Abstract
Despite substantial advances in the diagnosis of cardiovascular disease, 18F-labeled positron emission tomography (PET) radiopharmaceuticals remain necessary to diagnose heart disease because clinical use of current PET tracers is limited by their short half-life. Lipophilic cations such as phosphonium salts penetrate the mitochondrial membranes and accumulate in mitochondria of cardiomyocytes in response to negative inner-transmembrane potentials. Radiolabeled tetraphenylphosphonium cation derivatives have been developed as myocardial imaging agents for PET. In this review, a general overview of these radiotracers, including their radiosynthesis, in vivo characterization, and evaluation is provided and clinical perspectives are discussed.
Keywords: 18F-Fluoroalkylphosphonium cations, Mitochondrial voltage sensors, Myocardial imaging agents, Positron emission tomography, Tetraphenylphosphonium cation derivatives
Introduction
Since the 1980s, single photon emission computed tomography (SPECT) has played a key role in assessing myocardial blood flow (MBF) and in diagnosing coronary artery disease (CAD) [1–3]. Notably, SPECT agents such as 99mTc-sestamibi, 99mTc-tetrofosmin, or 201Tl are the mainstay of myocardial perfusion imaging (MPI) [4, 5]. However, the technical limitations of SPECT imaging, such as the absence of a standardized method for correction of photon attenuation and significant uptake of SPECT tracers in organs adjacent to the heart, may compromise the delineation of small lesions and limit the diagnostic accuracy of SPECT [6]. Positron emission tomography (PET) has several technical advantages over SPECT. Not only does PET imaging have higher spatial resolution and a standardized method for the correction of photon attenuation, but PET also enables quantitative measurements of MBF because it allows for accurate determination of rapidly changing tissue counts over time. Therefore, PET allows for quantitative measures of myocardial tracer uptake and regional MBF [7, 8]. However, the PET tracers currently used for MPI have a short half-life (13N-NH3: 9.97 min; 82Rb: 1.27 min; 15O-H2O: 2.04 min), which limits widespread clinical use of PET because of the need for an on-site cyclotron or generator [9, 10]. Thus, use of a radioisotope that has proper half-life for clinical use to label myocardial perfusion agents could overcome these limitations and simplify clinical protocols [7, 11].
The Rationale for 18F-Labeled Tetraphenylphosphonium Cation Derivatives
The mitochondrial membrane potential (MMP) is higher in cardiomyocytes than in normal epithelial cells, and loss of MMP is an early event in cell death caused by myocardial ischemia [12, 13]. Similar to SPECT agents such as 99mTc-sestamibi and 99mTc-tetrofosmin, tetraphenylphosphonium cation (TPP) derivatives penetrate the hydrophobic barriers of the plasma and mitochondrial membranes and accumulate in mitochondria of cardiomyocytes [14–18]. This accumulation occurs because the 99mTc-sestamibi, 99mTc-tetrofosmin, and TPP derivatives move into mitochondrium in response to its high density and the negative inner transmembrane potentials (−150 to −170 mV, negative inside) in cardiomyocytes [17]. Therefore, radiolabeled TPP derivatives are promising candidates for myocardial imaging [19, 20].
Table 1 shows the radiolabeled TPP derivatives that have been developed [15, 16, 21–36]. The chemical structures of radiolabeled TPP derivatives are shown in Fig. 1. Among them, the first reported was 11C-triphenylmethylphosphonium cation (11C-TPMP), which serves as a mitochondrial membrane voltage sensor detectible via PET [21]. However, 11C-TPMP use is limited to use widely because of the short half-life of 11C (20 min) despite good in vivo characteristics. To provide a tracer similar to 11C-TPMP but with a longer half-life, the synthesis and evaluation of 18F-labeled TPP derivatives were investigated. Ravert et al. reported that 3-18F-fluoropropyltriphenylphosphonium, 4-18F-fluorobenzyltriphenylphosphonium (18F-FBnTP), and 4-18F-fluorobenzyltris 4-dimethylaminophenylphosphonium cations could be synthesized in multistep reactions with no carrier added (nca) 18F [37]. Among these cations, 18F-FBnTP showed promise as a myocardial perfusion agent. 18F-FBnTP is metabolically stable and performs well as a cardiac imaging agent in healthy and CAD models [15, 16, 27]. In a separate study, direct fluorination of TPP was investigated [23]. However, these 18F-labeled tracers needed improved in vivo characteristics, such as higher uptake and longer retention of tracer in the heart and more rapid wash out from liver to be used as myocardial imaging agents. Thus we developed novel 18F-labeled tetraphenylphosphonium cation derivatives, fluoroalkyltriphenylphosphonium cations (FATPs). Alkyl chain conjugated triphenylphosphonium cation, have improved characteristics through lipophilicity control [28–32].
Table 1.
The radiolabeled tetraphenylphosphonium cation derivatives
| Radioisotope | Radiotracer | Target | Reference |
|---|---|---|---|
| 11C | 11C-triphenylmethylphosphonium cation (11C-TPMP) | heart, brain tumor | 21, 22 |
| 18F | (4-18F -fluorophenyl)triphenylphosphonium (18F-TPP) | heart | 23, 24, 25 |
| 4-18F-fluorobenzyltriphenylphosphonium (18F-FBnTP) |
heart, brown adipose tissue | 15, 16, 26, 27 | |
| (5-18F-fluoropentyl)triphenylphosphonium cation (18F-FPTP) |
heart | 31, 33, 34 | |
| (6-18F-fluorohexyl)triphenylphosphonium cation (18F-FHxTP) |
heart | 29 | |
| (7-18F-fluoroheptyl)triphenylphosphonium cation (18F-FHtTP) |
heart | 32 | |
| (8-18F-fluorooctyl)triphenylphosphonium cation (18F-FOTP) |
heart | 32 | |
| (2-(2-18F-fluoroethoxy)ethyl)triphenylphosphonium cation (18F-FETP) |
heart | 28 | |
| (2-(2-18F-fluoroethoxy)ethyl)tris (4-methoxyphenyl)phosphonium cation (18F-FETMP) | heart | 30 | |
| 64Cu | 64Cu-(4-((4,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1 yl)methyl)benzyl)triphenylphosphonium cation | glioma | 35 |
| 64Cu-(2-(diphenylphosphoryl)ethyl)diphenyl(4-((4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl)methyl)benzyl)phosphonium cation | glioma | 36 |
Fig. 1.
Schematic structure of radioisotope labeled tetraphenylphosphonium cation derivatives. a 11C-triphenylmethylphosphonium cation (11C-TPMP), b (4-18F-fluorophenyl)triphenylphosphonium cation (18F-TPP), c (3-18F-fluoropropyl)triphenylphosphonium cation, d 4-18F-fluorobenzyltriphenylphosphonium cation (18F-FBnTP), e 4-18F-fluorobenzyltris 4-dimethylaminophenylphosphonium cation, f (5-18F-fluoropentyl)triphenylphosphonium cation (18F-FPTP), g (6-18F-fluorohexyl)triphenylphosphonium cation (18F-FHxTP), h (7-18F-fluoroheptyl)triphenylphosphonium cation (18F-FHtTP), i (8-18F-fluorooctyl)triphenylphosphonium cation (18F-FOTP), j (2-(2-18F-fluoroethoxy)ethyl)triphenylphosphonium cation (18F-FETP), k (2-(2-18F-fluoroethoxy)ethyl)tris(4-methoxyphenyl)phosphonium cation (18F-FETMP), l 64Cu-(4-((4,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1 yl)methyl)benzyl)triphenylphosphonium cation, m 64Cu-(2-(diphenylphosphoryl)ethyl)diphenyl(4-((4,7,10-tris(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl)methyl)benzyl)phosphonium cation
Myocardial selectivity and hepatic clearance depend on the lipophilicity and functional groups of the radiotracer. Although little is known regarding the optimal lipophilicity needed for high myocardial selectivity, cationic radiotracers with log P values in the range of 0.5–1.3 are particularly useful for imaging organs with high mitochondrial density, as a result of their fast membrane-penetration kinetics [36]. Furthermore, the lipophilic interaction between the triphenylphosphonium cation and the lipid layer is attractive because the alkyl group increases lipophilicity [38, 39].
Radiosynthesis of 18F-Labeled Tetraphenylphosphonium Cation Derivatives
We described radiolabeling procedures of representative radiolabeled phosphonium salts including 11C-TPMP, 18F-labeled TPP, 18F-labeled TPP derivatives and 18F-labeled fluoroalkylphosphonium cations (18F-FATPs) among reported radiolabeled phosphonium salts. Because 11C-TPMP and 18F-labeled TPP showed the potential of phosphonium salts as MPI agents for PET, the first structure of 18F-FATPs was modified based on 18F-labeled TPP derivatives.
11C-TPMP was prepared by methylation of triphenylphosphonium with 11C-methyl iodide (11C-CH3I) [21]. 11C-CH3I is a very reactive structure and generally is used in 11C chemistry. 11C-TPMP was synthesized with a 9.4–12.5 % radiochemical yield (RCY, non-decay corrected) within 30 minutes and the specific activity was 14.8-33.3 GBq/μmol.
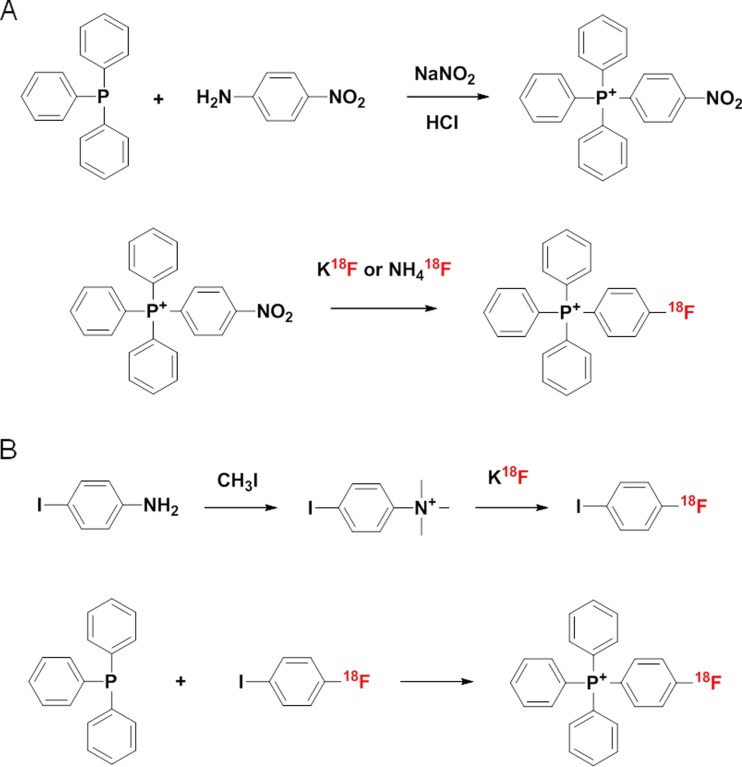
Direct 18F-labeling of TPP (18F-TPP) has been achieved by two methods (Fig. 2) [23–25]. The first method used 4-nitrophenyltriphenylphosphonium as the 18F-labeling precursor for a one step nucleophilic substitution reaction. This precursor was prepared first by following the procedure of Rieke et al. [40]. The diazonium chloride intermediate was formed by slowly adding hydrochloric acid to 4-nitroaniline. The formation of 4-nitrophenyltriphenylphosphonium was obtained by the addition of triphenylphosphine to the diazonium chloride intermediate, which was then extracted in aqueous solution and precipitated with sodium iodide (NaI) to generate the pure precursor. Cheng used potassium carbonate (K2CO3) and the phase transfer agent Kryptofix 222, and Shoup used ammonium hydroxide after counter ion exchange of 4-nitrophenyltriphenylphosphonium changed nitrate with 18F for labeling [23, 25]. The second method used 4-iodophenyltrimethylammonium iodide as a precursor [24]. After 18F-labeling of 4-iodophenyltrimethylammonium iodide, 1-18F-fluoro-4-iodobenzene was made to react with triphenylphosphine to yield the final labeled compound. The RCY of the two methods was 10–15 % and the specific activity was >18.5 TBq/mmol at the end of synthesis. The total synthesis time was less than 60 minutes, and the radiochemical purity above 95 %.
Fig. 2.
Different radiosynthetic methods of 18F-TPP. a Direct 18F labeling method using 4-nitrophenyltriphenylphosphonium b Two step 18F labeling method using 4-iodophenyltrimethylammonium iodide
18F-labeling of TPP derivatives was reported by Ravert et al. He created a functional group between the triphenylphosphonium and 18F [37]. The radiosynthesis of 3-18F-fluoropropyltriphenylphosphonium cation was performed in a single vessel. After drying the nca 18F in the presence of Kryptofix and K2CO3, propylene glycol ditosylate in acetonitrile was added and the solution heated. Triphenylphosphine in toluene was added subsequently. After the solution was heated to boiling, the toluene evaporated and purified by high pressure liquid chromatography (HPLC). They used 0.1 M ammonium formate as an HPLC eluent, but a buffer change was needed, such as injectable water, saline or phosphate buffer saline to use the cation for in vivo experiments due to the toxicity of ammonium formate. The synthesis, purification and formulation were completed in 56 minutes (n = 10). The RCY was 12 % (non-decay corrected) and the specific activity was 15.5 GBq/μmol.
The radiosynthesis of 18F-FBnTP was more complicated than that of the (3-18F-fluoropropyl)triphenylphosphonium cation. 18F-FBnTP was synthesized using a four-step procedure. As a precursor, 4-trimethylammoniumbenzaldehyde was used to create a third intermediate, 4-18F-fluorobenzyl bromide, which was reacted with triphenylphosphine to yield 18F-FBnTP. Finally, 18F-FBnTP was purified using a preparative HPLC system. A radiochemically pure product (>99 %) was obtained with a 6 % yield (non-decay corrected) with an average synthesis time of 82 minutes (n = 20) and a specific radioactivity of 16.7 GBq/μmol. The radiosynthesis of the 4-18F-fluorobenzyltris-4-dimethylaminophenylphosphonium cation was performed in the same manner as the 4−18F-FBnTP described above except that tris-dimethylaminophosphine was substituted for triphenylphosphine in the last synthetic step. The radiochemically pure radioproduct (>99 %) was obtained with a 15 % yield (non-decay corrected) with an average synthesis time of 80 minutes (n = 6) and an average specific radioactivity of 12.6 GBq/μmol.
Finally, 18F-FATPs, including (5-18F-fluoropentyl)triphenylphosphonium cation (18F-FPTP), (6-18F-fluorohexyl)triphenylphosphonium cation (18F-FHxTP), (7-18F-fluoroheptyl)triphenylphosphonium cation (18F-FHtTP), (8-18F-fluorooctyl)triphenylphosphonium cation (18F-FOTP), (2-(2-18F-fluoroethoxy)ethyl)triphenylphosphonium cation (18F-FETP), and (2-(2-18F-fluoroethoxy)ethyl)tris(4-methoxyphenyl)phosphonium cation (18F-FETMP), were investigated to assess the appropriate range of lipophilicity suitable for PET myocardial imaging agents. 18F-FATPs were prepared as described previously [28–32]. It took a total reaction time of less than 60 minutes for 18F-FATPs to be ready for tracer injection. A radiochemically pure radioproduct (>98 %) was obtained with a 10-20 % yield (non-decay corrected) and the average specific radioactivity was >5.9 Tbq/μmol.
Biodistribution studies of 18F-Labeled Tetraphenylphosphonium Cation Derivatives
Biodistribution results of radioisotope labeled TPP derivatives are summarized in Table 2. 14C-TPMP distribution was evaluated in mice and rats. 14C-TPMP had maximum cardiac uptake two minutes after injection (16.53 ± 1.90 % ID/g) in mice and its level remained nearly constant for 60 min. The heart-to-blood ratios were 31, 54, 86, and 153 at 2, 10, 30, and 60 min, respectively. The small intestine, liver, and muscle values also were relatively constant for 60 min, but lower than that of the heart. The kidney showed an extremely high concentration at 2 min, which then decreased rapidly over time. In rat studies, the heart concentration was also high at two minutes (3.05 ± 0.16 % ID/g) and remained almost constant for 60 min. The heart-to-blood ratios were 93 and 154 at 30 and 60 min, respectively. The distribution pattern was nearly identical to that of the murine study. The in vivo biodistribution of 14C-TPMP demonstrated that the cation first became concentrated in the myocardium and then remained constant with high heart to-blood ratios. The 14C-TPMP concentration in the kidney was initially high and then decreased rapidly [21]. High radioactivity was observed in the urinary bladder, and relatively high activity in the lumen of the intestine. These results suggest that 14C-TPMP is excreted mainly through the kidney and, to some extent, through the hepatobiliary system, because a high gallbladder concentration was observed in the preliminary study. Hepatobiliary secretion of the cation may interfere with interpretation of heart images adjacent to the liver. However, the heart-to-liver ratios were higher than two in murine studies with heart imaging.
Table 2.
Normal biodistribution at 60 minutes after i.v. injection of radioisotope labeled TPP derivatives into mice
| 14C-TPMP* | 18F-TPP* | 18F-FBnTP | 18F-FPTP | 18F-FHxTP | 18F-FETP | 18F-FETMP | |
|---|---|---|---|---|---|---|---|
| Blood | 0.027 ± 0.002 | 0.02 ± 0.003 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.19 ± 0.03 | 0.23 ± 0.02 | 0.73 ± 0.63 |
| Heart | 4.144 ± 0.827 | 1.57 ± 0.18 | 12.16 ± 2.4 | 21.23 ± 3.60 | 23.20 ± 2.70 | 19.96 ± 2.08 | 29.88 ± 7.59 |
| Lung | 1.071 ± 0.108 | 0.38 ± 0.11 | 1.82 ± 0.3 | 2.96 ± 0.63 | 3.70 ± 0.12 | 4.01 ± 0.74 | 6.38 ± 1.21 |
| Liver | 1.049 ± 0.269 | 0.17 ± 0.03 | 8.09 ± 3.9 | 3.05 ± 0.92 | 1.30 ± 0.12 | 2.03 ± 0.17 | 11.22 ± 1.48 |
| Kidney | 1.288 ± 0.185 | 1.75 ± 0.38 | 24.70 ± 4.1 | 11.68 ± 3.73 | 12.68 ± 3.13 | 16.86 ± 2.73 | 41.10 ± 9.94 |
| Muscle | 0.648 ± 0.111 | 0.26 ± 0.11 | 1.27 ± 0.4 | 6.16 ± 1.78 | 4.53 ± 0.54 | 4.42 ± 1.15 | 5.41 ± 1.93 |
| Heart-to-liver | 3.95 | 8 | 1.50 | 7.25 | 17.83 | 9.87 | 2.74 |
| Heart-to-lung | 3.89 | 4 | 6.68 | 7.28 | 6.27 | 5.05 | 4.75 |
| Heart-to-blood | 153.48 | 75 | 243 | 523.34 | 126.76 | 86.78 | 61.07 |
*: biodistribution in Sprague–Dawley rats, Data are expressed as the percentage of administered activity (injected dose) per gram of tissue
18F-TPP demonstrated an 11-fold higher accumulation in the heart at five minutes (1.64 % ID/g) relative to the blood and a five-fold higher accumulation relative to the liver [25]. At 30 min, the radioactivity in the heart was 1.51 % ID/g and the heart-to-blood ratio was 75. Blood activity changed significantly from 5 to 60 minutes, dropping from 0.15 to 0.02 % ID/g. At 5, 30, and 60 minutes, lung activity was 0.69, 0.36, and 0.38 % ID/g, respectively, whereas liver uptake was 0.34, 0.18, and 0.17 % ID/g, respectively. Heart-to-lung ratios at 5, 30, and 60 minutes were 2, 5, and 4, respectively. Bone accumulation, an indication of defluorination, was minimal: 0.33 % ID/g at 5 minutes and 0.39 % ID/g at 60 minutes.
Among 3-18F-fluoropropyltriphenylphosphonium, 18F-FBnTP, and 4-18F-fluorobenzyltris 4-dimethylaminophenylphosphonium cations, murine biodistribution was reported only for 18F-FBnTP [16]. 18F-FBnTP biodistribution in mice was organ specific, with the strongest uptake in the kidney, followed by the heart and liver, and very low uptake in the lung at 60 min. 18F-FBnTP demonstrated a 240-fold higher accumulation in the heart at 60 minutes (12.16 % ID/g) relative to the blood (0.05 % ID/g) and a 1.5-fold higher accumulation relative to the liver (8.09 % ID/g).
18F-FATPs showed superior heart-specific uptake and wash out from liver than those reported for radioisotope labeled TPP derivatives with modifications of lipophilicity and functional groups of 18F-FATPs. The lipophilicity and functional group changes between triphenylphosphonium and 18F of 18F-FATPs are summarized in Table 3. Although there is little information available with regard to the optimal lipophilicity needed for high myocardial selectivity, cationic radiotracers with log P values in the range of 0.5–1.3 are particularly useful for imaging organs with high mitochondrial density as a result of their fast membrane-penetration kinetics [36]. Furthermore, the lipophilic interaction between the triphenylphosphonium cation and the lipid layer is attractive because the alkyl group increases lipophilicity [38, 39]. In vivo biodistribution of 18F-FATPs was examined in BALB/c mice at 10, 30, 60, and 120 minutes after intravenous injection. A high level of radioactivity accumulation in the heart with rapid clearance from the liver and blood. The myocardial uptake of 18F-FATPs was more than 20 % ID/g at 10, 30, 60, and 120 minutes after radiotracer injection. The heart-to-liver ratio and heart-to-blood ratio was over 4 and 30, respectively, at 10 minutes. However, clearance of 18F-FETMP from the liver occurred later than for other organs due to the methoxy functional group of triphenylphosphonium (heart-to-liver ratio at 10 min: 2.33 ± 0.36).
Table 3.
Comparison of lipophilicity and functional groups between triphenylphosphonium and 18F of 18F-FATPs
PET imaging studies of 18F-Labeled Tetraphenylphosphonium Cation Derivatives
PET imaging studies of 11C-TPMP were performed in normal dog models [21, 41]. 11C-TPMP concentrated in the myocardium within a few minutes with very rapid clearance from the blood. The concentration varied from 0.038 to 0.082 % dose/ml and the heart-to-blood ratios were 28–53 and 49–105 at 20 and 60 minutes, respectively. In addition, these studies measured the 11C-TPMP concentration in the heart after injection of potassium chloride, which resulted in a reduced heart rate, arrhythmia, and ventricular fibrillation. When ventricular fibrillation occurred, a decrease of about 40-50 % in the 11C-TPMP concentration was observed. The rapid decrease of 11C-TPMP might indicate an irreversible depolarization of the myocardium.
In vivo characterization of 18F-TPP was performed in normal rat and coronary occlusion rabbit models. 18F-TPP showed intense cardiac uptake with an initial spike in activity corresponding to blood flow followed by a plateau after 1–2 min. The cardiac uptake was 1.5 % ID/g in rats, which is in the range for that of 99mTc-tetrofosmin or -sestamibi. The time-activity curve (TAC) generated from PET imaging of rats showed that 18F-TPP uptake by the heart wall was approximately two-fold higher than blood uptake for 60 minutes. Similarly, rabbit images indicated rapid blood clearance and clear delineation of the plateau of heart activity for the hour-long scanning period. The uptake and kinetics of 18F-FBnTP were evaluated in murine and normal canine models. 18F-FBnTP is metabolically stable and demonstrated excellent characteristics as a cardiac imaging agent in both models. The myocardium-to-liver uptake ratio in the TAC reached 1 at approximately 25 minutes after intravenous injection of 18F-FBnTP in mice. Furthermore, the myocardium-to-liver uptake ratio was 1 in dog models for 90 minutes after 18F-FBnTP injection.
18F-FATPs showed excellent heart-background contrast in microPET imaging of murine models at each time point and afforded better in vivo characterization than 13N-NH3, which is the clinical gold standard myocardial imaging agent for PET [28–31, 34]. Static microPET images of normal rats after intravenous (i.v.) injection of 18F-FATPs or 13N-NH3 are shown in Fig. 3. The 18F-FATP images demonstrated good visualization of the heart, with excellent heart-to-liver and heart-to-lung discrimination and high contrast between the myocardium and liver or lung. By contrast, 13N-NH3 images showed higher liver uptake than heart uptake until 30 minutes after tracer injection. Intense liver uptake, caused by prominent hepatobiliary excretion, is frequently observed on 99mTc-based myocardial imaging [42, 43]. High liver uptake is the cause of photon scatter that may mask the detection of flow abnormalities, particularly in the inferior and inferoapical left ventricular wall [43, 44]. The 18F-FATP TACs demonstrated rapid accumulation in the myocardium (1–2 minutes), with stable retention for at least 60 minutes. The myocardium-to-liver ratios of 18F-FPTP, 18F-FHTP, and 18F-FETP were 1.67 ± 0.21, 2.49 ± 0.08, and 2.6 ± 0.85, respectively, whereas myocardium-to-lung ratios were 3.65 ± 0.2, 5.67 ± 0.52, and 6.31 ± 0.8, respectively, 1 minutes after i.v. injection. By contrast, 13N-NH3 TAC revealed that the myocardium-to-liver ratio and the myocardium-to-lung ratio were 0.8 ± 0.14 and 0.93 ± 0.07, respectively at 5 minutes after injection. Thereafter, liver and lung uptake of 13N-NH3 was higher than that of the myocardium. The myocardium-to-liver ratios of 18F-FPTP, 18F-FHTP, and 18F-FETP were over seven-, 17-, and ten-fold higher, whereas myocardium-to-lung ratios were two-, three-, and three-fold higher, respectively, than those of 13N-NH3 at 30 minutes after injection. On the basis of the TAC from microPET images, 18F-FATPs demonstrated high heart-to-liver and heart-to-lung ratios 10 minutes after injection. These results are consistent with the high-quality myocardial images obtained with 18F-FATPs.
Fig. 3.
Coronal microPET images of normal rats after intravenous injection of 37 MBq of a 13N-NH3, b 18F-FPTP, c 18F-FHTP, d 18F-FETP and e 18F-FETMP. The heart is visible, with excellent heart-to-background contrast at each time point after radiotracer injection. H: heart; L: liver; SUV: standardized uptake value. (Reprinted with permission of Kim et al. [28–31, 34])
In addition, 18F-FATPs were compared with 13N-NH3 in myocardial infarction (MI) models. Representative images of MI rats acquired after acute ligation of the left coronary artery (LCA) in the short-, vertical long-, and horizontal long-axes and a polar map collected 10 and 30 minutes after injection of 18F-FATPs or 13N-NH3 are shown in Fig. 4. Sharply defined myocardial defects were present with 18F-FATPs or 13N-NH3 (10 min). However, the 13N-NH3 images significantly underestimated infarct size early after tracer injection (0–10 min, P = 0.027), which might be due to spillover of radioactivity into the left ventricular or right ventricular myocardium [34]. To confirm the spillover effect of 13N-NH3 early after i.v. injection, we compared polar map images acquired from zero to three minutes and three to six minutes. The defect size and contrast ratio of 13N-NH3 images at zero to three minutes were significantly lower than those at three to six minutes (P = 0.043), whereas 18F-FATPs showed similar values for defect size and contrast ratio at zero to three and three to six minutes. The defect size for MI generated from microPET polar map imaging of 18F-FPTP or 13N-NH3 was compared with 2,3,5-triphenyltetrazolium chloride (TTC) staining to measure the coefficient of determination [33]. The correlation coefficient between TTC staining and the polar map of 18F-FPTP or 13N-NH3 was 0.89 and 0.84, respectively (threshold: 60 %). The strong correlation demonstrated that noninvasive imaging results obtained by 18F-FPTP or 13N-NH3 small-animal PET can serve as a surrogate for histology to quantify infarct size.
Fig. 4.
Short-, vertical long-, and horizontal long-axis and polar map images of a 13N-NH3, b 18F-FPTP, c 18F-FHTP, or d 18F-FETP in each representative MI model. Data were collected between 0 to 10 and 20 to 30 minutes after radiotracer injection (37 MBq). (Reprinted with permission of Kim et al. [34])
Perfused Isolated Rat Heart Study
The first-pass extraction fraction (EF) values of 18F-FATPs or 13N-NH3 were measured in isolated rat hearts perfused by the Langendorff method [34]. Despite the limitations of the Langendorff method, including the absence of normal humoral influences and neuronal regulation, a variety of cardiovascular researchers still use this technique because it has many advantages, such as simplicity, measurement accuracy, high reproducibility, and relatively low cost [45]. EF values for all flow rates are shown in Table 4. Lower EFs were found at higher flow rates, which was true for both 18F-FATPs and 13N-NH3. The EF values of 18F-FPTP, 18F-FHTP, and 18F-FETP were significantly higher than those of 13N-NH3 at flow rates of 4, 8, and 16 mL/minute (P < 0.05). At the lower flow rate of 0.5 mL/min, there were no significant differences between 18F-FPTP, 18F-FHTP, and 13N-NH3. Only 18F-FETP demonstrated a significantly lower EF value than 13N-NH3 at a flow velocity of 0.5 mL/min. The flow rate tested in this study (0.5–16 mL/min) would be relevant because the expected flow rate of the Langendorff method is 7–9 mL/minutes for rats and the MBF of normal rats under isoflurane anesthesia is 4.2 ± 0.9 mL/minutes [46]. Generally, flow rates in isolated rat hearts are considerably higher than those under physiologic conditions and lower EFs have been found at higher flow rates. Thus, perfusion tracers showed considerably lower values in the isolated heart model [7]. The SPECT agent 99mTc-sestamibi demonstrated an average myocardial EF of 38 % (SD = 9 %) for a flow range of 0.52–3.19 mL/min/g in isolated rabbit hearts. In another study, 82Rb exhibited a value of 42 % (SD = 6 %), which was observed after injection into the femoral vein of mongrel dogs at normal resting flow rates (0.75–1.5 mL/min/g) [47, 48]. The 18F-FATPs demonstrated similar EF values to 99mTc-sestamibi and 82Rb.
Table 4.
First-pass extraction fraction values (EF, %) of 18F-FATPs and 13N-NH3 at different flow rates
| 18F-FPTP | 18F-FHTP | 18F-FETP | 13N-NH3 | |
|---|---|---|---|---|
| 0.5 mL/min (n = 2) |
41.26 ± 11.51 | 45.30 ± 8.78 | 33.89 ± 10.30* | 49.30 ± 4.05 |
| 4.0 mL/min (n = 2) |
30.18 ± 8.90† | 31.59 ± 8.73† | 24.35 ± 4.38* | 15.18 ± 7.29 |
| 8.0 mL/min (n = 2) |
23.21 ± 6.62† | 22.57 ± 6.47* | 19.70 ± 3.05* | 12.89 ± 6.30 |
| 16 mL/min (n = 2) |
18.50 ± 4.65† | 18.43 ± 3.57† | 13.94 ± 1.72† | 7.38 ± 3.80 |
Data are expressed as mean ± SD. EF values were measured three times in each isolated heart. * P < 0.05 vs 13N-NH3 by Mann–Whitney test. † P < 0.01 vs 13N-NH3 by Mann–Whitney test. (Reprinted with permission of Kim et al. [34])
Limitations
Some limitations of radiolabeled TPP derivatives should be considered in view of the results so far achieved. First, although the results using radiolabeled TPP derivatives suggest that the agents used in the current preclinical studies may be suitable for clinical studies (because of the stable uptake and excellent pharmacokinetics), these results were obtained only in an experimental study using small animal models. Further preclinical application is being evaluated using a pig model that more closely resembles the human heart in its size, heart rate, myocardial blood flow, and mitochondrial density. Second, these results were limited to acute MI with permanent LCA ligation. This model is well-suited to determine myocardial defects but is not identical to the clinical situation in which hemodynamically relevant stenosis is unmasked by a stress-induced increase of myocardial blood flow. Further studies are needed to validate radiolabeled TPP derivatives for the detection of small areas of myocardial ischemia and scars from chronic infarctions.
Clinical Perspective and Conclusion
Despite numerous groundbreaking advances in the field, cardiovascular disease (CVD) remains the leading cause of mortality and morbidity worldwide [49, 50]. Cardiac PET imaging with novel radiotracer can display biological processes directly at the cellular and molecular level within the intact and living heart, and is becoming an indispensable tool in CVD diagnosis. The excellent performance of radiolabeled TPP derivatives as mitochondrial voltage sensors suggests their suitability for detecting CVD. This hypothesis is supported by previously reported studies that evaluated in vivo characterization of these agents in animal models. Among radiolabeled TPP derivatives, 18F-FATPs provided excellent cardiac PET image quality. In vivo characteristics of 18F-FATPs was significantly better than the current gold standard tracer, 13N-NH3. In addition, 18F-FATPs overcame the limitation to widespread use of tracers in clinical PET because the half-life of 18F is 109.8 min. Further studies are needed to find the best 18F-FATP derivative. Nevertheless, 18F-FATPs are promising 18F-labeled radiopharmaceuticals for the evaluation of myocardial perfusion by PET. The use of 18F-FATP will support high-throughput clinical protocols as well as the widespread application of PET MPI in hospitals that do not have a cyclotron.
Acknowledgment
This research was funded by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0163), and was supported in part by the National Research Foundation of Korea (NRF-2015M2B2A4031795 and NRF-2015M2B2A9031798)
Compliance with Ethical Standards
Conflict of Interest
Dong-Yeon Kim and Jung-Joon Min declare that they have no conflict of interest.
Ethical Statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Contributor Information
Dong-Yeon Kim, Email: blueburr@gmail.com.
Jung-Joon Min, Phone: 82-61-379-8476, Email: jjmin@jnu.ac.kr.
References
- 1.Small GR, Wells RG, Schindler T, Chow BJ, Ruddy TD. Advances in cardiac SPECT and PET imaging: overcoming the challenges to reduce radiation exposure and improve accuracy. Can J Cardiol. 2013;29:275–84. doi: 10.1016/j.cjca.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Camici PG, Rimoldi OE. The clinical value of myocardial blood flow measurement. J Nucl Med. 2009;50:1076–87. doi: 10.2967/jnumed.108.054478. [DOI] [PubMed] [Google Scholar]
- 3.Ohira H, Mc Ardle B, Cocker MS, Dekemp RA, Dasilva JN, Beanlands RS. Current and future clinical applications of cardiac positron emission tomography. Circ J. 2013;77:836–48. doi: 10.1253/circj.CJ-13-0213. [DOI] [PubMed] [Google Scholar]
- 4.Fovino LN, Saladini G, Mormino GP, Saladini F, Razzolini R, Evangelista L. Risk stratification and prognostic assessment by myocardial perfusion-gated SPECT in patients with left bundle-branch block and low-intermediate cardiac risk. Ann Nucl Med. 2012;26:559–70. doi: 10.1007/s12149-012-0613-4. [DOI] [PubMed] [Google Scholar]
- 5.Schwaiger M, Melin J. Cardiological applications of nuclear medicine. Lancet. 1999;354:661–6. doi: 10.1016/S0140-6736(99)06057-2. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons RJ, Valeti US, Araoz PA, Jaffe AS. The quantification of infarct size. J Am Coll Cardiol. 2004;44:1533–42. doi: 10.1016/j.jacc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 7.Huisman MC, Higuchi T, Reder S, Nekolla SG, Poethko T, Wester HJ, et al. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49:630–6. doi: 10.2967/jnumed.107.044727. [DOI] [PubMed] [Google Scholar]
- 8.Knuuti J, Bengel FM. Positron emission tomography and molecular imaging. Heart. 2008;94:360–7. doi: 10.1136/hrt.2007.118992. [DOI] [PubMed] [Google Scholar]
- 9.Siegrist PT, Husmann L, Knabenhans M, Gaemperli O, Valenta I, Hoefflinghaus T, et al. 13N-Ammonia myocardial perfusion imaging with a PET/CT scanner: impact on clinical decision making and cost-effectiveness. Eur J Nucl Med Mol Imaging. 2008;35:889–95. doi: 10.1007/s00259-007-0647-3. [DOI] [PubMed] [Google Scholar]
- 10.Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol. 2007;49:1052–8. doi: 10.1016/j.jacc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Guaraldi MT, Mistry M, Kagan M, McDonald JL, Drew K, et al. BMS-747158-02: a novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14:789–98. doi: 10.1016/j.nuclcard.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Summerhayes IC, Lampidis TJ, Bernal SD, Nadakavukaren JJ, Nadakavukaren KK, Shepherd EL, et al. Unusual retention of rhodamine 123 by mitochondria in muscle and carcinoma cells. Proc Natl Acad Sci U S A. 1982;79:5292–6. doi: 10.1073/pnas.79.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LB. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–81. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi T, Fukushima K, Rischpler C, Isoda T, Javadi MS, Ravert H, et al. Stable delineation of the ischemic area by the PET perfusion tracer 18F-fluorobenzyl triphenyl phosphonium after transient coronary occlusion. J Nucl Med. 2011;52:965–9. doi: 10.2967/jnumed.110.085993. [DOI] [PubMed] [Google Scholar]
- 15.Madar I, Ravert H, Dipaula A, Du Y, Dannals RF, Becker L. Assessment of severity of coronary artery stenosis in a canine model using the PET agent 18F-fluorobenzyl triphenyl phosphonium: comparison with 99mTc-tetrofosmin. J Nucl Med. 2007;48:1021–30. doi: 10.2967/jnumed.106.038778. [DOI] [PubMed] [Google Scholar]
- 16.Madar I, Ravert H, Nelkin B, Abro M, Pomper M, Dannals R, et al. Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur J Nucl Med Mol Imaging. 2007;34:2057–65. doi: 10.1007/s00259-007-0500-8. [DOI] [PubMed] [Google Scholar]
- 17.Min JJ, Biswal S, Deroose C, Gambhir SS. Tetraphenylphosphonium as a novel molecular probe for imaging tumors. J Nucl Med. 2004;45:636–43. [PubMed] [Google Scholar]
- 18.Murphy MP. Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol. 1997;15:326–30. doi: 10.1016/S0167-7799(97)01068-8. [DOI] [PubMed] [Google Scholar]
- 19.Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 2003;304:433–5. doi: 10.1016/S0006-291X(03)00614-4. [DOI] [PubMed] [Google Scholar]
- 20.Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 2005;70:222–30. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda H, Syrota A, Charbonneau P, Vallois J, Crouzel M, Prenant C, et al. Use of 11C-triphenylmethylphosphonium for the evaluation of membrane potential in the heart by positron-emission tomography. Eur J Nucl Med. 1986;11:478–83. doi: 10.1007/BF00252793. [DOI] [PubMed] [Google Scholar]
- 22.Madar I, Anderson JH, Szabo Z, Scheffel U, Kao PF, Ravert HT, et al. Enhanced uptake of 11C-TPMP in canine brain tumor: a PET study. J Nucl Med. 1999;40:1180–5. [PubMed] [Google Scholar]
- 23.Cheng Z, Subbarayan M, Chen X, Gambhir SS. Synthesis of (4-18F-fluorophenyl)triphenylphosphonium as a potential imaging agent for mitochondrial dysfunction. J Label Compd Radiopharm. 2005;48:131–7. doi: 10.1002/jlcr.906. [DOI] [Google Scholar]
- 24.Kim D, Yu K, Bom H, Min J. Synthesis of (4-18F-Fluorophenyl)triphenylphosphonium as a mitochondrial voltage sensor for PET. Nucl Med Mol Imaging. 2007;41:561–5. [Google Scholar]
- 25.Shoup TM, Elmaleh DR, Brownell AL, Zhu A, Guerrero JL, Fischman AJ. Evaluation of (4-18F-fluorophenyl)triphenylphosphonium ion. A potential myocardial blood flow agent for PET. Mol Imaging Biol. 2011;13:511–7. doi: 10.1007/s11307-010-0349-2. [DOI] [PubMed] [Google Scholar]
- 26.Madar I, Isoda T, Finley P, Angle J, Wahl R. 18F-Fluorobenzyl triphenyl phosphonium: a noninvasive sensor of brown adipose tissue thermogenesis. J Nucl Med. 2011;52:808–14. doi: 10.2967/jnumed.110.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madar I, Ravert HT, Du Y, Hilton J, Volokh L, Dannals RF, et al. Characterization of uptake of the new PET imaging compound 18F-fluorobenzyl triphenyl phosphonium in dog myocardium. J Nucl Med. 2006;47:1359–66. [PubMed] [Google Scholar]
- 28.Kim DY, Kim HJ, Yu KH, Min JJ. Synthesis of 18F-labeled (2-(2-fluoroethoxy)ethyl)triphenylphosphonium cation as a potential agent for myocardial imaging using positron emission tomography. Bioorg Med Chem Lett. 2012;22:319–22. doi: 10.1016/j.bmcl.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Kim DY, Kim HJ, Yu KH, Min JJ. Synthesis of 18F-labeled (6-fluorohexyl)triphenylphosphonium cation as a potential agent for myocardial imaging using positron emission tomography. Bioconjug Chem. 2012;23:431–7. doi: 10.1021/bc2004439. [DOI] [PubMed] [Google Scholar]
- 30.Kim DY, Kim HJ, Yu KH, Min JJ. Synthesis of 18F-labeled (2-(2-fluoroethoxy)ethyl)tris(4-methoxyphenyl)phosphonium cation as a potential agent for positron emission tomography myocardial imaging. Nucl Med Biol. 2012;39:1093–8. doi: 10.1016/j.nucmedbio.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Kim DY, Kim HS, Le UN, Jiang SN, Kim HJ, Lee KC, et al. Evaluation of a mitochondrial voltage sensor, (18F-fluoropentyl)triphenylphosphonium cation, in a rat myocardial infarction model. J Nucl Med. 2012;53:1779–85. doi: 10.2967/jnumed.111.102657. [DOI] [PubMed] [Google Scholar]
- 32.Kim DY, Kim HS, Min JJ. Radiosynthesis and evaluation of 18F-labeled aliphatic phosphonium cations as a myocardial imaging agent for positron emission tomography. Nucl Med Commun. 2015;36:747–54. doi: 10.1097/MNM.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 33.Kim DY, Kim HS, Jang HY, Kim JH, Bom HS, Min JJ. Comparison of the cardiac microPET images obtained using 18F-FPTP and 13N-NH3 in rat myocardial infarction models. ACS Med Chem Lett. 2014;5:1124–8. doi: 10.1021/ml500251z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DY, Kim HS, Reder S, Zheng JH, Herz M, Higuchi T, et al. Comparison of 18F-labeled fluoroalkylphosphonium cations with 13N-NH3 for PET myocardial perfusion imaging. J Nucl Med. 2015;56:1581–5. doi: 10.2967/jnumed.115.156794. [DOI] [PubMed] [Google Scholar]
- 35.Kim YS, Yang CT, Wang J, Wang L, Li ZB, Chen X, et al. Effects of targeting moiety, linker, bifunctional chelator, and molecular charge on biological properties of 64Cu-labeled triphenylphosphonium cations. J Med Chem. 2008;51:2971–84. doi: 10.1021/jm7015045. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Liu S. 64Cu-labeled phosphonium cations as PET radiotracers for tumor imaging. Bioconjug Chem. 2011;22:1459–72. doi: 10.1021/bc200106p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravert HT, Madar I, Dannals RF. Radiosynthesis of 3-18F-fluoropropyl and 4-18F-fluorobenzyl triarylphosphonium ions. J Label Compd Radiopharm. 2004;47:469–76. doi: 10.1002/jlcr.835. [DOI] [Google Scholar]
- 38.Demura M, Kamo N, Kobatake Y. Binding of lipophilic cations to the liposomal membrane: thermodynamic analysis. Biochim Biophys Acta. 1987;820:303–8. doi: 10.1016/0005-2736(87)90220-3. [DOI] [PubMed] [Google Scholar]
- 39.Ono A, Miyauchi S, Demura M, Asakura T, Kamo N. Activation energy for permeation of phosphonium cations through phospholipid bilayer membrane. Biochemistry. 1994;33:4312–8. doi: 10.1021/bi00180a027. [DOI] [PubMed] [Google Scholar]
- 40.Rieke RD, White CK, Milliren CM. Electrochemical and electron paramagnetic resonance studies of a series of ammonium and phosphonium compounds. J Am Chem Soc. 1976;98:6872–7. doi: 10.1021/ja00438a018. [DOI] [Google Scholar]
- 41.Krause BJ, Szabo Z, Becker LC, Dannals RF, Scheffel U, Seki C, et al. Myocardial perfusion with 11C-methyl triphenyl phosphonium: measurements of the extraction fraction and myocardial uptake. J Nucl Biol Med. 1994;38:521–6. [PubMed] [Google Scholar]
- 42.Nakajima K, Taki J, Shuke N, Bunko H, Takata S, Hisada K. Myocardial perfusion imaging and dynamic analysis with technetium-99m tetrofosmin. J Nucl Med. 1993;34:1478–84. [PubMed] [Google Scholar]
- 43.Nuyts J, Dupont P, Van den Maegdenbergh V, Vleugels S, Suetens P, Mortelmans L. A study of the liver-heart artifact in emission tomography. J Nucl Med. 1995;36:133–9. [PubMed] [Google Scholar]
- 44.Kailasnath P, Sinusas AJ. Comparison of Tl-201 with Tc-99m-labeled myocardial perfusion agents: technical, physiologic, and clinical issues. J Nucl Cardiol. 2001;8:482–98. doi: 10.1067/mnc.2001.115078. [DOI] [PubMed] [Google Scholar]
- 45.Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff---still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55:113–26. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Croteau E, Benard F, Bentourkia M, Rousseau J, Paquette M, Lecomte R. Quantitative myocardial perfusion and coronary reserve in rats with 13N-ammonia and small animal PET: impact of anesthesia and pharmacologic stress agents. J Nucl Med. 2004;45:1924–30. [PubMed] [Google Scholar]
- 47.Leppo JA, Meerdink DJ. Comparison of the myocardial uptake of a technetium-labeled isonitrile analogue and thallium. Circ Res. 1989;65:632–9. doi: 10.1161/01.RES.65.3.632. [DOI] [PubMed] [Google Scholar]
- 48.Mullani NA, Goldstein RA, Gould KL, Marani SK, Fisher DJ, O’Brien HA, Jr, et al. Myocardial perfusion with rubidium-82. I. Measurement of extraction fraction and flow with external detectors. J Nucl Med. 1983;24:898–906. [PubMed] [Google Scholar]
- 49.Knapper JT, Ghasemzadeh N, Khayata M, Patel SP, Quyyumi AA, Mendis S, et al. Time to change our focus: defining, promoting, and impacting cardiovascular population health. J Am Coll Cardiol. 2015;66:960–71. doi: 10.1016/j.jacc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Du W, Tao H, Zhao S, He ZX, Li Z. Translational applications of molecular imaging in cardiovascular disease and stem cell therapy. Biochimie. 2015;116:43–51. doi: 10.1016/j.biochi.2015.06.021. [DOI] [PubMed] [Google Scholar]