Abstract
Drug induced pulmonary toxicity is not uncommon with the use of various chemotherapeutic agents. Cyclophosphamide is a widely used chemotherapeutic drug in the treatment of breast cancer. Although rare, lung toxicity has been reported with cyclophosphamide use. Detection of bleomycin induced pulmonary toxicity and pattern of 18F-fluorodeoxyglucose (18F-FDG) uptake in lungs on fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) has been elicited in literature in relation to lymphoma. However, limited data is available regarding the role of 18F-FDG PET-CT in monitoring drug induced pulmonary toxicity in breast cancer. We here present two cases of cyclophosphamide induced drug toxicity. Interim 18F-FDG PET-CT demonstrated diffusely increased tracer uptake in bilateral lung fields in both these patients. Subsequently there was resolution of lung uptake on 18F-FDG PET-CT scan post completion of chemotherapy. These patients did not develop significant respiratory symptoms during chemotherapy treatment and in follow up.
Keywords: Carcinoma breast, Cyclophosphamide, Drug induced, 18F-FDG PETCT, Pulmonary toxicity
Introduction
Drug induced pulmonary toxicity is not uncommon with the use of chemotherapeutic agents in cancer treatment. It can have variable clinical presentation ranging from mild respiratory discomfort to life threatening complications. Early effects of drug toxicity might be reversible if diagnosed promptly. Therefore, early detection and timely intervention is important to ensure favorable outcome. 18F-FDG PET-CT have been proven to be useful in evaluation of patients with drug induced pulmonary toxicity in lymphoma post chemotherapy treatment, both in symptomatic and asymptomatic patients [1–3]. We here present two cases of drug induced pulmonary toxicity in two patients treated with cyclophosphamide as a neoadjuvent treatment for breast cancer and resolution of pulmonary findings post completion of chemotherapy along with corticosteroids treatment.
Case 1
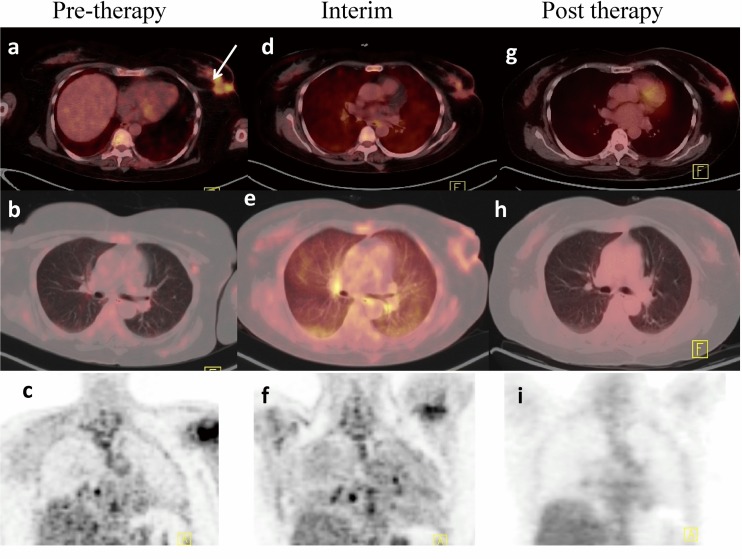
A 65 years old lady presented with lump in right breast for 2 years. On examination there was an approximately 6 x 5 cm sized lump in the upper outer quadrant of right breast with a few centimeter sized lymph nodes in right axilla. Mammography showed a Breast Imaging-Reporting and Data System (BI-RADS) VI lesion at 11 o’clock position in right breast. Biopsy from the breast lump revealed invasive ductal carcinoma. The patient was referred to the department of nuclear medicine for 18F-FDG PET-CT. It demonstrated intense 18F-FDG uptake in primary disease in right breast, right axillary lymph nodes, and a few subcentimeter sized bilateral pulmonary nodules with mild 18F-FDG uptake. With this clinical information diagnosis of metastatic breast carcinoma was considered and the neoadjuvent chemotherapy with combination of epirubicine (75 mg/m2), cyclophosphamide (600 mg/m2), and 5 fluorouracil (5FU -600 mg/m2) was planned. The patient received 4 cycles of chemotherapy. She again underwent 18F-FDG PET-CT to evaluate the treatment response to chemotherapy. Interim 18F-FDG PET-CT showed a decrease in size and metabolic activity of primary lesion in breast and a decrease in size as well as number of pulmonary nodules suggestive of partial response to treatment. In addition to this, there was mild ground glass haziness in bilateral lung fields with diffusely increased 18F-FDG uptake. In view of the decrease in primary lesion and axillary lymph nodes as well as resolution of earlier seen pulmonary nodules, these 18F-FDG PET-CT findings in lungs were attributed to an inflammatory etiology and suspicion for drug toxicity was raised. The patient was asymptomatic at this time and clinical examination was unremarkable. In view of partial response, it was planned to give four more cycles of docetaxel (100 mg/m2) based chemotherapy along with corticosteroids (dexamethasone 8 mg IV followed by oral prednisolone 20 mg BD 5 days). Follow up 18F-FDG PET-CT done after completion of chemotherapy showed no significant interval changes in right breast lesion and axillary lymph nodes. However, there was resolution of 18F-FDG uptake in bilateral lungs which was noted in mid therapy 18F-FDG PET-CT (Fig. 1). Thus, in the given clinical context appearance of diffuse 18F-FDG uptake in lungs post chemotherapy and complete resolution in the follow up reconfirmed the diagnosis of lung toxicity due to cyclophosphamide based chemotherapy. At follow up after 8 months, patient was asymptomatic and doing well.
Fig. 1.
18F-FDG PET-CT: pretherapy 18F-FDG PET-CT (a,b,c) showed primary lesion in the right breast with intense 18F-FDG uptake. There was no abnormal 18F-FDG uptake in bilateral lungs. Interim 18F-FDG PET-CT (d,e,f) demonstrated decrease in size and radiotracer uptake in the primary with diffuse 18F-FDG uptake in the lungs more prominent in bilateral in subpleural areas. Post therapy 18F-FDG PET-CT showed persistent primary in right breast (g), with resolution of uptake in the bilateral lung field (h, i)
Case 2
A 58 years old female patient presented with a lump in left breast since 2 years. Mammography showed BI-RADS V lesion in supero-lateral quadrant of left breast with extension into retroareolar region and involvement of overlying skin. Biopsy from the left breast mass revealed infiltrating duct carcinoma. The patient was then referred for 18F-FDG PET-CT to evaluate the disease extent. Baseline 18F-FDG PET-CT demonstrated 18F-FDG avid mass in left breast, left axillary lymph nodes, multiple skeletal sites, and right lung nodules. In view of metastatic disease, neoadjuvent chemotherapy was planned. She received three cycles of epirubicine (75 mg/m2), cyclophosphamide (600 mg/m2), and 5FU (600 mg/m2) based chemotherapy. The patient underwent 18F-FDG PET-CT to monitor the chemotherapy response. Interim 18F-FDG PET-CT showed residual disease involving left breast and multiple skeletal sites. However compared to her staging 18F-FDG PET-CT there was a decrease in size of left breast lesion, a decrease in number of lymph nodes, and resolution of lung nodules with no significant change in skeletal lesions. Along with these findings, there was diffusely increased 18F-FDG uptake in bilateral lung fields with mild underlying haziness. This lung uptake was not seen in baseline 18F-FDG PET-CT scan. In the given clinical context, with overall partial response on 18F-FDG PET-CT, no significant respiratory symptoms, and a history of cyclophosphamide based chemotherapy, these pulmonary findings pointed toward the possibility of lung toxicity due to cyclophosphamide. As per institute protocol the patient was then treated with docetaxel (100 mg/m2) and epirubicine (75 mg/m2), chemotherapy with corticosteroids (dexamethasone 8 mg IV followed by oral prednisolone 20 mg BD 5 days). She also received palliative radiotherapy to pelvis and lumbar region for skeletal metastasis. Post 2 months of completion of chemotherapy and radiotherapy the patient underwent 18F-FDG PET-CT scan. It showed persistent breast and skeletal lesions. However diffuse lung uptake which was seen in the interim 18F-FDG PET-CT was resolved (Fig. 2). Thus, our diagnosis of cyclophosphamide induced lung toxicity was reconfirmed. In view of persistent disease the patient was further planned for palliative chemotherapy and radiotherapy.
Fig. 2.
18F-FDG PET-CT: pretherapy 18F-FDG PET-CT (a,b,c) showed primary in left breast with intense 18F-FDG uptake. There was no abnormal 18F-FDG uptake in bilateral lungs. Interim 18F-FDG PET-CT (d,e,f) demonstrated decrease in size and radiotracer uptake in the primary lesion with diffusely increased 18F-FDG uptake in the lungs. Post therapy 18F-FDG PET-CT (g, h, i) showed persistent primary in left breast with resolution of uptake in the bilateral lung fields
Discussion
Drug toxicity in lung is not unusual with use of chemotherapeutic drugs. Many agents may be toxic to the lungs. These include cytotoxic drugs, such as bleomycin, methotrexate, and cyclophosphamide, and non-cytotoxic drugs, such as nitrofurantoin, sulfasalazine, and amiodarone [4–6]. Patients presented here were treated with cyclophosphamide, 5FU, and epirubicine chemotherapy regimen initially. Lung toxicity caused by 5FU or epirubicine is uncommon. Only a few cases of lung toxicity with combination of 5FU and oxaliplatin and in patients treated with irinotecan having a history of previous treatment with 5FU [7] have been reported. However to the best of our knowledge no literature is available on pulmonary toxicity caused by 5FU or epirubicine alone. Cyclophosphamide has been implicated in lung toxicity with an incidence of less than 1 %. With this background pulmonary toxicity in these patients was contributed to cyclophosphamide. Patients with cyxlophosphamide induced lung toxicity usually present between 2 weeks to many years from initiation of treatment with dyspnea on exertion, cough, and in some cases fever. Further, on examination late inspiratory crackles is a common finding. Endogenous release of reactive oxygen species by cyclophosphamide leads to lung injury. The two patterns of lung injury seen in cyclophosphamide injury are early-onset pneumonitis, which occurs within 1–6 months after exposure. This pattern is not only reversible but may also respond to corticosteroids as well. Another pattern described with cyclophosphamide is late-onset pneumonitis, which may appear years after drug discontinuation, has a chronically progressive course, and is unresponsive to corticosteroids [8]. In early stages peripheral ground-glass opacity is a common radiological finding, however bilateral reticular or nodular diffuse opacities are noted in both early-onset and late-onset pulmonary toxicity.
Clinical manifestations, laboratory, and radiological findings of drug induced lung toxicity (DILD) are non-specific [9–12]. Therefore, the diagnosis of drug induced toxicity is difficult and is often a diagnosis of exclusion. Typical clinical findings of drug toxicity are the gradual appearance of dyspnea and non-productive cough. Diminished total lung capacity, vital capacities, and DLCO (diffusing capacity of lung for carbon monoxide) have been reported on pulmonary function tests. The common findings on CT are scattered or diffuse areas of ground-glass opacity, interlobular septal thickening, fibrosis, or consolidation [4]. Our patients showed diffusely increased 18F-FDG uptake in both lungs, slightly more prominent in subpleural location on interim 18F-FDG PET-CT. This abnormal 18F-FDG uptake in lungs developed in 3–4 months post chemotherapy. Thereafter the patients were treated with docetaxel, epirubicine along with corticosteroids and there was resolution of these 18F-FDG PET-CT pulmonary findings in 3–4 months post chemotherapy.
Although, not much data is available in literature on diffuse homogenous 18F-FDG uptake in bilateral lungs, this pattern could be seen in sarcoidosis, tuberculosis, drug induced pneumonitis, lymphangitis carcinomatosis, diffuse parenchymal lung disease, idiopathic pulmonary fibrosis, intravascular large B cell lymphoma, pulmonary fibrosis, and post radiotherapy [13–17]. It is not always possible to differentiate between different causes of diffuse 18F-FDG uptake in lungs. In the present cases, in view of the absence of any clinical symptoms, lack of evidence for an underlying infectious process, no prior treatment history of radiotherapy, and the spontaneous resolution of abnormal imaging findings during the follow-up, these findings were considered convincing of a non-neoplastic nature and it was decided not to perform a confirmatory tissue biopsy. Furthermore, on the basis of the temporal association with preceding chemotherapy, the absence of any systemic or respiratory symptoms pointing toward a possible infectious aetiology or neoplastic etiology, and the favorable clinical course of this patient, we considered that this linear pattern of moderately increased 18F-FDG uptake in the lungs was most likely inflammatory in nature and represent treatment-related pulmonary toxicity, possibly due to cyclophosphamide.
The role of 18F-FDG PET-CT imaging in monitoring the therapy response in patients with breast cancer is well known. Limited data is available on DILD in breast cancer patients post cyclophosphamide therapy. Kalkanis et al. reported rituximab lung toxicity in five patients with non Hodgkin’s lymphoma with no pulmonary symptoms [2]. Similarly both the patients with breast cancer presented here did not develop significant respiratory symptoms during treatment with cyclophosphamide based chemotherapy. However, there was drug induced pulmonary toxicity detected on interim 18F-FDG PET-CT with resolution after completion of chemotherapy along with corticosteroids treatment.
To conclude, 18F-FDG PET-CT can detect drug induced lung toxicity at an earlier stage even before development of symptoms which can help to prevent serious complications of pulmonary toxicity by early intervention and also aid in the management by monitoring the treatment response. Awareness of this radiographic finding on 18F-FDG PET-CT is important for the clinician and it should neither be considered as neoplastic in nature nor be confused with disease progression. However, the importance of this pattern of pulmonary 18F-FDG uptake needs validation by larger prospective studies.
Compliance with Ethical Standards
Conflict of Interest
Authors Sameer Kamalakar Taywade, Chandrasekhar Bal and Rakesh Kumar declare that they have no conflict of interest.
The manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Ethical Statement
Manuscript contains a statement that the study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
Contributor Information
Sameer Kamalakar Taywade, Email: sameertaywade@gmail.com.
Rakesh Kumar, Phone: 91-11-26594472, Email: rkphulia@yahoo.com.
Sainath Bhethanabhotla, Email: bsainath101@yahoo.co.in.
Chandrasekhar Bal, Email: csbal@hotmail.com.
References
- 1.Kazama T, Faria SC, Uchida Y, Ito H, Macapinlac HA. Pulmonary drug toxicity: FDG-PET findings in patients with lymphoma. Ann Nucl Med. 2008;22:111–114. doi: 10.1007/s12149-007-0089-9. [DOI] [PubMed] [Google Scholar]
- 2.Kalkanis D, Stefanovic A, Paes F, Escalon MP, Serafini A, Lossos IS. [18F]-fluorodeoxyglucose positron emission tomography combined with computed tomography detection of asymptomatic late pulmonary toxicity in patients with non-Hodgkin lymphoma treated with rituximab-containing chemotherapy. Leuk & Lymphoma. 2009; 50:904–11. [DOI] [PubMed]
- 3.Song BI, Lee SW, Lee HJ, Kang S, Jeong SY, Seo JH, et al. Rituximab-induced pneumonitis on F-18 FDG PET/CT in patient with non-Hodgkin lymphoma. Clin Nucl Med. 2010;35:601–603. doi: 10.1097/RLU.0b013e3181e4da5c. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: cytotoxic drugs. Am Rev Respir Dis. 1986;133:321–340. doi: 10.1164/arrd.1986.133.2.321. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JA, Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 2: noncytotoxic drugs. Am Rev Respir Dis. 1986;133:488–505. doi: 10.1164/arrd.1986.133.3.488. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulou I, Bamias A, Lyberopoulos P, Dimopoulos MA. Pulmonary toxicity from novel antineoplastic agents. Ann Oncol. 2006;17:372–379. doi: 10.1093/annonc/mdj057. [DOI] [PubMed] [Google Scholar]
- 7.Trisolini R, Lazzari Agli L, Tassinari D, Rondelli D, Cancellieri A, Patelli M, et al. Acute lung injury associated with 5-fluorouracil and oxaliplatin combined chemotherapy. Eur Respir J. 2001;18:243–245. [PubMed] [Google Scholar]
- 8.Malik SW, Myers JL, DeRemee RA, Specks U. Lung toxicity associated with cyclophosphamide use. Two distinct patterns. Am J Respir Crit Care Med. 1996;154:1851–1856. doi: 10.1164/ajrccm.154.6.8970380. [DOI] [PubMed] [Google Scholar]
- 9.Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000;20:1245–1249. doi: 10.1148/radiographics.20.5.g00se081245. [DOI] [PubMed] [Google Scholar]
- 10.Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 11.Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528–538. doi: 10.1378/chest.07-0851. [DOI] [PubMed] [Google Scholar]
- 12.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120:617–624. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- 13.Wagnerae T. Diffuse pulmonary uptake on FDG-PET with normal CT diagnosed as intravascular large B-cell lymphoma: a case report and a discussion of the causes of diffuse FDG uptake in the lungs. Cancer Imaging. 2012; 12:7–12. [DOI] [PMC free article] [PubMed]
- 14.Groves AM, Win T, Screaton NJ, Berovic M, Endozo R, Booth H, et al. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med. 2009;50:538–545. doi: 10.2967/jnumed.108.057901. [DOI] [PubMed] [Google Scholar]
- 15.Prakash P, Kalra MK, Sharma A, Shepard JA, Digumarthy SR. FDG PET/CT in assessment of pulmonary lymphangitic carcinomatosis. AJR Am J Roentgenol. 2010;194:231–236. doi: 10.2214/AJR.09.3059. [DOI] [PubMed] [Google Scholar]
- 16.Yu JQ, Kumar R, Xiu Y, Alavi A, Zhuang H. Diffuse FDG, uptake in the lungs in aspiration pneumonia on positron emission tomographic imaging. Clin Nucl Med. 2004;29:567–568. doi: 10.1097/01.rlu.0000134986.58984.5f. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues RS, Miller PR, Bozza FA, Marchiori E, Zimmerman GA, Hoffman JM, et al. FDG-PET in patients at risk for acute respiratory distress syndrome: a preliminary report. Intensive Care Med. 2008;34:2273–2278. doi: 10.1007/s00134-008-1220-7. [DOI] [PubMed] [Google Scholar]