Abstract
Purpose
To compare sequential glaucoma drainage device (GDD) implantation with transscleral diode cyclophotocoagulation (CPC) following failure of a primary GDD.
Materials and Methods
Retrospective review of all patients who underwent GDD implantation at a single institution over ten years. Patients who required an additional GDD and/or CPC were analyzed. Success was defined as absence of: loss of light perception, reoperation for glaucoma, and IOP >21 or < 6 at two consecutive visits after an initial 3-month period.
Results
Thirty-two patients received sequential GDD. Twenty-one underwent CPC. Cohorts were statistically similar in regards to age, sex, race, and number of previous surgeries. Preoperatively, the GDD cohort had a lower IOP and better visual acuity. The mean length of follow-up was 37.9 months for the GDD group and 46.3 months for CPC. Both procedures significantly reduced IOP, however CPC led to a greater reduction (p=0.0172). Survival analysis found the 5-year probability of surgical success to be 65.3% for sequential GDD and 58.0% for CPC (p=0.8678). No cases of phthisis occurred in either group. There were 2 cases of endophthalmitis (6.3%) in the GDD group, and none in the CPC group. In eyes without pre-existing corneal edema, estimated corneal decompensation probability at 3-years was 31.6% for GDD and 6.7% for CPC (p=0.0828).
Conclusion
Sequential GDD and CPC are both effective at reducing IOP following the failure of a primary GDD. CPC after GDD failure warrants further investigation as it led to a greater reduction in IOP with fewer serious adverse events.
Keywords: Glaucoma Drainage Device, Transscleral Cyclophotocoagulation, Glaucoma Surgery
Background
Glaucoma drainage devices (GDD) are increasingly being used in the management of glaucoma1. Despite their efficacy, at times GDD fail to adequately control intraocular pressure (IOP). After five years, the Tube Versus Trabeculectomy Study reported treatment failure in 33% of the tube group2. In the Ahmed Versus Baerveldt Study, the cumulative probability of failure was 51% in the Ahmed group and 34% in the Baerveldt group at three years3.
In cases of primary GDD failure, treatment options include revision or replacement of the GDD, placement of a sequential GDD, or cyclodestruction4. Diode-laser transscleral cyclophotocoagulation (CPC) is one of the most commonly used procedures to ablate the ciliary body5 and decrease production of aqueous humor.
Several studies have found transscleral cyclophotocoagulation to be effective in controlling IOP5–9. CPC has the advantage of being a relatively quick procedure that can be performed in an office setting in eyes with conjunctival scarring from prior surgery. However, concerns about chronic hypotony, phthisis and vision loss have frequently relegated CPC to a treatment of last resort for eyes that fail medical and surgical interventions and those with limited visual potential6.
The management of a patient with uncontrolled IOP following implantation of a GDD is challenging. Little evidence is available in the literature to support one treatment modality over another. The purpose of this retrospective study is to compare the outcomes of sequential GDD implantation with transscleral diode CPC following failure of a primary drainage device.
Materials and Methods
A retrospective chart review was performed of the medical records of all adult patients who underwent GDD implantation at the Emory Eye Center between January 1, 2000 and December 31, 2010. The records pertaining to 702 primary GDD were obtained. Review of these records identified patients who later underwent implantation of a sequential GDD and/or CPC. Eyes that underwent implantation of a sequential GDD or CPC after removal of the primary GDD were not included for analysis. This study was approved by Emory University’s Institutional Review Board. Analyses include data beginning at each patient’s last visit prior to the intervention and continuing through September 1st, 2012. Baseline clinical data included age, sex, race, type of glaucoma, number of glaucoma medications, number of prior surgeries, and visual acuity (VA). Semi-quantitative visual acuities were converted to LogMAR acuities using values described previously in the literature10.
All procedures were performed at the Emory Eye Center in Atlanta, GA. The choice of procedure, GDD model, and CPC laser settings were made at the discretion of the surgeon.
Outcome data included both efficacy of IOP reductions and major complications. Success was defined as an absence of all of the following: IOP > 21 or < 6 at two consecutive visits after an initial 3-month period, reoperation for glaucoma, and/or loss of light perception. Reoperation for glaucoma included additional GDD surgery or CPC. IOP, vision and number of glaucoma medications were recorded at the last available clinic visit. In cases where reoperation for glaucoma was necessary, data collection was stopped at the time of the additional procedure.
GDD implants included Ahmed FP7 and S2 (New World Medical, Rancho Cucamonga, CA), Baerveldt-350 (Advanced Medical Optics, Santa Ana, CA), and Molteno-3 (IOP Inc, Costa Mesa, CA). The sequential GDD was placed into another quadrant with a reinforcing graft of donor cornea or sclera. The Iridex-G probe was used in conjunction with an Iridex 810nm SLx diode laser (Iridex, Mountain View, CA) to perform transscleral cyclophotocoagulation procedures. Typical CPC parameters at our institution include 1250–1450 milliwatts, 3500–4000 microseconds, and 180–270 degrees of treatment. CPC in this study included a median of 15 applications per treatment session (range 12–21).
Corneal decompensation was chosen as a complication of interest. Corneal status was noted preoperatively. Eyes were excluded from this secondary analysis if they were noted to have corneal failure or the presence of a corneal graft prior to undergoing a sequential GDD or CPC.
Baseline characteristics were compared between groups using chi-square tests for categorical variables and t-tests for continuous variables. Survival analysis was conducted using the Kaplan-Meier survival estimate and log-rank test.
Results
A total of 56 eyes were identified that met the inclusion criteria. Three eyes were excluded from analysis in the CPC group: one that underwent CPC on the first post-operative day following implantation of a primary GDD and two blind eyes in which CPC was performed for comfort care. Fifty-three eyes were included for analysis in this study; Thirty-two received sequential GDD and 21 underwent CPC.
Pre-operative clinical variables can be found in Table 1. There was no statistically significant difference in age, gender, or number of previous surgeries between the groups. The average number of prior incisional surgeries was 3.2 in the GDD group and 3.0 in the CPC group, reflecting the complexity of these cases.
Table 1.
Pre-operative clinical and demographic variables
| Pre-Op Variable | GDD (n=32) | CPC (n=21) | p-value† |
|---|---|---|---|
| Age (years) | 58.0 ± 13.1 | 62.7 ± 12.0 | 0.1927 |
| Gender – Male | 12 (37.5%) | 10 (47.6%) | 0.4646 |
| # Previous Surgeries | 3.2 ± 1.2 | 3.0 ± 1.7 | 0.6381 |
| IOP | 27.8 ± 8.5 | 33.2 ± 8.6 | 0.0275* |
| logMAR Visual Acuity | 0.95 ± 0.73 | 1.40 ± 0.81 | 0.0414* |
Chi-square test for categorical variables, t-test for continuous variables
For glaucoma diagnosis, the GDD group included 9 (28.1%) patients with chronic angle closure glaucoma, 8 (25.0%) with open angle glaucoma, 6 (18.8%) with neovascular glaucoma, 5 (15.6%) with inflammatory glaucoma, 2 (6.3%) with traumatic glaucoma, and 2 (6.3%) with a diagnosis of “other” which included mixed mechanism glaucoma. The CPC group included 6 (28.6%) with chronic angle closure glaucoma, 5 (23.8%) patients with open angle glaucoma, 5 (23.8%) with neovascular glaucoma, 1 (4.8%) with traumatic glaucoma, 1 (4.8%) with inflammatory glaucoma, and 3 (14.3%) with a diagnosis of “other”. In the sequential GDD group, choice of implant included Ahmed-S2 (15.6%), Ahmed-FP7 (62.5%) Baerveldt-350 (18.8%), and Moteno-3 (3.1%).
Significant differences were noted in pre-operative IOP and LogMAR VA. Eyes that were chosen to undergo CPC had on average higher IOP (33.2 vs. 27.8, p=0.0275) and worse LogMAR VA (1.40 vs. 0.95, Snellen equivalent 20/502 vs 20/178, p=0.0414). Both groups included several patients with Count Fingers or worse visual acuity at baseline (25.0% in the GDD group and 38.1% in the CPC group). Final visual acuity was 1.80 in the CPC group (Snellen 20/1262) and 1.18 (Snellen 20/302) for sequential GDD.
Mean length of follow up for the GDD and CPC groups was 37.9 months and 46.3 months, respectively. The survival probabilities for the GDD and CPC groups, respectively, were 80.3% vs. 85.0% at 1 year and 65.3% vs 58.0% at 5 years. Kaplan-Meier survival analysis (Figure 1) did not demonstrate a statistically significant difference in survival between groups (p=0.8678). In both groups, uncontrolled intraocular pressure was the most common reason for surgical failure. A list of the percentage of patients meeting each failure criterion is presented in Table 2. Univariate analysis of risk factors for surgical failure found only higher pre-operative IOP to be statistically significant (Table 3).
Figure 1.
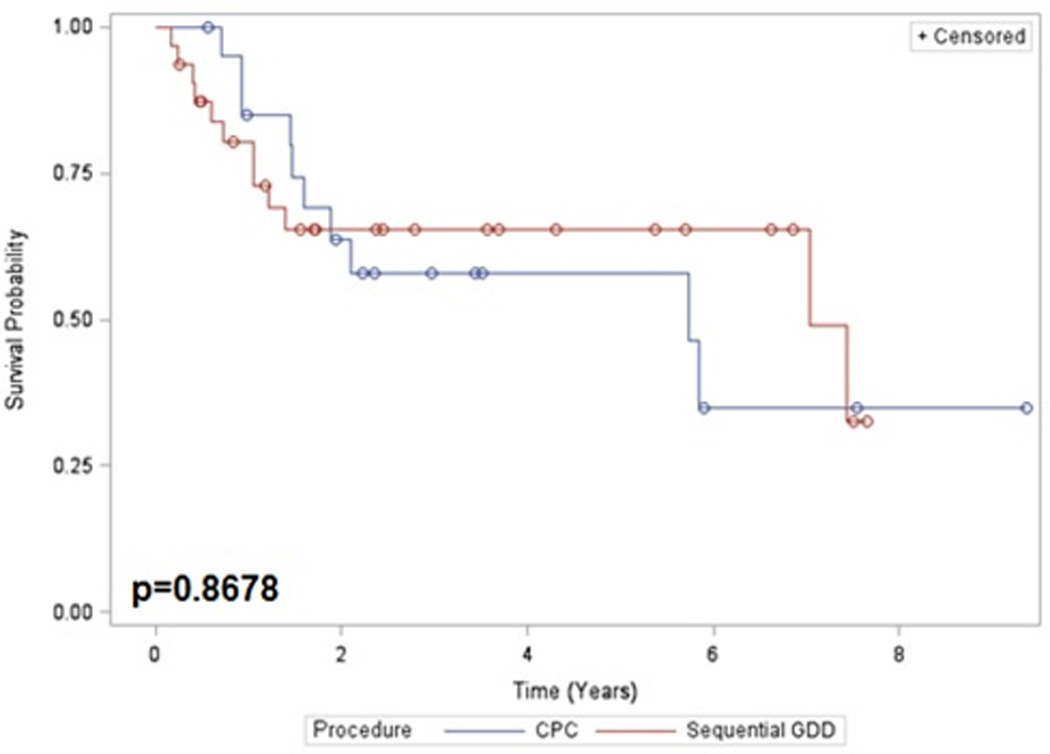
Kaplan-Meier survival curve: Sequential GDD versus CPC.
Table 2.
Reasons for surgical failure
| Outcome | GDD (n=32) | CPC (n=21) |
|---|---|---|
| Failure | 12 (37.5%) | 10 (47.6%) |
| IOP < 6 | 2 (6.3%) | 3 (14.3%) |
| IOP > 21 | 8 (25.0%) | 6 (28.6%) |
| Re-operation for Glaucoma | 5 (15.6%) | 3 (14.3%) |
| Loss of LP | 1 (3.1%) | 2 (9.5%) |
Percentages sum to greater than total failure percentage as several patients met multiple criteria for surgical failure
Table 3.
Univariate analysis of risk factors for surgical failure
| Pre-Op Variable | p-value† |
|---|---|
| Age | 0.4042 |
| Race | 0.7176 |
| Sex | 0.4184 |
| Procedure Choice | 0.8673 |
| # Previous Surgeries | 0.8094 |
| Pre-op IOP | 0.0199* |
| Pre-op VA | 0.4388 |
conducted with Wald χ2 Tests
Both procedures were effective in reducing intraocular pressure (Table 4). IOP was recorded at the pre-operative and last follow up exams. IOP decreased on average 40.7% (27.8 vs 16.5 mmHg, p<0.0001) in the GDD group and 56.3% (33.2 vs 14.5 mmHg, p<0.0001) in the CPC group. CPC led to a greater reduction in IOP than sequential GDD (p=0.0172). Both groups required fewer ocular hypotensive agents at the final follow up visit compared to pre-operatively.
Table 4.
Intraocular Pressure Management
| Variable | GDD | CPC | p-value† |
|---|---|---|---|
| IOP | |||
| Pre-op | 27.8 ± 8.5 | 33.2 ± 8.6 | 0.0172* |
| Final | 16.5 ± 8.7 | 14.5 ± 8.5 | |
| Reduction | 11.3 ± 9.8 | 18.7 ± 12.0 | |
| % Reduction* | 40.7% (p < 0.0001) | 56.3% (p < 0.0001) | |
| Glaucoma Meds | |||
| Pre-op | 3.88 ± 0.98 | 3.57 ± 1.03 | 0.3906 |
| Final | 2.56 ± 1.32 | 2.62 ± 1.60 | |
| Reduction | 1.31 ± 1.31 (p < 0.0001) | 0.95 ± 1.72 (p=0.0194) | |
Tests for the difference in IOP reduction between treatment groups.
Test for difference in pre-operative and final IOP within the same treatment group
T-tests were used for continuous variables; a Cochran-Armitage Trend Test was used for the ordinal IOP Category variable
Post-operative complications are listed in Table 5. Special interest was paid to the development of corneal decompensation. Eyes with corneal grafts or pre-operative corneal decompensation were excluded from this analysis. Corneal decompensation occurred in 19.2% (5/26) of the GDD group and 5.6% (1/18) of the CPC group. Kaplan-Meier estimates of the probability of corneal failure at 3-years were 31.6% and 6.7% in the GDD and CPC groups, respectively (p=0.0828, Figure 2). Also of note, there were 2 cases of endophthalmitis (6.3%) in the GDD group and none in the CPC group.
Table 5.
Surgical Complications
| Complication | GDD | CPC |
|---|---|---|
| Shallow Anterior Chamber | 4 (12.5%) | 1 (4.8%) |
| Serous Choroidal Detachment | 4 (12.5%) | 3 (14.3%) |
| Hemorrhagic Choroidal Detachment | 0 (0.0%) | 0 (0.0%) |
| Phthisis | 0 (0.0%) | 0 (0.0%) |
| Re-operation for Glaucoma | 5 (15.6%) | 3 (14.3%) |
| Loss of LP | 1 (3.1%) | 2 (9.5%) |
| Endophthalmitis | 2 (6.3%) | 0 (0.0%) |
| Enucleation | 1 (3.1%) | 0 (0.0%) |
| GDD Tube Occlusion | 3 (9.4%) | N/A |
| GDD Exposure | 3 (9.4%) | N/A |
Figure 2.
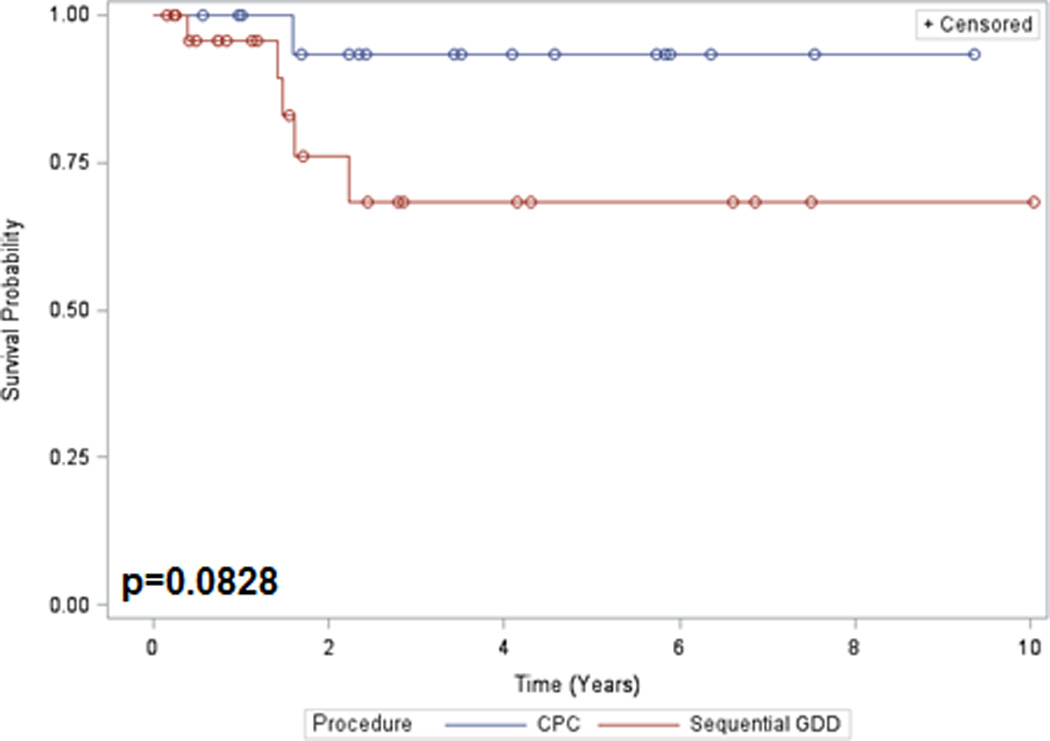
Kaplan-Meier survival curve. Corneal survival following Sequential GDD versus CPC
Discussion
This study retrospectively reviewed the surgical outcomes of sequential GDD versus transscleral CPC following failure of a primary drainage device. Both procedures were found to be effective in lowering intraocular pressure and decreasing ocular hypotensive medications. No statistical difference was found in surgical success rates between groups. CPC resulted in a greater reduction in IOP, though this was influenced by a higher pre-operative IOP in the CPC group.
Published data regarding surgical outcomes for sequential GDD are limited to the results of small, retrospective studies11–14. These studies found sequential GDD to be a viable option for controlling IOP with surgical success rates ranging from 37–83%. Complications of GDD include overfiltration with hypotony, corneal decompensation, exposure of the device through the conjunctiva, endophthalmitis, and failure to adequately control IOP15.
Semchyshyn et al. retrospectively studied the outcomes of CPC following failure of a primary GDD in adults and children. CPC significantly reduced IOP and mean number of glaucoma medications16. Later studies by Panarelli et al.17 and Ness et al18 also found transscleral CPC to be an effective treatment following shunt failure.
Only one study could be identified that directly compared the outcomes of sequential GDD and CPC following failure of a primary drainage device. Sood and Beck4 retrospectively reviewed outcomes in a small case series with pediatric glaucoma. Successful outcomes were achieved in 62.5% of patients who underwent implantation of a sequential GDD and 66.7% of those receiving CPC at 24 months.
The most serious complications with CPC are phthisis and loss of light perception. It is notable that no cases of phthisis occurred in either treatment group during the study period. Loss of light perception occurred in one eye that underwent sequential GDD and two eyes that underwent CPC. All three patients that lost light perception in this study had Count Fingers or worse vision pre-operatively, and no cases of loss of light perception occurred in the immediate post-operative period.
Exposure of a GDD through the conjunctiva has been shown to be a risk factor for endophthalmitis19. GDD exposure was noted in three cases in our series including two cases associated with endophthalmitis. No cases of endophthalmitis occurred in the CPC group, and no primary GDD exposed following CPC. Another well-known complication of GDD implantation is the loss of corneal endothelial cells resulting in corneal edema20. The incidence of corneal edema following sequential GDD has been reported to be as high as 43%13. The rate of corneal decompensation in our study was higher in the GDD group, though this difference did not reach statistical significance (p=0.0828) likely due to the limited statistical power of the study.
This study has several limitations, most notably its retrospective nature and small sample size. Data was collected from multiple surgeons without standardization of implant model or laser parameters.
CPC has traditionally been used a treatment of last resort in refractory glaucoma, and that has been the practice at our center as well. It is, therefore, not surprising that the patients chosen to undergo CPC in this study had higher pre-operative IOP and worse pre-operative visual acuity on average. This selection bias is significant in the interpretation of our results as higher pre-operative IOP was found to be a predictor of surgical failure. It is possible that the success rate in the CPC group would have been improved if performed on a group similar to those that underwent sequential GDD implantation. Other studies have shown favorable results when CPC has been used as a primary glaucoma treatment in eyes with preserved visual potential7,8,21. However, it is also possible that more vision loss would have been noted in the CPC group had the preoperative acuity been better, as cystoid macular edema is a well-known risk of CPC.
In comparing rates of corneal decompensation between treatment groups, eyes with pre-existing corneal edema or corneal grafts were excluded from analysis. Measurement of pre- and post-operative endothelial cell counts in a prospective study would be helpful to control for pre-operative differences between groups and more accurately assess the relative risk of corneal decompensation for each intervention.
In summary, both sequential GDD and transscleral CPC are effective in reducing IOP following failure of a primary drainage device. CPC after primary GDD failure warrants further investigation for use in eyes with preserved central vision as it may provide similar IOP-lowering effects with a lower risk of serious adverse events.
Acknowledgements
Supported by NIH Departmental Core Grant EY006360 and Research to Prevent Blindness, Inc, New York, New York. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest
References
- 1.Ramulu PY, Corcoran KJ, Corcoran SL, et al. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114:2265–2270. doi: 10.1016/j.ophtha.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christakis PG, Tsai JC, Kalenak JW, et al. The Ahmed versus Baerveldt study: three-year treatment outcomes. Ophthalmology. 2013;120:2232–2240. doi: 10.1016/j.ophtha.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma. J AAPOS. 2009;13:379–383. doi: 10.1016/j.jaapos.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mistlberger A, Liebmann JM, Tschiderer H, et al. Diode laser transscleral cyclophotocoagulation for refractory glaucoma. J Glaucoma. 2001;10:288–293. doi: 10.1097/00061198-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Ishida K. Update on results and complications of cyclophotocoagulation. Curr Opin Ophthalmol. 2013;24:102–110. doi: 10.1097/ICU.0b013e32835d9335. [DOI] [PubMed] [Google Scholar]
- 7.Egbert PR, Fiadoyor S, Budenz DL. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Arch Ophthalmol. 2001;119:345–350. doi: 10.1001/archopht.119.3.345. [DOI] [PubMed] [Google Scholar]
- 8.Lai JS, Tham CC, Chan JC, et al. Diode laser transscleral cyclophotocoagulation as primary surgical treatment for medically uncontrolled chronic angle closure glaucoma: long-term clinical outcomes. J Glaucoma. 2005;14:114–119. doi: 10.1097/01.ijg.0000151890.41239.c5. [DOI] [PubMed] [Google Scholar]
- 9.Schlote T, Derse M, Rassmann K. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma. 2001;10:294–301. doi: 10.1097/00061198-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lange C, et al. "Resolving the clinical acuity categories "hand motion" and "counting fingers" using the Freiburg Visual Acuity Test (FrACT) Graefes Arch Clin Exp Ophthalmol. 2009;247:137–142. doi: 10.1007/s00417-008-0926-0. [DOI] [PubMed] [Google Scholar]
- 11.Burgoyne JK, WuDunn D, Lakhani V, et al. Outcomes of sequential tube shunts in complicated glaucoma. Ophthalmology. 2000;107:309–314. doi: 10.1016/s0161-6420(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey DG, Krishna R, Greenfield DS, et al. Implantation of second glaucoma drainage devices after failure of primary devices. Ophthalmic Surg Lasers. 2002;33:37–43. [PubMed] [Google Scholar]
- 13.Shah AA, WuDunn D, Cantor LB. Shunt revision versus additional tube shunt implantation after failed tube shunt surgery in refractory glaucoma. Am J Ophthalmol. 2000;129:455–460. doi: 10.1016/s0002-9394(99)00410-9. [DOI] [PubMed] [Google Scholar]
- 14.Anand A, Tello C, Sidoti PA, et al. Sequential glaucoma implants in refractory glaucoma. Am J Ophthalmol. 2010;149:95–101. doi: 10.1016/j.ajo.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Sarkisian SR., Jr Tube shunt complications and their prevention. Curr Opin Ophthalmol. 2009;20:126–130. doi: 10.1097/ICU.0b013e328323d519. [DOI] [PubMed] [Google Scholar]
- 16.Semchyshyn TM, Tsai JC, Joos KM. Supplemental transscleral diode laser cyclophotocoagulation after aqueous shunt placement in refractory glaucoma. Ophthalmology. 2002;109:1078–1084. doi: 10.1016/s0161-6420(02)01019-9. [DOI] [PubMed] [Google Scholar]
- 17.Panarelli JF, Bannitt MR, Sidoti PA. Transscleral Diode Laser Cyclophotocoagulation After Baerveldt Glaucoma Implant Surgery. J Glaucoma. 2014;23:405–409. doi: 10.1097/IJG.0b013e318279c957. [DOI] [PubMed] [Google Scholar]
- 18.Ness PJ, Khaimi MA, Feldman RM, et al. Intermediate term safety and efficacy of transscleral cyclophotocoagulation after tube shunt failure. J Glaucoma. 2012;21:83–88. doi: 10.1097/IJG.0b013e31820bd1ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Torbak AA, Al-Shahwan S, Al-Jadaan I, et al. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2004;89:454–458. doi: 10.1136/bjo.2004.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hau S, Barton K. Corneal complications of glaucoma surgery. Curr Opin Ophthalmol. 2009;20:131–136. doi: 10.1097/ICU.0b013e328325a54b. [DOI] [PubMed] [Google Scholar]
- 21.Rotchford AP, Jayasawal R, Madhusudhan S. Transscleral diode laser cycloablation in patients with good vision. Br J Ophthalmol. 2010;94:1180–1183. doi: 10.1136/bjo.2008.145565. [DOI] [PubMed] [Google Scholar]


