Abstract
Approximately 1 in 6 infants are born prematurely each year. Typically, these infants spend 25 days in the Neonatal Intensive Care Unit (NICU) where they experience 10–18 painful and inflammatory procedures each day. Remarkably, pre-emptive analgesics and/or anesthesia are administered less than 25% of the time. Unalleviated pain during the perinatal period is associated with permanent decreases in pain sensitivity, blunted cortisol responses and high rates of neuropsychiatric disorders. To date, the mechanism(s) by which these long-term changes in stress and pain behavior occur, and whether such alterations can be prevented by appropriate analgesia at the time of insult, remains unclear. Work in our lab using a rodent model of early life pain suggests that inflammatory pain experienced on the day of birth blunts adult responses to stress- and pain-provoking stimuli, and dysregulates the hypothalamic pituitary adrenal (HPA) axis in part through a permanent upregulation in central endogenous opioid tone. This review focuses on the long-term impact of neonatal inflammatory pain on adult anxiety- and stress-related responses, and underlying neuroanatomical changes in the context of endogenous pain control and the HPA axis. These two systems are in a state of exaggerated developmental plasticity early in postnatal life, and work in concert to respond to noxious or aversive stimuli. We present empirical evidence from animal and clinical studies, and discuss historical perspectives underlying the lack of analgesia/anesthetic use for early life pain in the modern NICU.
Keywords: Hypothalamic pituitary adrenal axis, Amygdala, Periaqueductal gray, Corticosterone, Glucocorticoid receptor, Corticotrophin releasing factor receptors, Endogenous opioids, Enkephalin, Endorphin, Morphine
1.1 Premature Birth And Pain Treatment In The Neonatal Intensive Care Unit
Premature Birth
Each year, 18% of infants worldwide and 11.4% of infants in the United States are born prior to 37 weeks gestation and are considered preterm (Martin et al., 2015; WHO, 2012). The etiologies underlying preterm birth are complex and not completely understood, but risk factors include maternal diabetes, hypertension, smoking, prenatal substance use, lack of prenatal care and assisted reproductive therapies (PeriStats, 2011).
Following birth, the majority of premature infants are admitted into the Neonatal Intensive Care Unit (NICU), where they spend an average of 25 days (PeriStats, 2011). During their stay in the NICU, preterm infants undergo 10–18 invasive procedures each day, including repeated heel lance, endotracheal intubation, surgery, and respiratory and gastric suctioning, (Barker and Rutter, 1995; Carbajal et al., 2008; PeriStats, 2011; Simons et al., 2003). Despite the fact that the majority of these procedures produce pain and inflammation, pre- and/or post-emptive analgesia or anesthesia is used in only 2–21% of invasive procedures performed in the NICU (Carbajal et al., 2008). As the perinatal period is a time of sensitive developmental plasticity, any perturbation during this period presents a risk for immediate and long-term negative consequences (Anand et al., 1987b; Carbajal et al., 2008; McGrath, 2011; Rodkey and Pillai Riddell, 2013).
Modern Absence Of Pain Treatment Early In Life And Historical Origins
The rationale for withholding pain treatment is complex and multifaceted. Historically, infants and children were considered lower ‘castes’ of people, unable to engage in sensory processing beyond the brainstem and thalamus, and lacking the cognitive capacity to remember early life events (Rodkey and Pillai Riddell, 2013). With this assumption, investigations of the 19th and 20th centuries interpreted infant responses to surgery, pin-pricks or electric shock as reflexive or non-specific to the procedures (Rodkey and Pillai Riddell, 2013). These perspectives guided medical training and practices, and by the 21st century, respiratory support and paralytics were deemed sufficient for preterm infants undergoing surgery (Wesson, 1982). The validity and ethics of such practices were strongly called into question in the 1980s (Anand et al., 1987a; Purcell-Jones et al., 1988). However, issues of reliable pain assessment, age-appropriate dosing, opioid tolerance and long-term consequences associated with pharmacologic intervention became points of concern that continue to hinder neonatal therapies for pain (Anand, 2000; Anand et al., 2005a; Anand and Hickey, 1987; Anand et al., 2005b; Anand et al., 1987a; Bellieni, 2012; Cignacco et al., 2009; Qiu, 2006).
Evidence over the last 30 years has demonstrated that premature and term infants can indeed discriminate noxious stimuli. NICU procedures induce robust secretion of stress hormones (Anand et al., 1987b), elevated heart rate and facial reactivity (Grunau et al., 2005; Grunau et al., 2010). Further, preterm infants as young as 25 gestational weeks display evoked cortical activity in response to noxious stimulation (Bartocci et al., 2006; Slater et al., 2006). While insufficient and sporadic administration of pain therapy persists in the modern NICU (Carbajal et al., 2008), the vast majority of modern pediatric physicians acknowledge that preterm infants feel pain (Purcell-Jones et al., 1988).
Efficacy Of Neonatal Analgesia
A number of clinical studies have demonstrated the benefit of acute opioid analgesia for infants undergoing invasive procedures in the NICU. For example, administration of opioid analgesics significantly decreases cortisol, norepinephrine, epinephrine and β-endorphin release, decreases sepsis and prevents death, both operatively and post-operatively in comparison to controls (Anand and Hickey, 1992; Anand et al., 1987b). Morphine administration before endotracheal suctioning, central venous catheterization, or heel lance reduces blood flow to the skin and decreases facial responses to procedural pain (McCulloch et al., 1995; Moustogiannis et al., 1996; Scott et al., 1999). These studies together suggest that specific and appropriate analgesia has immediate antinociceptive benefits for preterm infants.
Despite the effectiveness of opioid analgesics for infant pain, concerns of tolerance, dependence and side effects such as bradycardia, hypotension, apnea, urinary retention and reduced gastrointestinal motility (Anand et al., 2011; Walker, 2014) make their use controversial. A number of studies have aimed to test the long-term impact of opioid analgesia in the NICU. However, the majority of these efforts have been challenged by small sample sizes, inclusion of infants with illnesses such as hypotension or pre-existing neurological impairment, or dosing that is age-inappropriate for the infant (Anand et al., 2004; Bouwmeester et al., 2001; de Graaf et al., 2011; MacGregor et al., 1998; Norman et al., 2013; Roze et al., 2008). Therefore, it is unclear what, if any, long-term consequences are associated with early life opioid analgesia. As a precaution, the International Association for the Study of Pain (IASP) currently recommends judicious use of morphine and its derivatives, and slow-speed infusions of fentanyl and other potent synthetic opioids, for premature and term infants experiencing moderate to severe procedural pain, surgical pain and post-operative care (Anand et al., 2011). In combination with opioids or alone, topical nerve block agents, acetaminophen, NSAIDs, ketamine or dexmedetomidine can also be used, while nonpharmacologic (e.g. massage, suckling, oral sweeteners) interventions are appropriate for acute or ongoing pain (Hall and Anand, 2014). While caution with opioids is prudent, it is important to note recent follow-up studies reported that former preterm infants at 8–9 years old who received morphine in the NICU for pain management had improved executive functioning, reduced externalization problems and no adverse effects on somatosensory perception, pain thresholds, incidence of chronic pain or neurological functioning relative to infants that received placebo (de Graaf et al., 2013; Valkenburg et al., 2015).
1.2 Early-Life Pain Impairs Stress And Pain Responding
Clinical Studies
Clinical studies show early life pain has an immediate impact on response to stress- and pain-evoking stimuli. For example, preterm infants undergoing surgical procedures without analgesic treatment have significantly higher concentrations of catecholamines and cortisol during and after surgery as compared with infants receiving analgesia (Anand et al., 1987b). Heart rate, facial reactivity and cortisol levels of preterm infants are initially high in response to procedural pain. However, these behavioral, neuroendocrine and autonomic responses become significantly blunted as the number of invasive procedures experienced in the NICU increases (Grunau et al., 2005; Grunau et al., 2010). This suggests that repeated, unalleviated pain results in immediate changes or ‘adaptations’ in systems mediating pain and stress that may become permanent.
At 4 and 8 months, former preterm infants display decreased facial responsiveness to immunization pain in comparison to full-term peers (Oberlander et al., 2000). Toddlers born prematurely into the NICU exhibit blunted nociceptive responses and are rated by parents as less sensitive to pain in comparison to term-born controls (Grunau et al., 1994a). At 9–12 years of age, children that experienced infant cardiac surgery with limited pain therapy display global alterations in both mechanical and thermal somatosensory processing (Schmelzle-Lubiecki et al., 2007). Further, male and female adolescents that spent time in the NICU as infants are less sensitive to thermal pain (Hermann et al., 2006; Walker et al., 2009b) and display attenuated stress-induced analgesia in comparison to controls (Wollgarten-Hadamek et al., 2011).
A number of studies have now associated NICU treatment with long-term changes in autonomic and cortisol reactivity. For example, basal and immunization pain-induced cortisol release is blunted for boys and girls at 3 months, and boys at 4 months, respectively, in former preterm infants as compared with term controls (Grunau et al., 2007; Grunau et al., 2010). At 6 months, tighter coordination of stress-related cortisol, heart rate and vagal tone is observed, suggesting an altered autonomic response pattern relative to term peers (Haley et al., 2010). And by 7 years, greater neonatal procedural pain predicts lower levels of acute, cumulative and diurnal cortisol relative to term peers, (emphasized in boys) (Brummelte et al., 2015; Grunau et al., 2013). Increased rates of behavior internalization have also been reported (Ranger et al., 2014). As physiological changes in stress responding are associated with disorders of anxiety, depression, obessive compulsion, panic and post-traumatic stress (Chrousos, 2009; Heim et al., 2000; Heim et al., 2001), such findings indicate that preterm infants are at higher risk for developing later-life changes in affective functioning, and potentially in a sex-dependent manner.
Indeed, altered cortisol reactivity for former preterm infants at age five is significantly associated with issues of internalization, emotional reactivity, anxiety, depression, inattention, and higher rates of negative verbalization during mother-child interactions similarly for boys and girls (Bagner et al., 2010). At age 7, blunted cortisol reactivity predicts attention problems and maternal years of education in both sexes as well (Brummelte et al., 2015). By middle school, these children are at least 28% more likely than term peers to have clinical symptoms of anxiety (exaggerated in girls), depression, and inattention (exaggerated in boys) (Botting et al., 1997; Hayes and Sharif, 2009). Psychological social-stress testing evokes blunted cortisol responses similarly in 8–14 yr old male and female former preterm infants relative to age- and gender-matched term controls (Buske-Kirschbaum et al., 2007). Lastly, parents and teachers of former NICU patients report significantly higher rates of neurobehavioral and neuropsychiatric impairments at 20 years of age relative to term peers, including issues with internalization and externalization, reduced cognitive and behavioral flexibility, and higher rates of anxiety and depression (Aarnoudse-Moens et al., 2009; Hack et al., 2004; Hayes and Sharif, 2009; Sullivan et al., 2012).
Early life pain is also associated with changes in brain development and later-life functioning. For example, magnetic resonance imaging spectroscopy and diffusion tensor imaging of preterm infants at 32 and 40 gestational weeks shows significant decreases in white and gray matter maturation for both boys and girls that is positively correlated with the number of skin breaking procedures experienced (Brummelte et al., 2012). Pain-related stress in the NICU is associated with reduced spontaneous cortical gamma-to-alpha ratio oscillations during perceptual reasoning in childhood at 7–8 yrs of age that is independent of illness severity, days on mechanical ventilation, cumulative morphine exposure, general intelligence, and sex (Doesburg et al., 2013). Notably, gamma and alpha band activity are thought to occur during active recruitment of brain regions for perception (Doesburg et al., 2008; Jensen et al., 2007) and resting-state (Doesburg et al., 2013; Klimesch et al., 2007; Pfurtscheller et al., 1996), respectively. As alterations in gamma band oscillations are significantly correlated with neurological and psychiatric disorders (Uhlhaas and Singer, 2006), these findings further suggest that early life pain represents a significant risk factor for later-life pathology.
Animal Studies
Animal models of early life pain support clinical findings demonstrating a long-term impact on subsequent responses to pain and stress (Schwaller and Fitzgerald, 2014). Acute or repeated exposure to early life pain induced by foot shock, surgery, or inflammatory agents results in general thermal or mechanical hypoalgesia (i.e. decreased pain sensitivity) in adult rodents (Bhutta et al., 2001; LaPrairie and Murphy, 2007, 2009; Shimada et al., 1990; Sternberg et al., 2005). Consistent with clinical findings showing blunted stress hormones in former preterm infants, early life inflammatory pain in male rats reduces adult release of the stress hormones corticotrophin releasing factor (CRF), arginine vasopressin (VAS), and adrenocorticotrophin releasing hormone (ACTH) following acute swim stress (Anseloni et al., 2005). Anatomically, early life pain results in cortical thinning and increases the number of apoptotic cells throughout the brain, specifically in the cortex, septum, hypothalamus and hippocampus. Reduced expression of cortical and thalamic proteins that control neuronal differentiation, migration and synaptic connections have also been reported (Duhrsen et al., 2013). Notably, administration of morphine at the time of early life injury prevents long-term reductions in pain sensitivity and alterations in brain development (Duhrsen et al., 2013; LaPrairie et al., 2008). While the mechanisms underlying these long-term changes in pain and stress responding and brain development are not well understood, recent evidence suggests that decreased sensitivity to pain later in life results from upregulated endogenous opioid tone in key brain regions mediating pain (LaPrairie and Murphy, 2009). As endogenous opioids have organizational effects on the developing brain (Zagon and McLaughlin, 1983, 1991) and contribute to the perception of stress (Akil et al., 1984), it is possible that the injury-induced increase in opioid tone underlies the long-term reduction in stress sensitivity as well.
1.3 The Stress System Early In Life
The hypothalamic-pituitary-adrenal (HPA) axis is used by the nervous system to mount physiological responses to stressors, promote survival in the presence of physical threats, and mediate psychological perturbations successfully, with the ultimate goal of reinstating homeostasis. In response to stressors, a variety of forebrain and brainstem regions are recruited to stimulate the release of CRF from the paraventricular nucleus of the hypothalamus (PVN) (Vale et al., 1981). Through the hypophyseal portal system, CRF stimulates the anterior pituitary gland to release ACTH (Dallman et al., 1987). In turn, ACTH acts on the cortex of the adrenal gland to promote release of glucocorticoids (cortisol in humans; corticosterone in rats: CORT)(Dallman and Jones, 1973; Guillemin et al., 1977). CORT then feeds up to the pituitary and PVN to terminate further release of neurohormones, and to the hippocampus where CORT binding to the glucocorticoid receptor (GR) reinstates inhibition of the PVN (Dallman et al., 1987; Sapolsky et al., 1984; Ulrich-Lai and Herman, 2009).
All components of the HPA axis are functional and interfaced by mid-gestation for both humans and rodents (Kandel, 2000). In rodents, stressors such as ether or laparotomy stimulate the release of fetal CRF (Hiroshige and Sato, 1971) and CORT (Negellen-Perchellet and Cohen, 1975), indicating that stress activates the HPA axis during gestation. At birth and over the first postnatal week, adrenal gland weight and circulating CORT concentrations are elevated (Corbier and Roffi, 1978), but gradually decrease to barely detectable levels, resulting in what is referred to as the stress hypo-responsive period (SHRP). The SHRP spans approximately P2-P14 in rat pups (Sapolsky and Meaney, 1986; Walker et al., 1986); for humans a similar process occurs over the first few months of life (Grunau et al., 2007; Mantagos et al., 1998). Although stressors such as ether, electric shock or hypoxia can activate the HPA axis, this period of adrenal quiescence allows glucocorticoid levels to remain low and promote neurogenesis, axonal outgrowth, synaptogenesis, myelination, and rise of endogenous CRF and ACTH levels (Baud et al., 2005; Sapolsky and Meaney, 1986; Walker et al., 1986) (Antonow-Schlorke et al., 2009; Du et al., 2009; Liston and Gan, 2011).
HPA axis dysfunction and the manifestation of neuropsychiatric disorders have been significantly associated with exposure to trauma early in life (Heim et al., 2008; Heim et al., 2001). As such, the influence of early life perturbation on later-life outcomes has been studied extensively in rodents. These studies have revealed that the type of stress (e.g. acute, chronic, mild, severe, homotypic, heterotypic), developmental stage at presentation (e.g. prenatal, postnatal, peripubertal), and in some cases the sex of the offspring, programs the HPA axis to be hyper- or hypo-responsive. For example, male but not female, offspring born to mothers exposed to mild chronic variable stress during the first week of gestation show adult increases in CRF expression, stress-induced CORT, and an increase in depression-related behaviors in both the forced swim (FST) and tail suspension tests (Mueller and Bale, 2008). Hyperactivity of the HPA axis is also observed in adult rats whose mothers were given the corticosteroid dexamethasone chronically during the last week of gestation (Shoener et al., 2006). In contrast, chronic restraint stress from gestational day 14–21 increases CRF expression and CORT output for adult female offspring, whereas CRF expression decreases, ACTH increases, and no change in CORT is observed in male offspring (Garcia-Caceres et al., 2010). Postnatal stress on P3 (acute peripheral inflammation) significantly decreases adult anxiety- and depression-related behaviors in the elevated plus maze (EPM) and FST, respectively, and blunts stress-induced CRF and ACTH release (Anseloni et al., 2005), indicative of HPA hypo-sensitivity. In contrast, chronic reduction of maternal resources for nest building on P2-P9 significantly increases basal CORT for offspring as adults (Rice et al., 2008). Although the mechanisms underlying the maintenance of these changes in gene expression and behavior are not completely understood, modification to the methylation and histone profiles of CRF and GR system genes are commonly observed in adult offspring exposed to early life stress (Elliott et al., 2010; Mueller and Bale; Rodgers et al., 2013; Weaver et al., 2004). In addition, epigenetic influence of miRNAs on the embryonic germ line have sex-specific, transgenerational effects on adult stress-related profiles (Morgan and Bale, 2011, 2012; Rodgers et al., 2013), suggesting that production of sexually dimorphic phenotypes is more complex than the organizational influence of sex steroids (Carruth et al., 2002; Konkle and McCarthy, 2011; McCarthy et al., 2012).
1.4 Interaction Between Systems Mediating Pain And Stress Responding
Notably, the endogenous opioid system works in concert with classic systems regulating HPA axis activity. For example, in the hypothalamus, thalamus, septum, hippocampus, amygdala, locus coeruleus, cortex, periaqueductal gray and dorsal horn, CRF and CRF receptors (CRFR) co-localize and are co-expressed with endogenous opioids (Chalmers et al., 1995; Dumont et al., 2000; Larsen and Mau, 1994; Marchant et al., 2007; Mousa et al., 2007; Rivalland et al., 2005; Sakanaka and Magari, 1989). Acute or chronic stressors such as restraint, inflammatory pain, or osmotic distress increase hypothalamic expression and release of both CRF and enkephalin, as well as circulating β-endorphin, ACTH and CORT (Guillemin et al., 1977; Lightman and Young, 1989; Shippenberg et al., 1991; Taylor et al., 1998). Dexamethasone or CORT completely blocks stress-induced increases in CRF, preproenkephalin and proopiomelanocortin (POMC) mRNA (Beaulieu et al., 1988; Harbuz and Lightman, 1989), and GR transcriptionally regulates expression of both the endogenous opioid and CRFR systems (Schoneveld et al., 2004). Together these data suggest that the stress system recruits and regulates endogenous opioids to aid in the response to homeostatic perturbations. As enkephalin or morphine administration significantly reduces cortisol concentrations (McDonald et al., 1959; Stubbs et al., 1978), and opioid receptor antagonists naloxone or naltrexone increase plasma β-endorphin, ACTH and cortisol (al’Absi et al., 2004; Wand and Schumann, 1998), such findings support the role of opioids in reducing stress reactivity.
While the CRFR system is well known for regulating responses to anxiety and stress (Bale et al., 2000; Coste et al., 2000; Smith et al., 1998; Weaver et al., 2004), it participates in antinociception as well. For example, administration of CRF in either humans or rodents stimulates the release of β-endorphin from the anterior pituitary to produce analgesia in response to noxious thermal heat (Hargreaves et al., 1990; Hargreaves et al., 1987). Adrenalectomy does not abolish CRF antinociception, indicating that analgesia results from central rather than peripheral release of CRF (Vit et al 2006). In addition, dexamethasone attenuates the antinociceptive effects of either DAMGO or β-endorphin (icv) (Pieretti et al., 1994), suggesting that endogenous glucocorticoids can modulate opioid analgesia.
Enkephalin and endorphin dampen responses to anxiety and stress-related behavior (Akil et al., 1984). For example, pharmacological activation of µ- or δ-opioid receptors, to which enkephalin and endorphin bind, significantly reduces fear-potentiated startle (Glover and Davis, 2008) and decreases stress-induced anxiety in the EPM (Randall-Thompson et al., 2010). In contrast, blockade of endogenous opioid signaling with naltrexone decreases activity in the center of the OF (de Cabo de la Vega et al., 1995). Preproenkephalin knockout mice display a heightened stress response, as indicated by reduced time in the open area of the light-dark test, decreased entries and time spent in the inner area of the OF and increased startle amplitudes relative to wild-types. (Bilkei-Gorzo et al., 2008; Konig et al., 1996; Kung et al., 2010). Significant reductions in basal CORT and prolonged recovery time from stress are also observed in these mice (Bilkei-Gorzo et al., 2008), suggesting that enkephalin is an important regulator for neuroendocrine response to, and recovery from, stress. Similar to mice lacking enkephalin, β-endorphin knockout mice show decreases in total time and percent entries into the open arm of the EPM (Grisel et al., 2008). However, loss of β-endorphin increases stress-induced ACTH, blunts basal and peak stress-induced CORT while maintaining CORT recovery time similarly to wild-types (Bilkei-Gorzo et al., 2008). Together these data suggest that although enkephalin and endorphin reduce anxiety, they have independent and unique rolls in regulating stress hormone responses.
In contrast to enkephalin and β-endorphin, dynorphin promotes anxiety and stress through binding to the K-opioid receptor (KOR). For example, activation of KOR with U-50488H in mice significantly decreases time in the center of the OF and percent time in the open arms of the EPM; anxiogenic effects are reversed by the KOR antagonist nor-Binaltorphimine (Wittmann et al., 2009). Similarly, loss of prodynorphin increases time in the center of the OF, time in the open arms of the EPM, and immobility in the tail suspension test (Kastenberger et al., 2012; Wittmann et al., 2009). Prodynorphin knockout mice show accelerated CORT peak following stress, yet negative feedback is prolonged in comparison to wild-type animals (Bilkei-Gorzo et al., 2008). These data suggest that dynorphin promotes anxiety, stress and has a specific role in stress hormone regulation. Collectively, the above findings suggest interplay between pain and stress systems, such that neuropeptides of the stress system have the ability to modulate pain, while endogenous opioids can dampen or increase sensitivity to anxiety and stress.
1.5 Experimental questions
Clinical findings indicate that early life exposure to repetitive pain and its associated stress in the NICU results in polysystemic changes in future responses to pain-, stress- and anxiety-provoking stimuli. These changes emerge early in development and persist in children, teens and young adults indicating that “adaptations” associated with neonatal pain and stress are permanent. The underlying mechanism(s) in humans, and preventability by appropriate analgesia at the time of injury, remains to be investigated.
Animal studies indicate that early life injury results in a permanent upregulation of endogenous opioids that is necessary for hypoalgesia in response to noxious stimuli observed in adult rats (LaPrairie and Murphy, 2007, 2009). In contrast, mechanisms whereby long-term changes in anxiety- and stress-related behavior and HPA axis regulation occur as a result of early life pain remain unclear. Our lab has investigated the long-term impact of a single neonatal inflammatory insult on adult stress-related responses and neuroanatomy using a clinically relevant rodent model of early life pain. In this model, P0 rat pups receive a single hind paw injection of the inflammatory agent carrageenan (CGN; 1%), resulting in 24–72 hrs of inflammation. As the endogenous pain control system and HPA axis are in a state of exaggerated developmental plasticity at this time point, and these systems work in concert to respond to noxious or aversive stimuli, we investigated whether early life pain: (1) produces deficits in adult stress-related behavior and alters stress-related neuroanatomy through an opioid-dependent mechanism, and (2) alters receptor systems regulating the activation and termination of the stress response in adulthood. Additional experiments assessed if stress- and pain-associated neurohormones are altered within the first week following early life inflammatory pain, and if this early activation of the pain system was necessary for the long-term changes in anxiety and stress-related behavior.
1.6 Early Postnatal Perturbations Result In Long-Term Adaptations In Later-Life Stress, Anxiety And Pain
Summary Of Behavioral Findings
Across all studies, we consistently find that early life pain blunts adult sensitivity to acute anxiety- and stress-provoking stimuli (Victoria et al., 2013b; Victoria et al., 2015). Neonatally injured adults spend significantly more time in the center of the OF and display significantly longer latencies to immobility in the FST. Our data also show that neonatally injured adults have significantly attenuated stress-induced analgesia (Victoria et al., 2013b). Blockade of endogenous opioid signaling with the broad-spectrum opiate antagonist naloxone reverses the injury induced changes in response to the FST and stress-induced analgesia, suggesting that the observed behavioral hyposensitivity to stress in neonatally injured adults is mediated by an opioid-dependent mechanism (Victoria et al., 2013b). Such findings are consistent with previous reports from our lab that neonatal injury decreases adult nociceptive sensitivity through an opioid-dependent mechanism (LaPrairie and Murphy, 2007, 2009).
We also tested the impact of early life injury on responses to stress following 7 days of mild chronic variable stress (mCVS) (Victoria et al., 2015). In contrast to the hypo-sensitivity observed in response to acute stress, neonatally injured adults exposed to chronic stress show a hyper-sensitive profile, such that latencies to immobility decrease and time spent immobile increases in the FST. Morphine administration at the time of injury reverses both the hypo- and hyper-sensitive profiles in response to acute and chronic stress, respectively, indicating that these changes result from early life noxious stimulation.
Summary Of Anatomical and Physiological Findings
Anatomically, we observe that neonatal injury significantly increases central expression of the opioids met-enkephalin and β-endorphin, as well as plasma corticosterone within 24 hrs of injury (Victoria et al., 2014). These markers of both pain and stress remain elevated by the end of the first postnatal week as compared with controls, suggesting aberrant regulation of critical developmental processes. Elevated levels of met-enkephalin, β-endorphin, and CORT likely have significant consequences for the developing organism. In adulthood, increases in endogenous opioid-peptide expression persist into adulthood in regions underlying responses to pain, anxiety and stress, including the ventrolateral PAG (vlPAG), central amygdala (CeA) and lateral septum (LS) (Victoria et al., 2013b). Alterations in CRFR1 and CRFR2 binding were also noted in the vlPAG, several amygdalar nuclei and the LS (Victoria et al., 2013a), suggesting a common circuit whereby injury alters adult perception and response to noxious stimulation.
Neonatal injury also alters adult GR expression in the PVN and hippocampus (Victoria et al., 2013b), key sites of HPA axis activation and termination (Cullinan et al., 1993; Dallman et al., 1987). Although basal CORT levels do not differ between injured and uninjured adults, CORT levels are reduced in response to acute stressors and return to baseline more rapidly. By contrast, repeated HPA activation by 7 days of mCVS results in prolonged corticosterone reactivity (Victoria et al., 2013b; Victoria et al., 2015). Together with our behavioral findings, these data suggest that injury confers resilience to acute stress but vulnerability to chronic stress. As with behavioral adaptations, morphine treatment for early life pain mitigates changes in corticosterone release in response to both acute and chronic stressors, suggesting that long-term changes resulting from neonatal injury are preventable with appropriate and specific pain therapy (Victoria et al., 2015). As a whole, these findings provide valuable insight into the long-term consequences of early life injury and have the potential to influence pain treatment regimens for human infants in the NICU.
Neonatal Injury In Relation To Behavioral Findings From Models Of Postnatal Perturbations
Early life perturbations have been shown previously to result in long-term changes in response to anxiety- and stress-provoking stimuli. In general, animals who have experienced either acute or mild perturbations during the perinatal period, including increased handling, licking and grooming, show decreased responsiveness to stress-provoking stimuli and reduced HPA reactivity (Bhatnagar and Meaney, 1995; Boufleur et al., 2013; Caldji et al., 2000; Weaver et al., 2005). By contrast, the opposite behavioral profile is observed in adults exposed to severe stress (e.g. maternal separation and maternal isolation) as neonates (Coutinho et al., 2002; Marais et al., 2008) for recent reviews of perinatal stress consequences: (LaPrairie and Murphy, 2010; Macri et al., 2011; Weinstock, 2008).
In our behavioral studies we observe that a single painful experience early in life results in behavioral and hormonal hypo-sensitivity to acute anxiety-, stress-, and pain-provoking stimuli, but hyper-sensitivity upon exposure to chronic perturbations. Other studies testing the long-term consequences of postnatal perturbation observe a similar dichotomy in adult responses to acute versus chronic/severe stressors. For example, early life inflammatory pain reduces basal pain sensitivity (hypoalgesia) in adult rats exposed to acute thermal or mechanical noxious stimuli. However, following exposure to a chronic and more intense noxious stimulus, these rats display significant increases in pain sensitivity (hyperalgesia) (LaPrairie and Murphy, 2007; Ren et al., 2004). Similarly, neonatal endotoxin exposure on P4 increases sucrose preference and social interaction, and decreases CORT following acute tail shock (Bilbo et al., 2008). However, exposure to chronic stress or administration of LPS in these animals increases anxiogenic behavior in EPM and OF, increases acoustic startle amplitude, and elevates CORT release (Bilbo et al., 2008; Walker et al., 2009a). Interestingly, some models of chronic maternal separation (3 hrs/day on P2-P14) have shown basal hyperactivation of the HPA axis in response to acute air puff startle stress, but reduced ACTH and CORT following chronic stress (Ladd et al., 2005), suggesting that HPA axis has flexibility for dichotomous dysregulation in both directions.
This hypo- versus hyper-sensitive response profile in response to acute versus chronic/severe stimuli is consistent with clinical findings in former preterm infants. For example, children, teens and young adults born prematurely are rated as less sensitive to pain by both their parents and physicians, (Grunau et al., 1994b; Hermann et al., 2006; Johnston et al., 1996; Oberlander et al., 2000), display reduced stress-induced analgesia (Wollgarten-Hadamek et al., 2011) and show blunted cortisol reactivity to psychological stress testing (Buske-Kirschbaum et al., 2007). In contrast, hyperalgesia is observed following surgery in the same dermatome, as well as increased negative verbalization and increased catastrophizing related to painful interventions. These former preterm infants also have higher rates of anxiety, depression and emotional reactivity following a more pronounced stressor (Aarnoudse-Moens et al., 2009; Bagner et al., 2010; Hack et al., 2004; Hayes and Sharif, 2009; Peters et al., 2005; Sullivan et al., 2012). Together, these data suggest that this hypo-/hyper-sensitive response profile may be a common adaptation that results from early life trauma. However, such extreme physiological and psychological coping strategies increase the risk for manifestation of disorders such as post-traumatic stress (PTSD) (Taylor and Stanton, 2007).
Neonatal Perturbations Affect Common Neurocircuits And Neurotransmitters Systems
Animal models of early life perturbations suggest that the amygdala, septum, hypothalamus and hippocampus are common sites where long-term changes in expression and function occur for the CRFR and GR systems (Bhatnagar and Meaney, 1995; Ladd et al., 2005; Proulx et al., 2001; Shanks et al., 1995). Changes in the vlPAG and endogenous opioid system have also been reported (Victoria et al., 2013a, b; Victoria et al., 2014). For example, we have previously reported that early life pain significantly increases adult enkephalin expression in the LS, CeA and vlPAG. Neonatal injury also decreases high-affinity CRFR1 binding in the basolateral amygdala (BLA) and vlPAG, and increases low-affinity CRFR2 binding in LS and cortical amygdala (CoA) in adults. These findings overlap with observations in adult rats that experienced maternal separation (decreases in BLA CRFR1 binding, stress-induced increases in CRFR2 binding in the LS; (Ladd et al., 2005), suggesting a common circuit for postnatal adaptations to early life perturbations.
In our studies, GR expression increases significantly in the PVN but decreases in both dorsal and ventral CA1 of the hippocampus. Lack of neonatal handling and exposure to adult chronic stress also decreases GR mRNA expression in the septum and hippocampus of rats (Bhatnagar and Meaney, 1995). Further, metabolic perturbation with leptin on P2-P9 in rodents decreases GR expression in the hippocampus, increases GR expression in the PVN and accelerates dexamethasone suppression of CORT (Proulx et al., 2001). Meanwhile, early life immune challenge in rats (P3-P5) decreases adult GR expression in both the hippocampus and hypothalamus (Shanks et al., 1995).
In humans, changes in GR are associated with early life trauma as well. For example, suicide victims that experienced childhood abuse show decreases in GR mRNA expression in post-mortem hippocampal samples (McGowan et al., 2009). Individuals that suffer from depression or PTSD also show increases in CRF from plasma and CSF (Bremner et al., 1997; Catalan et al., 1998; McEwen, 2002). Interestingly, former NICU patients suffer from a higher incidence of anxiety and depression (Aarnoudse-Moens et al., 2009; Botting et al., 1997; Hack et al., 2004; Hayes and Sharif, 2009; Levy-Shiff et al., 1994; Sullivan et al., 2012), suggesting changes in GR and CRF systems for former preterm infants may contribute to the increased rate of mood disorders. Together, these findings support the hypothesis that traumatic early life experience impacts later-life susceptibility to affective dysfunction that is associated with changes in CRFR and GR systems in the septum, amygdala, hippocampus, hypothalamus. Our results suggest a role for the endogenous opioid system, as well as the PAG, in this susceptibility.
1.7 Potential Mechanisms Underlying Long-Term Changes In Stress-, Anxiety-And Pain-Related Responding
Opioids In Behavior And Anatomy
Our pharmacological manipulations show that neonatal injury changes adult behaviors through an opioid-dependent mechanism. In particular, we observed that naloxone reverses neonatal injury-induced changes in FST and stress-induced analgesia behaviors (Victoria et al., 2013b), as well as hypoalgesia in response to noxious stimulation (LaPrairie et al., 2008); suggesting opioids are necessary for injury-induced behavioral changes. We also found that early life injury changes expression of endogenous opioids in stress-, anxiety- and pain-related brain regions (Victoria et al., 2013b). Collectively, our data suggest that neonatal injury dampens basal responses to acute noxious and stress-provoking stimuli, while exacerbating responses to chronic perturbations.
Animal studies: Analgesia
In our studies, we observe that early life pain increases adult basal pain thresholds and impairs stress-induced analgesia (LaPrairie and Murphy, 2007; Victoria et al., 2013b). Our anatomical and pharmacological data suggest that this hypoalgesia is due to increased endogenous opioid-tone in pain-related brain regions (LaPrairie et al., 2008; LaPrairie and Murphy, 2007, 2009; Victoria et al., 2013b; Victoria et al., 2014; Victoria et al., 2015). In particular, we show that enkephalin and endorphin immunoreactivity increase significantly in vlPAG of adults that were injured on the day of birth (LaPrairie and Murphy, 2009). Systemic blockade of opioid receptors with naloxone, or vlPAG-specific antagonization of µ- and δ-, but not κ-receptor attenuated the injury-induced increase in basal pain thresholds. Neonatally injured adults also exhibit significant upregulation of met-enkephalin mRNA and protein in the CeA and LS.
Stress-induced analgesia is also impaired in neonatally-injured male and female adult rats (Victoria et al., 2013b). In particular, 30 min of restraint stress significantly increases paw withdrawal latencies in control, but not neonatally injured adults. Naloxone administration before restraint completely blocks stress-induced analgesia in controls, implicating an opioid-dependent mechanism. Others have shown that stress-induced analgesia increases nociceptive thresholds through µ-opioid receptor signaling in the amygdala and PAG (Stein et al., 1992), and is prevented by pretreatment with naloxone (Lewis et al., 1980). In our studies, the inability of prolonged restraint (30 min) to alter sensory thresholds in injured rats suggests that enkephalin or endorphin could not increase beyond the 2-fold elevations in basal levels (Victoria et al., 2013a, b). Notably, stress-induced CORT levels peak at 30 min making it possible that any opioid surge above baseline is mitigated by CORT activation of GR, which negatively regulates enkephalin and β-endorphin expression (Schoneveld et al., 2004). Impairments in stress-induced analgesia suggests opioid-dependent vulnerability to more severe stressors, and likely contributes to the hyper-responsive profile observed in the FST following chronic variable stress (Victoria et al., 2015). Our data are consistent with clinical reports hypothesizing that changes in the endogenous opioid system mediate elevations in thermal pain sensitivity and impairments in stress-induced analgesia observed in adolescence and teens burned early in infancy (Goffaux et al., 2008; Hermann et al., 2006; Peters et al., 2005).
Animal studies: Anxiety- and stress-related behavior and anatomy
Based on our early studies (LaPrairie et al., 2008; LaPrairie and Murphy, 2007, 2009), we hypothesized that the neonatal injury-induced increase in endogenous opioidtone decreases adult anxiogenesis in the OF and sensitivity to forced swim stress (Victoria et al., 2013b). Indeed, overexpression of enkephalin reduces anxiety-like behavior in the EPM and OF, whereas loss of enkephalin has the opposite effect (Kang et al., 2000; Konig et al., 1996; Randall-Thompson et al., 2010). We observe that morphine treatment at the time of injury prevents the injury-induced suppression in response to acute anxiety- and stress-provoking stimuli. In response to mCVS, neonatally injured adults initiate floating rapidly and spend significantly more time immobile relative to controls, suggesting that high levels of opioids interact with chronic stress to result in depression-related behavior (Victoria et al., 2015). Indeed, others have shown that morphine treatment of adult rats during 7 days of chronic variable stress increases immobility in the FST and decreases sucrose preference (Molina et al., 1994; Zurita et al., 2000). Daily naloxone or naltrexone administration before stressors rescues these behaviors (Molina et al., 1994; Zurita et al., 2000), suggesting that chronic opioid signaling is necessary for FST behaviors in response to repeated stress. They further suggest that opioid blockade prevents vulnerability to chronic stress.
In our anatomical studies, opioid concentrations change in the brain and spinal cord immediately after injury and over the first postnatal week (Victoria et al., 2014). We hypothesize the anatomical basis for our opioid-dependent changes in anxiety- and stress-related behaviors are due to significant elevation of enkephalin mRNA and protein in the LS, CeA and vlPAG (Victoria et al., 2013b). As the CeA and vlPAG reciprocally connect and collaborate to cope with noxious stimuli (e.g. inflammatory pain), and the LS inhibits HPA activation (Behbehani, 1995; Herman, 2010; Manning and Mayer, 1995; Rizvi et al., 1991; Zubieta et al., 2001), enkephalin in these regions may dampen the noxious perception associated with neonatal pain.
Human studies: Analgesia, affect, stress-related anatomy and physiology
While our model of early life pain employs rodents, the effect of trauma on analgesia, affect, and stress are similar in humans. For example, opioid-dependent hypo-sensitivity to pain is observed in individuals suffering from PTSD (McCubbin, 1993), such that exposure to combat videos produces naloxone-reversible decreases in pain sensitivity (McCubbin, 1993). Morphine administration at the time of trauma, during resuscitation or early during treatment protects veterans from developing PTSD (Holbrook et al., 2010). Notable, these effects were specific to morphine, as benzodiazepines or serotonin reuptake inhibitors were not effective. Consistent with this finding, children given morphine for burn injuries are significantly less likely to show signs of PTSD in a 6-month follow up assessment (Saxe et al., 2001). Together these data suggest that morphine administration is effective for preventing long-term psychological consequences associated with trauma, potentially through decreasing sensory-affective perception of pain and injury severity. In addition, these studies support our findings that morphine treatment for neonatal injury confers protection against extreme behavioral responses to acute or chronic perturbations.
Changes in the endogenous opioid system directly impact human HPA physiology. For example, morphine treatment dampens CORT output from the HPA axis (McDonald et al., 1959; Zis et al., 1984). Adult men with the µ-opioid receptor gene A118G polymorphism show significant increases in plasma ACTH and CORT in response to naloxone, suggesting tighter endogenous opioid regulation over HPA functioning (Wand et al., 2002). The A-G substitution has been linked to a 3-fold increase in β-endorphin binding to µ-opioid receptor and is significantly associated with decreases in personality factors related to planning, task completion and organization (Wand et al., 2002). Adult females suffering from major depressive disorder show general decreases for in vivo µ-receptor binding potential in the amygdala, thalamus and PFC, in addition to decreases in ACTH and CORT, following autobiographical recall of a neutral or sad story relative to healthy controls (Kennedy et al., 2006). While these studies were not performed in former preterm infants, they support the hypothesis that dysregulation of the endogenous opioid and stress systems are inter-related, and impact subsequent responses to environmentally relevant stimuli that can culminate in affective dysfunction.
Injury Is Sufficient To Create A New HPA Profile In Presence Of Acute And Chronic Stress
Prolonged pain and inflammation during the first postnatal week provides the opportunity for sustained activation and reprogramming of the HPA axis. Our studies show that in adulthood, GR mRNA and protein levels increase significantly in the PVN but decrease in the hippocampus, suggesting compensatory adaptations to facilitate termination of the HPA axis. As affinity for GR is elevated in the first postnatal weeks, high levels of CORT likely result in more frequent negative feedback, and therefore more efficient stress recovery (Sapolsky and Meaney, 1986). Based on early studies examining the development of the GR system and HPA axis functioning (Meaney et al., 1985; Sapolsky and Meaney, 1986; Walker et al., 1986), we hypothesize that as CORT continues to feed up to the hippocampus, GR becomes downregulated (Victoria et al., 2013a) and less able to terminate stress. To compensate, GR in the PVN becomes upregulated to promote HPA axis inhibition. Indeed, models of early life stress, immune challenge and metabolic perturbations also observe long-term changes in hypothalamic and hippocampal GR expression, supporting the hypothesis that this system is malleable early in life (Bilbo et al., 2008; Ladd et al., 2005; Proulx et al., 2001; Sapolsky and Meaney, 1986). This is further supported by human studies showing that postmortem hippocampal GR expression is significantly decreased in severely depressed adults who were abused as children (McGowan 2009).
In addition to the HPA axis-specific changes we observe in the GR system, we reported that neonatal injury significantly decreases adult CRFR1 binding only in the BLA and vlPAG (Victoria et al., 2013a). This suggests that injury on the day of birth selectively impacts circuits underlying the activation of stress (Bhatnagar et al., 2004), autonomic tone (Bandler and Shipley, 1994; Floyd et al., 1996) and processing of noxious stimuli (Behbehani and Fields, 1979).
Factors In Addition To Pain May Change Adult HPA Reactivity
The pain assay employed in our studies (intraplantar CGN) results in a prolonged inflammatory response (approximately 24–72 hrs); therefore, the role of immune factors on HPA activity cannot be ruled out. In response to inflammation, peripheral CRF and cytokine IL-1β are released and act on immune cells to stimulate the release of endogenous opioids, β-endorphin or met-enkephalin, to produce antinociception (Schafer et al., 1994; Schafer et al., 1997). Centrally, IL-1β is known to stimulate norepinephrine (NE) and activate the HPA along with CRF (Brunton et al., 2005). While IL-1β may contribute to the injury-induced changes in HPA reactivity, it is worth noting that enkephalin rapidly reduces stress-induced release of NE (Tanaka et al., 1989), suggesting that the injury-induced surge in enkephalin would mitigate the effects of IL-1β on HPA activity. In addition, peripheral application of enkephalin mimics the anti-inflammatory effects of CRF (Schafer et al., 1994; Schafer et al., 1997), suggesting neonatal CGN may instead upregulate anti-inflammatory factors. In fact, a similar model of early life inflammation results in adult upregulation of IL-10 and proenkephalin gene expression in the spinal cord with no observed changes in pro-inflammatory markers (Ren et al., 2005). Certainly, this does not rule out the contribution of other immune system mediators, such as TNFα, which can stimulate the HPA axis and is also negatively regulated by GR (Tsigos and Chrousos, 2002).
Working Hypothesis
Our working hypothesis is that neonatal pain experienced during a critical neurodevelopmental period (P0-P8; LaPrairie and Murphy, 2007) increases afferent nociceptive drive to supraspinal regions responsive to noxious input, including the thalamus, hypothalamus, amygdala and PAG (Fitzgerald, 2005; LaPrairie and Murphy, 2009; Victoria et al., 2013b; Walker et al., 1986) (Figure 1). Endogenous opioids, including met-enkephalin and β-endorphin, are released to dampen pain (Hurley and Hammond, 2001; Konig et al., 1996; Loh et al., 1976) and stress responses (Bilkei-Gorzo et al., 2008; Lightman and Young, 1987; Rivier et al., 1982; Rossier et al., 1977). Concurrently, neurohormones from the HPA axis, including CRF, ACTH and CORT, are released to mount appropriate physiological responses and promote recovery from the physical threat associated with inflammation (Dallman et al., 1987; Taylor et al., 1998; Vale et al., 1981). As the inflammation associated with intraplantar carrageenan persists for 24–72 hours, endogenous opioids are released in regions mediating descending pain inhibition, perception of pain and HPA regulation (e.g. vlPAG, CeA, LS). Sustained activation of the HPA axis in the first postnatal week following injury (Victoria et al., 2014) likely results in sustained elevation of CRF, which in turn downregulates highaffinity CRFR1, while increasing low-affinity CRFR2 in regions mediating stress activation and perception of noxious stimuli (e.g. vlPAG, amygdala, LS) to program circuits such that future insults are less potent or aversive. As CORT levels remain high and continue to feed up to the hippocampus, GR becomes downregulated and the ability to terminate stress is impaired (Boyle et al., 2005); GR in the PVN becomes upregulated to compensate and promote HPA axis inhibition (Proulx et al., 2001; Victoria et al., 2013a). In turn, CORT negative feedback becomes more efficient to facilitate recovery (Sapolsky and Meaney, 1986; Victoria et al., 2013a). As these perturbations occur during a highly plastic developmental period, and GR transcriptionally regulates itself as well as CRFRs and endogenous opioids (Schoneveld et al., 2004), it is probable that this new “production profile” becomes epigenetically programmed as the basal state and persists throughout the life span. Notably, opioids and CRFRs regulate inhibition and excitation, respectively, and changes in their expression occur in regions that regulate HPA-tone and responses to stress-, anxiety- and pain-provoking stimuli (Victoria et al., 2013a, b). As such, the neuroanatomical changes we observe likely interact to produce the behavioral phenotypes that result from injury: hypo-sensitive responses to acute stress-, anxiety- and pain-provoking stimuli, and hyper-sensitive responses to chronic, unpredictable stressors (Victoria et al., 2015) (Figure 2). Morphine treatment at the time of injury reverses these behavioral changes, suggesting that pain therapy mitigates the activation of circuits responsive to pain and stress. We hypothesize that morphine reverses the site-specific changes in neurohormone and receptor profiles, however, partial rescue or additional compensatory changes are possible and are a potential starting point for future studies.
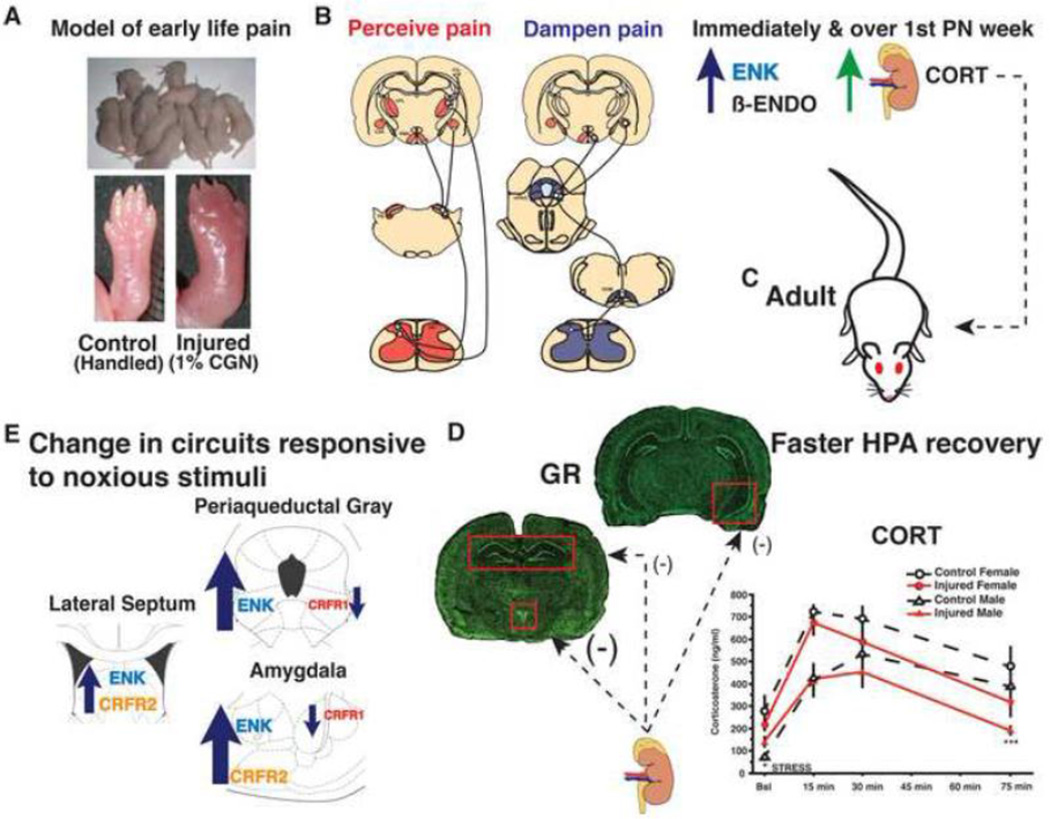
Figure 1. The lasting impact of a single neonatal injury on HPA activity and circuits responsive to noxious stimuli.
A. Preterm infants experience numerous painful, inflammatory procedures in the Neonatal Intensive Care Unit often in the absence of pain therapy. To model a single painful experience on the day of birth, rat pups receive an injection of the inflammatory agent carrageenan (CGN; 1%) into the intraplantar surface of the right hind paw. B. Inflammatory pain increases afferent nociceptive drive to supraspinal brain regions responsive to noxious stimuli (e.g. septum, thalamus, hypothalamus, amygdala and periaqueductal gray (PAG); diagram and brain plates adapted from (Hunt and Mantyh, 2001) and (Paxinos and Watson, 2005), respectively). Met-enkephalin (ENK), β-endorphin (β-ENDO) and corticosterone (CORT) are released to dampen pain perception and stress associated with inflammation. ENK and plasma CORT remain elevated at the end of the first postnatal week, suggesting permanent changes in the endogenous opioid and stress systems. C. In neonatally injured adults (D) CORT levels return to baseline more rapidly following acute stress stimulation of the hypothalamic pituitary adrenal (HPA) axis. In parallel, glucocorticoid receptor (GR) mRNA and protein are increased in the paraventricular nucleus of the hypothalamus but decreased in the dorsal and ventral hippocampus, suggesting support for accelerated negative feedback. E. Anatomical changes in corticotrophin releasing factor receptor (CRFR) 1 and 2, and ENK are observed in circuits that process anxiety-, stress-, and pain-provoking stimuli, contribute to stimulation of the HPA axis and homeostasis. CRFR1 binding is significantly decreased in the amygdala and ventrolateral (vl) PAG. CRFR2 binding was increased in the amygdala and lateral septum (LS). ENK mRNA and protein expression are significantly increased in the vlPAG, amygdala and LS.
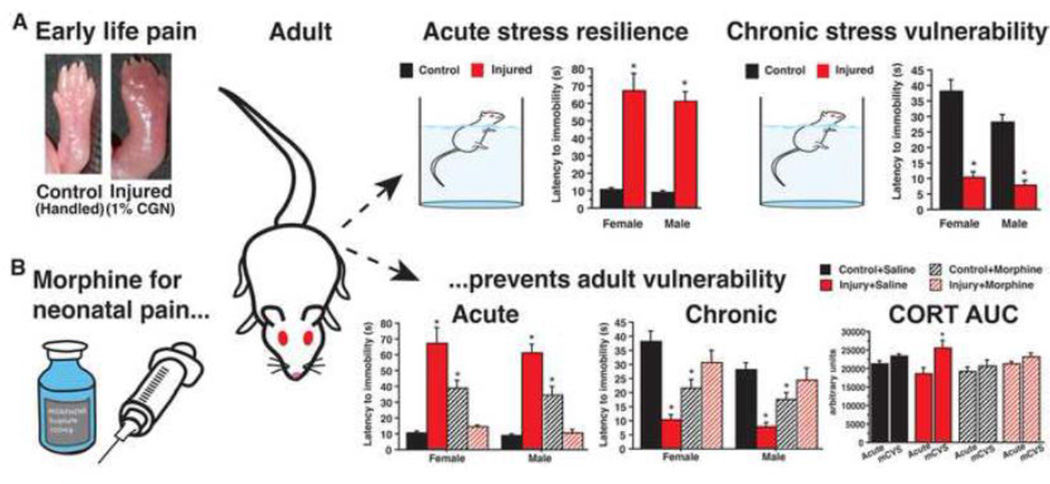
Figure 2. The lasting impact of a single neonatal injury on anxiety and stress responding.
A. In response to acute stressors (forced swim), neonatally injured adults take significantly longer to initiate floating. By contrast, adults injured early in life float rapidly after exposure to 7 days of mild chronic variable stress (mCVS). B. Hypo-sensitivity to acute stress-provoking stimuli and hyper-sensitivity to sequential, unpredictable stress are rescued if male and female rats are given morphine for early life pain, suggesting that 1) injury-induced behavioral and hormonal vulnerability are preventable, 2) neonatal pain is necessary for the long-term changes in stress responding. (Abbreviations: corticosterone, CORT; area under the curve, AUC).
1.8 Final Remarks
Numerous clinical studies have shown that exposure to unalleviated pain and stress in the NICU has immediate and long-lasting consequences for sensory perception, neuroendocrine stress responses and emotional health in former preterm infants. In humans, the mechanism(s) by which these long-term changes in stress and pain behavior and physiology occur, and whether such changes can be prevented by analgesic intervention at the time of injury, is not known. The goal of our studies was to delineate the specific impact of a single injury early in life on adult behavioral responses to anxiety- and stress-provoking stimuli, stress axis functioning, and associated changes in underlying neuroanatomical circuits. In addition, we have tested whether blockade of inflammatory pain with morphine administration attenuate the previously observed behavioral and hormonal responses. Our studies show for the first time that a single injury on the day of birth significantly reduces sensitivity to acute anxiety- and stress-provoking stimuli, yet increases sensitivity to prolonged and unpredictable stressors (Victoria et al., 2013b). Administration of morphine at the time of injury prevents injury-induced changes in stress and anxiety behavior. In the absence of treatment for early life pain, injury increases endogenous opioid tone within 24 hrs of injury, and opioid levels remain elevated at the end of the first postnatal week, suggesting a time point by which changes in the opioid system become programmed. In adulthood enkephalin levels are upregulated in brain regions responsive to stress, anxiety and pain, and blockade of opioid signaling with naloxone reverses the observed changes in stress-related behavior. These findings suggest that opioid dysregulation underlies the injury-induced changes in behavioral sensitivity, which is consistent with clinical reports in former preterm infants.
Our studies further show that neonatal injury significantly altered classic stress-receptor systems in regions modulating the HPA axis (Victoria et al., 2013a). HPA functioning is dampened in response to acute stressors, consistent with dampened hormonal responses to stress observed in former preterm infants. Immediately following injury, HPA activity is significantly elevated suggesting disruption of developmentally sensitive HPA maturation, and establishing a mechanism supporting the observed adult changes in brain receptor systems mediating stress responses. Morphine administration at the time of injury reverses these alterations in the HPA signaling arguing strongly that the experience of pain associated with early life injury is necessary for the long-term changes in stress-related behavior and hormone responses. Collectively, our studies are the first to provide evidence that early life injury-induced dysregulation of the opioid system alters adult responses to stress-related stimuli, and show that such changes are preventable with analgesic treatment. As former preterm infants are at risk for disorders of stress, including anxiety, depression and PTSD, these studies provide imperative evidence for the development and use of specific and appropriate analgesic regimes for human infants undergoing procedures resulting in pain.
Acknowledgments
This work was supported by National Institutes of Health grant DA16272 awarded to AZM and a GSU Brains & Behavior seed grant. Morphine sulfate and naloxone were kindly provided by the National Institute on Drug Abuse (NIDA) drug supply program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med. 2000;6:971–973. doi: 10.1038/79658. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo WA, Hummel P, Lantos J, Johnston CC, Lehr VT, Lynn AM, Maxwell LG, Oberlander TF, Raju TN, Soriano SG, Taddio A, Walco GA. Analgesia and anesthesia for neonates: study design and ethical issues. Clin Ther. 2005a;27:814–843. doi: 10.1016/j.clinthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Bergqvist LL, Hall RW, Carbajal R. Acute pain management in newborn infants. Pain: Clinical Updates. 2011;XIX:1–6. [Google Scholar]
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA, Group NTI. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321–1329. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9. doi: 10.1056/NEJM199201023260101. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Johnston CC, Oberlander TF, Taddio A, Lehr VT, Walco GA. Analgesia and local anesthesia during invasive procedures in the neonate. Clinical Ther. 2005b;27:844–876. doi: 10.1016/j.clinthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Sippell WG, Aynsley-Green A. Pain, anaesthesia, and babies. Lancet. 1987a;2:1210. doi: 10.1016/s0140-6736(87)91347-x. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet. 1987b;1:243–248. doi: 10.1016/s0140-6736(87)90065-1. [DOI] [PubMed] [Google Scholar]
- Anseloni VC, He F, Novikova SI, Turnbach Robbins M, Lidow IA, Ennis M, Lidow MS. Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult. Neuroscience. 2005;131:635–645. doi: 10.1016/j.neuroscience.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Helgert A, Gey C, Coksaygan T, Schubert H, Nathanielsz PW, Witte OW, Schwab M. Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet Gynecol. 2009;113:142–151. doi: 10.1097/AOG.0b013e3181924d3b. [DOI] [PubMed] [Google Scholar]
- Bagner DM, Sheinkopf SJ, Vohr BR, Lester BM. A preliminary study of cortisol reactivity and behavior problems in young children born premature. Dev Psychobiol. 2010;52:574–582. doi: 10.1002/dev.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch Dis Child Fetal Neonatal Ed. 1995;72:F47–F48. doi: 10.1136/fn.72.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122:109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Baud O, Verney C, Evrard P, Gressens P. Injectable dexamethasone administration enhances cortical GABAergic neuronal differentiation in a novel model of postnatal steroid therapy in mice. Pediatr Res. 2005;57:149–156. doi: 10.1203/01.PDR.0000148069.03855.C4. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Gagne B, Barden N. Glucocorticoid regulation of proopiomelanocortin messenger ribonucleic acid content of rat hypothalamus. Mol Endocrinol. 1988;2:727–731. doi: 10.1210/mend-2-8-727. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Bellieni CV. Pain assessment in human fetus and infants. AAPS J. 2012;14:456–461. doi: 10.1208/s12248-012-9354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Meaney MJ. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and non handled rats. J Neuroendocrinol. 1995;7:97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73:51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Yirmiya R, Amat J, Paul ED, Watkins LR, Maier SF. Bacterial infection early in life protects against stressor-induced depressive-like symptoms in adult rats. Psychoneuroendocrinology. 2008;33:261–269. doi: 10.1016/j.psyneuen.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33:425–436. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38:931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Boufleur N, Antoniazzi CT, Pase CS, Benvegnu DM, Dias VT, Segat HJ, Roversi K, Roversi K, Nora MD, Koakoskia G, Rosa JG, Barcellos LJ, Burger ME. Neonatal handling prevents anxiety-like symptoms in rats exposed to chronic mild stress: behavioral and oxidative parameters. Stress. 2013;16:321–330. doi: 10.3109/10253890.2012.723075. [DOI] [PubMed] [Google Scholar]
- Bouwmeester NJ, Anand KJ, van Dijk M, Hop WC, Boomsma F, Tibboel D. Hormonal and metabolic stress responses after major surgery in children aged 0–3 years: a double-blind, randomized trial comparing the effects of continuous versus intermittent morphine. Brit J Anaesth. 2001;87:390–399. doi: 10.1093/bja/87.3.390. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Chau CM, Cepeda IL, Degenhardt A, Weinberg J, Synnes AR, Grunau RE. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology. 2015;51:151–163. doi: 10.1016/j.psyneuen.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71:385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J Neurosci. 2005;25:5117–5126. doi: 10.1523/JNEUROSCI.0866-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Krieger S, Wilkes C, Rauh W, Weiss S, Hellhammer DH. Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. J Clin Endocrinol Metab. 2007;92:3429–3435. doi: 10.1210/jc.2006-2223. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, de Saint Blanquat L, Boelle P, Annequin D, Cimerman P, Anand KJS, Breart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- Catalan R, Gallart JM, Castellanos JM, Galard R. Plasma corticotropin-releasing factor in depressive disorders. Biol Psychiatry. 1998;44:15–20. doi: 10.1016/s0006-3223(97)00539-8. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature reviews. Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cignacco E, Denhaerynck K, Nelle M, Buhrer C, Engberg S. Variability in pain response to a non-pharmacological intervention across repeated routine pain exposure in preterm infants: a feasibility study. Acta Paediatrica. 2009;98:842–846. doi: 10.1111/j.1651-2227.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- Corbier P, Roffi J. Increased adrenocortical activity in the newborn rat. Biol Neonate. 1978;33:72–79. doi: 10.1159/000241054. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Jacobson L, Levin N, Cascio CS, Shinsako J. Characterization of corticosterone feedback regulation of ACTH secretion. Ann N Y Acad Sci. 1987;512:402–414. doi: 10.1111/j.1749-6632.1987.tb24976.x. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Jones MT. Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone ssecretion on subsequent stress responses in the rat. Endocrinology. 1973;92:1367–1375. doi: 10.1210/endo-92-5-1367. [DOI] [PubMed] [Google Scholar]
- de Cabo de la Vega C, Pujol A, Paz Viveros M. Neonatally administered naltrexone affects several behavioral responses in adult rats of both genders. Pharmacol Biochem Behav. 1995;50:277–286. doi: 10.1016/0091-3057(94)00314-9. [DOI] [PubMed] [Google Scholar]
- de Graaf J, van Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, Roofthooft DW, Groot Jebbink LJ, Veenstra RR, Tibboel D, van Dijk M. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain. 2011;152:1391–1397. doi: 10.1016/j.pain.2011.02.017. [DOI] [PubMed] [Google Scholar]
- de Graaf J, van Lingen RA, Valkenburg AJ, Weisglas-Kuperus N, Groot Jebbink L, Wijnberg-Williams B, Anand KJ, Tibboel D, van Dijk M. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain. 2013;154:449–458. doi: 10.1016/j.pain.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Chau CM, Cheung TP, Moiseev A, Ribary U, Herdman AT, Miller SP, Cepeda IL, Synnes A, Grunau RE. Neonatal pain-related stress, functional cortical activity and visual-perceptual abilities in school-age children born at extremely low gestational age. Pain. 2013 doi: 10.1016/j.pain.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Roggeveen AB, Kitajo K, Ward LM. Large-scale gamma-band phase synchronization and selective attention. Cerebral cortex. 2008;18:386–396. doi: 10.1093/cercor/bhm073. [DOI] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS, Manji HK. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhrsen L, Simons SH, Dzietko M, Genz K, Bendix I, Boos V, Sifringer M, Tibboel D, Felderhoff-Mueser U. Effects of repetitive exposure to pain and morphine treatment on the neonatal rat brain. Neonatology. 2013;103:35–43. doi: 10.1159/000341769. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Kinkead R, Trottier JF, Gosselin I, Drolet G. Effect of chronic psychogenic stress exposure on enkephalin neuronal activity and expression in the rat hypothalamic paraventricular nucleus. J Neurochem. 2000;75:2200–2211. doi: 10.1046/j.1471-4159.2000.0752200.x. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Keay KA, Arias CM, Sawchenko PE, Bandler R. Projections from the ventrolateral periaqueductal gray to endocrine regulatory subdivisions of the paraventricular nucleus of the hypothalamus in the rat. Neurosci Lett. 1996;220:105–108. doi: 10.1016/s0304-3940(96)13240-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Caceres C, Lagunas N, Calmarza-Font I, Azcoitia I, Diz-Chaves Y, Garcia-Segura LM, Baquedano E, Frago LM, Argente J, Chowen JA. Gender differences in the long-term effects of chronic prenatal stress on the HPA axis and hypothalamic structure in rats. Psychoneuroendocrinology. 2010;35:1525–1535. doi: 10.1016/j.psyneuen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Glover EM, Davis M. Anxiolytic-like effects of morphine and buprenorphine in the rat model of fear-potentiated startle: tolerance, cross-tolerance, and blockade by naloxone. Psychopharmacology (Berl) 2008;198:167–180. doi: 10.1007/s00213-008-1112-0. [DOI] [PubMed] [Google Scholar]
- Goffaux P, Lafrenaye S, Morin M, Patural H, Demers G, Marchand S. Preterm births: can neonatal pain alter the development of endogenous gating systems? Eur J Pain. 2008;12:945–951. doi: 10.1016/j.ejpain.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Bartels JL, Allen SA, Turgeon VL. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl) 2008;200:105–115. doi: 10.1007/s00213-008-1161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Cepeda IL, Chau CM, Brummelte S, Weinberg J, Lavoie PM, Ladd M, Hirschfeld AF, Russell E, Koren G, Van Uum S, Brant R, Turvey SE. Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PLoS One. 2013;8:e73926. doi: 10.1371/journal.pone.0073926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Tu MT, Whitfield MF, Oberlander TF, Weinberg J, Yu W, Thiessen P, Gosse G, Scheifele D. Cortisol, behavior, and heart rate reactivity to immunization pain at 4 months corrected age in infants born very preterm. Clin J Pain. 2010;26:698–704. doi: 10.1097/AJP.0b013e3181e5bb00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RV, Whitfield MF, Petrie JH. Pain sensitivity and temperament in extremely low-birth-weight premature toddlers and preterm and full-term controls. Pain. 1994a;58:341–346. doi: 10.1016/0304-3959(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Grunau RV, Whitfield MF, Petrie JH, Fryer EL. Early pain experience, child and family factors, as precursors of somatization: a prospective study of extremely premature and fullterm children. Pain. 1994b;56:353–359. doi: 10.1016/0304-3959(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Guillemin R, Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, Bloom F. beta-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977;197:1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor HG, Flannery D, Klein N, Borawski E. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics. 2004;114:932–940. doi: 10.1542/peds.2003-1017-L. [DOI] [PubMed] [Google Scholar]
- Haley DW, Grunau RE, Weinberg J, Keidar A, Oberlander TF. Physiological correlates of memory recall in infancy: vagal tone, cortisol, and imitation in preterm and full-term infants at 6 months. Infant Behav Dev. 2010;33:219–234. doi: 10.1016/j.infbeh.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RW, Anand KJ. Pain management in newborns. Clin Perinatol. 2014;41:895–924. doi: 10.1016/j.clp.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Glucocorticoid inhibition of stress-induced changes in hypothalamic corticotrophin-releasing factor messenger RNA and proenkephalin A messenger RNA. Neuropeptides. 1989;14:17–20. doi: 10.1016/0143-4179(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Flores CM, Dionne RA, Mueller GP. The role of pituitary beta-endorphin in mediating corticotropin-releasing factor-induced antinociception. Am J Physiol. 1990;258:E235–E242. doi: 10.1152/ajpendo.1990.258.2.E235. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Mueller GP, Dubner R, Goldstein D, Dionne RA. Corticotropin-releasing factor (CRF) produces analgesia in humans and rats. Brain Res. 1987;422:154–157. doi: 10.1016/0006-8993(87)90550-6. [DOI] [PubMed] [Google Scholar]
- Hayes B, Sharif F. Behavioural and emotional outcome of very low birth weight infants--literature review. J Matern Fetal Neona. 2009;22:849–856. doi: 10.1080/14767050902994507. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Herman JP. Stress Response: Neural and Feedback Regulation of the HPA axis. In: Fink G, editor. Stress Science: Neuroendocrinology. San Diego, CA, USA: Academic Press; 2010. pp. 75–81. [Google Scholar]
- Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–285. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Hiroshige T, Sato T. Changes in hypothalamic content of corticotropin-releasing activity following stress during neonatal maturation in the rat. Neuroendocrinology. 1971;7:257–270. [PubMed] [Google Scholar]
- Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J Neurosci. 2001;21:2536–2545. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Johnston CC, Stevens B, Yang F, Horton L. Developmental changes in response to heelstick in preterm infants: a prospective cohort study. Dev Med Child Neurol. 1996;38:438–445. doi: 10.1111/j.1469-8749.1996.tb15101.x. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Principles of Neural Science. (4th) 2000 [Google Scholar]
- Kang W, Wilson SP, Wilson MA. Overexpression of proenkephalin in the amygdala potentiates the anxiolytic effects of benzodiazepines. Neuropsychopharmacology. 2000;22:77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Kastenberger I, Lutsch C, Herzog H, Schwarzer C. Influence of sex and genetic background on anxiety-related and stress-induced behaviour of prodynorphin-deficient mice. PLoS One. 2012;7:e34251. doi: 10.1371/journal.pone.0034251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatr. 2006;63:1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC. Anxiety-and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci. 2010;17:29. doi: 10.1186/1423-0127-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–533. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- LaPrairie JL, Johns ME, Murphy AZ. Preemptive morphine analgesia attenuates the long-term consequences of neonatal inflammation in male and female rats. Pediatr Res. 2008;64:625–630. doi: 10.1203/PDR.0b013e31818702d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain 132 Suppl. 2007;1:S124–S133. doi: 10.1016/j.pain.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci. 2009;3:31. doi: 10.3389/neuro.08.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ. Long-term impact of neonatal injury in male and female rats: Sex differences, mechanisms and clinical implications. Front Neuroendocrinol. 2010;31:193–202. doi: 10.1016/j.yfrne.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Mau SE. Effect of acute stress on the expression of hypothalamic messenger ribonucleic acids encoding the endogenous opioid precursors preproenkephalin A and proopiomelanocortin. Peptides. 1994;15:783–790. doi: 10.1016/0196-9781(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Levy-Shiff R, Einat G, Mogilner MB, Lerman M, Krikler R. Biological and environmental correlates of developmental outcome of prematurely born infants in early adolescence. J Pediatr Psychol. 1994;19:63–78. doi: 10.1093/jpepsy/19.1.63. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., 3rd Changes in hypothalamic preproenkephalin A mRNA following stress and opiate withdrawal. Nature. 1987;328:643–645. doi: 10.1038/328643a0. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., 3rd Influence of steroids on the hypothalamic corticotropin-releasing factor and preproenkephalin mRNA responses to stress. Proc Natl Acad Sci. 1989;86:4306–4310. doi: 10.1073/pnas.86.11.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh HH, Tseng LF, Wei E, Li CH. beta-endorphin is a potent analgesic agent. Proc Natl Acad Sci. 1976;73:2895–2898. doi: 10.1073/pnas.73.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R, Evans D, Sugden D, Gaussen T, Levene M. Outcome at 5–6 years of prematurely born children who received morphine as neonates. Arch Dis Child Fetal Neonatal Ed. 1998;79:F40–F43. doi: 10.1136/fn.79.1.f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neurosci Biobehav Rev. 2011;35:1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Manning BH, Mayer DJ. The central nucleus of the amygdala contributes to the production of morphine antinociception in the rat tail-flick test. J Neurosci. 1995;15:8199–8213. doi: 10.1523/JNEUROSCI.15-12-08199.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantagos S, Moustogiannis A, Vagenakis AG. Diurnal variation of plasma cortisol levels in infancy. J Pediatric Endocrinol MetabJ. 1998;11:549–553. doi: 10.1515/jpem.1998.11.4.549. [DOI] [PubMed] [Google Scholar]
- Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WM. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res. 2008;61:106–112. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;64:1–65. [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin JA. Stress and endogenous opioids: behavioral and circulatory interactions. Biological Psychol. 1993;35:91–122. doi: 10.1016/0301-0511(93)90008-v. [DOI] [PubMed] [Google Scholar]
- McCulloch KM, Ji SA, Raju TN. Skin blood flow changes during routine nursery procedures. Early Hum Dev. 1995;41:147–156. doi: 10.1016/0378-3782(95)01617-c. [DOI] [PubMed] [Google Scholar]
- McDonald RK, Evans FT, Weise VK, Patrick RW. Effect of morphine and nalorphine on plasma hydrocortisone levels in man. J Pharmacol Exp Ther. 1959;125:241–247. [PubMed] [Google Scholar]
- McEwen BS. The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. Psychiatr Clin N Am. 2002;25:469–494. doi: 10.1016/s0193-953x(01)00009-0. ix. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PJ. Science is not enough: the modern history of pediatric pain. Pain. 2011;152:2457–2459. doi: 10.1016/j.pain.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. II. An autoradiographic study. Brain Res. 1985;350:165–168. doi: 10.1016/0165-3806(85)90260-3. [DOI] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology (Berl) 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol Sex Diff. 2012;3:22. doi: 10.1186/2042-6410-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa SA, Bopaiah CP, Richter JF, Yamdeu RS, Schafer M. Inhibition of inflammatory pain by CRF at peripheral, spinal and supraspinal sites: involvement of areas coexpressing CRF receptors and opioid peptides. Neuropsychopharmacology. 2007;32:2530–2542. doi: 10.1038/sj.npp.1301393. [DOI] [PubMed] [Google Scholar]
- Moustogiannis AN, Raju TN, Roohey T, McCulloch KM. Intravenous morphine attenuates pain induced changes in skin blood flow in newborn infants. Neurol Res. 1996;18:440–444. doi: 10.1080/01616412.1996.11740448. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negellen-Perchellet E, Cohen A. Effect of ether inhalation by adrenalectomized pregnant rats on the adrenal corticosterone concentration in norma, decapitated, and encephalectomized fetuses. Neuroendocrinology. 1975;17:225–235. doi: 10.1159/000122358. [DOI] [PubMed] [Google Scholar]
- Norman E, Wikstrom S, Rosen I, Fellman V, Hellstrom-Westas L. Premedication for intubation with morphine causes prolonged depression of electrocortical background activity in preterm infants. Pediatr Res. 2013;73:87–94. doi: 10.1038/pr.2012.153. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP. Biobehavioral pain responses in former extremely low birth weight infants at four months’ corrected age. Pediatrics. 2000;105:e6. doi: 10.1542/peds.105.1.e6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain atlas in stereotaxic coordinates. 5th. Elsevier Academic Press; 2005. pp. 1–166. [Google Scholar]
- PeriStats March of Dimes Perinatal Data Center/Quality Analytic Services: Special Care Nursery Adminssions. 2011 [Google Scholar]
- Peters JW, Schouw R, Anand KJ, van Dijk M, Duivenvoorden HJ, Tibboel D. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain. 2005;114:444–454. doi: 10.1016/j.pain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band--an electrophysiological correlate of cortical idling: a review. Int Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Pieretti S, Di Giannuario A, Domenici MR, Sagratella S, Capasso A, Sorrentino L, Loizzo A. Dexamethasone-induced selective inhibition of the central mu opioid receptor: functional in vivo and in vitro evidence in rodents. Br J Pharmacol. 1994;113:1416–1422. doi: 10.1111/j.1476-5381.1994.tb17155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx K, Clavel S, Nault G, Richard D, Walker CD. High neonatal leptin exposure enhances brain GR expression and feedback efficacy on the adrenocortical axis of developing rats. Endocrinology. 2001;142:4607–4616. doi: 10.1210/endo.142.11.8512. [DOI] [PubMed] [Google Scholar]
- Purcell-Jones G, Dormon F, Sumner E. Paediatric anaesthetists’ perceptions of neonatal and infant pain. Pain. 1988;33:181–187. doi: 10.1016/0304-3959(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Qiu J. Infant pain: does it hurt? Nature. 2006;444:143–145. doi: 10.1038/444143a. [DOI] [PubMed] [Google Scholar]
- Randall-Thompson JF, Pescatore KA, Unterwald EM. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology (Berl) 2010;212:585–595. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger M, Synnes AR, Vinall J, Grunau RE. Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur J Pain. 2014;18:844–852. doi: 10.1002/j.1532-2149.2013.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ren K, Novikova SI, He F, Dubner R, Lidow MS. Neonatal local noxious insult affects gene expression in the spinal dorsal horn of adult rats. Mol Pain. 2005;1:27. doi: 10.1186/1744-8069-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalland ET, Iqbal J, Clarke IJ, Turner AI, Tilbrook AJ. Co-localization and distribution of corticotrophin-releasing hormone, arginine vasopressin and enkephalin in the paraventricular nucleus of sheep: a sex comparison. Neuroscience. 2005;132:755–766. doi: 10.1016/j.neuroscience.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Rivier C, Brownstein M, Spiess J, Rivier J, Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, beta-endorphin, and corticosterone. Endocrinology. 1982;110:272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkey EN, Pillai Riddell R. The infancy of infant pain research: the experimental origins of infant pain denial. J Pain. 2013;14:338–350. doi: 10.1016/j.jpain.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rossier J, French ED, Rivier C, Ling N, Guillemin R, Bloom FE. Foot-shock induced stress increases beta-endorphin levels in blood but not brain. Nature. 1977;270:618–620. doi: 10.1038/270618a0. [DOI] [PubMed] [Google Scholar]
- Roze JC, Denizot S, Carbajal R, Ancel PY, Kaminski M, Arnaud C, Truffert P, Marret S, Matis J, Thiriez G, Cambonie G, Andre M, Larroque B, Breart G. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: results from the EPIPAGE cohort. Arch Pediatr Adolesc Med. 2008;162:728–733. doi: 10.1001/archpedi.162.8.728. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Magari S. Reassessment of enkephalin (ENK)-containing afferents to the rat lateral septum with reference to the fine structures of septal ENK fibers. Brain Res. 1989;479:205–216. doi: 10.1016/0006-8993(89)91621-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Saxe G, Stoddard F, Courtney D, Cunningham K, Chawla N, Sheridan R, King D, King L. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatr. 2001;40:915–921. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- Schafer M, Carter L, Stein C. Interleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci. 1994;91:4219–4223. doi: 10.1073/pnas.91.10.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Mousa SA, Stein C. Corticotropin-releasing factor in antinociception and inflammation. Eur J Pharmacol. 1997;323:1–10. doi: 10.1016/s0014-2999(97)00057-5. [DOI] [PubMed] [Google Scholar]
- Schmelzle-Lubiecki BM, Campbell KA, Howard RH, Franck L, Fitzgerald M. Long-term consequences of early infant injury and trauma upon somatosensory processing. Eur J Pain. 2007;11:799–809. doi: 10.1016/j.ejpain.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Schoneveld OJ, Gaemers IC, Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. 2004;1680:114–128. doi: 10.1016/j.bbaexp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Schwaller F, Fitzgerald M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur J Neurosci. 2014;39:344–352. doi: 10.1111/ejn.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CS, Riggs KW, Ling EW, Fitzgerald CE, Hill ML, Grunau RV, Solimano A, Craig KD. Morphine pharmacokinetics and pain assessment in premature newborns. J Pediatr. 1999;135:423–429. doi: 10.1016/s0022-3476(99)70163-0. [DOI] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada C, Kurumiya S, Noguchi Y, Umemoto M. The effect of neonatal exposure to chronic footshock on pain-responsiveness and sensitivity to morphine after maturation in the rat. Behav Brain Res. 1990;36:105–111. doi: 10.1016/0166-4328(90)90165-b. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A, Nikolarakis K. Prolonged inflammatory pain modifies corticotropin-releasing factor-induced opioid peptide release in the hypothalamus. Brain Res. 1991;563:209–214. doi: 10.1016/0006-8993(91)91535-9. [DOI] [PubMed] [Google Scholar]
- Shoener JA, Baig R, Page KC. Prenatal exposure to dexamethasone alters hippocampal drive on hypothalamic-pituitary-adrenal axis activity in adult male rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1366–R1373. doi: 10.1152/ajpregu.00757.2004. [DOI] [PubMed] [Google Scholar]
- Simons SH, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospectie study of procedural pain and analgesia in neonates. Arch Pediatric Adolesc Med. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Stein EA, Hiller JM, Simon EJ. Effects of stress on opioid receptor binding in the rat central nervous system. Neuroscience. 1992;51:683–690. doi: 10.1016/0306-4522(92)90307-n. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Scorr L, Smith LD, Ridgway CG, Stout M. Long-term effects of neonatal surgery on adulthood pain behavior. Pain. 2005;113:347–353. doi: 10.1016/j.pain.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Stubbs WA, Delitala G, Jones A, Jeffcoate WJ, Edwards CR, Ratter SJ, Besser GM, Bloom SR, Alberti KG. Hormonal and metabolic responses to an enkephalin analogue in normal man. Lancet. 1978;2:1225–1227. doi: 10.1016/s0140-6736(78)92100-1. [DOI] [PubMed] [Google Scholar]
- Sullivan MC, Msall ME, Miller RJ. 17-year outcome of preterm infants with diverse neonatal morbidities: Part 1--Impact on physical, neurological, and psychological health status. J Spec Pediatr Nurs. 2012;17:226–241. doi: 10.1111/j.1744-6155.2012.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Ida Y, Tsuda A, Tsujimaru S, Shirao I, Oguchi M. Met-enkephalin, injected during the early phase of stress, attenuates stress-induced increases in noradrenaline release in rat brain regions. Pharmacol Biochem Behav. 1989;32:791–795. doi: 10.1016/0091-3057(89)90035-x. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Akana SF, Peterson MA, Dallman MF, Basbaum AI. Pituitary-adrenocortical responses to persistent noxious stimuli in the awake rat: endogenous corticosterone does not reduce nociception in the formalin test. Endocrinology. 1998;139:2407–2413. doi: 10.1210/endo.139.5.5993. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Stanton AL. Coping resources, coping processes, and mental health. Ann Rev Clin Psych. 2007;3:377–401. doi: 10.1146/annurev.clinpsy.3.022806.091520. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valkenburg AJ, van den Bosch GE, de Graaf J, van Lingen RA, Weisglas-Kuperus N, van Rosmalen J, Groot Jebbink LJ, Tibboel D, van Dijk M. Long-term effects of neonatal morphine infusion on pain sensitivity: Follow-up of a randomized controlled trial. J Pain. 2015 doi: 10.1016/j.jpain.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Victoria NC, Inoue K, Young LJ, Murphy AZ. Long-term dysregulation of brain corticotrophin and glucocorticoid receptors and stress reactivity by single early-life pain experience in male and female rats. Psychoneuroendocrinology. 2013a;38:3015–3028. doi: 10.1016/j.psyneuen.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Victoria NC, Inoue K, Young LJ, Murphy AZ. A single neonatal injury induces life-long deficits in response to stress. Dev Neurosci. 2013b;35:326–337. doi: 10.1159/000351121. [DOI] [PubMed] [Google Scholar]
- Victoria NC, Karom MC, Eichenbaum H, Murphy AZ. Neonatal injury rapidly alters markers of pain and stress in rat pups. Dev Neurobiol. 2014;74:42–51. doi: 10.1002/dneu.22129. [DOI] [PubMed] [Google Scholar]
- Victoria NC, Karom MC, Murphy AZ. Analgesia for early-life pain prevents deficits in adult anxiety and stress in rats. Dev Neurosci. 2015;37:1–13. doi: 10.1159/000366273. [DOI] [PubMed] [Google Scholar]
- Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009a;34:1515–1525. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Walker SM. Neonatal pain. Paediatric anaesthesia. 2014;24:39–48. doi: 10.1111/pan.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009b;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wand GS, Schumann H. Relationship between plasma adrenocorticotropin, hypothalamic opioid tone, and plasma leptin. J Clin Endocrinol Metab. 1998;83:2138–2142. doi: 10.1210/jcem.83.6.4900. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wesson SC. Ligation of the ductus arteriosus: anesthesia management of the tiny premature infant. AANA J. 1982;50:579–582. [PubMed] [Google Scholar]
- WHO. March of Dimes, PMNCH, Save the Children, WHO. In: Howson CP, Lawn MKJE, editors. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012. [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollgarten-Hadamek I, Hohmeister J, Zohsel K, Flor H, Hermann C. Do school-aged children with burn injuries during infancy show stress-induced activation of pain inhibitory mechanisms? Eur J Pain. 2011;15(423):e421–e410. doi: 10.1016/j.ejpain.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Naltrexone modulates growth in infant rats. Life Sci. 1983;33:2449–2454. doi: 10.1016/0024-3205(83)90639-2. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res. 1991;542:318–323. doi: 10.1016/0006-8993(91)91585-o. [DOI] [PubMed] [Google Scholar]
- Zis AP, Haskett RF, Albala AA, Carroll BJ. Morphine inhibits cortisol and stimulates prolactin secretion in man. Psychoneuroendocrinology. 1984;9:423–427. doi: 10.1016/0306-4530(84)90050-7. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- Zurita A, Martijena I, Cuadra G, Brandao ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: reversal by naltrexone pretreatment. Behav Brain Res. 2000;117:163–171. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]