Summary
The effects of preceding endoscopic mucosal resection (EMR) on the efficacy and safety of radiofrequency ablation (RFA) for treatment of nodular Barrett’s esophagus (BE) is poorly understood. Prior studies have been limited to case series from individual tertiary care centers. We report the results of a large, multicenter registry. We assessed the effects of preceding EMR on the efficacy and safety of RFA for nodular BE with advanced neoplasia (high-grade dysplasia or intramucosal carcinoma) using the US RFA Registry, a nationwide study of BE patients treated with RFA at 148 institutions. Safety outcomes included stricture, gastrointestinal bleeding, and hospitalization. Efficacy outcomes included complete eradication of intestinal metaplasia (CEIM), complete eradication of dysplasia (CED), and number of RFA treatments needed to achieve CEIM. Analyses comparing patients with EMR before RFA to patients undergoing RFA alone were performed with Student’s t-test, Chi-square test, logistic regression, and Kaplan–Meier analysis. Four hundred six patients were treated with EMR before RFA for nodular BE, and 857 patients were treated with RFA only for non-nodular BE. The total complication rates were 8.4% in the EMR-before-RFA group and 7.2% in the RFA-only group (P = 0.48). Rates of stricture, bleeding, and hospitalization were not significantly different between patients treated with EMR before RFA and patients treated with RFA alone. CEIM was achieved in 84% of patients treated with EMR before RFA, and 84% of patients treated with RFA only (P = 0.96). CED was achieved in 94% and 92% of patients in EMR-before-RFA and RFA-only group, respectively (P = 0.17). Durability of eradication did not differ between the groups. EMR-before-RFA for nodular BE with advanced neoplasia is effective and safe. The preceding EMR neither diminished the efficacy nor increased complication rate of RFA treatment compared to patients with advanced neoplasia who had RFA with no preceding EMR. Preceding EMR is not associated with poorer outcomes in RFA.
Keywords: ablation technique, Barrett’s esophagus, safety, treatment efficacy
INTRODUCTION
Barrett’s esophagus (BE) is a premalignant condition characterized by the replacement of normal stratified squamous epithelium by intestinalized columnar epithelium.1–4 BE is associated with a markedly increased risk of esophageal adenocarcinoma, and the incidence of this cancer has increased approximately sixfold over the past four decades.5–9 Radiofrequency ablation (RFA) is safe and effective for eradication of the non-nodular dysplastic BE.10,11 For BE with nodular lesions, endoscopic mucosal resection (EMR) is commonly used to remove focal, nodular areas before performing RFA to eradicate the remaining intestinal metaplasia or dysplasia.12,13
The effects of preceding EMR on the efficacy and safety of RFA remains poorly understood. Theoretically, the scarring of the esophagus and the associated change in compliance of the esophageal wall caused by the preceding EMR may impact the efficacy of RFA and increase the risk of stricture or other complication.14 Previous work has reported a markedly diminished complete eradication of intestinal metaplasia (CEIM) rate and an increased stricture rate among patients who underwent EMR before RFA.14 However, two other studies have shown no increase in stricture rate with preceding EMR, nor decrease in rates of complete ablation of intestinal metaplasia.4,15 Despite the common performance of EMR prior to RFA in clinical practice, our understanding of the safety and efficacy of this treatment is limited, especially as it pertains to experience outside academic tertiary referral centers.
The aims of this study were to assess the effects of preceding EMR on the efficacy and safety of RFA for nodular BE, and to compare outcomes of EMR followed by RFA to those of RFA alone, in a nationwide, multicenter registry of patients treated with RFA for BE.
MATERIAL AND METHODS
US RFA patient registry
The US RFA Patient Registry, a multicenter study reporting processes and outcomes of care for patients treated with RFA for BE, enrolled patients at 148 institutions in the United States (113 community based, 35 academic affiliated). The registry assessed clinical outcomes of patients undergoing treatment of BE with RFA using the HALO Ablation Systems (GI Solutions, Sunnyvale, CA, a subsidiary of Covidien), and was funded by Covidien, Inc. The registry did not mandate protocols for care but provided a framework for treatment and follow-up of patients with Barrett’s esophagus. All physicians participating in this registry (n = 320) either elected to use Western institutional review board (IRB) approval, for those institutions with their own IRB, obtained IRB approval through their respective institutions.
Patient eligibility
Patients were enrolled from July 2007 to July 2011. Patients were eligible for inclusion in the registry if: (i) they had endoscopic evidence of columnar metaplasia in the tubular esophagus with accompanying biopsies demonstrating intestinal metaplasia (IM), and (ii) they were candidates for RFA for BE. Histology was classified using standardized grading, including non-dysplastic BE (NDBE), indefinite for dysplasia (IND), low-grade dysplasia (LGD), high-grade dysplasia (HGD), intramucosal carcinoma (IMC), and invasive adenocarcinoma.12,16 Those patients who had previously received one or more treatments prior to enrollment had collection of retrospective data, with subsequent prospective collection of data for ensuing visits. Patients who had not yet undergone treatment were prospectively enrolled. For purposes of this analysis, we included all eligible registry patients with confirmed HGD or IMC.
Data collection and record retention
Demographic data, baseline histology, endoscopic findings, number of treatment sessions, ablation outcomes, and complications were collected. Data were entered on standardized case report forms through an Internet-based, secured data entry and processing system. Data were analyzed by investigators in the clinical epidemiology program at the University of North Carolina Center for Gastrointestinal Biology and Disease (T32 DK07634), who had complete access to the data.
Treatment protocol
Data collated from previous clinical trials were distributed to the sites as a guideline for the treatment and follow-up protocol. However, because this is a registry study, institutions and individual physicians were free to deviate from the treatment protocols suggested in the literature, depending on patient requirements and the institution’s standard of care. The suggested treatment protocol provided to sites has been previously described.3 Our standardized protocol suggested medical therapy with twice-daily proton pump inhibitors (PPIs) to minimize any baseline inflammatory changes of the esophageal mucosa and to decrease acid reflux prior to and throughout RFA treatment, unless the patient had a documented history of antireflux surgery. The endoscopic resection device used to resect visible lesions varied by institution. RFA was recommended at 2 months after all visible lesions underwent successful EMR.
At the initial visit, patients were treated with one of two ablation devices: the HALO360 Circumferential Ablation System or the HALO90 Focal Ablation System. The decision regarding device was based on the burden of disease (Barrett’s segments of >3 cm being generally best treated with the circumferential catheter), as well as operator preference. Recommended treatment protocols were based on previously published data.10
Follow-up protocol
Following the initial RFA treatment, repeat endoscopy at 2–3 month intervals was recommended, with additional circumferential or focal RFA treatment for any visible residual BE. If no visible BE was observed, four-quadrant biopsies every cm were recommended throughout the length of the pretreatment BE. If these biopsies demonstrated no BE on pathologic review, the patients entered endoscopic surveillance. Initial surveillance was recommended at 3 months for patients with HGD or 6 months for patients with NDBE, IND, or LGD. If follow-up biopsies revealed IM or dysplasia, recurrent treatment with RFA was recommended.
Adverse events were reported using standardized forms and terminology. Each site also complied with reporting guidelines for their institution regarding reporting adverse events to their IRB and Food and Drug Administration under the Medical Device Reporting regulation in 21 C.F.R. Part 803.
Outcomes
Safety outcomes include stricture formation, gastrointestinal (GI) bleeding, and hospitalization. A stricture was defined as a narrowing of the lumen requiring dilation. GI bleeding was considered clinically significant if it resulted in hospitalization or blood transfusion. All treated patients were included in the safety analysis. Complication rates were reported per patient for both patients who received EMR before RFA and for patients who received RFA only.
The rates of CEIM, complete eradication of dysplasia (CED), and the number of RFA sessions were determined to assess treatment efficacy. CEIM was defined as at least one biopsy session negative for IM at least 12 months after initial RFA treatment. CED was defined as the absence of dysplasia from biopsy specimens at least 12 months after initial RFA treatment. All review was performed by local pathologists; results were reported on a standardized pathology form which specifically queried for the presence of intestinal metaplasia and dysplasia.
The efficacy analysis included patients who had a biopsy performed 12 months or more after initial RFA treatment. Efficacy outcomes were reported for patients who received the EMR before RFA treatment and for patients who received RFA only.
Statistical analysis
Statistical analysis was performed using Stata software (version 13.0; StataCorp LP, College Station, TX). For descriptive statistics, mean and standard deviations were reported for continuous variables, and percentages were reported for continuous variables. Comparative analyses were performed with Student’s t-test or the Wilcoxon rank sum test for continuous variables, and Pearson Chi-square test or Fisher exact test for categorical variables. Logistic regression analysis was performed to compare the efficacy and safety between the two groups. Kaplan–Meier analysis was used to generate the curves of durability of CEIM. Log-rank test was used to compare the differences in durability between the two treatment groups. P-values less than 0.05 were considered statistically significant.
RESULTS
Demographics and clinical characteristics
A total of 5521 patients with BE were enrolled in the US RFA Patient Registry. The patients were treated with RFA by 320 physicians at 148 institutions. Of these patients, 1263 (23%) had pretreatment histology HGD or IMC, and thus were included in the safety analysis. One thousand eighty-five (86%) were male, 1190 (94%) were Caucasian, and 1054 (83%) had HGD, and 209 (17%) had IMC. Of these patients, 406 (32%) patients underwent EMR before RFA, and 857 (68%) patients had RFA only.
Compared to patients with RFA alone, patients with EMR before RFA had worse pretreatment histology (IMC, 38% vs. 6%, P < 0.001), shorter BE segments (mean, 4.6 vs. 5.4 cm, P < 0.001), were less likely to be taking twice-daily PPIs (74% vs. 81%, P < 0.001), and were more likely to be treated at academic settings (62% vs. 53%, P = 0.003) (Table 1). Other baseline characteristics, such as age, gender, and race, were not statistically different between the two groups.
Table 1.
Safety cohort baseline characteristics
| EMR before RFA (n = 406) | RFA alone (n = 857) | P-value | |
|---|---|---|---|
| Age (mean ± SD, years) | 67.2 ± 10.2 | 66.3 ± 10.4 | 0.14 |
| Race, n (%) | |||
| Caucasian | 388 (95.6) | 802 (93.6) | 0.43 |
| Black | 5 (1.2) | 8 (0.9) | |
| Hispanic | 5 (1.2) | 13 (1.5) | |
| Asian/Pacific Islander/Other | 1 (0.3) | 5 (0.6) | |
| Unknown | 7 (1.7) | 29 (3.4) | |
| Male gender, n (%) | 350 (86) | 735 (86) | 0.83 |
| Length of BE segment (mean ± SD, cm) | 4.6 ± 3.6 | 5.4 ± 3.6 | <0.001 |
| Pre-treatment fundoplication, n (%) | 15 (3.7) | 31 (3.6) | 0.95 |
| Pre-treatment histology, n (%) | |||
| High-grade dysplasia | 252 (62) | 802 (94) | <0.001 |
| Intramucosal carcinoma | 154 (38) | 55 (6) | |
| Taking twice daily PPI, n (%) | 299 (74) | 693 (81) | 0.003 |
| Treatment at an academic medical center, n (%) | 252 (62) | 457 (53) | 0.003 |
| Mean follow-up time (mean ± SD, years) | 2.86 ± 1.53 | 2.76 ± 1.66 | 0.31 |
BE, Barrett’s esophagus; EMR, endoscopic mucosal resection; PPI, proton pump inhibitor; RFA, radiofrequency ablation; SD, standard deviation.
Safety outcomes
Complication rates among patients who had EMR before RFA were not different than RFA only (8.4% vs. 7.2%; P = 0.48). Stricture occurred in 29 patients (7.1%) treated with EMR before RFA compared with 52 patients (6.1%) treated with RFA only (P = 0.47). Three patients (0.7%) in the EMR-before-RFA group experienced clinically significant GI bleeding compared with eight patients (0.9%) in the RFA-only group. Of patients who underwent EMR before RFA, seven patients (1.7%) were hospitalized, compared with 11 patients (1.3%) who underwent RFA only. Rates of stricture, bleeding, and hospitalization were not significantly different between the two groups. No treatment-related deaths occurred in either group. Stratified by pretreatment histology, the complication rates among patients who had EMR before RFA and RFA only were also similar (Table 2). In the safety cohort, patients in the EMR-before-RFA group underwent slightly fewer total RFA treatment sessions compared with RFA-only group (2.8 vs. 3.2, P < 0.001). Logistic regression analysis was conducted to control for BE length, baseline histology, PPI compliance, and practice setting. Results showed that the stricture rate was not significantly different between the two groups (odds ratios [ORs] with 95% confidence intervals [CIs] were 1.4 (0.9–2.4), using RFA-only group as reference).
Table 2.
Safety outcomes
| EMR before RFA | RFA alone | P-value | |
|---|---|---|---|
| All patients, n (%) | 406 | 857 | |
| Any complication† | 34 (8.4) | 62 (7.2) | 0.48 |
| Stricture | 29 (7.1) | 52 (6.1) | 0.47 |
| Bleeding | 3 (0.7) | 8 (0.9) | 0.73 |
| Hospitalization‡ | 7 (1.7) | 11 (1.3) | 0.54 |
| High-grade dysplasia, n (%) | 252 | 802 | |
| Any complication† | 26 (10.3) | 60 (7.5) | 0.15 |
| Stricture | 21 (8.3) | 50 (6.2) | 0.25 |
| Bleeding | 3 (1.2) | 8 (1.0) | 0.79 |
| Hospitalization‡ | 7 (2.8) | 11 (1.4) | 0.13 |
| Intramucosal carcinoma, n (%) | 154 | 55 | |
| Any complication† | 8 (5.2) | 2 (3.6) | 0.64 |
| Stricture | 8 (5.2) | 2 (3.6) | 0.64 |
| Bleeding | 0 | 0 | – |
| Hospitalization‡ | 0 | 0 | – |
| Total RFA treatments (mean ± SD) | 2.8 ± 1.9 | 3.2 ± 2.0 | <0.001 |
| Circumferential treatments | 0.7 ± 0.8 | 0.9 ± 0.9 | 0.08 |
| Focal treatments | 2.1 ± 1.6 | 2.4 ± 1.8 | 0.29 |
Some patients experience >1 complication;
Hospitalizations were secondary to post-procedural hemorrhage. EMR, endoscopic mucosal resection; RFA, radiofrequency ablation; SD, standard deviation.
Efficacy outcomes
Of the 1263 patients with HGD or IMC at baseline, 994 had biopsies performed 12 months or more after initial treatment and thus were included in the efficacy analysis. Of these patients, 331 had EMR before RFA, and 663 had RFA only. Patients treated with EMR before RFA had similar rates of CEIM and similar rates of CED, compared with those treated with RFA only (see Table 3). CEIM was achieved in 277 of patients (84%) who had EMR before RFA and 554 of patients (84%) who received RFA only (P = 0.96). CED was achieved in 312 patients (94%) who had EMR before RFA and 609 patients (92%) who received RFA only (P = 0.17). CEIM and CED rates were also stratified by pretreatment histology. When stratified, the CEIM and CED rates were comparable between the two treatment groups (Table 3). In the efficacy cohort, the number of total RFA sessions required for the EMR-before-RFA group was fewer than that for the RFA-only group (mean, 3.0 vs. 3.5, P < 0.001). Controlling for BE length, baseline histology, PPI compliance and practice setting, neither CEIM rate nor CED rate was significantly different between the two groups (ORs with 95% CIs are 1.1 [0.8–1.5] and 1.3 [1.0–1.7], for CEIM and CED rate, respectively, using RFA-only as reference group.
Table 3.
Efficacy outcomes
| EMR before RFA | RFA alone | P-value | |
|---|---|---|---|
| All patients, n (%) | 331 | 663 | |
| CED | 312 (94) | 609 (92) | 0.17 |
| CEIM | 277 (84) | 554 (84) | 0.96 |
| High-grade dysplasia, n (%) | 204 | 628 | |
| CED | 192 (94) | 574 (92) | 0.21 |
| CEIM | 173 (85) | 521 (83) | 0.54 |
| Intramucosal carcinoma, n (%) | 127 | 35 | |
| CED | 120 (95) | 35 (100) | 0.16 |
| CEIM | 104 (82) | 33 (94) | 0.07 |
| Total RFA treatments, (mean ± SD) | 3.0 ± 1.9 | 3.5 ± 2.1 | <0.001 |
| Circumferential treatments | 0.7 ± 0.8 | 0.9 ± 0.9 | 0.001 |
| Focal treatments | 2.3 ± 1.7 | 2.6 ± 1.8 | 0.46 |
CED, complete eradication of dysplasia; CEIM, complete eradication of intestinal metaplasia; EMR, endoscopic mucosal resection; RFA, radiofrequency ablation; SD, standard deviation.
Five out of 331 patients (1.5%) who had EMR before RFA progressed to invasive adenocarcinoma, whereas 24 out of the 663 patients (3.6%) who had RFA-only developed invasive adenocarcinoma (P = 0.07). Among the five patients in the EMR-before-RFA group, two (1.0%) patients progressed from HGD and three (2.4%) patients progressed from IMC. Among the 24 patients in the RFA-only group, 23 (3.7%) patients progressed from HGD and one (2.9%) patients progressed from IMC.
Durability outcomes
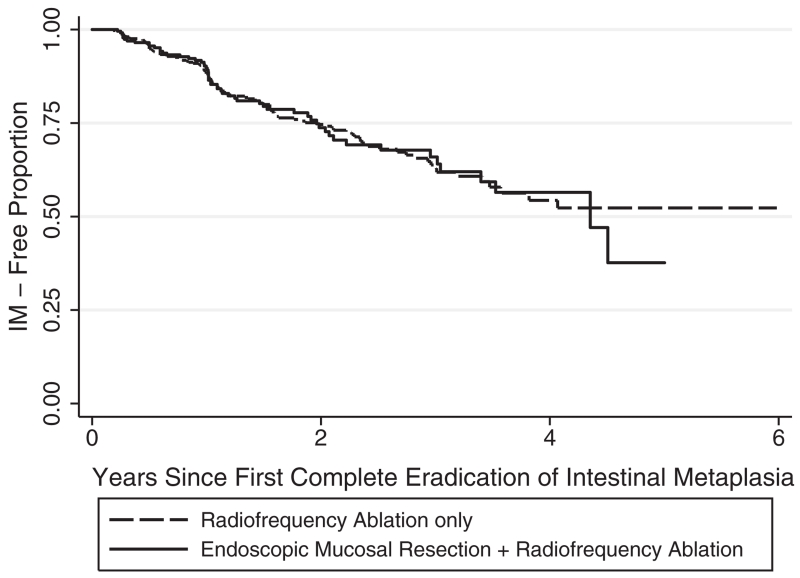
Among the patients who achieved CEIM, 165 out of 831 (19.9%) experienced a recurrence of IM, including 57 of 277 patients (21%) who were treated with EMR prior to RFA, and 108 of 554 patients (19%) who were treated with RFA alone. The recurrence of IM were not significantly different (log-rank P = 0.96). The Kaplan Meier curves were shown in Figure 1.
Fig. 1.
Kaplan–Meier analysis of durability of complete eradication of intestinal metaplasia (CEIM).
DISCUSSION
We used the US RFA Registry to assess the effects of preceding EMR on the efficacy and safety of RFA for BE with advanced neoplasia, and to compare these patients to those who had RFA only for non-nodular BE with advanced neoplasia, in an effort to understand the impact of performing EMR prior to RFA. Both treatment techniques were found to be equally effective and safe. Among the 1263 patients in the safety group, approximately 7% of patients experienced complications, the most common being strictures. In the efficacy analysis of the 994 patients, the efficacy of EMR/RFA treatment was comparable to RFA alone with approximately 84% of patients achieving CEIM and >90% of patients achieving CED.
The impact of the preceding EMR on the efficacy and safety of the RFA treatment remains unclear, and previous data are conflicting. Several studies investigating the safety and efficacy of RFA for BE have included patients undergoing EMR, however, the lack of two distinct groups (EMR-before-RFA and RFA alone) prevented comparison of their effects on the safety and efficacy.17–20 A study from the Mayo Clinic has reported a CEIM rate of only 43% in EMR-before-RFA treatment group (n = 44) compared with 74% in RFA-only group (n = 46), suggesting that preceding EMR may diminish the efficacy of RFA.14 A second study from the Netherlands found that EMR was associated with a poor initial response to circumferential RFA, if that EMR resulted in regeneration of tissue that was Barrett’s, as opposed to a squamous island (OR 4.7; 95% CI 1.1–20.0).21 In contrast, a study from the University of North Carolina (UNC) reported no statistically significant differences in CEIM rates between EMR-before-RFA and RFA-only groups (n = 148; 88.0% vs. 77.6%, P = 0.13).4 Studies from other western countries also reported comparable efficacy between the two groups.15,22 A recent study from the UK National Halo RFA Registry reported that the dysplasia clearance among patients underwent EMR before RFA to be comparable to that among patients treated with RFA alone (79% vs. 71%, P = 0.20).22 The results from a tertiary center in France, although limited by small number of patients (16 and 18 patients, respectively in each group), also suggested that EMR before RFA has not diminished the efficacy of RFA (56% vs. 44%, P = 0.73).15
The inconsistencies in the published results may be attributed to the limits of tertiary care single-center studies. These studies are subject to local expertise and individual assessment standards, were varied in pretreatment histology, and some were limited by small study populations. Because nodularity is more common among advanced neoplasia, we restricted our analysis to patients with pretreatment histology of high-grade dysplasia and intramucosal carcinoma to improve comparability between the nodular and non-nodular groups. In addition, we compared the efficacy and safety between the two groups by pre-treatment histology to avoid potential confounding.
Complication rates of EMR performed in conjunction with RFA in the literature are varied. The Mayo Clinic study reported a stricture rate of 14% for EMR-before-RFA group and 9% for RFA-only group.14 The UK registry study also found that patients who underwent EMR before RFA were more likely to develop strictures compared to those who underwent RFA alone (12% vs. 5.9%; P = 0.04).22 However, in the UNC study, the stricture rate was not higher in the group with preceding EMR (4.6% in EMR/RFA vs. 7.7% in RFA alone, P = 0.53).4 Our results are most consistent with the findings from this study, in that preceding EMR did not increase the risk of stricture or other complications.
Our study reported comparable durability of CEIM between the two groups (21% vs. 19%, P = 0.96). Consistent with our results, a US multicenter consortium study also reported durability of CEIM in subjects who had an EMR prior to RFA to be comparable to that of subjects who had RFA alone after controlling for age and gender (n = 229, OR = 1.18 [0.60, 2.34], P = 0.62).23 Another US multicenter follow-up study reported that patients who underwent an EMR prior to RFA were actually more likely to maintain durable eradication of IMC/dysplasia (P = 0.03).24 However, these results may be subject to the small patient number (n = 36).
Our data have important implications for patient management. If patients with advanced neoplasia in BE and nodularity had only a 43% rate of CEIM, as reported by Okoro et al.,14 clinicians might be more likely to consider this therapy inadequate, opting instead for esophagectomy in patients with advanced neoplasia and nodularity. On the other hand, the higher rates of eradication reported in this study are reassuring that EMR, with resection of neoplasia to rule out the presence of submucosal disease, lymphovascular invasion or other characteristics of locally advanced disease, followed by RFA, is adequate therapy for these patients.
We found that the original BE length of patients treated with EMR before RFA was somewhat shorter than that of patients treated with RFA only (4.6 vs. 5.4 cm, P < 0.001). This small difference in baseline BE length was unlikely to have confounded our results. Recent studies have demonstrated that EMR with RFA was effective and safe in the treatment of BE independent of BE length, although more treatment sessions were required for treating longer BE segments.25,26 Consistent with these findings, our results have shown that fewer RFA sessions were performed in the EMR-before-RFA group compared with the RFA-only group (2.8 vs. 3.2, P < 0.001). Our multivariate regression analysis also suggest that both safety and efficacy are comparable between the two groups after controlling for BE length and other unbalanced distribution of demographic/clinical characteristics observed in Table 1.
Our study’s limitations must be noted. This study was strictly observational and treatment paradigms could not be mandated. While BE with nodularity and non-nodular BE are different conditions, comparison of the two groups allows inference of the effect of preceding EMR on subsequent RFA treatment. Additionally, given the size and nature of our study, no re-interpretation of pathological specimens by a central lab was possible, and local practices as to the histological interpretation were used. Another limitation of this study is the lack of information on the total mucosal area removed by EMR, which may have a potential effect on efficacy and stricture rates. Additionally, a small proportion of patients were enrolled retrospectively, which may potentially result in underestimation of complication rates and misclassification errors. However, given that the proportion of subjects with retrospective enrollment was non-differential between the nodular and non-nodular groups, such errors would also be expected to be non-differential.
There are several strengths to our study. Our study is the largest cohort to date to assess the effects of EMR before RFA on the efficacy and safety outcomes of RFA. Our study is a nationwide, multicenter registry study, including both academic-affiliated and community-based institutions. Therefore, our results are more representative of real-life practices and provide increased generalizability. Study definitions were a priori, and data were collected in a standardized fashion. Furthermore, our study addressed the effect of initial EMR on the efficacy and safety of RFA, an area with conflicting prior reports.
In summary, EMR-before-RFA for nodular BE is effective and safe for the treatment of BE with advanced neoplasia, and comparable in safety, efficacy and durability to RFA only for non-nodular BE in this largest reported cohort of patients.
Acknowledgments
Financial support: This research was funded by T32 DK07634 from the National Institutes of Health and GI Solutions, a subsidiary of Covidien Medical. Dr Shaheen receives research funding from Covidien Medical, NeoGenomics, CSA Medical, Takeda Pharmaceuticals and Oncoscope, and is a consultant for Oncoscope. Drs Pruitt, Komanduri, Wolfsen, Chmielewski, Corbett, and Chang receive research funding from Covidien Medical.
Footnotes
Author contributions: Conception and design: Nan Li, Sarina Pasricha, William J. Bulsiewicz, Ron E. Pruitt, Srinadh Komanduri, Herbert C. Wolfsen, Gary W. Chmielewski, F. Scott Corbett, Kenneth J. Chang, Nicholas J. Shaheen.
Analysis and interpretation of data: Nan Li, Sarina Pasricha, William J. Bulsiewicz, Nicholas J. Shaheen.
Drafting of the manuscript: Nan Li, Sarina Pasricha, Nicholas J. Shaheen.
Editing and critical revision of the manuscript for important intellectual content: Nan Li, Sarina Pasricha, William J. Bulsiewicz, Ron E. Pruitt, Srinadh Komanduri, Herbert C. Wolfsen, Gary W. Chmielewski, F. Scott Corbett, Kenneth J. Chang, Nicholas J. Shaheen.
Conflicts of interest: The other authors have no conflicts to declare.
References
- 1.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373:850–61. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ, Association AG. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen NJ, Kim HP, Bulsiewicz WJ, et al. Prior fundoplication does not improve safety or efficacy outcomes of radiofrequency ablation: results from the U.S. RFA registry. J Gastrointest Surg. 2013;17:21–8. doi: 10.1007/s11605-012-2001-8. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 4.Kim HP, Bulsiewicz WJ, Cotton CC, et al. Focal endoscopic mucosal resection before radiofrequency ablation is equally effective and safe compared with radiofrequency ablation alone for the eradication of Barrett’s esophagus with advanced neoplasia. Gastrointest Endosc. 2012;76:733–9. doi: 10.1016/j.gie.2012.04.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iftikhar SY, James PD, Steele RJ, Hardcastle JD, Atkinson M. Length of Barrett’s oesophagus: an important factor in the development of dysplasia and adenocarcinoma. Gut. 1992;33:1155–8. doi: 10.1136/gut.33.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 7.Cook MB, Wild CP, Everett SM, et al. Risk of mortality and cancer incidence in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2090–6. doi: 10.1158/1055-9965.EPI-07-0432. [DOI] [PubMed] [Google Scholar]
- 8.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 9.Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070–4. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 11.Sharma VK, Jae Kim H, Das A, Wells CD, Nguyen CC, Fleischer DE. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol. 2009;104:310–17. doi: 10.1038/ajg.2008.142. [DOI] [PubMed] [Google Scholar]
- 12.Wang KK, Sampliner RE. Gastroenterology PPCotACo. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Fernández-Sordo J, Parra-Blanco A, García-Varona A, et al. Endoscopic resection techniques and ablative therapies for Barrett’s neoplasia. World J Gastrointest Endosc. 2011;3:171–82. doi: 10.4253/wjge.v3.i9.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okoro NI, Tomizawa Y, Dunagan KT, Lutzke LS, Wang KK, Prasad GA. Safety of prior endoscopic mucosal resection in patients receiving radiofrequency ablation of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2012;10:150–4. doi: 10.1016/j.cgh.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caillol F, Bories E, Pesenti C, et al. Radiofrequency ablation associated to mucosal resection in the oesophagus: experience in a single centre. Clin Res Hepatol Gastroenterol. 2012;36:371–7. doi: 10.1016/j.clinre.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Voltaggio L, Montgomery EA, Lam-Himlin D. A clinical and histopathologic focus on Barrett esophagus and Barrett-related dysplasia. Arch Pathol Lab Med. 2011;135:1249–60. doi: 10.5858/arpa.2011-0019-RA. [DOI] [PubMed] [Google Scholar]
- 17.Perry KA, Walker JP, Salazar M, Suzo A, Hazey JW, Melvin WS. Endoscopic management of high-grade dysplasia and intramucosal carcinoma: experience in a large academic medical center. Surg Endosc. 2014;28:777–82. doi: 10.1007/s00464-013-3240-9. [DOI] [PubMed] [Google Scholar]
- 18.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for Barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23–9. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Pouw RE, Gondrie JJ, Sondermeijer CM, et al. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;12:1627–36. doi: 10.1007/s11605-008-0629-1. discussion 36–7. [DOI] [PubMed] [Google Scholar]
- 20.Herrero LA, van Vilsteren FG, Pouw RE, et al. Endoscopic radiofrequency ablation combined with endoscopic resection for early neoplasia in Barrett’s esophagus longer than 10 cm. Gastrointest Endosc. 2011;73:682–90. doi: 10.1016/j.gie.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 21.van Vilsteren FG, Alvarez Herrero L, Pouw RE, et al. Predictive factors for initial treatment response after circumferential radiofrequency ablation for Barrett’s esophagus with early neoplasia: a prospective multicenter study. Endoscopy. 2013;45:516–25. doi: 10.1055/s-0032-1326423. [DOI] [PubMed] [Google Scholar]
- 22.Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic Barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145:87–95. doi: 10.1053/j.gastro.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 23.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US multicenter consortium. Gastroenterology. 2013;145:79–86. e1. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss AC, Agoston AT, Dulai PS, Srivastava A, Rothstein RI. Radiofrequency ablation for Barrett’s-associated intramucosal carcinoma: a multi-center follow-up study. Surg Endosc. 2014;28:3366–72. doi: 10.1007/s00464-014-3629-0. [DOI] [PubMed] [Google Scholar]
- 25.Dulai PS, Pohl H, Levenick JM, Gordon SR, MacKenzie TA, Rothstein RI. Radiofrequency ablation for long- and ultralong-segment Barrett’s esophagus: a comparative long-term follow-up study. Gastrointest Endosc. 2013;77:534–41. doi: 10.1016/j.gie.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Infantolino A, Bulsiewicz W, Ertan A, et al. Length of Barrett’s esophagus predicts likelihood of complete eradication of intestinal metaplasia and number of treatment sessions of radiofrequency ablation (RFA): results from the U.S. RFA registry. Gastrointest Endosc. 2012;75:AB121. [Google Scholar]