Abstract
Jaundice in the newborn period can be physiologic and is often due to benign causes. Jaundice due to conjugated hyperbilirubinemia extending beyond the second week of life may be an early sign of several cholestatic or metabolic liver diseases, and it requires logical and timely analysis so that specific treatments can be initiated. A1AT deficiency is the most common genetic cause of pediatric liver disease and transplantation, and it must be considered when evaluating cholestatic infants. Here, we present an unusual case of A1AT deficiency with severe infantile cholestasis and rapid decompensation in the first 4 months of life, where in-depth but timely diagnosis was crucial for the appropriate intervention to take place.
Keywords: alpha-1 antitrypsin deficiency, neonatal cholestasis, liver transplantation, hyperbilirubinemia, cirrhosis, metabolic disease
Introduction
Direct hyperbilirubinemia reflects impaired bile flow, and it may be an early sign of idiopathic neonatal hepatitis, biliary atresia, A1AT deficiency, Alagille syndrome, tyrosinemia, disorders of energy metabolism, and bile acid synthetic defects. Cholestasis affects 1 in 2500 infants, and it requires logical and timely analysis so that specific treatments can be initiated. Infants with biliary atresia, the most common cause of pediatric liver transplantation, have better outcomes if Kasai hepatic portoenterostomy is performed before 45-60 days of life (1). Other, less straight-forward diagnoses are potentially delayed in a well-appearing infant. A classic example of such clinical heterogeneity is A1AT deficiency (A1ATD), the most common inherited cause of liver disease in children. In A1ATD, most cases of neonatal cholestasis gradually recover. We report an unusual case of infantile A1ATD, with severe persistent cholestasis and rapid deterioration in the first 4 months of life, requiring early liver transplantation.
Case Presentation
The patient is a term 2807g infant girl born via uncomplicated SVD. There was no history of neonatal jaundice. Newborn screen was normal. Family history was unremarkable. At ~DOL 42, the family observed intermittent yellowing of her skin, with pigmented stools. At DOL 109, her pediatrician noted jaundice. Initial labs revealed cholestatasis, with elevated transaminases (ALT 190 IU/L, ref 14-54; AST 360 IU/L, ref 20-63), direct hyperbilirubinemia (TSB 8.3 mg/dL, ref 0.2-1.3; direct bilirubin 4.5 mg/dL, ref 0.1-0.5), and elevated GGT (781 IU/L, ref <121). There was mildly impaired hepatic synthetic function (serum albumin 2.9 g/dL, ref 3.8-5.4; ammonia 61 μmol/L, ref 12-38), but coagulation studies were preserved (PT 16.1 sec, ref 11.5-16.1; PTT 31 sec, ref 27-39.4; INR 1.4, ref 0.9-1.3). She was referred to our center for urgent evaluation.
On exam, she was alert and afebrile, but icteric, with hepatosplenomegaly, prominent abdominal vessels, and no appreciable ascites. There were no obvious dysmorphic features. Several acholic stools were observed. Ultrasound showed a normal gallbladder without ductal dilatation, heterogeneous liver echotexture, and normal Doppler flow. Labs during this admission showed normal electrolytes, glucose, thyroid studies, lactate, pyruvate, and uric acid. Infectious work-up was negative. She had a right posterior embryotoxon on fundoscopic exam, raising the question of Alagille syndrome. Spine films showed no vertebral anomalies. Echocardiogram was normal.
HIDA scan on DOL 115 showed no excretion in the intestine at 24 hours, concerning for biliary atresia. She soon developed significant abdominal distention and intermittent hypoglycemia. Repeat labs were unchanged. The patient was readmitted for intraoperative cholangiogram and liver biopsy on DOL 116. A hard nodular liver was noted with normal flow into tiny bile ducts, favoring Alagille syndrome over biliary atresia. A large volume of dark yellow ascites fluid was drained. This appeared transudative, but due to leukocytosis, antibiotics were started for presumed cholangitis. Acylcarnitine profile, urine succinylacetone, serum iron, and sweat chloride were normal. Serum amino acids and urine organic acids showed non-specific abnormalities. Of note, her serum A1AT level was low (47 mg/dL, ref 88-174), with a PiZZ phenotype.
In the weeks following the biopsy, she progressively decompensated. She had recurrent ascites while on diuretics, requiring two rounds of paracentesis. She was unable to maintain euglycemia without IVF or continuous feeds. She had marked coagulopathy (peak INR 5.7) that did not correct with FFP or vitamin K. Liver indices acutely worsened, requiring urgent listing for liver transplantation. There was a dramatic rise in her bilirubin (Supplemental Figure 1), with TSB 47.5 mg/dL and direct bilirubin 23.4 mg/dL just 4 weeks after biopsy. These elevations were highly unusual for A1ATD. Due to this severe cholestasis, other etiologies were re-considered; however, no specific causes were identified. At age 5 mos, she underwent orthotopic liver transplantation from a 4-mo deceased donor 5 weeks after biopsy. Her liver function and hyperbilirubinemia rapidly improved after transplant (Supplemental Figure 1). Post-operative course was complicated by parainfluenza virus requiring intubation, and a pericardial effusion which resolved. She was discharged home on POD #35, and continues to do well 12 months post-transplant. She is the youngest patient we have transplanted for A1ATD at our center.
Pathology
Liver biopsy
The wedge biopsy showed marked nodularity of the liver parenchyma, with regenerative nodules separated by bands of fibrous tissue bridging portal areas (Figure 1A). There was marked ductular proliferation within portal areas with abnormal forms, including some mimicking the lesions of ductal plate malformation, as seen on both the CK7 (Figure 1B) and AE1/3 (Figure 1C) cytokeratin stains. The AE1/3 stain showed an occasional well-preserved interlobular bile duct. The exuberant bile ductular reaction made it difficult to differentiate the interlobular ducts from the ductular reaction in many portal areas. Overall, there appeared to be decreased numbers of interlobular bile ducts suggesting an element of paucity, though difficult to assess. There was some aberrant CK7 staining in hepatocytes. Bile ductular as well as canalicular plugs (Figure 1D) were noted with prominent acinar transformation of hepatocytes. Giant cell transformation with ballooning of hepatocytes was present. Portal areas showed minimal inflammation with mainly lymphocytes and macrophages. The PASD stain (Figure 1E) showed prominent granules in periportal hepatocytes as well as in Kupffer cells and portal macrophages. An A1AT stain (Figure 1F) showed a high background with staining of all hepatocytes, but accentuated cytoplasmic staining in many periportal and zone 2 hepatocytes. The trichrome stain (Figure 1G) showed marked bridging fibrosis, regenerative nodules, and fibrosis within nodules dissecting groups of hepatocytes. A few residual lobules with central veins and sinusoidal fibrosis were also seen. EM (Figure 2) confirmed multiple globular inclusions within dilated ER cisterns, near secondary lysosomes.
Figure 1. Histopathology of liver biopsy (A-G) and explanted liver (H-I) in A1ATD.
A. H&E stain, 40x. B. CK7 immunohistochemistry, 20x. C. AE1/3 immunohistochemistry, 200x. D. H&E stain, 400x. E. PASD stain, 400x. F. A1AT immunohistochemistry, 200x. G. Trichrome stain, 20x. H. H&E stain, 4x. I. H&E stain, 20x.
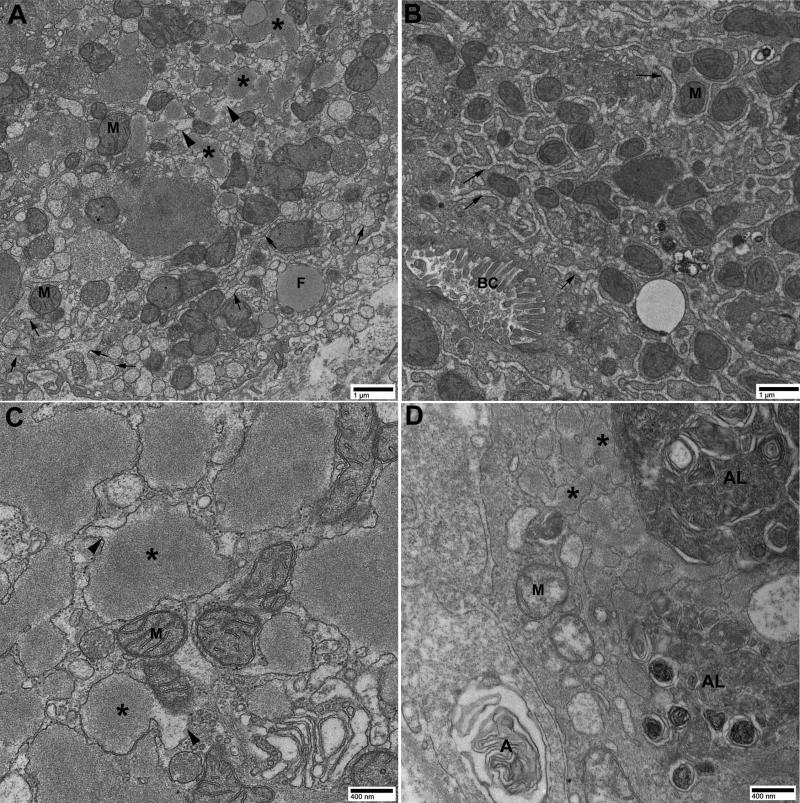
Figure 2. Electron microscopy of liver biopsy.
Analysis of hepatocyte cytoplasm shows dilated smooth and rough ER cisterns containing multiple deposits of globular, amorphous and heterogeneous bodies of different sizes, displacing mitochondria (M) and other organelles. In Zone 1 hepatocytes (panel A), there was predominantly dilated ER containing polymerized ATZ inclusions (*). There was also some dilated ER with little or no ATZ inclusions (arrows), and dilated ER with less polymerized intermediate ATZ protein (arrowheads). In zones 2-3 hepatocytes (panel B), there was predominantly dilated ER with little or no ATZ inclusions (arrows). Panel C clearly shows higher magnification of dilated ER containing polymerized ATZ inclusions (*) connected to dilated ER with less polymerized intermediate ATZ protein (arrowheads). Panel D shows autophagolysosomes (AL) and autophagosomes (A) in zone 1 adjacent to dilated ER containing polymerized ATZ inclusions (*). These changes are characteristic of A1ATD. Panels A and B = 15000x. Panels C and D = 40000x. F = fat. BC = bile canaliculus.
Liver Explant
The liver explant showed significant worsening and marked cholestasis, with canalicular and bile ductular plugs, bridging fibrosis, regenerative nodules, and cirrhosis (Figures H and I). Prominent ballooning and intracellular cholestasis was noted. The trichrome stain highlighted bridging fibrosis and sinusoidal fibrosis. It showed marked ductular proliferation, better seen in the CK7 stain. The AE1/3 cytokeratin stain also showed ductular proliferation and portal areas with larger caliber interlobular ducts, though this was not evident in all portal areas. The degree of ductular proliferation made this distinction difficult in many areas. No ectopic hepatocyte staining was noted with CK7, a feature frequently seen with paucity of bile ducts. An A1AT stain showed diffuse staining of nodules with an accentuated staining in the periportal hepatocytes. Mixed micro- and macrovesicular steatosis was found in 80% of the liver parenchyma.
Discussion
Our case highlights several important lessons in the evaluation and progression of infantile cholestasis. Although this patient was initially well-appearing, she did have subtle signs of feeding issues and poor weight gain, with intermittent skin and stool color changes prior to our evaluation at DOL 109. A heightened awareness for signs of possible cholestasis in the first 2 months of life is essential for both clinicians and families, especially to avoid delayed diagnosis of biliary atresia, since only 20% of infants achieve successful biliary drainage if Kasai is performed after DOL 90 (2). Successful anticipatory guidance for hypopigmented stools continues to be an area of quality improvement (3-5).
A precise cause of infantile hepatitis and cirrhosis is often difficult to define. The elevated GGT placed our patient in a category that includes biliary atresia, Alagille's syndrome, PFIC3, and possibly cystic fibrosis. This differential is usually resolved by a combination of clinical observation, imaging, determination of A1AT protein phenotype, liver biopsy, and/or genetic screening. The delay of a clear-cut diagnosis in this infant is partly the result of decisions made decades ago in the US to abandon newborn screening for A1ATD because of low yield and sparse clinical effects in childhood. Of note, a recent workshop recommended a series of pilot studies focused on generating new data on the risks and benefits of newborn screening for A1ATD (6). Our case exemplifies the diagnostic dilemmas often encountered when evaluating infantile cholestasis. This patient had signs of cholestasis and portal hypertension at a very young age, requiring inpatient admission to expedite her work-up. Since she deteriorated so rapidly after initial presentation, concurrent investigations led to some conflicting results. She had findings suspicious for biliary atresia and Alagille's syndrome, and while many screening labs were still pending, the liver biopsy performed at the time of intra-operative cholangiogram was essential in both diagnosing A1ATD and staging her disease, as well as ruling out other etiologies. Her initial liver histology was fairly impressive, since ATZ globules may not be readily apparent in biopsies from PiZZ infants under the age of 3 months (7).
Our patient's subsequent clinical course is also quite unusual, since she progressed to decompensated cirrhosis in a month's time, with a rapid rise in her bilirubin atypical for A1ATD, and worsening fibrosis at time of liver transplant only 1 month later. Based on our single center experience, only 14 children have undergone orthotopic liver transplantation in the past 13 years, and the mean age at transplant was 4 years old. In the majority of cases diagnosed, infantile cholestasis gradually improves in A1ATD (8, 9). Persistently elevated bilirubin has been shown to be a marker of poor prognosis (10). A recent study reported that PiZZ infants with a mean deviation in total bilirubin of ~5 mg/dL above the normal mean will have a bad outcome requiring liver transplantation (10). Our patient was almost 10x this deviation. As with any rapidly progressive organ failure, there exists the possibility of unidentified comorbidities, such as infection, medications, or multiple metabolic/genetic insults. Coexisting paucity in A1ATD could explain this severe degree of cholestasis to some extent, but could not be proven beyond a doubt on both the biopsy and the explant. There have been isolated reports of rapid deterioration and acute liver failure in infants with A1ATD and concomitant infections, such as congenital CMV viremia, which was ruled out in our patient (11, 12). Since she required early liver transplantation, we were unable to send whole-exome sequencing, which may have revealed a “second hit”. Nevertheless, her post-transplant course has thus far been reassuring and does not suggest co-existing systemic disease.
A1ATD is the most common inherited pediatric liver disorder, and the most frequent genetic cause of liver transplantation in children (reviewed in (13)). In its classical form, A1ATD is an autosomal co-dominant disorder that affects as many as 1 in 3000 live births in the United States and Europe. The genetic defect in A1ATD involves homozygosity for the Z allele (PiZZ) in the SERPINA1 gene on human chromosome 14, encoding a single base pair substitution at Glu342Lys. The resulting toxic gain-of-function mutation generates misfolded ATZ protein that accumulates in the ER of hepatocytes, forming insoluble globules that are PAS+/diastase-resistant. Chronic hepatocyte injury can progress over time to cirrhosis and hepatocellular carcinoma in some individuals. Liver transplantation remains the only treatment for patients with severe liver disease; however, it is often not required in childhood. In fact, prospective studies of a Swedish cohort of PiZZ newborns reported that only ~8% of homozygotes develop clinically significant liver disease in the first 4 decades of life (14, 15). This suggests the role of other genetic and/or environmental modifiers of disease susceptibility; however, it is difficult to predict which factors predispose some patients to develop liver disease while sparing others. Interestingly, severe neonatal A1ATD may be associated with infants having low birth weight or poor weight gain (8, 15). In summary, manifestations and prognosis of A1ATD are highly variable. Our patient represents an unusual case of aggressive A1ATD-related liver disease leading to end-stage cirrhosis in the first 4 months of life, and this suggests very early onset of chronic hepatocellular injury, possibly during neonatal or even fetal development.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by 5P01DK0969901 (DBS), 1T32HD071834 (ZK), 5K12HD052892 (ZK), the Hillman Foundation (ZK), and the Alpha-1 Foundation (ZK). We also appreciate technical assistance from the University of Pittsburgh's Center for Biologic Imaging Core A and the Division of Pediatric Pathology.
Abbreviations
- A1AT
alpha-1 antitrypsin
- A1ATD
alpha-1 antitrypsin deficiency
- DOL
day of life
- EM
electron microscopy
- ER
endoplasmic reticulum
- FFP
fresh frozen plasma
- H&E
hematoxylin and eosin
- HIDA
hepatobiliary iminodiacetic acid
- IVF
intravenous fluids
- PASD
Periodic acid–Schiff/diastase stain
- SVD
spontaneous vaginal delivery
- TSB
total serum bilirubin
Footnotes
DISCLOSURES/CONFLICT OF INTEREST:
The authors do not have any disclosures or conflicts of interest.
REFERENCES
- 1.Serinet MO, Wildhaber BE, Broue P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123:1280–1286. doi: 10.1542/peds.2008-1949. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RA, Barker CC, Roberts EA, et al. Biliary atresia: the Canadian experience. J Pediatr. 2007;151:659–665. 665, e651. doi: 10.1016/j.jpeds.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 3.Gu YH, Yokoyama K, Mizuta K, et al. Stool color card screening for early detection of biliary atresia and long-term native liver survival: a 19-year cohort study in Japan. J Pediatr. 2015;166:897–902. e891. doi: 10.1016/j.jpeds.2014.12.063. [DOI] [PubMed] [Google Scholar]
- 4.Mogul D, Zhou M, Intihar P, Schwarz K, Frick K. Cost-effective analysis of screening for biliary atresia with the stool color card. J Pediatr Gastroenterol Nutr. 2015;60:91–98. doi: 10.1097/MPG.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 5.Franciscovich A, Vaidya D, Doyle J, et al. PoopMD, a Mobile Health Application, Accurately Identifies Infant Acholic Stools. PLoS ONE. 2015;10:e0132270. doi: 10.1371/journal.pone.0132270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teckman J, Pardee E, Howell RR, et al. Appropriateness of newborn screening for alpha1-antitrypsin deficiency. J Pediatr Gastroenterol Nutr. 2014;58:199–203. doi: 10.1097/MPG.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talbot IC, Mowat AP. Liver disease in infancy: histological features and relationship to alpha-antitrypsin phenotype. J Clin Pathol. 1975;28:559–563. doi: 10.1136/jcp.28.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aagenaes O, M TF, Elgjo K, Munthe E, Hovig T. Pathology and pathogenesis of liver disease in alpha-1-antitrypsin deficient individuals. Postgrad Med J. 1974;50:365–375. doi: 10.1136/pgmj.50.584.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McPhie JL, Binnie S, Brunt PW. Alpha 1-antitrypsin deficiency and infantile liver disease. Arch Dis Child. 1976;51:584–588. doi: 10.1136/adc.51.8.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pferdmenges DC, Baumann U, Muller-Heine A, Framke T, Pfister ED. Prognostic marker for liver disease due to alpha1-antitrypsin deficiency. Klin Padiatr. 2013;225:257–262. doi: 10.1055/s-0033-1347196. [DOI] [PubMed] [Google Scholar]
- 11.Arias P, Kerner J, Christofferson M, Berquist W, Park KT. Misdiagnosis of alpha-1 antitrypsin phenotype in an infant with CMV infection and liver failure. Dig Dis Sci. 2014;59:1710–1713. doi: 10.1007/s10620-014-3094-6. [DOI] [PubMed] [Google Scholar]
- 12.Potocnjak I, Tesovic G, Kuna AT, Stefanovic M, Zaja O. Unusually difficult clinical presentation of an infant suffering from congenital Cytomegalovirus (CMV) infection combined with alpha 1-antitrypsin (A1AT) deficiency. Biochem Med (Zagreb) 2014;24:396–402. doi: 10.11613/BM.2014.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghouse R, Chu A, Wang Y, Perlmutter DH. Mysteries of alpha1-antitrypsin deficiency: emerging therapeutic strategies for a challenging disease. Dis Model Mech. 2014;7:411–419. doi: 10.1242/dmm.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piitulainen E, Carlson J, Ohlsson K, Sveger T. Alpha1-antitrypsin deficiency in 26-year-old subjects: lung, liver, and protease/protease inhibitor studies. Chest. 2005;128:2076–2081. doi: 10.1378/chest.128.4.2076. [DOI] [PubMed] [Google Scholar]
- 15.Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294:1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.