflict of interest
No authors have a conflict of interest.
References
- 1.Reeder SB, Yu H, Johnson JW, Shimakawa A, et al. T1- and T2-weightedfast spin-echo imaging of the brachial plexus and cervical spine with IDEAL water-fat separation. J Magn Reson. Imaging. 2006;24:825–832. doi: 10.1002/jmri.20721. [DOI] [PubMed] [Google Scholar]
- 2.Krinsky G, Rofsky NM, Weinreb JC. Nonspecificy of short inversion time inversion recovery (STIR) as a technique of fat suppression: pitfalls in image interpretation. AJR. 1996;166:523–526. doi: 10.2214/ajr.166.3.8623620. [DOI] [PubMed] [Google Scholar]
- 3.Bydder GM, Hajnal JV, Young IR. MRI: use of the inversion recovery pulse sequence. Clin. Radiol. 1998;53:159–176. doi: 10.1016/s0009-9260(98)80096-2. [DOI] [PubMed] [Google Scholar]
- 4.Block W, Pauly J, Kerr A, Nishimura D. Consistent fat suppression with compensated spectral-Spatial pulses. Magn. Reson. Med. 1997;38:198–206. doi: 10.1002/mrm.1910380207. [DOI] [PubMed] [Google Scholar]
- 5.Fuller S, Reeder S, Shimakawa A, et al. Iterative decomposition of water and fat with echo asymmetry and least-Squares estimation (IDEAL) fast spin-Echo imaging of the ankle initial clinical experience. AJR. 2006;187(6):1442–1447. doi: 10.2214/AJR.05.0930. [DOI] [PubMed] [Google Scholar]
- 6.Hu HH, Kim H-W, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxe MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity. 2010;18(4):841–847. doi: 10.1038/oby.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa DN, Pedrosa I, McKenzie C, Reeder SB, Rofsky NM. Body MRI using IDEAL. AJR. 2008;190(4):1076–1084. doi: 10.2214/AJR.07.3182. [DOI] [PubMed] [Google Scholar]
- 8.Dixon W. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 9.Glover G. Multipoint dixon technique for water and fat proton and susceptibility imaging. J Magn Reson. Imaging. 1991;1:521–530. doi: 10.1002/jmri.1880010504. [DOI] [PubMed] [Google Scholar]
- 10.Glover GH, Schneider E. Three-point dixon technique for true water/fat decomposition with B0 in homogeneity correction. Magn. Reson. Med. 1991;18:371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Reeder SB, Shimakawa A, Brittain JH, Pelc NJ. Field map estimation with a region growing scheme for iterative 3-point water-fat decomposition. Magn. Reson. Med. 2005;54:1032–1039. doi: 10.1002/mrm.20654. [DOI] [PubMed] [Google Scholar]
- 12.Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn. Reson. Med. 2005;54:636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 13.Brau AC, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data-driven parallel imaging methods. Magn. Reson. Med. 2008 Feb 2;59:382–395. doi: 10.1002/mrm.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Midha R. Epidemiology of brachial plexus injuries in a multitrauma population. Neurosurgery. 1997;40(6):1182–1189. doi: 10.1097/00006123-199706000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Castillo M. Imaging anatomy of the brachial plexus. Am. J. Roentgenol. 2005;185:S196–S204. doi: 10.2214/AJR.05.1014. [DOI] [PubMed] [Google Scholar]
- 16.Nacey NC, Almira Suarez MI, Mandell JW, Anderson MW, Gaskin CM. Intraneural perineurioma of the sciatic nerve: an under-recognized nerve neoplasm with characteristic MRI findings. Skelet. Radiol. 2014;43:375–379. doi: 10.1007/s00256-013-1733-1. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Sohn SK, Ahn BC, et al. Sarcomatous transformation of neurofibromas. Clin. Nucl. Med. 1997;22:610–614. doi: 10.1097/00003072-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis. JAMA. 1997;278(1):51–57. [PubMed] [Google Scholar]
- 19.Coleman BG, Arger PH, Dalinka MK, Obringer AC, Raney BR, Meadows AT. CT of sarcomatous degeneration in neurofibromatosis. Am. J. Roentgenol. 1983;140:383–387. doi: 10.2214/ajr.140.2.383. [DOI] [PubMed] [Google Scholar]
- 20.Benz MR, Czernin J, Dry SM, et al. Quantitative F18-fluorodeoxyglucos positron emission tomography accurately characterizes peripheral nerve sheath tumors as malignant or benign. Cancer. 2010;116(2):451–458. doi: 10.1002/cncr.24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon SB, Dogan AS, Nicol TL, Campbell JN, Pomper MG. Positron emission tomography in the detection and management of sarcomatous transformation in neurofibromatosis. Clin. Nucl. Med. 2001;26(6):525–528. doi: 10.1097/00003072-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sharma SD, Artz NS, Hernando D, Horng DE, Reeder SB. Improving chemical shift encoded water-fat separation using object-based information of the magnetic field in homogeneity. Magn. Reson. Med. 2015;73:597–604. doi: 10.1002/mrm.25163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggers H, Bornert P. Chemical shift encoding-based water-fat separation techniques. J Magn Reson. Imaging. 2014;40:251–268. doi: 10.1002/jmri.24568. [DOI] [PubMed] [Google Scholar]